Introduction
Balo’s Concentric Sclerosis is a fulminant type of Multiple Sclerosis which is an autoimmune disorder. It causes demyelination and neuronal degeneration via autoantibodies [1]. Multiple sclerosis was first defined by Jean-Martin Charcot, a neurologist [2]. It forms alternate demyelinating and remyelinating lesions, giving it an “onion ring” look in MRI [3]. Demyelination causes formation of oligoclonal bands which can be seen by MRI. Increased IgG levels is also a diagnostic finding of Multiple Sclerosis [4]. It can occur in any neuronal fibre at any time. Symptoms are generally relapsing and remitting and it can be exaggerated by increased temperature. Hot showers or exercise can lead to Multiple sclerosis attacks. It is most commonly seen in women of 20-30 years [5] and in whites.
Wolff-Parkinson-White Syndrome is a heart condition occurring due to formation of accessory pathway abnormality leading to arrhythmia [6]. It is generally presented with tachycardia. It can even be asymptomatic in some patients. It is diagnosed by the presence of Delta waves in ECG [6]. Formation of accessory pathways disrupts the regular rhythm of the heart's electrical impulse conduction. It leads to transmission of fast impulse before the original slow impulse from atria to ventricles. It can be diagnosed via an ECG or Electrophysiological studies.
Case Presentation
This is the case presentation of a 38-year-old male patient who came to the hospital with complaints of numbness in the left-sided upper and lower limbs, slippage of slippers from the foot, slurring of speech and left-sided headache for 15-20 days. The patient did not have vision loss, loss of consciousness, giddiness, fever, cough, cold chest pain, or decreased urine output. On further questioning the patient gave the history of left upper limb weakness 5 years back.
Physical Examination
Physical examination showed normal temperature, pulse rate of 120/min, blood pressure of 118/78, and SpO2 98% on room air. Respiratory examination showed a respiratory rate of 18/min and bilateral air entry was present and clear. S1S2 were present on cardiovascular examination. The patient was conscious and oriented to person, place and time. Pupil was bilaterally reactive to light. Power in both the upper limb was 5/5 and each lower limb was 4/4 with normal tone in all the limbs. The plantar reflex was flexed in both legs.
History of Past Illness
The patient had no relevant previous medical history.
Laboratory Investigation
Lab investigations include CBC, which showed haemoglobin count of 14, WBC count of 7000 and platelet count of 2.05 lakh. In liver function test (LFT) SGPT score was 16. Renal function test (RFT) showed creatinine- 1.08, sodium- 140, potassium- 4.04, ionized calcium- 1.14. The patient’s IgG test was also positive. CSF examination showed R/M 4 cells, 10% Polymorphonuclear cells and 90% Lymphocytes, a protein level of 57 mg/dl and a glucose level of 64 mg/dl.
| CBC |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| HEMOGLOBIN |
14 |
mg/dl |
12.3–17 mg/dL |
| WBC |
7000 |
cells/μL |
4,500 to 11,000 cells/μL |
| PLATELET |
2.05 |
lakh/μL |
1.5-4.5 cells/μL |
| |
| LFT |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| SGPT |
16 |
units/liter |
7-56 units/liter |
| |
| RFT |
OBSERVED VALUE |
UNITS |
REFERENCE RANGE |
| CREATININE |
1.08 |
mg/dl |
0.74 to 1.35 mg/dL |
| SODIUM |
140 |
mmol/L |
135-145 mmol/L |
| POTASSIUM |
4.04 |
mmol/L |
3.5-5 mmol/L |
| IONISED CALCIUM |
1.14 |
mmol/L |
2-2.6 mmol/L |
Electrocardiogram (ECG) Report
The patient’s ECG showed a normal sinus rhythm with a heart rate of 84 beats per minute. There were no significant ST-T wave changes or arrhythmias noted. ‘Delta wave’ was detected along with narrowed PR intervals and widened QRS complexes on ECG. This gives evidence that the patient is suffering from Wolff Parkinson White syndrome. Additionally, there were no signs of conduction abnormalities, suggesting a generally healthy cardiac status.
Figure 1.
ECG showing Delta Wave.
Figure 1.
ECG showing Delta Wave.
