1. Introduction
The global agricultural system is facing additional difficulties in the twenty-first century, including a drop in productivity and a deterioration in the sustainability of agricultural ecosystems. According to United Nations forecasts, nine billion people will inhabit the planet by 2050 (Wood, 2001), and food demand has been steadily rising while the available supply has been constrained (Kumar et al., 2017a). Due to climate change, major cereal crops have seen significant production losses, with yield decreases of about 3.8 percent and five percent in reference to corn and wheat separately (Lipper et al.,2014). Temperature rises have brought major rises in global temperatures and the appearance of various abiotic factors that have a negative impact on agricultural output (Pareek et al., 2020). According to an FAO assessment from 2009, a seventy percent increase in agricultural output is required by 2050 as a means of achieving anticipated demand. The importance of sustainable farming practices is also becoming more widely recognized in order to satisfy the future economic needs of the world (Altieri, 2004).
It is crucial for sustainable agriculture to retain the soil's dynamic nature in order to provide enough food and ensure global long-term environmental health for the next generation (Kumar et al., 2017b). Because it is very hard to expand agricultural areas with fertile soil, researchers and experts have focused their efforts on developing more effective and safe farming procedures. Exploring unconventional resources is thus urgently needed to protect our ecology from further degradation and slow down the ever-increasing population's appetite for resources. PGPR can be used to boost plant vitality and promote development without releasing harmful byproducts into the atmosphere (Calvo et al., 2014).
Crop production requires PGPR to boost plant nutrient availability. PGPR directly or indirectly affects plant development. The phytomicrobiome—a group of beneficial bacteria—improves plant resilience to biotic and abiotic stress and agricultural productivity, according to studies. (Backer et al., 2018). PGPR, a beneficial phyto microbiome component, promotes plants' responses to biotic stress by producing chemical messengers, boosting their intake of nutrients, and releasing antibiotics (Lyu et al., 2020).
2. Benefits of Rhizobial Associations for Plant Growth:
Two to five percent of rhizobacteria have a major impact on plant development when reintroduced by plants inoculating in soil containing competing microorganisms; these are known as PGPR. Additionally, evidence suggests that PGPRs are efficient, and a tightly organized microbial community constantly resides in the plant's immediate environment.
A plant growing in the wild is not an isolated entity but rather part of a dynamic and stable social network (Lundberg et al., 2012). The colony of microorganisms surrounding the plant is always organized and maintained (Smith et al., 2017). PGPRs are rhizobacteria that help plants flourish. The soil's rhizosphere, its lowermost layer near plant roots, is where a group of helpful bacteria are found. These bacteria establish a cooperative relationship with plants and offer a variety of benefits that promote plant growth. Microbiome interactions exist in all multicellular creatures and, presumably, all eukaryotes. In actuality, these may date back to the time before the region was settled by plants. Many different kinds of microbes can be found in soil, most of which are bacteria. Where soil bacteria and plant roots come into contact with one another is in the rhizosphere, which frequently has a higher microbial density. The beneficial bacteria either live in the soil freely or work in symbiotic relationships with the plant, but they are present nearby or even inside the plant (van et al.). Numerous living creatures can be found in the root-tip environment (or rhizosphere). The root vital activities of breathing and root secretion are both quantitatively and qualitatively influenced (Lazarovits). For each of these regions, the word "rhizosphere" is used. The plant, soil, bacteria, and soil microbes all interact significantly and widely in the rhizosphere. According to Berg et al. (2014), these predate the arrival of plants in the region. It's possible that these connections matter greatly and affect plant development and crop yield. When two or more types of microflora are present, PGPR can multiply and colonize every ecological niche on the roots at every stage of plant development. The age, stage of growth, species of plant, soil tissue, and ecological circumstances all have an effect on the activities of the microbes in the rhizosphere. Because of their quick development and adaptability to a bacteria can take up carbon and nitrogen from a make up the bulk of the rhizosphere's microbial population.
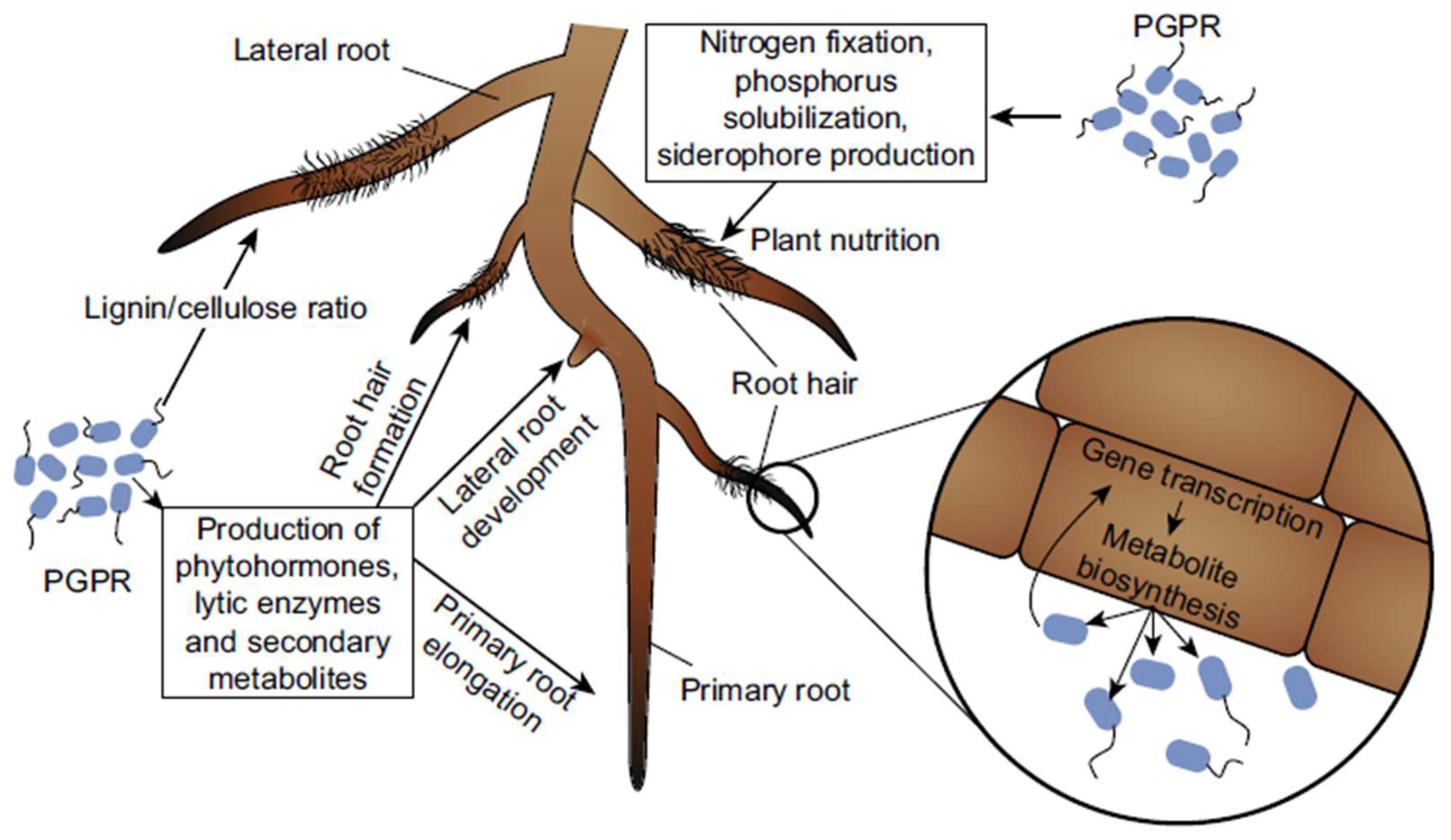
Figure 1.
