Submitted:
22 October 2023
Posted:
24 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
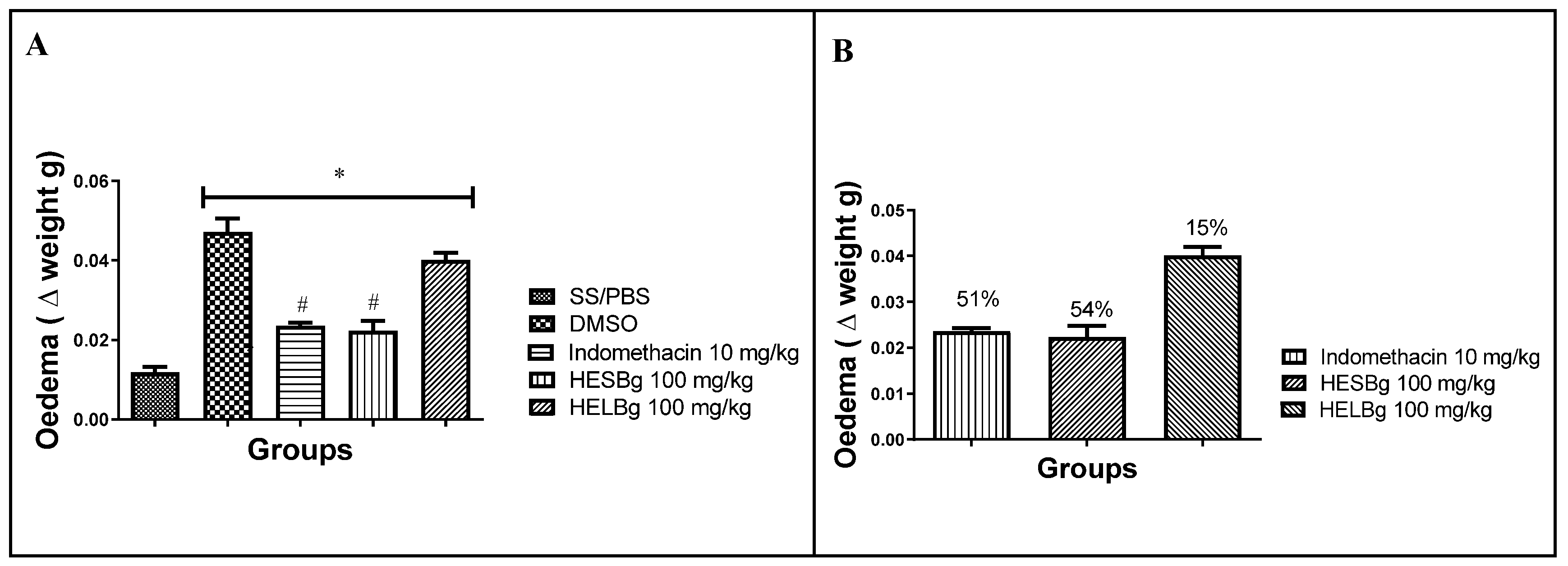
2.1. Anti-inflammatory potential of HESBg and HELBg in the inhibition of edema by carrageenan in Danio rerio
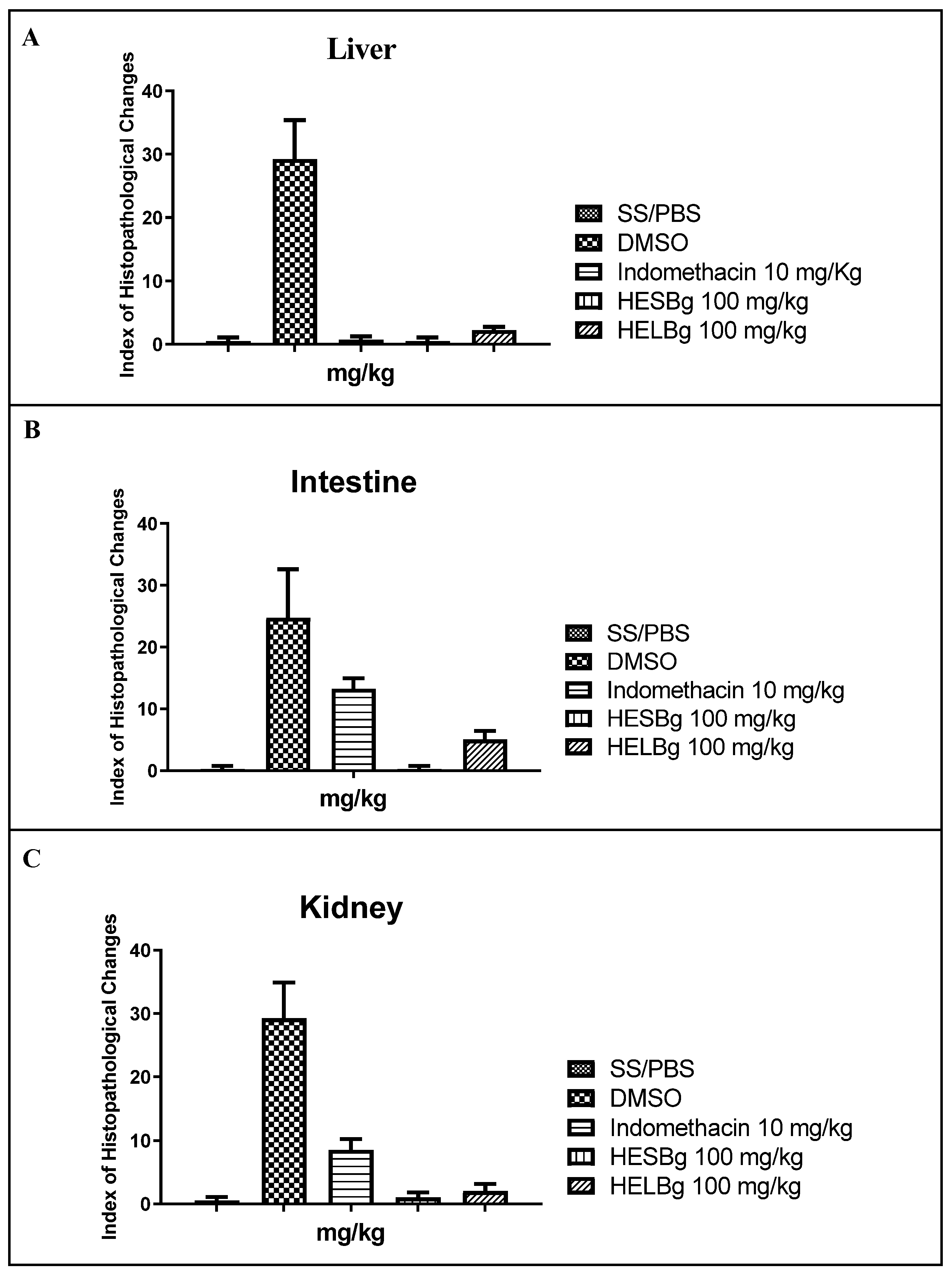
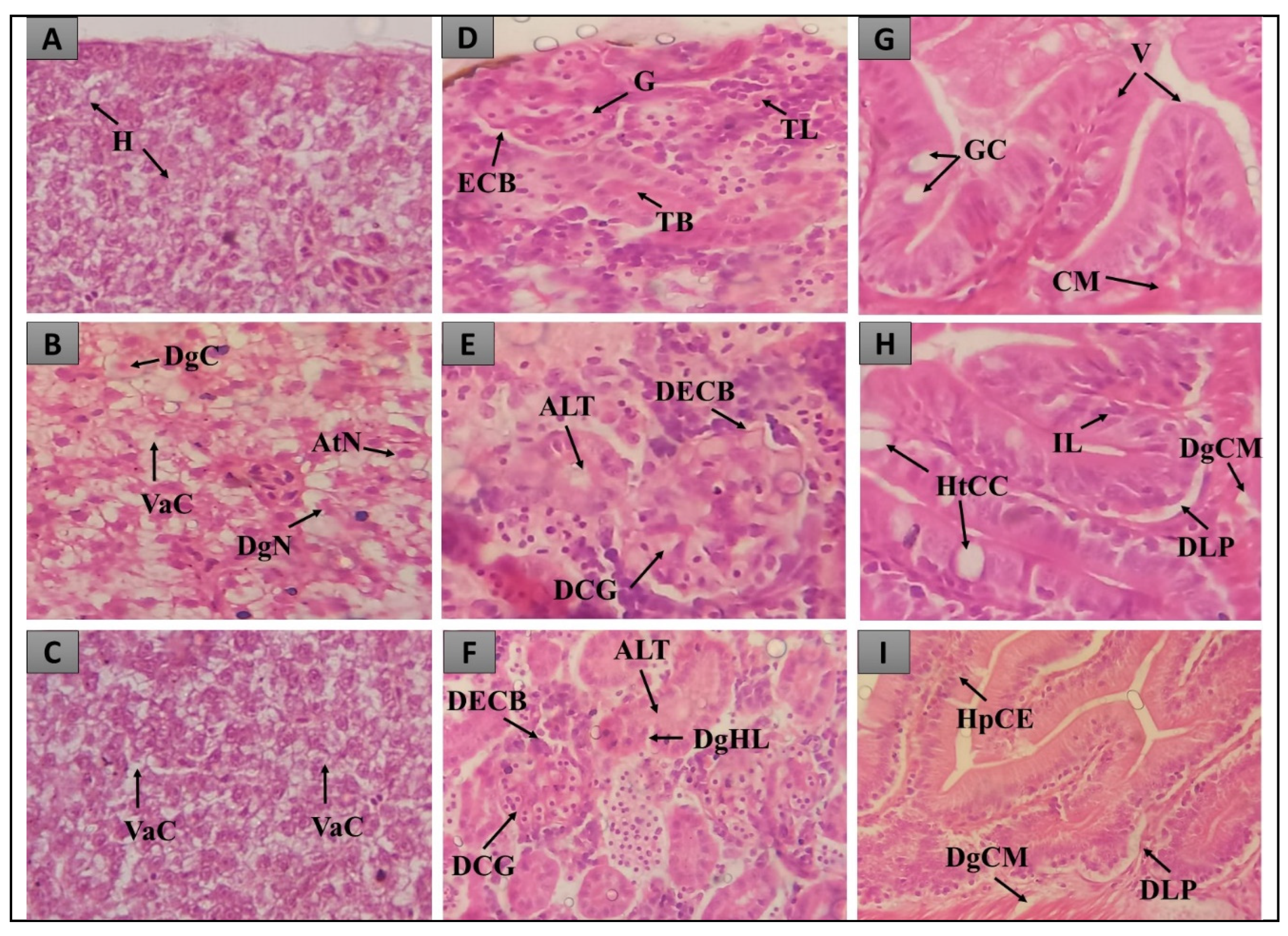
2.2. Histopathological evaluation of the inflammation test in Danio rerio
2.2.1. Histopathological evaluation of the liver
2.2.2. Histopathological evaluation of the intestine
2.2.3. Histopathological evaluation of the Kidneys
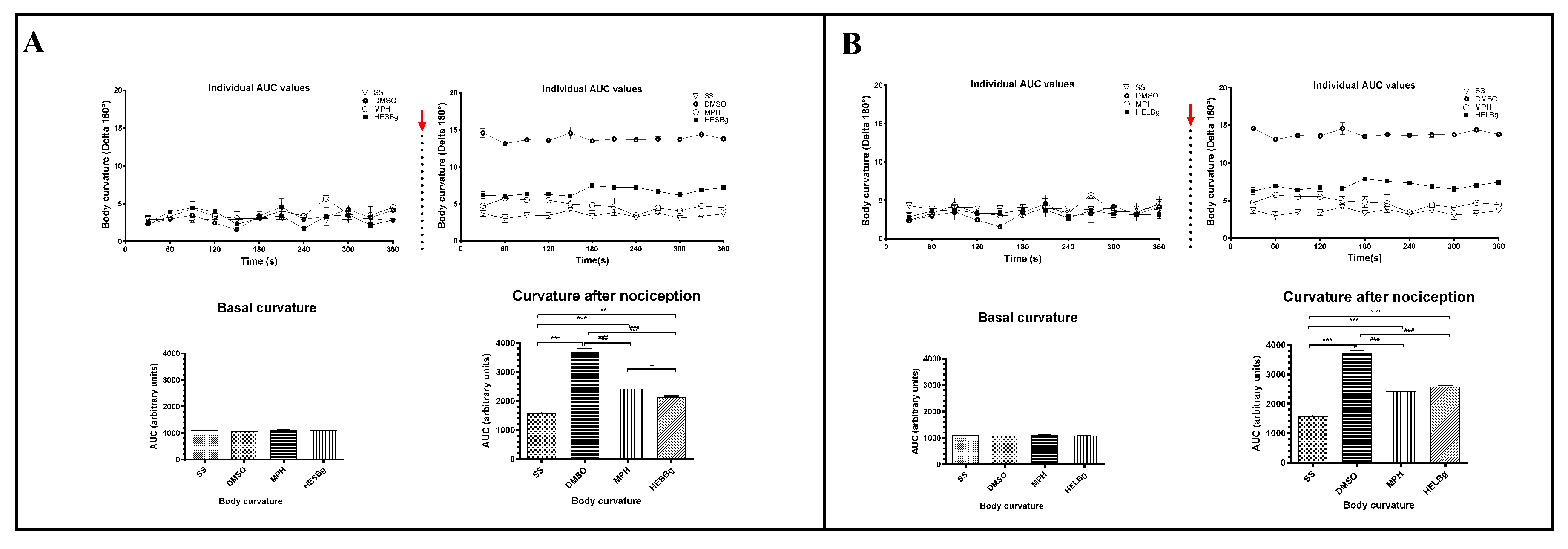
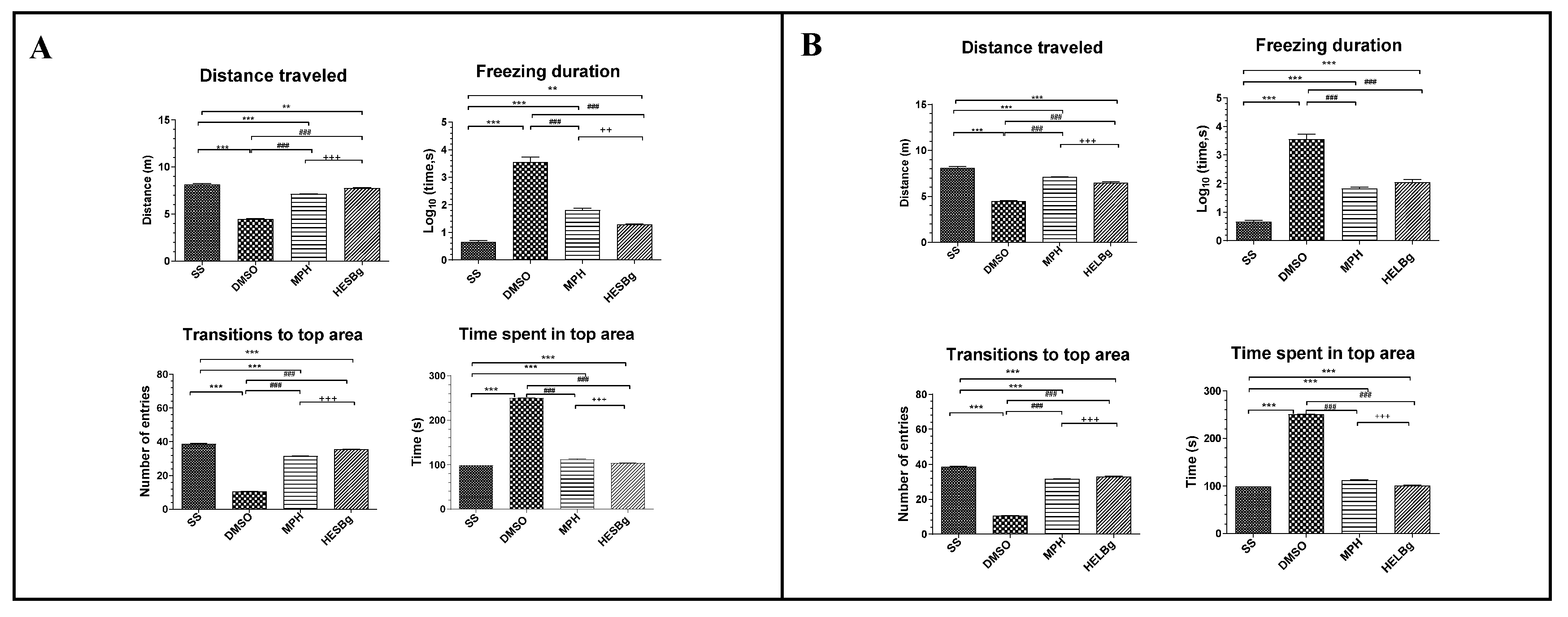
2.3. Antinociceptive potential of HESBg and HELBg in the modulation of behavioral phenotypes after intraperitoneal injection of acetic acid in Danio rerio
3. Discussion
4. Materials and Methods
4.1. Drugs and reagents
4.2. Experimental animals
4.2.1. Treatment groups and routes of administration
4.3. Inflammation induction protocol with carrageenan and measurement of inflammatory edema Danio rerio
4.3.1. Histopathological analysis of the inflammation test in Danio rerio
4.3.2. Evaluation of histopathological alterations of the inflammation test in Danio rerio
4.4. Hyperalgesia induction protocol with acetic acid and behavioral analysis
4.4.1. Behavioral parameters
4.5. Statistical analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lisa, S.R.; Islam, M.K.; Qais, N. Plants and Plant Constituents with Analgesic and Anti-Inflammatory Activities: A Systematic Review. Dhaka University Journal of Pharmaceutical Sciences 2020, 19, 207–224. [Google Scholar] [CrossRef]