Radiological Imaging
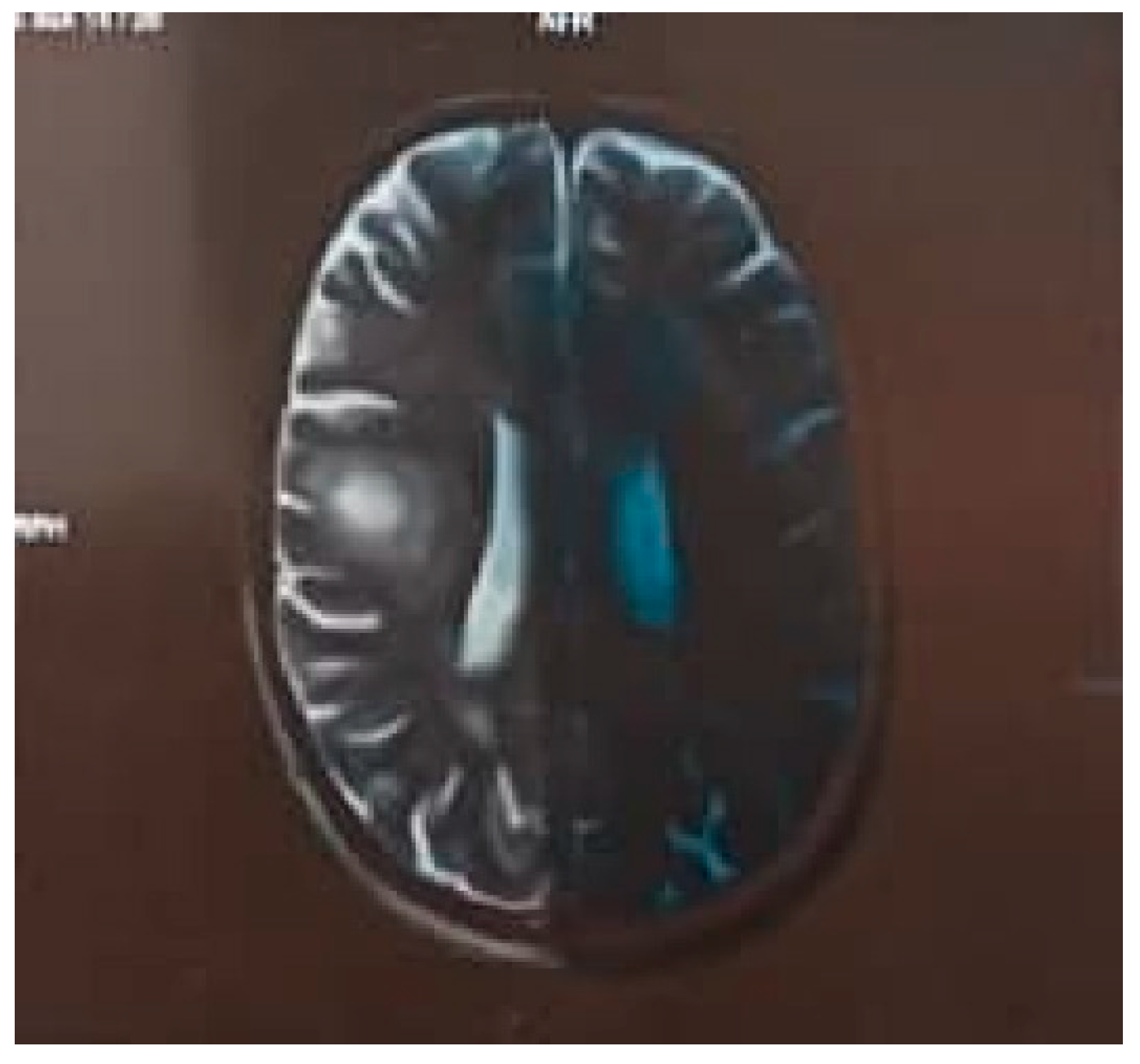
MRI brain findings showed a few well-defined non-enhancing intra-axial altered signal intensity lesions at calloso-septal interface adjacent to both lateral ventricles (left > right), juxtacortical aspect of bilateral high frontal, occipital and postero-lateral portion of both temporal lobes right middle cerebellar peduncle showing patchy area of peripheral diffusion restriction at few places with mild peri-lesional white matter oedema without significant ventricular compression or midline shift.
The largest lesion in the right supra-sylvian frontal lobe is 13 x 13 mm and in the left anterior frontal periventricular white matter is 13 x 12.5 mm. Mild cerebral atrophy is noted. The rest of the cerebral parenchyma appears normal.
The cerebellum, brainstem structures and pituitary gland are normal. Susceptibility-weighted images do not reveal any "blooming" to suggest intracranial haemorrhage evident bony or vascular abnormality is seen. Cervical-medullary junction does not reveal any abnormality. Screening of both orbits reveals no evident intrinsic hyperintensity in either optic nerves or optic chiasma. Findings s/p/o Demyelinating Etiology- Balo’s concentric sclerosis likely.
Figure 2.
MRI Brain Axial Section Showing Onion peel Rings Appearance.
Figure 2.
MRI Brain Axial Section Showing Onion peel Rings Appearance.