The probable modes of effect that (PGPR) employ in order to promote increased plant development based on work by Vacheron et al., (2013).
Figure 1.
The probable modes of effect that (PGPR) employ in order to promote increased plant development based on work by Vacheron et al., (2013).
The positive impacts of PGPR, a naturally occurring soil bacterium, on plant vitality and output have been thoroughly studied. In addition to protecting plants from pathogens and harsh conditions, they can also boost nutrient availability, spur plant growth, fortify root development, and more. Several different mechanisms are involved in how PGPR helps plants. Auxins, cytokinins, and gibberellins, which encourage root and shoot growth, are formed by some PGPR.
Additionally, they may convert nitrogen in the air into a plant-available form through a process called "fixation," which they can easily use to augment the plant's nitrogen needs. Phosphorus and other mineral nutrients can be solubilized by PGPR, making them more available to plants. Some PGPRs can create antimicrobial substances that stop the development of plant pathogens, shielding the plant from disease.
PGPR is comparable to dangerous microbes for oxygen, food, and room by colonizing the rhizosphere, which lowers the danger of pathogen invasion and boosts plant health in general. PGPR systemic effects that resistance in plants by defensive systems being put into action and improving acceptance to a broad range of environmental factors, including dryness, salinity, and extreme heat. Soil has many different kinds of bacteria and viruses, primarily bacteria.
In sustainable agricultural techniques, the application of PGPR as bio-fertilizers and bio-pesticides has attracted a lot of attention. The foundation of the world economy, agriculture is crucial in supplying food, fiber, bioenergy, and other necessities to the expanding human population. But chemical pesticides and fertilizers are frequently used in conventional agricultural practices, which can be bad for the health of humans, the environment, and the sustainability of agricultural output. Therefore, sustainable and environmentally friendly methods are becoming increasingly important to increase plant growth and improve agricultural productivity. In recent years, PGPR has surfaced as a viable option for long-term farming success.
As all the variables are considered, PGPR offers a workable method for fostering plant development, increasing crop output, and lessening the ecological damage caused by agricultural practices. PGPR mechanisms and their applications in microbial and plant interactions and sustainable agriculture are revealed.
3. The Growth-Promoting Mechanism in Plants PGPR as a mediator:
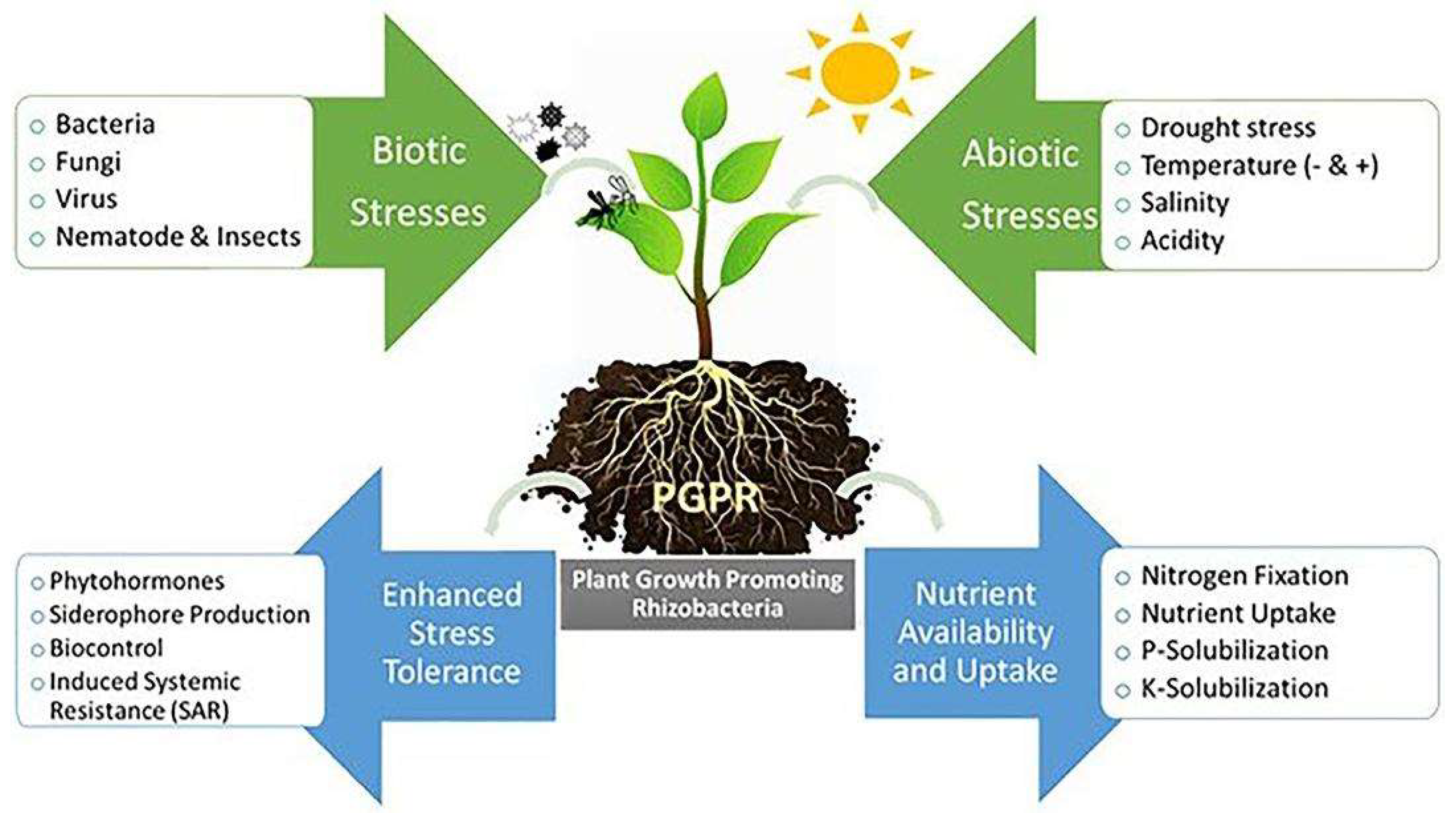
Rhizobacteria that promote plant growth (PGPR) are important growth stimulants for plants in sustainable agriculture. Rhizosphere microorganisms are beneficial microbes that use a range of strategies to promote plant growth. The following are significant contributions that PGPR makes to environmentally friendly farming: Direct and indirect means exist for PGPR to stimulate plant development.
Figure 2.
