Submitted:
24 October 2023
Posted:
24 October 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. General experimental procedures
2.2. Extraction and isolation
2.3. Cell culture
2.4. Cell viability
2.5. IL-6 induced STAT3 luciferase reporter assay.
2.5.1. Real-time PCR
2.5.2. Western blot analysis
2.6. Statistical Analysis
3. Results and Discussion
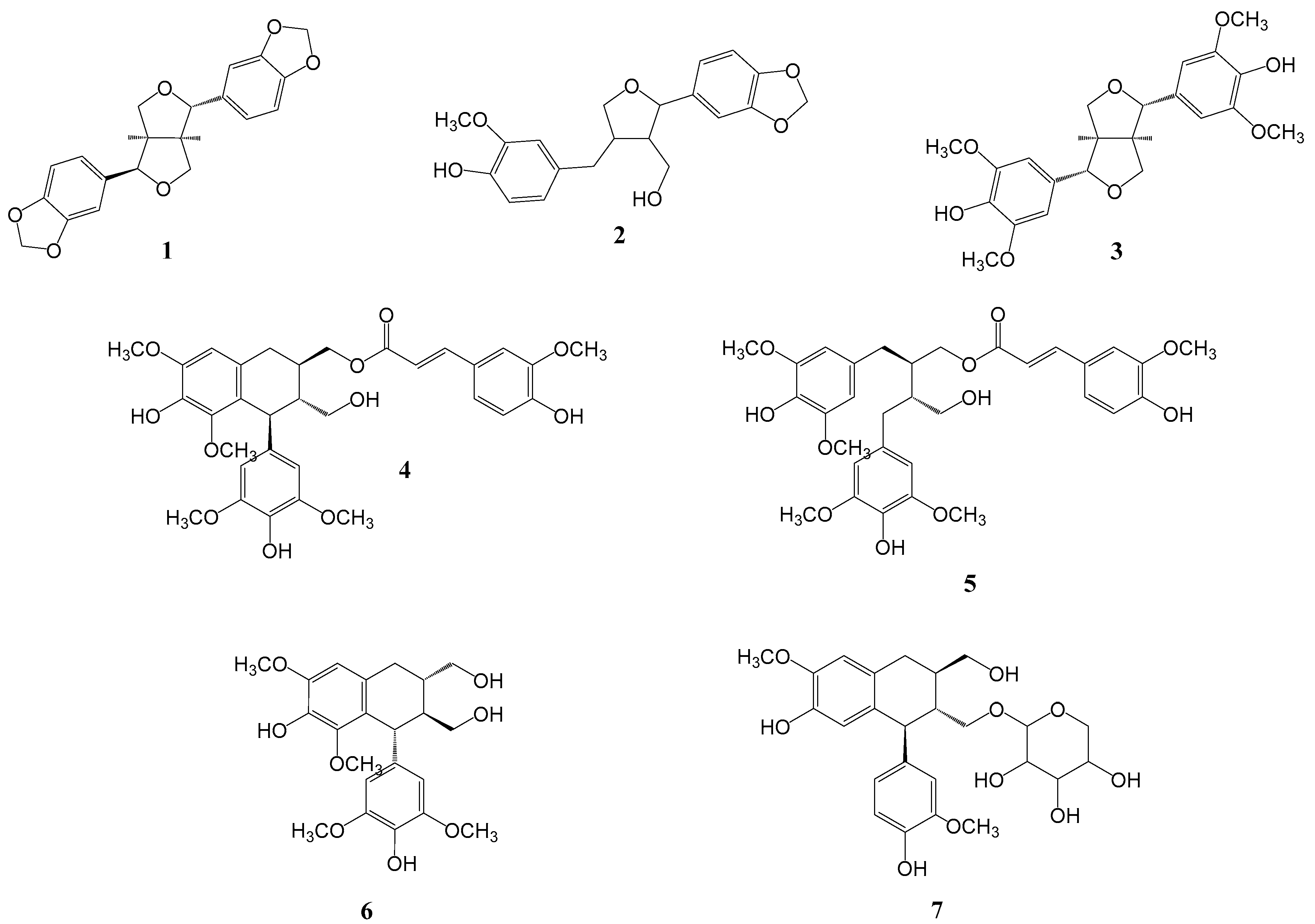
3.1. Identification of compounds from Lindera obtusiloba
3.2. L. Obtusiloba Blume extract and fractions inhibit IL-6-induced pSTAT3 luciferase activity.