- Conegundes, J.L.M.; Silva, J.M. da; Mendes, R. de F.; Fernandes, M.F.; Pinto, N. de C.C.; Almeida, M.A. de; Dib, P.R.B.; Andrade, R. de O.; Rodrigues, M.N.; Castañon, M.C.M.N.; et al. Anti-Inflammatory and Antinociceptive Activity of Siparuna Guianensis Aublet, an Amazonian Plant Traditionally Used by Indigenous Communities. J Ethnopharmacol 2021, 265, 113344. [Google Scholar] [CrossRef]
- Carvalho, A.C.B.; Lana, T.N.; Perfeito, J.P.S.; Silveira, D. The Brazilian Market of Herbal Medicinal Products and the Impacts of the New Legislation on Traditional Medicines. J Ethnopharmacol 2018, 212, 29–35. [Google Scholar] [CrossRef]
- Borges, R.S.; Keita, H.; Ortiz, B.L.S.; dos Santos Sampaio, T.I.; Ferreira, I.M.; Lima, E.S.; de Jesus Amazonas da Silva, M.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; da Conceição, E.C.; et al. Anti-Inflammatory Activity of Nanoemulsions of Essential Oil from Rosmarinus Officinalis L.: In Vitro and in Zebrafish Studies. Inflammopharmacology 2018, 26, 1057–1080. [Google Scholar] [CrossRef]
- Andrade, S.F.; Cardoso, L.G.V.; Carvalho, J.C.T.; Bastos, J.K. Anti-Inflammatory and Antinociceptive Activities of Extract, Fractions and Populnoic Acid from Bark Wood of Austroplenckia Populnea. J Ethnopharmacol 2007, 109, 464–471. [Google Scholar] [CrossRef]
- Koga, R. de C.R.; Teixeira dos Santos, A.V.T. de L.; Rodrigues Sarquis, R. do S.F.; Carvalho, J.C.T. Bauhinia Guianensis Aubl., a Plant from Amazon Biome with Promising Biologically Active Properties: A Systematic Review. Pharmacogn Rev 2021, 15, 76–81. [Google Scholar] [CrossRef]
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A Natural History of Botanical Therapeutics. Metabolism 2008, 57, S3–S9. [Google Scholar] [CrossRef]