Insights on plant-PGPR interactions in the rhizosphere source Shah et al., (2021).
Figure 2.
Insights on plant-PGPR interactions in the rhizosphere source Shah et al., (2021).
3.1. Direct Mechanism:
By fixing nitrogen, mineralizing organic compounds, solubilizing mineral nutrients, and producing phytohormones, PGPR can directly help plant growth and development through processes like nutrient intake or increased access to nutrition.
3.1.1. Nitrogen fixation
The majority of plant biochemical processes, like the synthesis of proteins and photosynthesis, depend on nitrogen (N), making it one of the most crucial minerals plants need to thrive (Alori et al., 2017). Dinitrogen, which comprises 79% of nitrogen in the atmosphere, has a triplet covalent bond and a relatively small amount of reactivity; therefore, plants cannot utilize it directly. As the most effective method of adding nitrogen, nitrogen fertilizers have become an essential component of the cultivation of crops and methods in agriculture. But their disproportionate and persistent use is polluting the environment, causing eutrophication, deadly discharges into the environment, poisonous deposition in water in the ground, and other water bodies—all of which are either directly or indirectly contributing to climate change. According to Bouchet et al. (2016), cropping systems only recover about half of the applied nitrogen; the other half either escapes from the soil by volatilization, leaching, or runoff, or it stays in the soil as organic complexes, which make up roughly 98% of the overall quantity of soil nitrogen. Symbiotic nitrogen-fixing organisms form a mutually beneficial connection with their host, as opposed to free-living nitrogen fixers and their host plants. Rhizobium, Azoarcus, Mesorhizobium, Frankia, Burkholderia, and several strains of Achromobacter are all symbiotic nitrogen fixers (Babalola, 2010; Pérez-Montao et al., 2014). These bacterial taxa, along with a few others, have earned their reputation as nitrogen-fixing PGPRs capable of significantly boosting plant growth and harvest (Bishnoi, 2015). A highly conserved and energy-demanding enzyme known as nitrogenase is responsible for fixing nitrogen. Two metalloprotein subunits make up the typical nitrogenase. The energy required by these bacteria to convert atmospheric nitrogen into usable forms is considerable. 16 moles of ATP are needed for every mole of nitrogen fixed, and the majority of this energy originates from oxidizing the organic molecules. Nitrogen fixers that don't use photosynthesis rely entirely on other organisms to get these energy-rich molecules, whereas microorganisms that can photosynthesise their own carbohydrates are called photoautotrophs.
Legumes are capable of symbiotic nitrogen fixation, which is responsible for 80 Tg of the 175 Tg of N2 fixed annually, with each legume fixing between twenty and two hundred kilograms of nitrogen annually, and industrial nitrogen fixation, which produces N fertilizers, accounts for about half (88 Tg) (Hillel, 2008). Seventy-plus percent of legumes form mutualistic relationships with rhizobia and are capable of fixing up to two hundred kg of nitrogen per square meter. Legumes can fix nitrogen from the atmosphere through symbiotic links with bacteria; therefore, they often don't need additional nitrogen until large amounts of N-fertilizers are applied. Using the provided fertilizer requires a lesser amount of energy than atmospheric N2 repair, which causes them to reduce or stop their nitrogen fixation. In order to increase growth, combat illness, and keep the soil's nitrogen content stable for farming, biologically N2-fixing PGPR is sprayed onto crops and crop fields.
3.1.2. Phosphate Dissolution:
The second essential ingredient for plants is phosphorus. Important functions that rely on it include breathing, signal transduction, and the transmission of energy. Photosynthesis and macromolecular biosynthesis (Hillel, 2008). Because 99 percent of the available phosphorus has been immobilized or precipitated, making it water-insoluble, plants have a hard time absorbing it. Since phosphorus is poorly soluble and has a preference for organic compounds and the soil matrix, only 0.1% of the combined phosphorus concentration in soil and plant availability has been estimated. Phosphorus is only absorbed by plants as monobasic (H2PO4) and dibasic (HPO42-) ions.
Phosphate deficiency can be remedied by applying phosphorus-based fertilizers to the soil, which replenishes a plant's quick access to the phosphorus already present in the substrate. Commercial fertilizer supplementation with phosphorus, however, is a costly process, and the phosphorus is frequently inhospitable to vegetation because the soil might readily lose it, mix with local waterways, and contaminate aquatic as well as terrestrial ecosystems (Adesemoye and Kloepper, 2009).
An essential characteristic that can be attained by PGPR is the phosphate-solubilizing bacteria's ability to dissolve and mineralize phosphate. According to Sundarara et al. (2002), phosphorus-solubilizing bacteria can reduce the P concentration that is essential by a quarter. Khan et al. (2009) found that PSB's influence rises when it is mixed with other PGPRs or AMFs. The primary method used by practically all bacteria that can dissolve metabolites, most often organic acids, requires phosphorus for their synthesis in the ketone and gluconic acid ketoforms,in which the cations are chelated and attached to their respective hydroxyl and carboxyl groups in phosphate (Heydari et al., 2007), so that they can be dissolved into the soil solution and made available for plant uptake (Riaz et al., 2021). Phosphorus-solubilizing PGPR bacteria are found in the following genera: Arthrobacter, Bacillus, Burkholderia, Enterobacter, Microbacterium, Pseudomonas, Rhizobium, Mesorhizobium, Flavobacterium, and Serratia. Two of these are the phosphate-solubilizing bacteria Mesomerhizobium ciceri and Mesomerhizobium mediterraneum, both of which have been isolated from chickpea nodules (Parmar and Sindhu, 2013). Despite the fact that these bacteria solubilize phosphorus, increasing soil fertility, there have been few investigations into their application as biofertilizers. Its availability in the soil is often limited due to its low solubility. Solubilizing insoluble phosphorus molecules in the soil is a special property of some PGPRs that makes them more accessible to plants.
3.1.3. Potassium Dissolution:
Potassium is the third essential component plants need. Since more than ninety percent of potassium is already in existence as insoluble rocks and mineral silicates, the soluble potassium content in soil is often quite low. The production of crops is now severely limited by the potassium deficit. Without enough potassium, plants produce fewer seeds, grow more slowly, and produce less of a crop.
Numerous microorganisms can solubilize potassium (K) in soil and interact closely with plants, particularly bacterial and fungal species (Setiawati and Mutmainnah, 2016) The PGPR's potential to produce and secrete organic acids capable of dissolving potassium rocks has been extensively investigated. Examples of PGPR that solubilize potassium include Acidothiobacillus sp., Bacillus edaphicus, Ferrooxidans sp., Bacillus mucilaginosus, Pseudomonas sp., and Burkholderia. Paenibacillus sp. has been found to convert potassium from inaccessible mineral forms found in soils.
According to research that suggests the resulting effects of plant-growth-stimulating microorganisms (PGPM), the formation of organic acids by K-solubilizing microbes improves potassium availability, which in turn promotes plant development. Soil microorganisms increase the acidity of the soil's rhizosphere by producing oxalate, the acid citrate, acetate, ferulic acid, and coumaric acid, all examples of organic acids. This accelerates the rate at which minerals dissolve and generate protons, resulting in the solubilization of the mineral K. Therefore, scientists have concluded that PGPM, like PSB, could be an efficient organic fertilizer, boosting the accessibility of plant nutrients and permitting less use of artificial fertilizers (Khan et al., 2019).