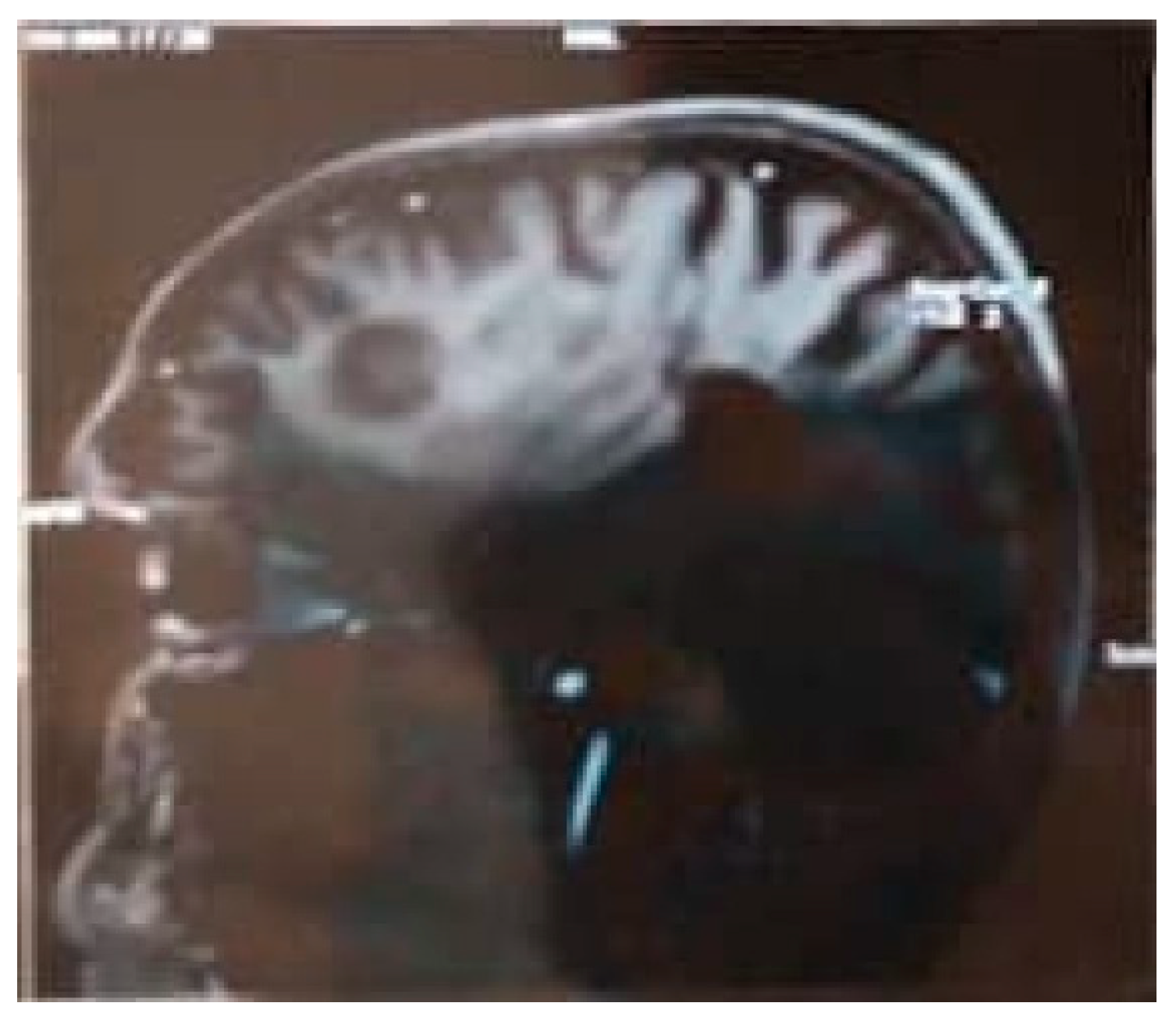
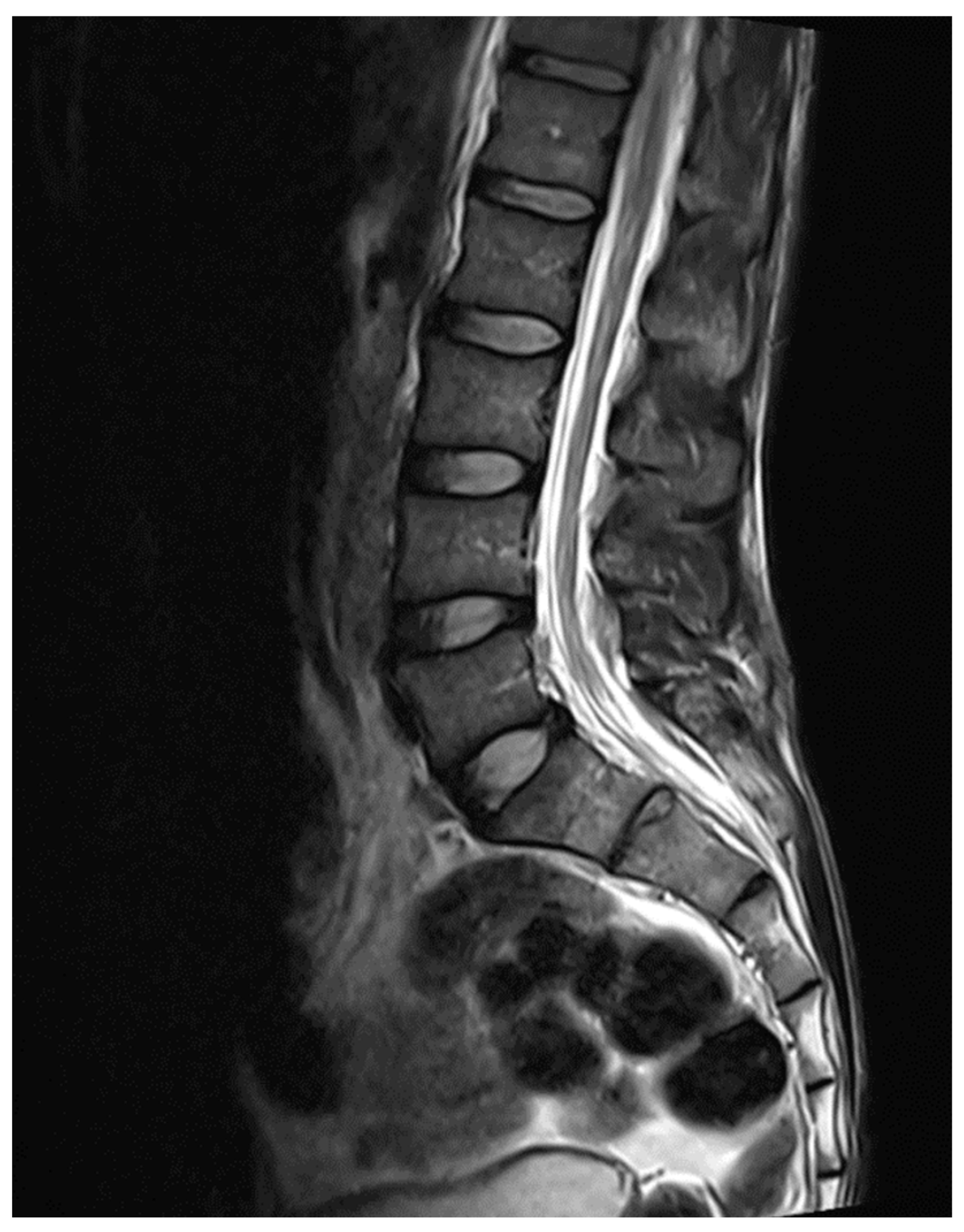
Figure 3.
MRI Brain Sagittal Section.
Figure 3.
MRI Brain Sagittal Section.
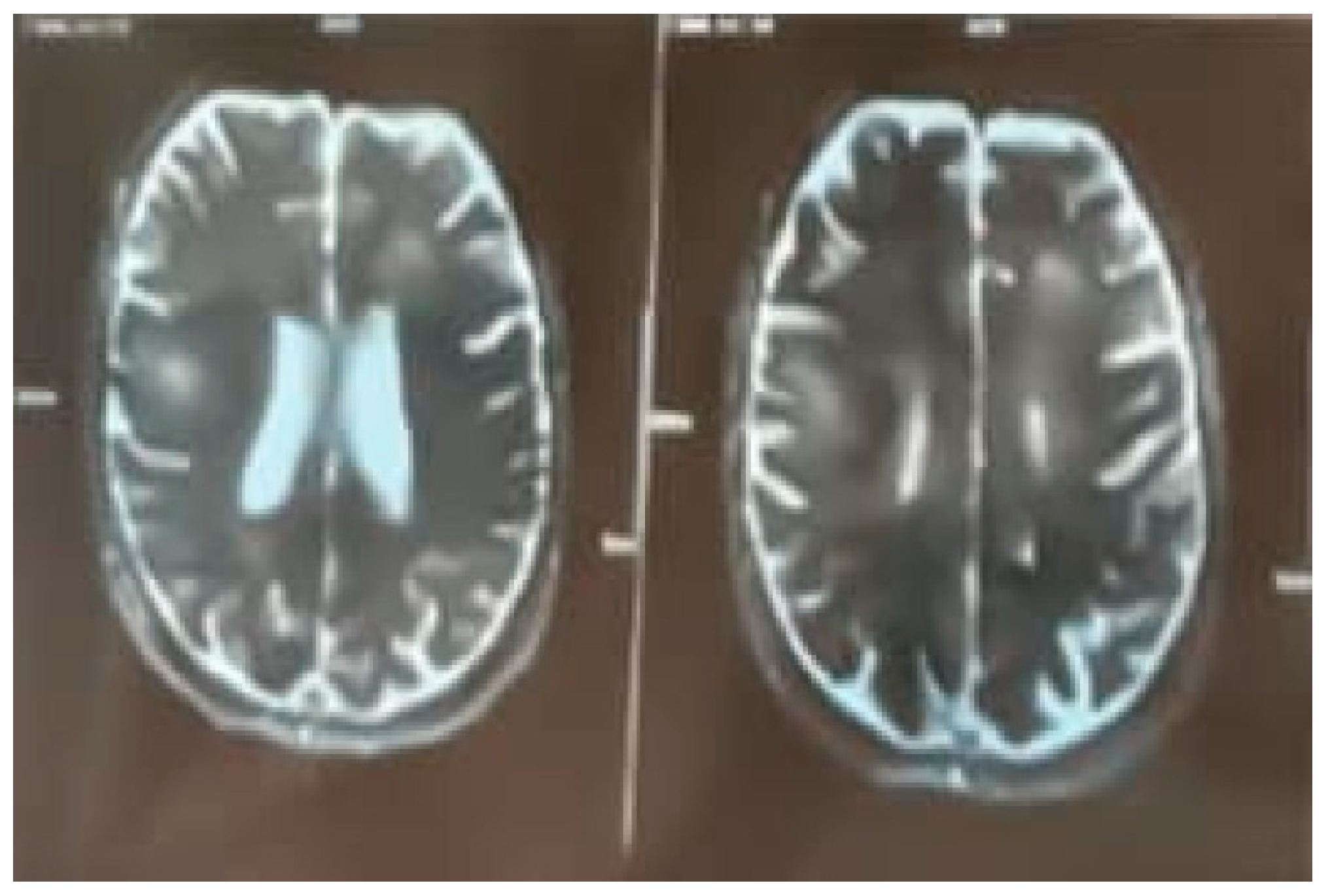
Figure 4.
MRI Brain Axial Section.
Figure 4.
MRI Brain Axial Section.