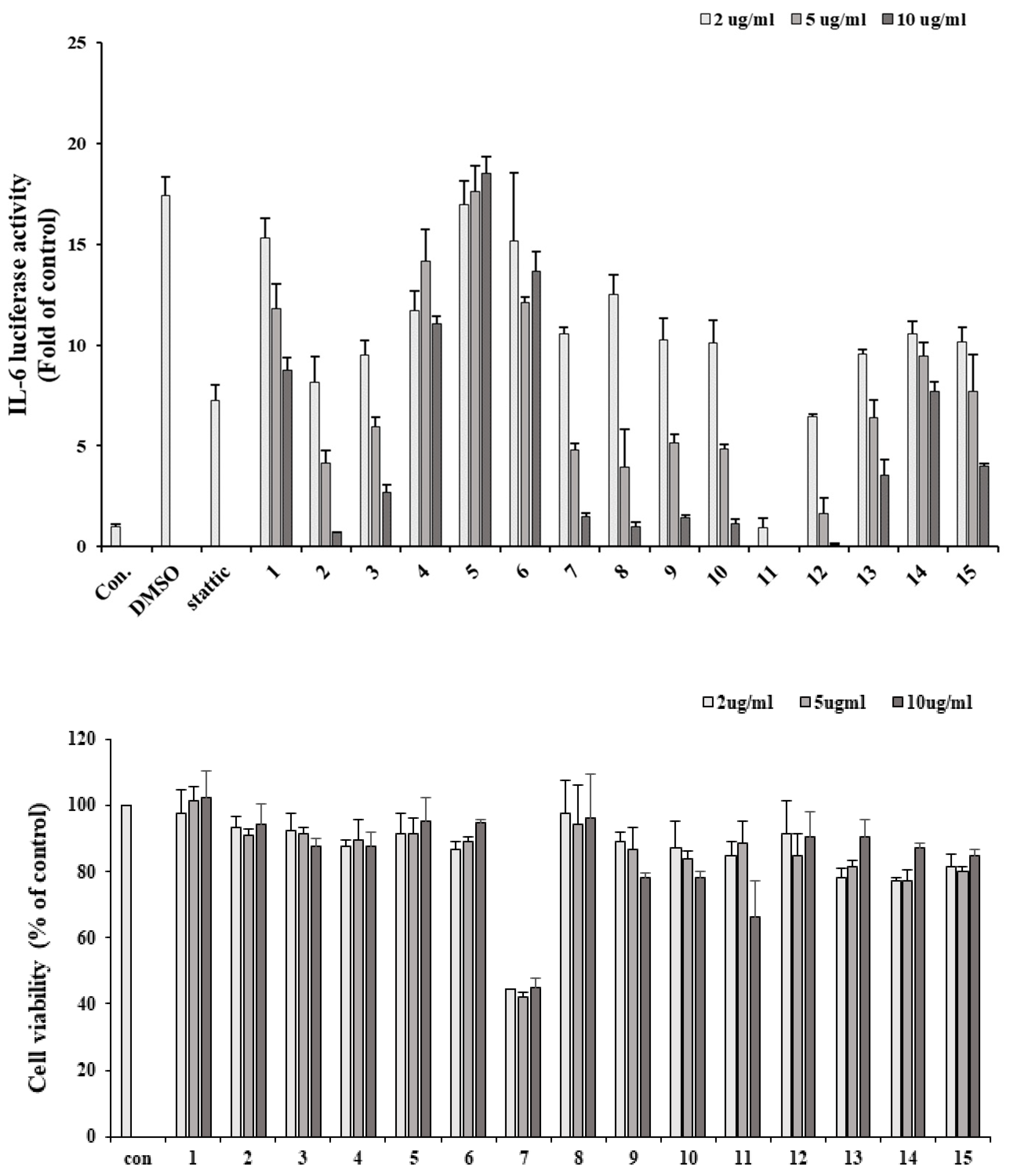
3.3. Inhibitory effects of isolated compounds on IL-6 induced pSTAT3 luciferase activity
| compounds | IC50(µM) |
|---|---|
| 1 | 14.3±2.67 |
| 2 | >50 |
| 3 | >50 |
| 4 | 40.39±3.71 |
| 5 | 10.83±2.40 |
| 6 | >50 |
| 7 | >50 |
| stattica | 0.27±0.03 |
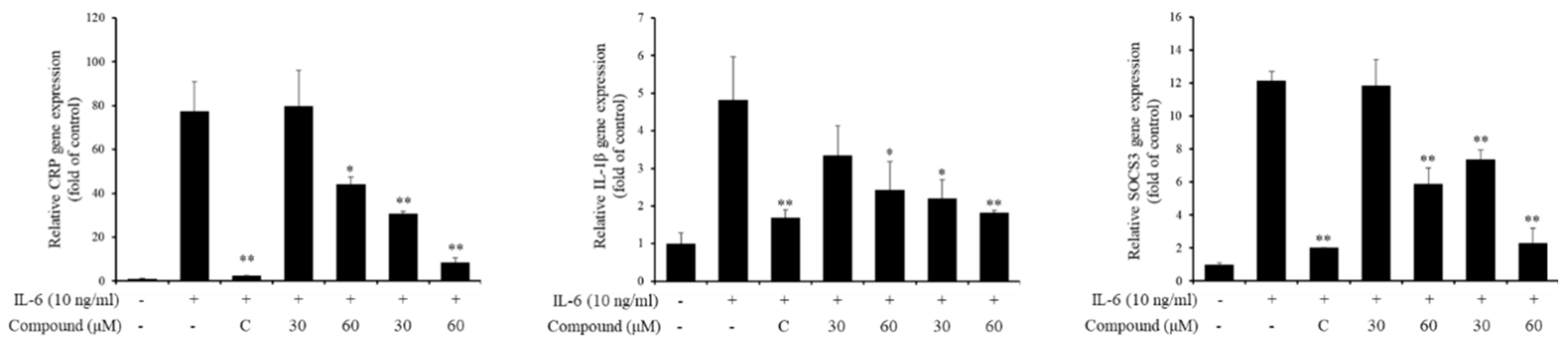
3.4. Inhibitory effects of active compounds on IL-6 induced gene expressions
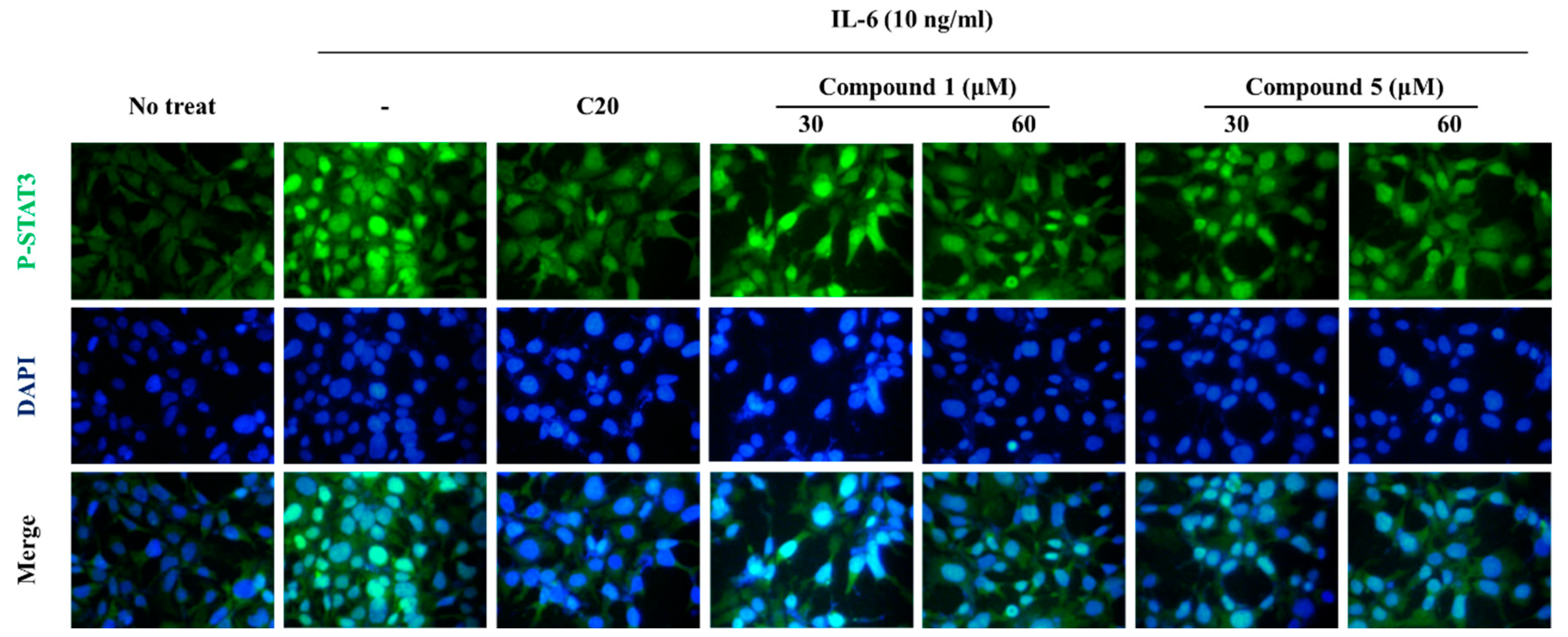
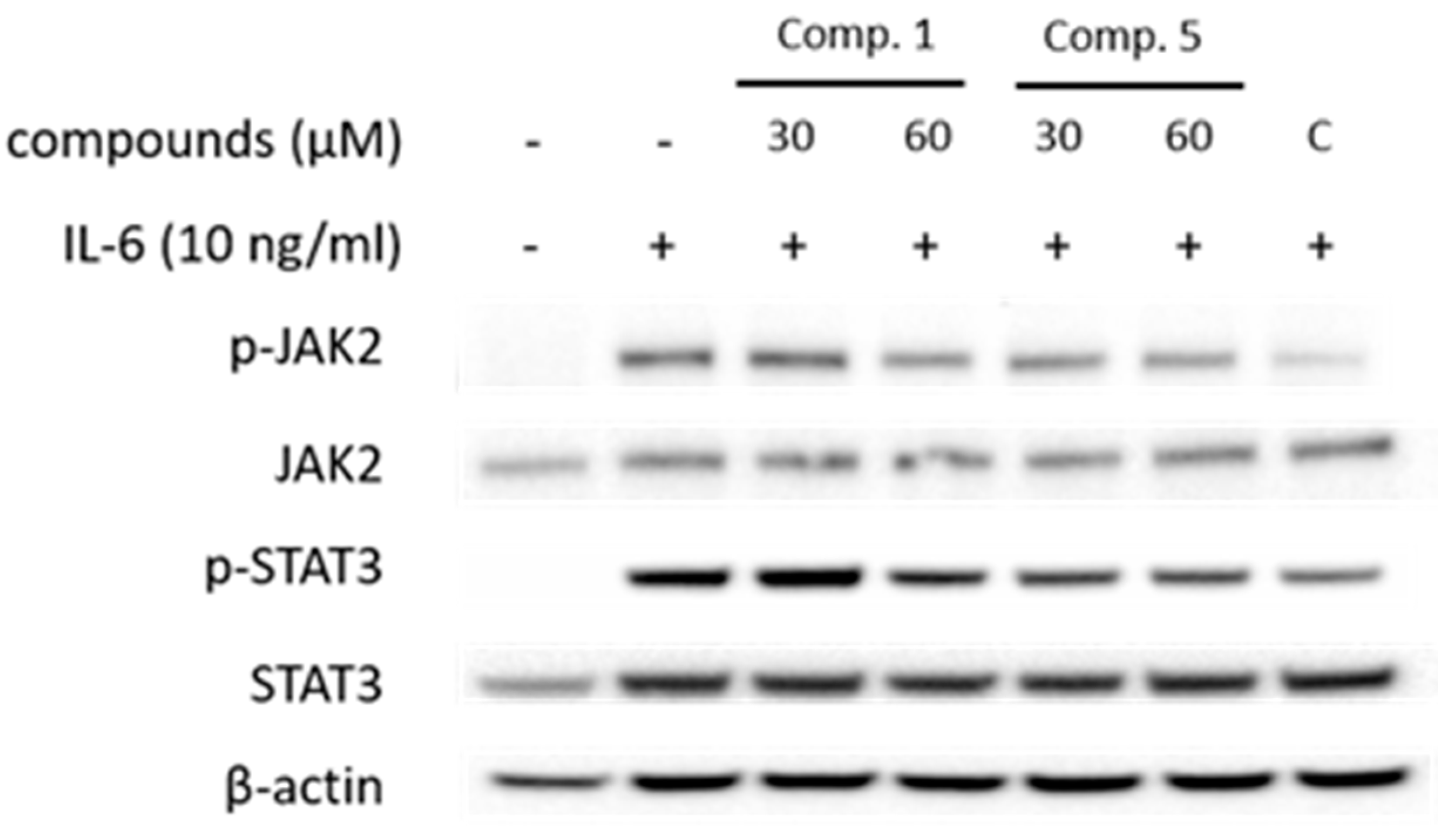
3.5. Effect of compound 1 and 5 on IL-6/STAT3 signaling molecules