3.1.3. Producing Siderophore:
Minor chemical molecules known as siderophores are made by bacteria in iron-restricted environments that increase the ability to absorb iron. Because it is a necessary enzyme cofactor for a number of metabolic processes, including the process of photosynthesis, amino acid synthesis, oxygen transfer, nitrogen fixation, and respiration, iron is crucial for all photosynthesis-based creatures. Iron, which normally exists in two oxidative states: Fe2+ and Fe3+, is a massive and common chemical compound on our planet. The latter state is significantly less accessible to plants because it forms insoluble iron oxides and hydroxides (Khoshru et al., 2020).
According to research, specific PGPB generate compounds with a low molecular weight (400–1,500 Da) that are capable of taking iron from the ground. Siderophores are iron-binding compounds that help make ferric ions (iron) more bioavailable to plants (Goswami et al., 2016).
Many microorganisms from both marine and terrestrial habitats have been separated and examined for their capacity to create siderophores (Rezanka et al., 2018). Siderophores have four primary chemical structures: phenolates, hydroxamates, pyoverdines, and carboxylates. Among the gram-negative bacteria, Enterobacter and Pseudomonas are the most prevalent producers of siderophores, while just 2% of gram-positive species, including Bacillus and Rhodococcus, are capable of making siderophores (Czarne et al., 2020). Beneficial soil and plant-associated microorganisms' production of siderophores is a crucial biological control mechanism because it prevents harmful plant pathogens from accessing iron sources by competing with them for those resources (Khoshru et al., 2020).
3.1.3. Zinc solubilization:
Zinc (Zn) is a necessary plant micronutrient that ranges in concentration from 5 to 100 mg kg1 and is vital for plant growth and development (Goteti et al., 2013). The production of chlorophyll, the activation of enzymes involved in auxin and glucose metabolism, and the biosynthesis of proteins, lipids, and nucleic acids are just a few of the important physiological processes that Zn is essential for in plants. It also aids plant survival in emerging climate circumstances, when plants must withstand increased temperatures and drought swings (Umair Hassan et al., 2020). According to FAO, zinc deficiency affects greater than fifty percent of global soils, mostly because Zn is associated with naturally occurring mineral forms that are often inaccessible to plants, like zincite, zinc silicates, willemite, and zinc sulfide (Lopes et al., 2021).
Application of inorganic fertilizers can correct a zinc shortage, although doing so causes some environmental harm and renders a significant portion of the fertilizer unavailable to plants. It would be preferable to use PGPR, which is noted for its ability to completely fill cation-containing minerals found in nature. Zinc exists in insoluble forms in the rhizosphere and is saturated by certain bacteria and fungi, increasing the available zinc nutrients. Inoculation with Zn-mobilizing PGPR has been proven in numerous experiments to significantly boost the yield of cereal crops like maize, wheat, and rice (Lopes et al., 2021).
Because of the rapidly expanding global population, there is a growing demand for staple foods, which in turn increases the demand for pesticides and artificial fertilizers. However, this could have a negative impact on the environment. Biofertilizers might be unable to completely replace mineral fertilizers at this time, but by adding beneficial bacteria to agricultural production, the rising number of people in the world necessitates more of the basic foods that are grown with the help of artificial fertilizers and pesticides.
3.1.3. Plant growth hormones production:
Phytohormones, as well as plant growth hormones, have an impact on Plant maturation and growth even at minimal doses (< 1 mM) (Damam et al., 2016). A microorganism, such as certain bacteria, is regarded as a plant growth regulator or phyto stimulator if it has the intrinsic ability to control the production of different growth regulator enzymes. It may even encourage plants to produce these phytohormones. The root cell can produce excessive amounts of auxins, cytokinins, abscisic acid, ethylene, brassinosteroids, and gibberellins that affect the beginning of the root, roots on either side, and hairs on roots, which are followed by a rise in nutrients and water intake. Symbiotic and endophytic bacteria, which live in close proximity to plants' roots, produce phytohormones that affect seed germination, root system expansion to increase nutrient absorption, development or elaboration of vascular tissues, shoot extension, flowering, and general plant growth (Antar et al., 2021a).
Indole Acetic Acid (IAA), cytokinins, gibberellins, and ethylene synthesis inhibitors are among the compounds that PGPRs generate. IAA phyto stimulators produced by PGPRs influence apical growth, phototropism, geotropism, cell division, root initiation, etc. in plants (Nath et al., 2017). An amino acid that is frequently present in root exudates is tryptophan. It serves as the primary precursor molecule in bacteria for the production of IAA (Etesami et al., 2009). IAA-producing microbes might be able to remove potentially toxic levels of tryptophan and tryptophan analogs from their cells. Plant cytokinins limit the expansion of the major root system and promote cell division, lateral root development, and root hair creation, whereas gibberellins promote stem tissue, root lengthening, and root elongation to the side. One important phytohormone, ethylene, has many procedures in biology that might influence plant growth and improvement. It accelerates seed germination, limits elongation of roots, aids in the maturation of fruit, cuts down on leaf withering, boosts drop production, and causes further plant hormone synthesis to increase. It's also crucial in the process of root formation. Bacillus, Pantoea, Arthrobacter, Pseudomonas, Enterobacter, Brevundimonas, and Burkholderia are some PGPRs linked to the production of phytostimulators (Kumar et al., 2014).
3.2. Indirect mechanism
By creating repressive chemicals that boost the host's inherent resistance, PGPR uses roundabout means to protect plants from or lessen the impact of phytopathogens. Hydrolytic enzyme synthesis (chitinases, cellulases, etc.), antibiotic synthesis in response to plant infectious agents or disease resistance, stimulation of systemic protection from a variety of threats from pathogens and pests, production of VOCs and exudates from photosynthesis, etc. are all examples of the roles played by PGPR in this process.