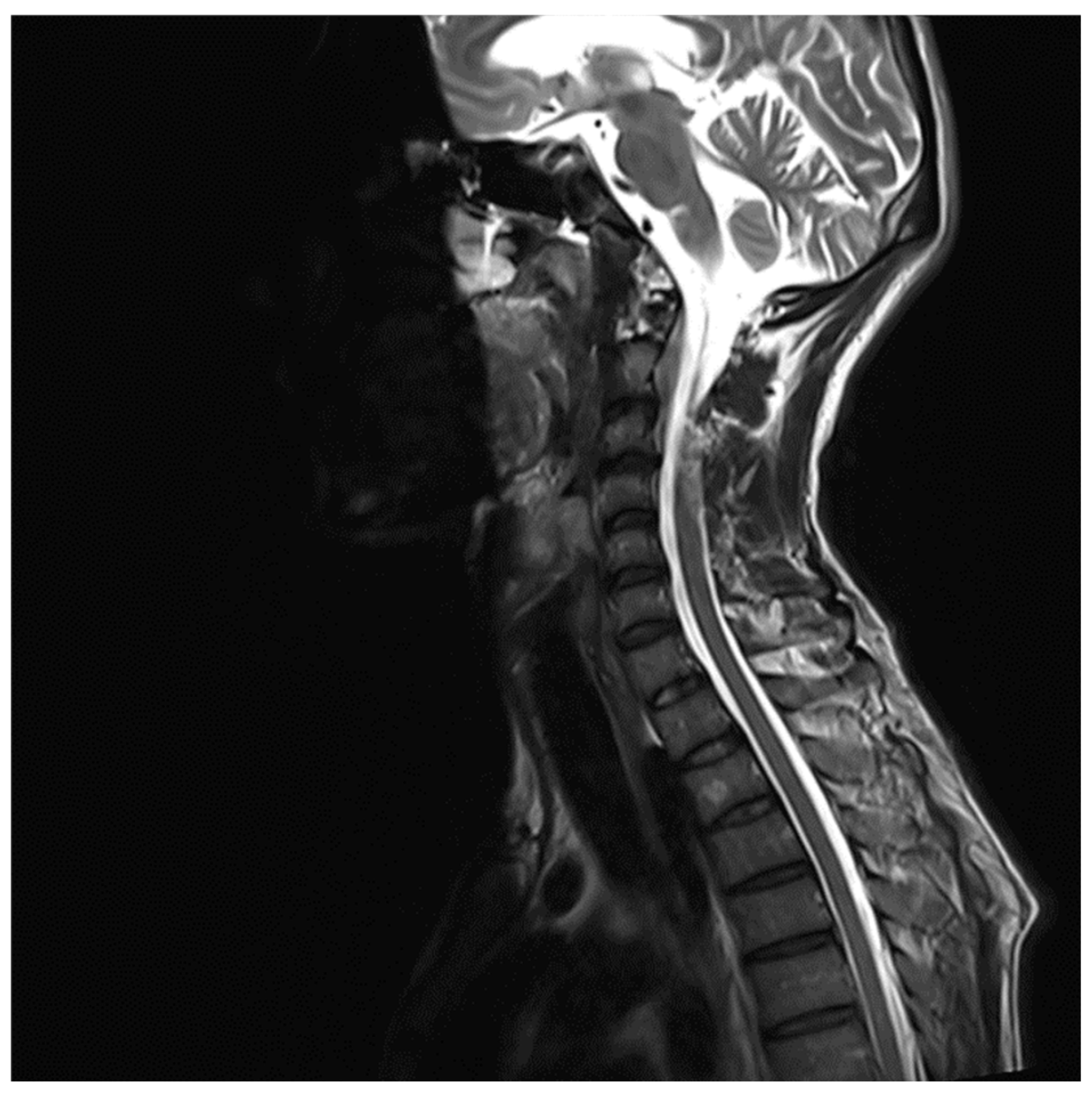
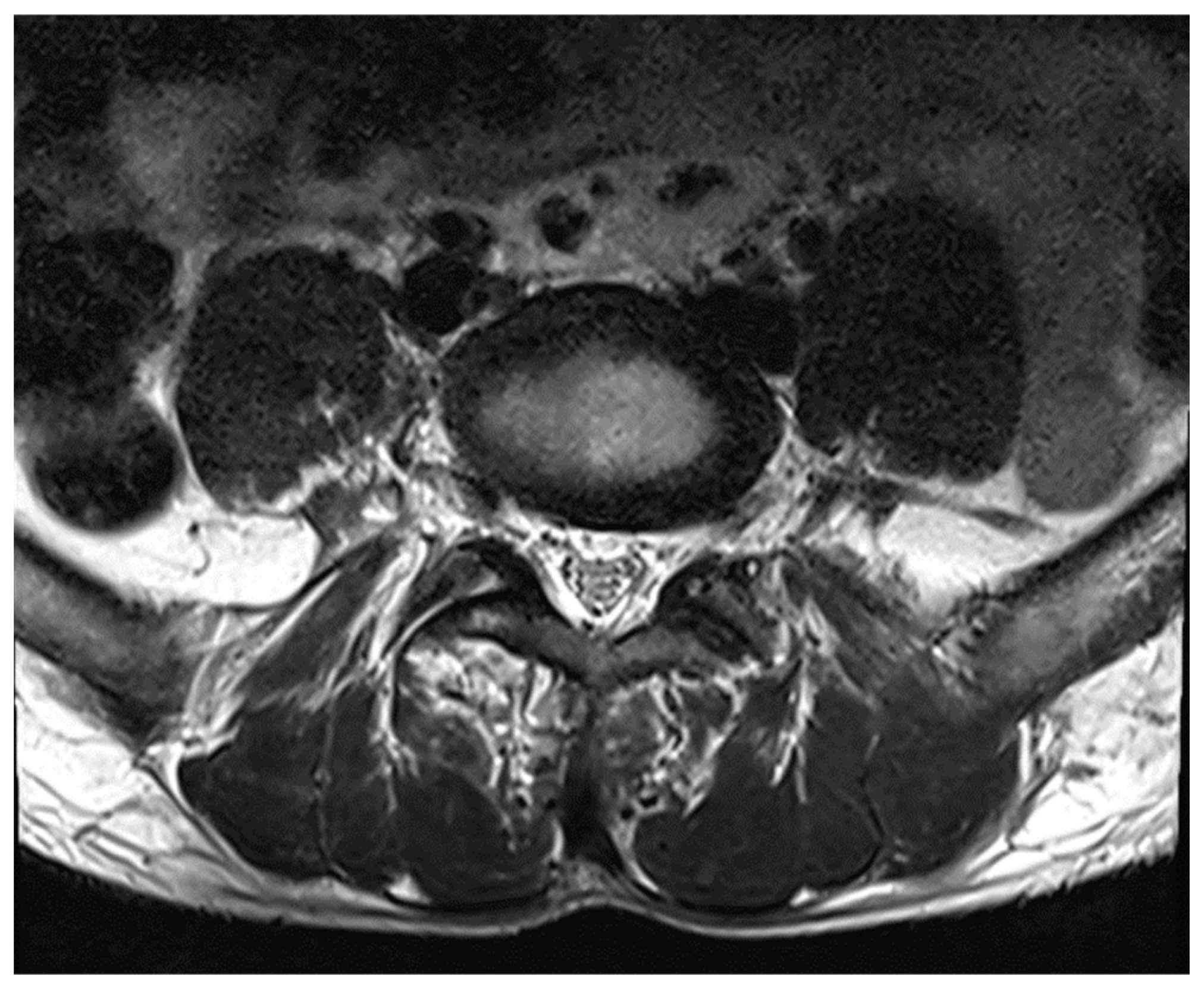
MRI whole spine screening showed early disc desiccation at upper cervical vertebral levels. Haemangioma was noted in the body of the D2 vertebra. C3-C4 level showed disc osteophytes complex causing indentation over anterior thecal sac without causing significant nerve root compression. Repeated MRI Spine showed an abutment over the right exiting nerve root at the above-mentioned level. L5-S1 level showed diffuse disc bulge causing bilateral lateral recess stenosis, neural foraminal narrowing and indentation over the anterior thecal sac with resultant abutment over bilateral exiting nerve roots. Above-stated findings give evidence of demyelinating Balo’s Concentric Sclerosis.