- de Souza, A.A.; Ortíz, B.L.S.; de Carvalho Rocha Koga, R.; Sales, P.F.; da Cunha, D.B.; Guerra, A.L.M.; de Souza, G.C.; Carvalho, J.C.T. Secondary Metabolites Found among the Species Trattinnickia Rhoifolia Willd. Molecules 2021, 26, 7661. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Begum, M.Y.; Chidambaram, K.; Sathasivam, K. v.; Jeyabalan, S.; et al. Kirenol: A Potential Natural Lead Molecule for a New Drug Design, Development, and Therapy for Inflammation. Molecules 2022, 27, 734. [Google Scholar] [CrossRef]
- Ferreira, M.C. Medicinal Knowledge and Plant Utilization in an Amazonian Coastal Community of Marudá, Pará State (Brazil). J Ethnopharmacol 2009, 126, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.T.; Santos, L.S.; Viana, E.P.; de Almeida, S.S.M.S.; Marconato, E.; Rodrigues, M.; Ferreira, L.R.; Van de Kamp, A. Anti-Inflammatory and Analgesic Activities of the Crude Extracts from Stem Bark of Bauhinia Guianensis. Pharm Biol 1999, 37, 281–284. [Google Scholar] [CrossRef]
- Quintans-Júnior L; Almeida R; Falcão A; Agra Maria de Fátima; Sousa Maria de Fátima Vanderlei; Barbosa-Filho José Maria Avaliação Da Atividade Anticonvulsivante de Plantas Do Nordeste Brasileiro. Acta Farmacêutica Bonaerense 2002, 21, 179–184.
- Soehnlein, O.; Lindbom, L. Phagocyte Partnership during the Onset and Resolution of Inflammation. Nat Rev Immunol 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a Model for Inflammation and Drug Discovery. Drug Discov Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef]
- Novoa, B.; Figueras, A. Zebrafish: Model for the Study of Inflammation and the Innate Immune Response to Infectious Diseases. In; 2012; pp. 253–275.