3.2.1. Stress management
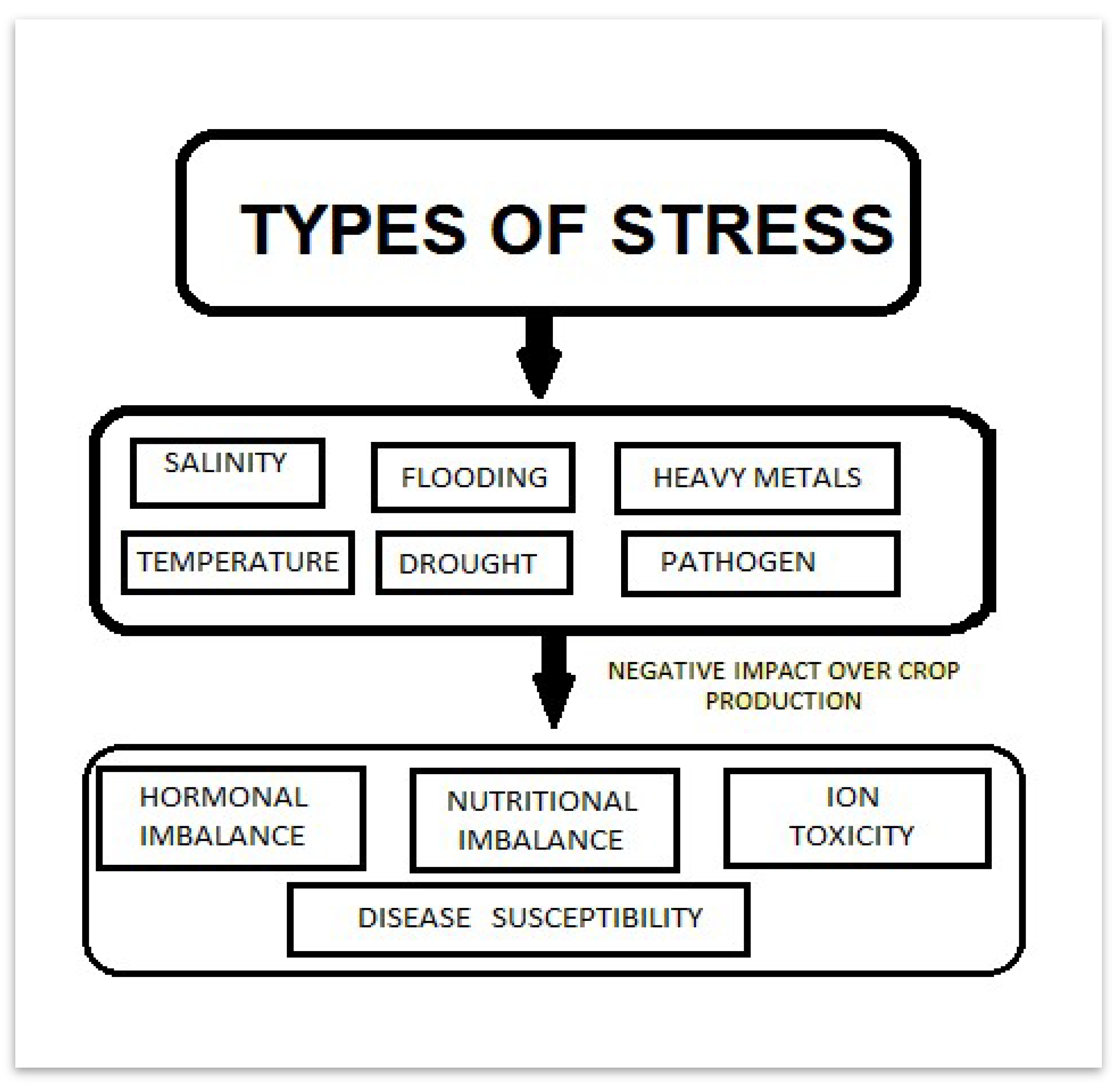
"Stress" refers to anything that acts as a barrier to development in a plant. A fundamental hurdle to long-term agricultural output is the multitude of stresses caused by the soil environment on plant growth. These pressures can be divided into biotic and abiotic groups. The main factor behind the more than 30% global crop loss is abiotic stress. The most common type of abiotic stress that prevents plants from growing and producing is drought or aridity stress, which is brought on by dryness, salinity, and high temperatures (Vejan et al., 2016).
Figure 3.
Graphical representation of Different stressors.
Figure 3.
Graphical representation of Different stressors.
Through the utilization of microorganism strains like Pseudomonas putida and Pseudomonas fluorescens, which significantly affect water salinity and other abiotic stresses and are capable of removing cadmium ions from the ground, PGPR's role in protecting plants from environmental stresses has been extensively researched (Baharlouei et al., 2011). Various pathogens, including bacteria, viruses, fungus, nematodes, protists, insects, and viroids, can produce biotic stress, which significantly lowers agricultural productivity (Haggag et al., 2015). Biotic stress has far-reaching consequences for plant health, including effects on plant health in greenhouses, natural habitat ecology, nitrogen cycling in ecological systems, and other horticultural concerns. PGPR could be used to address these issues and work with strains of Paenibacillus polymyxa B2, B3, and B4, Bacillus amyloliquefaciens HYD-B17, HYDGRFB19, P. favisporus HYTAPB30, and B. subtilis RMPB44.
3.2.2. Hydrolytic Enzymes Production
The action of PGPR in generating protective enzymes could be categorized as a biopesticide. PGPR stimulates plant growth by inhibiting phytopathogenic agents, principally to produce compounds with antibiosis and antifungal characteristics that are used as defense mechanisms. Two examples of hydrolyzing enzymes that would be created throughout the process include chitinase and glucanase. Bacteria that produce chitinases and betaglucanases would inhibit fungal growth because these components make up the bulk of a fungal cell wall. Sinorhizobium fredii KCC5 and Pseudomonas fluorescens LPK2 generate chitinase and beta-glucanase, respectively that prevent Fusarium udum from causing wilt (Kumar et al., 2010).
3.2.3. VOCs Formation
Inducing systemic resistance in plants against phytopathogens and suppressing bacterial, fungal, and nematode infections are only a few of the many benefits that result from the production of volatile organic compounds (VOCs) by PGPR. Several microbial families, like Pseudomonas, Bacillus, Arthrobacter, Stenotrophomonas, and Serratia, produce specific bacterial species that have an impact on plant growth. Bacillus spp. produce the best VOCs, including 2-butanediol and acetoin, for preventing the spread of fungi and encouraging plant development (Santoro et al., 2016).
3.2.4. Exopolysaccharides (EPSs) Production
Diverse types of bacteria, algae, and plants create biodegradable polymers called exopolysaccharides (EPSs). They are constructed from glucose residues and their analogues. (Sanlibaba and Cakmak, 2016). EPSs sustain the host under stress (from salty soil, drought, or too much moisture) by holding water reserves, aggregating soil particles, and facilitating obligatory rhizobacteria, which are bacteria that interact with plant roots. Soil fertility can be increased and sustainable agriculture can be supported thanks to EPS-producing PGPR such as Rhizobium leguminosarum, Azotobacter, Bacillus drentensis, Agrobacterium sp., Xanthomonas sp., and Rhizobium sp. (Mahmood et al., 2016).
3.2.5. Antibiotics production
It has been suggested that microbial antagonists can be used in place of traditional pesticides to combat plant diseases in agricultural crops. By producing antibiotics, PGPR is crucial in preventing the spread of disease-causing bacteria, together with Bacillus spp. and Pseudomonas sp. One of the most promising areas of plant sciences in the last 20 years has been the production of antibiotics by PGPR to fend off numerous plant-based pathogens and extensively researched bio-control mechanisms (Ulloa-Ogaz et al., 2015). Antibiotics such as oomycin A, cepaciamide A, ecomycins, and viscosin are produced by the vast majority of Pseudomonas species. Other medicines manufactured by Pseudomonas include pyrrolnitrin, pyoluteorin, 2,4-diacetylphloroglucinol, rhamnolipids, and pyoluteorin. Additionally, a number of lipopeptide antibiotics, including surfactin and bacillomycin, are produced by Bacillus species, along with an assortment of antibiotics and anti-fungal drugs. The antibiotics are divided into volatile and non-volatile substances for further classification. Polyketides, cyclic lipopeptides, aminopolyols, heterocyclic nitrogenous chemicals, etc. are all examples of non-volatile antibiotics. To name a few examples, volatile antibiotics include alcohols, aldehydes, ketones, hydrogen cyanide, and so on (Fouzia et al., 2015).