Figure 5.
MRI Spine C3-C4 level.
Figure 5.
MRI Spine C3-C4 level.
Figure 6.
MRI spine L5-S1 level.
Figure 6.
MRI spine L5-S1 level.
Figure 7.
MRI Spine Axial Section.
Figure 7.
MRI Spine Axial Section.
Discussion
Balo’s concentric sclerosis (BCS) is a subtype of Multiple Sclerosis(MS), which is a chronic inflammatory demyelinating disorder of the central nervous system. It causes gliosis and neuronal loss and can be relapsing or progressive. BCS is a concentric white matter lesion with alternating spheres of demyelination and remyelination.
Multiple sclerosis, an autoimmune disease that leads to demyelination and neuronal degeneration that damages the white matter. It most often affects females than males in their 20-30 years of age. Inflammation damages and increases permeability of blood brain barrier. T-lymphocytes, mainly cytotoxic CD 8 T cells, are the predominant cells that enter CNS via damaged blood brain barrier and cross reacts with myelin oligodendrocyte glycoprotein forming autoantibodies [7]. Autoantibodies causes demyelination of axons and plaque formation. It disrupts the saltatory pathway of neuronal transmission and deteriorates its functions. New plaques can be formed at different time and place.
Multiple sclerosis can be relapsing or progressive. 90% of Multiple sclerosis is mainly relapsing type. It is characterized by bouts of neurological attacks that resolve over days [8]. Over time it can change its clinical course to progressive type, known as Secondary Progressive Multiple Sclerosis (SPMS). SPMS leads to progressive deterioration of neuronal functions [9]. Primary Progressive Multiple Sclerosis (PPMS) accounts for only around 10% of cases. In PPMS there is a progressive decline in neuronal function from its onset. Patients are generally asymptomatic [10].
Multiple Sclerosis occurs due to both genetic as well as environmental causes. Familial causes are due to genetic predisposition. Multiple sclerosis is a polygenic disorder associated with HLA-DR1 and HLA-DR15A (HLA-DR2) [12].
Multiple sclerosis can damage both sensory and motor neurons. Most common symptoms are fatigue, depression, constipation. Around 90% of patients feel fatigue. Neuronal attacks can be felt for around 10s to 2 mins. It can be of different frequency. Patients can also lose consciousness during attacks. It can be precipitated by movement or change in temperature.
Symptoms are mainly associated with the nerve which is damaged. Optic neuritis is usually seen in Multiple sclerosis patients which occurs due to damage to the optic nerve [11]. It is painful vision loss and generally unilateral but can be bilateral. Other symptoms include trigeminal neuralgia associated with trigeminal nerve, sensory symptoms like paraesthesia, multifocal pain and motor symptoms like spastic weakness, ataxia. Damage to medial longitudinal fasciculus causes internuclear ophthalmoplegia whereas damage to 6th cranial nerve along with MLF lesion causes one and a half syndrome. UMNL can lose inhibitory control of bladder leading to detrusor hyper-reflexia or detrusor sphincter dyssynergia causing urine incontinence or urine retention respectively [12]. All the signs and symptoms are relapsing and remitting over time.
Investigation of choice is gadolinium enhanced MRI which shows dawson fingers (perivenous demyelination) in white matter. Lesions >6mm in white matter is diagnostic of MS. MRI shows an “onion ring” lesion in balo’s concentric sclerosis [13].