- Huang, S.-Y.; Feng, C.-W.; Hung, H.-C.; Chakraborty, C.; Chen, C.-H.; Chen, W.-F.; Jean, Y.-H.; Wang, H.-M.D.; Sung, C.-S.; Sun, Y.-M.; et al. A Novel Zebrafish Model to Provide Mechanistic Insights into the Inflammatory Events in Carrageenan-Induced Abdominal Edema. PLoS ONE 2014, 9, e104414. [Google Scholar] [CrossRef]
- Yatoo, Mohd.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders - A Review. Recent Pat Inflamm Allergy Drug Discov 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Wingerd, B.A.; Arakawa, T.; Smith, W.L. Cyclooxygenase-2 Gene Transcription in a Macrophage Model of Inflammation. The Journal of Immunology 2006, 177, 8111–8122. [Google Scholar] [CrossRef]
- Batlouni, M. Anti-Inflamatórios Não Esteroides: Efeitos Cardiovasculares, Cérebro-Vasculares e Renais. Arq Bras Cardiol 2010, 94, 556–563. [Google Scholar] [CrossRef]
- Perazzo, F.F.; Lima, L.M.; Padilha, M.de M.; Rocha, L.M.; Sousa, P.J.C.; Carvalho, J.C.T. Anti-Inflammatory and Analgesic Activities of Hypericum Brasiliense (Willd) Standardized Extract. Revista Brasileira de Farmacognosia 2008, 18. [Google Scholar] [CrossRef]
- Borges, R.S.; Lima, E.S.; Keita, H.; Ferreira, I.M.; Fernandes, C.P.; Cruz, R.A.S.; Duarte, J.L.; Velázquez-Moyado, J.; Ortiz, B.L.S.; Castro, A.N.; et al. Anti-Inflammatory and Antialgic Actions of a Nanoemulsion of Rosmarinus Officinalis L. Essential Oil and a Molecular Docking Study of Its Major Chemical Constituents. Inflammopharmacology 2018, 26, 183–195. [Google Scholar] [CrossRef]
- Holanda, F.H.; Ribeiro, A.N.; Sánchez-Ortiz, B.L.; de Souza, G.C.; Borges, S.F.; Ferreira, A.M.; Florentino, A.C.; Yoshioka, S.A.; Moraes, L.S.; Carvalho, J.C.T.; et al. Anti-Inflammatory Potential of Baicalein Combined with Silk Fibroin Protein in a Zebrafish Model (Danio Rerio). Biotechnol Lett 2023, 45, 235–253. [Google Scholar] [CrossRef]