4. PGPR used in vegetable production
The PGPR facilitates all beneficial soil processes, including crop residue breakdown and soil organic material production. Improvements in soil fertility and production can be attained by various processes involving the decomposition of organic substances into mineral form, the locking up of mineral nutrients, the dissolution of phosphate, the nitrification of nitrogen, and the synthesis of phytohormones. Rhizobacteria attached to roots produce a high quantity of biomolecules in the soil for further health benefits. Numerous volatile substances and additional compounds (such as enzymes, proteins, etc.) produced by PGPR improve soil health and encourage plant growth. Numerous bacteria from various genera, such as Bacillus, Pseudomonas, Arthrobacter, and Stenotrophomonas, have been identified as producers of volatile compounds (Vejan P. et al., 2016). As more is discovered about the plant growth-promoting mechanism of PGPR, it is anticipated that tomato production will rise while chemical inputs will fall. A study in Ethiopia on the effects of under greenhouse circumstances, root development in tomatoes and the role of Pseudomonas isolates, stems, and leaves were all measured differently, and leaves' dry weight was the most variable (Fenta L. et al., 2017). Additionally, Pseudomonas APF1 and B. subtilis B2G treatments resulted in the highest dry and fresh weight tomatoes ever reported (Lemessa F et al., 2007). Trichoderma spp. and Bacillus spp. established new benchmarks for growth rates, fruit production, and nutrient accessibility.
5. PGPR used in Maize
Corn, or maize (Zea mays L.), is a cereal grass belonging to the Poaceae family. It is one of the world's largest and most significant cereal crop species. The International Grains Council (2019) predicts that global maize consumption will ascend to even greater heights (throughout the years until 2024) and that maize will be used more frequently as animal feed. Providing about half of the energy needed each day by creatures in Africa and the Americas, maize is one of the three most significant species of crop in the world (FAOSTAT Food Balance Sheets, 2020). An increase in fertilizer use will be required to maintain gains in maize productivity, which will raise production costs and have a worsening effect on the environment. Rhizobacteria that promote plant growth offer a wide range of established advantages for crop growth and productivity. Researchers Kuan et al. (2016) suggest that PGPR could be the biological solution needed to improve crop production, rebalance the atmosphere's N2, and slow maize nitrogen recovery plants. Increased ear rates as high as 39 percent with lower fertilizer-N input are encouraged due to the discovery that plant-N remobilization is intimately associated with the aging of plants. Cereal crops, especially maize, can benefit from PGPR's ability to boost their development and grain yield. Several types of bacteria are capable of producing IAA, which has beneficial repercussions on maize's ability to take in nutrients through its leaves and roots. Additionally, phosphorus solubilization and other PGPR features that have not been examined promote plant growth. Also investigated is the bioprotective function of PGPR in maize crops. Fusarium is a harmful fungus that is closely connected with corn. While some forms of PGPR, Bacillus amyloliquefaciens and Microbacterium oleovorans, were successful in protecting maize from Fusarium verticillioides when used as seed coverings (Pereira et al., 2011), Certain PGPR species may help plants flourish by serving as biocontrol and biofertilizers simultaneously. For instance, B. cepacia strains with biocontrol abilities against Fusarium spp. have been noted. They can also encourage the growth of maize in settings with low levels of iron by creating siderophores (Bevivino et al., 1998).
6. PGPR Used in Sugarcane
Due to the numerous advantages connected with its industrial use, Sugarcane, a hybrid of the saccharum plant, is one of the earliest and most important agricultural crops. Tropical and subtropical regions are ideal for growing sugarcane, which is primarily used as a source of raw materials by the sugar industry to make sugar (Zhao and Li, 2015). Because of the advantages linked to the production of biofuel and biogas, sugarcane is also important on a global scale.
The benefits of PGPR over alternative strategies, which can increase sugarcane yield by reducing the amount of fertilizer needed, are both financial and environmental. Poor and inadequately rich soil, which does not fulfill its nutrient and growth demands and makes it challenging to produce high yields, is one of the main obstacles to sugarcane growth. When it comes to the soil, phosphorus (P) is by far the most influential element. Although it is not as crucial for sugarcane as N and K, it nevertheless has a significant effect on the plant's survival and root system development.
Insufficient phosphorus in the starting material, phosphorus absorption by clay, and phosphorus precipitation with iron and aluminum oxides and hydroxides all contribute to insufficient phosphorus availability. As a result, sugarcane cultivation uses a lot of P fertilizers, which raises the cost of production. While a significant percentage of the phosphorus fertilizer provided in the first year accumulates in the soil as fixed P, which is unavailable to plants, only around 10 to 30 percent of that quantity is absorbed by the roots of cane crops (Syers et al., 2008). Finding alternatives to phosphate fertilizers is therefore urgently needed.
In order to maximize the effectiveness of the use of mineral fertilizers, such as phosphate, and to produce sugarcane with little environmental impact, plant growth-promoting rhizobacteria (PGPR) are a possible alternative (Spolaor et al., 2016). Several studies, including Rosa et al. (2020), assessed the impact of inoculating sugarcane with three PGPR species and five dosages of P. They found that the inoculation had a positive impact on the crop and reduced fertilizer costs for the growers. These results showed that the best fertilizer management for sugarcane production involved a mixture of Azospirillum umbrasilense and Bacillus subtilis, along with inexpensive P2O5. The use of B. subtilis in conjunction with by-products can increase soil fertility parameters, lessen the negative effects of vinasse fertilization, promote shoot and root growth, and create a synergistic effect for high-yield sugarcane production that is also good for the environment (Santos et al. 2018).
According to research by Moura et al. (2018), the application of Azospirillum in sugarcane crops enhanced root systems, which improved water and nutrient uptake, which may positively affect yield. This study demonstrated the intricate interplay of several elements, including indigenous plant auxin pools, by demonstrating the considerable interaction of cultivar, water regime, and Azospirillum inoculation.
7. Conclusion:
In conclusion, plant growth-promoting rhizobacteria (PGPR) have emerged as promising tools for sustainable agriculture due to their ability to enhance plant growth and improve crop productivity. This review has provided an overview of the mechanisms through which PGPR exert their beneficial effects on plants, including the production of phytohormones, nitrogen fixation, phosphate solubilization, and biocontrol of plant pathogens.
The use of PGPR as a plant growth enhancer offers several advantages over traditional chemical-based approaches. PGPR are environmentally friendly as they reduce the need for synthetic fertilizers and pesticides, thus minimizing the negative impacts on soil and water resources. Additionally, the use of PGPR can contribute to the development of sustainable farming practices, promoting soil health and biodiversity.
Numerous studies have demonstrated the positive effects of PGPR on various crop plants, including cereals, legumes, and vegetables. These beneficial effects include increased seed germination, enhanced root and shoot growth, improved nutrient uptake, and increased resistance to biotic and abiotic stresses. The ability of PGPR to promote plant growth under adverse conditions, such as drought and salinity, makes it particularly valuable in the face of climate change and resource limitations.