Other diagnostic methods are, Evoked Potential and Cerebrospinal Fluid analysis. EP testing studies electric potentials of a nerve when repeatedly stimulated. CSF analysis shows increased levels of IgG immunoglobulins, pleocytosis. Electrophoresis of CSF fluid also measures oligoclonal bands useful in diagnosis of Multiple sclerosis [14].
Glucocorticoids are mainly the starting drug in MS. It is usually used during a first or acute attack. Disease modifying drugs are β-interferon, glatiramer, S1P receptor modulator, rituximab (anti CD-20 monoclonal antibodies), Natalizumab [13]. Natalizumab is highly effective and it reduces attacks and slows down the progression of disease.
Wolff-Parkinson White syndrome is an accessory pathway disorder of the heart. Accessory Pathway (Bundle of Kent) causes abnormal fast accessory conduction from atria to ventricles. It bypasses the rate slowing pathway and causes an early depolarization of ventricles [7].
Wolff-Parkinson-White (WPW) syndrome is a pathological accessory pathway formation disorder. Physiologically, the SA node in heart generates an impulse which travels to AV node, Bundle of His and Purkinje fibres. During this transmission, the pathway from the AV node to the Bundle of His is rate slowing pathway. In WPW, an accessory pathway called Bundle of Kent, bypasses these rate limiting pathways and generates an extra impulse disrupting a normal heart conduction.
Most common symptoms of WPW are palpitations, tachycardia, dizziness, chest pain. Patients can also faint due to arrhythmia.
WPW can be diagnosed mainly by a cardiogram study. Presence of ‘Delta waves’ and widened QRS complexes is a characteristic feature of WPW. It can also be diagnosed by Holter method, a portable ECG device method that records heart activity for a longer duration or an electrophysiology study. EP study is an invasive procedure in which a catheter is placed inside the heart to identify the location of accessory pathway.
WPW can be treated by catheter ablation which is the most common and effective treatment. Antiarrhythmic drugs, beta-blockers or adenosine can also be used for its treatment in patients for whom catheter ablation is not an option.
Conclusion
This case reports showed Balo’s Concentric sclerosis, a rare brain disorder and Wolf-Parkinson-White syndrome, a heart disorder coexisting. This medical condition though unrelated, existing in same patients at a time present complex challenges for diagnosis and treatment. Balo’s concentric sclerosis causes demyelination leading to brain lesions while WPW syndrome affects heart’s electrical signals. This case report emphasis on medical research and proper care in rare medical combinations to provide better treatments for patients with multiple coexisting health conditions.
References
- J. S. Xie, T. Jeeva-Patel, and E. Margolin, “Baló’s concentric sclerosis – A rare entity within the spectrum of demyelinating diseases,” J Neurol Sci 2021, 428, 117570. [CrossRef]
- B. Zalc, “One hundred and fifty years ago Charcot reported multiple sclerosis as a new neurological disease,” Brain 2018, 141, 3482–3488. [CrossRef]
- S. Arif, M. W. Wali, A. U. R. Slehria, H. Khalid, and H. Malik, “ONION PEEL APPEARANCE IN BALOS CONCENTRIC SCLEROSIS--A VARIANT OF MULTIPLE SCLEROSIS.,” J Ayub Med Coll Abbottabad 2015, 27, 236–8.
- Y. Zheng et al., “IgG Index Revisited: Diagnostic Utility and Prognostic Value in Multiple Sclerosis,” Front Immunol 2020, 11. [CrossRef]
- M. P. McGinley, C. H. Goldschmidt, and A. D. Rae-Grant, “Diagnosis and Treatment of Multiple Sclerosis,” JAMA 2021, 325, 765. [CrossRef]
- H. A. Mahamat, S. Jacquir, C. Khalil, G. Laurent, and S. Binczak, “Wolff-Parkinson-White (WPW) syndrome: The detection of delta wave in an electrocardiogram (ECG),” in 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, Aug. 2016, pp. 3809–3812. [CrossRef]
- J. Goverman, “Autoimmune T cell responses in the central nervous system,” Nat Rev Immunol 2009, 9, 393–407. [CrossRef]
- M. M. Goldenberg, “Multiple sclerosis review.,” P T 2012, 37, 175–84.
- B. A. C. Cree et al., “Secondary Progressive Multiple Sclerosis,” Neurology 2021, 97, 378–388. [CrossRef]
- D. H. Miller and S. M. Leary, “Primary-progressive multiple sclerosis,” Lancet Neurol 2007, 6, 903–912. [CrossRef]
- N. Kale, “Optic neuritis as an early sign of multiple sclerosis,” Eye Brain 2016, 8, 195–202. [CrossRef]
- S. J. Kumar and D. A. Biswas, “Anatomical Aspects of Neurogenic Bladder and the Approach in Its Management: A Narrative Review,” Cureus 2022. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).