- Quitian-Useche, Y.F.; Sánchez-Ortiz, B.L.; Borges, S.F.; Ramos, B.; de Souza, G.C.; Batista, M.A.; da Silva Hage Melim, L.I.; Ferreira, I.M.; Carvalho, J.C.T.; Borges, R.S. Fatty Ethanolamide of Bertholletia Excelsa Triglycerides (Brazil Nuts): Anti-Inflammatory Action and Acute Toxicity Evaluation in Zebrafish (Danio Rerio). Inflammopharmacology 2021, 29, 1519–1537. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; de Souza, G.C.; dos Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; de Oliveira Carvalho, H.; Silva, M.L. Effects of Bothrops Alternatus Venom in Zebrafish: A Histopathological Study. Inflammopharmacology 2018, 26, 273–284. [Google Scholar] [CrossRef]
- Santos, I; Souza, G; Santana, G; Duarte, J; Fernandes, C; Keita, H; Velázquez-Moyado, J; Navarrete, A; Ferreira, I; Carvalho, H; et al. Histopathology in Zebrafish (Danio Rerio) to Evaluate the Toxicity of Medicine: An Anti-Inflammatory Phytomedicine with Janaguba Milk (Himatanthus Drasticus Plumel). In Histopathology-An update; Hoboken: Wiley Blackwell, 2018; pp. 39–64. [Google Scholar]
- de Sá Hyacienth, B.M.; Tavares Picanço, K.R.; Sánchez-Ortiz, B.L.; Barros Silva, L.; Matias Pereira, A.C.; Machado Góes, L.D.; Sousa Borges, R.; Cardoso Ataíde, R.; dos Santos, C.B.R.; de Oliveira Carvalho, H.; et al. Hydroethanolic Extract from Endopleura Uchi (Huber) Cuatrecasas and Its Marker Bergenin: Toxicological and Pharmacokinetic Studies in Silico and in vivo on Zebrafish. Toxicol Rep 2020, 7, 217–232. [Google Scholar] [CrossRef]