Despite the tremendous potential of PGPR, there are still challenges that need to be addressed for its successful implementation in agriculture. These include the selection and optimization of PGPR strains for specific crops and environmental conditions, understanding the interactions between PGPR and plant hosts, and ensuring the compatibility of PGPR with existing agricultural practices.
In conclusion, the utilization of plant growth-promoting rhizobacteria (PGPR) as a plant growth enhancer holds great promise for achieving sustainable agriculture. By harnessing the beneficial interactions between PGPR and plants, farmers can reduce their reliance on chemical inputs, improve soil health, and increase crop productivity. Further research and field trials are needed to fully understand the potential of PGPR and develop practical strategies for its application on a large scale. With continued efforts, PGPR-based approaches have the potential to revolutionize agriculture and contribute to a more sustainable and resilient food production system.
References
- Adesemoye, A.O.; Kloepper, J.W. Plant–microbes interactions in enhanced fertilizer- use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. “Microbial inoculants for soil quality and plant health,” in Sustainable Agriculture Reviews, eds. E. Lichtfouse (Cham: Springer), 2017; 281–307. [CrossRef]
- Altieri, M.A. Linking ecologists and traditional farmers in the search for sustainable agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: an effective and alternative approach as bio-fertilizers. Int. J. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Antar, M.; Gopal, P.; Msimbira, L.A.; Naamala, J.; Nazari, M.; Overbeek, W.; et al. (2021a). “Inter-organismal signaling in the rhizosphere,” in Rhizosphere Biology: Interactions Between Microbes and Plants (Singapore: Springer), 255–293. [CrossRef]
- Archana, D.; Nandish, M.; Savalagi, V.; Alagawadi, A. Screening of potassium solubilizing bacteria (KSB) for plant growth promotionalactivity. Bioinfolet-A Q. J. Life Sci. 2012, 9, 627–630. [Google Scholar]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Baharlouei, J.; Pazira, E.; Solhi, M. Evaluation of inoculation of plant growthpromotingRhizobacteria on cadmium uptake by canola and barley, in Environmental Science and Technology : proceedings of the second IPCBEE conference, 2011; pp. 2832.
- Bevivino, A.; Sarrocco, S.; Dalmastri, C.; Tabacchioni, S.; Cantale, C.; Chiarini, L. Characterization of a free-living maize-rhizosphere population of Burkholderiacepacia: Effect of seed treatment on disease suppression and growth promotion of maize. FEMS Microbiol. Ecol. 1998, 27, 225–237. [Google Scholar] [CrossRef]
- Bouchet, A.-S.; Laperche, A.; Bissuel-Belaygue, C.; Snowdon, R.; Nesi, N.; Stahl, A. Nitrogen use efficiency in rapeseed. A review. Agronomy for Sustain. Dev. 2016, 36, 38. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Council, I.G. Five-year baseline projections of supply and demand for wheat, maize (corn), rice and soyabeans to 2023/24; International Grains Council: London, UK, 2019. [Google Scholar]
- Czarnes, S.; Mercier, P.-E.; Lemoine, D.G.; Hamzaoui, J.; Legendre, L. Impact of soil water content on maize responses to the plant growth-promoting rhizobacterium Azospirillumlipoferum CRT1. J. Agro. Crop Sci. 2020, 206, 1–12. [Google Scholar] [CrossRef]
- Damam, M.; Kaloori, K.; Gaddam, B.; Kausar, R. Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int. J. Pharm. Sci. Rev. 2016, 37, 130–136. [Google Scholar]
- Etesami, H.A.; Alikhani, H.A.; Akbari, A.A. Evaluation of plant growth hormones production (IAA) ability by Iranian soils rhizobial strains and effects of superior strains application on wheat growth indexes. World Applied Sciences Journal 2009, 6, 15761584. [Google Scholar]
- FAO. Human Vitamin and Mineral Requirements; Food and Agriculture Organization of the United Nations: Bangkok, 2002. [Google Scholar]
- FAOSTAT Food Balance Sheets (2020). Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 24 April 2020).
- Fenta, L.; Assefa, F. Isolation and characterization of phosphate solubilizing bacteria from tomato rhizosphere and their effect on growth and phosphorus uptake of the host plant under greenhouse experiment. Int J Adv Res. 2017, 3, 2320–5407. [Google Scholar]
- Fouzia, A.; Allaoua, S.; Hafsa, C.; Mostefa, G. Plant growth promoting and antagonistic traits of indigenous fluorescent Pseudomonas spp. Isolated from wheat rhizosphere and a thalamus endosphere. Eur. Sci. J. 2015, 11, 129–148. [Google Scholar]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agri. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Goteti, P.K.; Emmanuel LD, A.; Desai, S.; Shaik MH, A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef]
- Goteti, P.K.; Emmanuel LD, A.; Desai, S.; Shaik MH, A. Prospective zinc solubilising bacteria for enhanced nutrient uptake and growth promotion in maize (Zea mays L.). Int. J. Microbiol. 2013, 2013, 869697. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth promoting rhizobacteria (PGPR): current and future prospects for development of sustainable agriculture. J MicrobBiochemTechnol 2015, 7, 096–102. [Google Scholar]
- Haggag, W.M.; Abouziena, H.F.; Abd-El-Kreem, F.; El Habbasha, S. Agriculture biotechnology for management of multiple biotic and abiotic environmental stress in crops. J. Chem. Pharm. Res. 2015, 7, 882889. [Google Scholar]
- Heydari, A.; Misaghi, I.; Balestra, G. Pre-emergence herbicides influence the efficacy of fungicides in controlling cotton seedling damping-off in the field. Int. J. Agri. Res. 2007, 2, 1049–1053. [Google Scholar] [CrossRef]
- Hillel, D. (2008). “Soil biodiversity,” in Soil in the Environment, ed D. Hillel. (San Diego, CA: Academic Press), 163–174. [CrossRef]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi SM, S.; Rasheed, M. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci 2009, 1, 48–58. [Google Scholar]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.d.; et al. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicerarietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef] [PubMed]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: an economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Schippers, B.; Bakker, P.A.H.M. Proposed elimination of the term endorhizosphere. Phytopathol. 1992, 82, 726–727. [Google Scholar]
- Kuan, K.B.; Othman, R.; Rahim, K.A.; Shamsuddin, Z.H. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Pratush, A. Molecular diversity and functional variability of environmental isolates of Bacillus species. SpringerPlus 2014, 3, 312. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, B.R.; Raghuwanshi, R.; Meena, V.S.; Islam, M.T. Coinoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticumaestivum L.) in the Alluvial soil under Indo-Gangetic plain of India. J. Plant Growth Regul. 2017, 110. [Google Scholar] [CrossRef]