- Souza, G; Duarte, J; Fernandes, C; Moyado, J; Navarrete, A; Carvalho, J. Obtainment and Study of the Toxicity of Perillyl Alcohol Nanoemulsion on Zebrafish (Danio Rerio). J Nanomed Res 2016, 4. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; de Souza, G.C.; dos Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; de Oliveira Carvalho, H.; Silva, M.L. Effects of Bothrops Alternatus Venom in Zebrafish: A Histopathological Study. Inflammopharmacology 2018, 26, 273–284. [Google Scholar] [CrossRef]
- Poleksic V; Mitrovic-Tutundzic V. Fish Gills as a Monitor of Sublethal and Chronic Effects of Pollution. In Sublethal and chronic effects of pollutants on freshwater fish; Fishing New Books: Cambridge, 1994; pp. 339–352. [Google Scholar]
- Rigolin-Sá O Toxicidade Do Herbicida Roundup (Glifosato) e Do Acaricida Omite (Propargito) Nas Fases Iniciais Da Ontogenia Do Bagre, Rhandia Hilarii (Valenciennes, 1840) (Pimelodidade, Siluriformes). Doutorado em Recursos Naturais, Universidade Federal de São Carlos: São Carlos, 1998.
- V. Stankov, S. Definition of Inflammation, Causes of Inflammation and Possible Anti-Inflammatory Strategies. Open Inflamm J 2012, 5, 1–9. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Pavadai, S. Anti-Inflammatory Effect of Naravelia Zeylanica DC via Suppression of Inflammatory Mediators in Carrageenan-Induced Abdominal Oedema in Zebrafish Model. Inflammopharmacology 2017, 25, 147–158. [Google Scholar] [CrossRef]
- Barbalho, P.G.; Lopes-Cendes, I.; Maurer-Morelli, C.V. Indomethacin Treatment Prior to Pentylenetetrazole-Induced Seizures Downregulates the Expression of Il1b and Cox2 and Decreases Seizure-like Behavior in Zebrafish Larvae. BMC Neurosci 2016, 17, 12. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and Inflammation: Molecular Targets and Health Implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Carvalho J Fitoterápicos Anti-Inflamatórios; 2nd ed.; Pharmabooks: São Paulo, 2017; ISBN 139788589731805.
- David, J; Barreiros, A; David, J. Antioxidantes de Fontes Naturais. In Fitoterápicos anti-inflamatórios; Pharmabooks: São Paulo, 2017; pp. 105–137. [Google Scholar]
- Sun, W.; Meng, J.; Wang, Z.; Yuan, T.; Qian, H.; Chen, W.; Tong, J.; Xie, Y.; Zhang, Y.; Zhao, J.; et al. Proanthocyanidins Attenuation of H 2 O 2 -Induced Oxidative Damage in Tendon-Derived Stem Cells via Upregulating Nrf-2 Signaling Pathway. Biomed Res Int 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Kim, S.-H.; Bang, J.; Son, C.-N.; Baek, W.-K.; Kim, J.-M. Grape Seed Proanthocyanidin Extract Ameliorates Murine Autoimmune Arthritis through Regulation of TLR4/MyD88/NF-ΚB Signaling Pathway. Korean J Intern Med 2018, 33, 612–621. [Google Scholar] [CrossRef]
- Ma, X.; Wang, R.; Yu, S.; Lu, G.; Yu, Y.; Jiang, C. Anti-Inflammatory Activity of Oligomeric Proanthocyanidins Via Inhibition of NF-ΚB and MAPK in LPS-Stimulated MAC-T Cells. J Microbiol Biotechnol 2020, 30, 1458–1466. [Google Scholar] [CrossRef]
- Baumann, J.; v. Bruchhausen, F.; Wurm, G. Flavonoids and Related Compounds as Inhibitors of Arachidonic Acid Peroxidation. Prostaglandins 1980, 20, 627–639. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Asmawi, M.; Vuorela, P.; Vapaatalo, H.; Moilanen, E. Effects of Flavonoids on Prostaglandin E 2 Production and on COX-2 and MPGES-1 Expressions in Activated Macrophages. Planta Med 2011, 77, 1504–1511. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Lee, D.E.; Rogozin, E.A.; Bode, A.M.; Lee, H.J.; Dong, Z. Cocoa Procyanidins Suppress Transformation by Inhibiting Mitogen-Activated Protein Kinase Kinase. Journal of Biological Chemistry 2008, 283, 20664–20673. [Google Scholar] [CrossRef]
- Kundu, J.K.; Na, H.-K.; Chun, K.-S.; Kim, Y.-K.; Lee, S.J.; Lee, S.S.; Lee, O.-S.; Sim, Y.-C.; Surh, Y.-J. Inhibition of Phorbol Ester–Induced COX-2 Expression by Epigallocatechin Gallate in Mouse Skin and Cultured Human Mammary Epithelial Cells. J Nutr 2003, 133, 3805S–3810S. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Terra, X.; Richart, C.; Ardèvol, A.; Pinent, M.; Blay, M. Omega-3 Docosahexaenoic Acid and Procyanidins Inhibit Cyclo-Oxygenase Activity and Attenuate NF-ΚB Activation through a P105/P50 Regulatory Mechanism in Macrophage Inflammation. Biochemical Journal 2012, 441, 653–663. [Google Scholar] [CrossRef]
- Terra, X.; Valls, J.; Vitrac, X.; Mérrillon, J.-M.; Arola, L.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; et al. Grape-Seed Procyanidins Act as Antiinflammatory Agents in Endotoxin-Stimulated RAW 264.7 Macrophages by Inhibiting NFkB Signaling Pathway. J Agric Food Chem 2007, 55, 4357–4365. [Google Scholar] [CrossRef]
- Chu, J.; Sadler, K.C. New School in Liver Development: Lessons from Zebrafish. Hepatology 2009, 50, 1656–1663. [Google Scholar] [CrossRef]
- Brugman, S. The Zebrafish as a Model to Study Intestinal Inflammation. Dev Comp Immunol 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Swanhart, L.M.; Cosentino, C.C.; Diep, C.Q.; Davidson, A.J.; de Caestecker, M.; Hukriede, N.A. Zebrafish Kidney Development: Basic Science to Translational Research. Birth Defects Res C Embryo Today 2011, 93, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, J.H.; Giselbrecht, S.; Schmidts, M.; Schindler, S.; Beales, P.L.; Tönshoff, B.; Liebel, U.; Gehrig, J. Development of an Automated Imaging Pipeline for the Analysis of the Zebrafish Larval Kidney. PLoS ONE 2013, 8, e82137. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and Inflammation: Molecular Targets and Health Implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yuan, M.; Ji, R.-R. Inflammation and Pain. In Neuroimmune Interactions in Pain; Springer International Publishing: Cham, 2023; pp. 17–41. [Google Scholar]
- Costa, F. V.; Rosa, L. V.; Quadros, V.A.; Santos, A.R.S.; Kalueff, A. V.; Rosemberg, D.B. Understanding Nociception-Related Phenotypes in Adult Zebrafish: Behavioral and Pharmacological Characterization Using a New Acetic Acid Model. Behavioural Brain Research 2019, 359, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Goebel, A.; Andersson, D.; Shoenfeld, Y. The Biology of Symptom-Based Disorders – Time to Act. Autoimmun Rev 2023, 22, 103218. [Google Scholar] [CrossRef]