- Kumar, H.; Bajpai, V.K.; Dubey, R.C. Wilt disease management and enhancement of growth and yield of Cajanuscajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Protect. 2010, 29, 591–598. [Google Scholar] [CrossRef]
- Lazarovits, G.; Nowak, J. Rhizobacteria for improvement of plant growth and establishment. HortScience 1997, 32, 188–192. [Google Scholar] [CrossRef]
- Lemessa, F.; Zeller, W. Screening rhizobacteria for biological control of Ralstoniasolanacearum in Ethiopia. Biol Cont. 2007, 42, 336–44. [Google Scholar] [CrossRef]
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; et al. Climate-smart agriculture for food security. Nat. Clim. Change 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Lopes, M.J.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Lyu, D.; Zajonc, J.; Pagé, A.; Tanney, C.A.; Shah, A.; Monjezi, N.; et al. Plant Holobiont Theory: The Phytomicrobiome Plays a Central Role in Evolution and Success. Microorganisms 2021, 9, 675. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Jian-jiao, Z.; En-tao, W.; Qian, C.; Jing, X.; Jian-guang, S. Multiphasic characterization of a plant growth promoting bacterial strain, Burkholderia sp. 7016 and its effect on tomato growth in the field. J IntegrAgric 2014, 14, 1855–1863. [Google Scholar]
- Moura, R.T.D.A.; Garrido, M.D.S.; Sousa, C.D.S.; Menezes, R.S.C.; Sampaio, E.V.D.S.B. Comparison of methods to quantify soil microbial biomass carbon. Acta Sci. Agron. 2018, 40, 39451. [Google Scholar] [CrossRef]
- Nath, D.; Maurya, B.R.; Meena, V.S. Documentation of five potassium-and phosphorus-solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatalysis and Agricultural Biotechnology 2017, 10, 174181. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the Impact of Climate Change on Plant Productivity and Ecosystem Sustainability; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. Potassium solubilisation by Rhizosphere Bacteria: influence of nutritional and environmental conditions. J. Microbial. Res. 2013, 3, 25–31. [Google Scholar]
- Pereira, P.; Ibàñez, F.; Rosenblueth, M.; Etcheverry, M.; Martínez-Romero, E. Analysis of the bacterial diversity associated with the roots of maize (Zea mays L.) through culture-dependent and culture-independent methods. ISRN Ecol. 2011, 10. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.; Del Cerro, P.; Espuny, M.; Jiménez-Guerrero, I.; et al. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Prajapati, K.; Sharma, M.; Modi, H. Growth promoting effect of potassium solubilizing microorganisms on Abelmoscusesculantus. Int. J. Agric. Sci. 2013, 3, 181–188. [Google Scholar]
- Rezanka, T.; Palyzová, A.; Sigler, K. Isolation and identification of siderophores produced by cyanobacteria. Folia Microbiol. 2018, 63, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Riaz, U.; Murtaza, G.; Anum, W.; Samreen, T.; Sarfraz, M.; Nazir, M.Z. (2021). “Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers and biopesticides,:” in Microbiota and Biofertilizers: A Sustainable Continuum for Plant and Soil Health, eds K. R. Hakeem, G.H. Dar, M.
- Rosa PA, L.; Mortinho, E.S.; Jalal, A.; Galindo, F.S.; Buzetti, S.; Fernandes, G.C.; et al. Inoculation with growth-promoting bacteria associated with the reduction of phosphate fertilization in sugarcane. Front. Environ. Sci. 2020, 8, 32. [Google Scholar] [CrossRef]
- Sanlibaba, P.; Cakmak, G.A. Exo-polysaccharides production by lactic acid bacteria. Appl. Microbiol. 2016, 2, 1–5. [Google Scholar]
- Santoro, M.V.; Bogino, P.C.; Nocelli, N.; Cappellari, L.R.; Giordano, W.F.; Banchio, E. Analysis of plant growth promoting effects of Fluorescent pseudomonas strains isolated from MenthapiperitaRhizosphere and effects of their volatile organic compounds on essential oil composition. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Kandasamy, S.; Rigobelo, E.C. Sugarcane growth and nutrition levels are differentially affected by the application of PGPR and cane waste. Microbiologyopen 2018, 7, e00617. [Google Scholar] [CrossRef]
- Saravanan, V.; Kumar, M.R.; Sa, T. “Microbial zinc solubilization and their role on plants,” in Bacteria in Agrobiology: Plant Nutrient Management, eds. D. Maheshwari (Berlin; Heidelberg: Springer), 2011; 47–63. [CrossRef]
- Setiawati, T.C.; Mutmainnah, L. Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agri. Agri. Sci. Proc. 2016, 9, 108–117. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Nithya, K.; Harikrishnan, H.; Jayaprakashvel, M.; Balasubramanian, N. Biocontrol mechanisms of siderophores against bacterial plant pathogens. Sustain. Approach. Control. Plant Pathog. Bacteria 2015, 24, 167–190. [Google Scholar]
- Spolaor, L.T.; Gonçalves, L.S.A.; Santos, O.J.A.P.D.; Oliveira, A.L.M.D.; Scapim, C.A.; Bertagna, F.A.B.; et al. Plant growth-promoting bacteria associated with nitrogen fertilization at topdressing in popcorn agronomic performance. Bragantia 2016, 75, 33–40. [Google Scholar] [CrossRef]
- Sundara, B.; Natarajan, V.; Hari, K. Influence of phosphorus solubilizing bacteria on the changes in soil available phosphorus and sugarcane and sugar yields. Field Crops Res. 2002, 77, 43–49. [Google Scholar] [CrossRef]
- Syers, J., Johnston, A., and Curtin, D. (2008). Efficiency of Soil and Fertilizer Phosphorus Use. Roma: FAO Fertilizer and plant nutrition bulletin, 18.
- Tian, F.; Ding, Y.; Zhu, H.; Yao, L.; Du, B. Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Brazil. J. Microbiol. 2009, 40, 276–284. [Google Scholar] [CrossRef]
- Ulloa-Ogaz, A.L.; Munoz-Castellanos, L.N.; Nevarez-Moorillon, G.V. Biocontrol of phytopathogens: Antibiotic production as mechanism of control, the battle against microbial pathogens. In: In: Mendez Vilas, A. (Ed.), Basic Science, Technological advance and educational programs 1. pp. 305–309.
- Umair Hassan, M.; Aamer, M.; UmerChattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; et al. Plant growth-promoting rhizobacteria and root system functioning. Frontiers of Plant Science 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed]
- Vaid, S.K.; Kumar, B.; Sharma, A.; Shukla, A.; Srivastava, P. Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J. Soil Sci. Plant Nutr. 2014, 14, 889–910. [Google Scholar] [CrossRef]
- Van Peer, R.; Schippers, B. Plant growth responses to bacterization with selected Pseudomonas spp. strains and rhizosphere microbial development in hydroponic cultures. Can J Microbiol 1989, 35, 456–463. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq, B.A. Role of plant growth promoting rhizobacteria in agricultural sustainability - A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability-a review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef]
- Wood, N.T. Nodulation by numbers: the role of ethylene in symbiotic nitrogen fixation. Trends Plant Sci. 2001, 6, 501–502. [Google Scholar] [CrossRef]
- Zhao, D.L.; Li, Y.R. Climate change and sugarcane production: potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, F. Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 2011, 339, 83–95. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).