- Lewis, S. Inflammation and Pain. Nat Rev Neurosci 2014, 15, 630–630. [Google Scholar] [CrossRef]
- Gonzalez-Nunez, V.; Rodriguez, R.E. The Zebrafish: A Model to Study the Endogenous Mechanisms of Pain. ILAR J 2009, 50, 373–386. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, P.K.; Gupta, P.; Garabadu, D. Antinociceptive Activity of Standardized Extract of Bacopa Monnieri in Different Pain Models of Zebrafish. J Ethnopharmacol 2022, 282, 114546. [Google Scholar] [CrossRef]
- Steenbergen, P.J.; Bardine, N. Antinociceptive Effects of Buprenorphine in Zebrafish Larvae: An Alternative for Rodent Models to Study Pain and Nociception? Appl Anim Behav Sci 2014, 152, 92–99. [Google Scholar] [CrossRef]
- Cechinel Filho, V; Breviglieri, E; Willain Filho, A. Santos ARS Estudo Fitoquímico e Avaliação Preliminar Da Atividade Analgésica de Bauhinia Splendens. Revista Brasileira de Farmacologia 1995, 76, 115–117. [Google Scholar]
- Willain Filho, A.; Breviglieri, E.; Filho, V.C.; Santos, A.R.S. Antinociceptive Effect of the Hydroalcoholic Extract of Bauhinia Splendens Stems in Mice. Journal of Pharmacy and Pharmacology 2011, 49, 823–827. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Santos, A.R.S.; Meyre-Silva, C.; Schmeling, L.O.; Machado, C.; Liz, F.H.; Filho, V.C. Antinociceptive Action of the Extract and the Flavonoid Quercitrin Isolated from Bauhinia Microstachya Leaves. Journal of Pharmacy and Pharmacology 2010, 57, 1345–1351. [Google Scholar] [CrossRef]
- Lopez-Luna, J.; Al-Jubouri, Q.; Al-Nuaimy, W.; Sneddon, L.U. Impact of Stress, Fear and Anxiety on the Nociceptive Responses of Larval Zebrafish. PLoS ONE 2017, 12, e0181010. [Google Scholar] [CrossRef]
- Chagas, T.Q.; da Silva Alvarez, T.G.; Montalvão, M.F.; Mesak, C.; Rocha, T.L.; da Costa Araújo, A.P.; Malafaia, G. Behavioral Toxicity of Tannery Effluent in Zebrafish (Danio Rerio) Used as Model System. Science of The Total Environment 2019, 685, 923–933. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behavioural Brain Research 2009, 205, 38–44. [Google Scholar] [CrossRef]
- de Melo, E.L.; Pinto, A.M.; Baima, C.L.B.; da Silva, H.R.; da Silva Sena, I.; Sanchez-Ortiz, B.L.; de Lima Teixeira, A.V.T.; Pereira, A.C.M.; da Silva Barbosa, R.; Carvalho, H.O.; et al. Evaluation of the in Vitro Release of Isoflavones from Soybean Germ Associated with Kefir Culture in the Gastrointestinal Tract and Anxiolytic and Antidepressant Actions in Zebrafish (Danio Rerio). J Funct Foods 2020, 70, 103986. [Google Scholar] [CrossRef]
- Cady, R.J.; Hirst, J.J.; Durham, P.L. Dietary Grape Seed Polyphenols Repress Neuron and Glia Activation in Trigeminal Ganglion and Trigeminal Nucleus Caudalis. Mol Pain 2010, 6, 1744-8069-6-91. [Google Scholar] [CrossRef]
- Woo, Y.J.; Joo, Y. Bin; Jung, Y.O.; Ju, J.H.; Cho, M. La; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape Seed Proanthocyanidin Extract Ameliorates Monosodium Iodoacetate-Induced Osteoarthritis. Exp Mol Med 2011, 43, 561. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac : A Fast and Robust Software for Tracking Organisms. Methods Ecol Evol 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Rasband W, S. ImageJ 2018.
| Treatment groups | Histopathology 1 | ||
|---|---|---|---|
| Liver | Intestine | Kidneys | |
| SS | Normal | ||
| DMSO | Moderate to severe | ||
| Indomethacin | Normal | Mild to moderate | |
| HESBg | Normal | ||
| HELBg | Normal | ||
| Substance | Concentration/Dose | Potential Effect |
|---|---|---|
| Acetic Acid | 2.5% | Noxious stimulus |
| Carrageenan | 300 μg | Noxious stimulus |
| DMSO | 0,1% | - |
| HELBg | 100 mg/kg | Anti-inflammatory |
| HESBg | 100 mg/kg | Anti-inflammatory |
| Indomethacin | 10 mg/kg | Anti-inflammatory |
| Morphine | 2,5 mg/kg | Anti-inflammatory |
| PBS | 0,128 mg/mL | - |
| Saline solution | 0,9% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