4. Conclusions
Acknowledgments
References
- Haque, M.E.; Azam, S.; Balakrishnan, R.; Akther, M.; Kim, I.S. Therapeutic potential of Lindera obtusiloba: Focus on antioxidative and pharmacological properties. Plants. 2020, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.O.; Rhee, C.H.; Won, N.H.; Choi, H.D.; Lee, K.W. Protective effect of 70% ethanolic extract of Lindera obtusiloba Blume on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food and chemical toxicology. 2013, 53, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Yu, Y.B.; Lee, J.H. Isolation and structure elucidation of flavonoid glycosides from Lindera Obtusiloba BL. J Korean Soc Food Nutr. 1996, 25.1, 76–79. [Google Scholar]
- Kwon, H.C.; Choi, S.U.; Lee, J.O.; Kwon, H.C.; Choi, S.U.; Lee, J.O.; Bae, K.H.; Zee, O.P. Lee, K. R. Two New Lignan from Lindera obtusiloba blume. Arch Pharm Res. 1999, 22, 417–422. [Google Scholar] [CrossRef]
- Kwon, H.C.; Baek, N.I.; Choi, S.U.; Lee, K.R. New cytotoxic butanolides from Lindera obtusiloba BLUME. Chemical & Pharmaceutical Bulletin. 2000, 48.5, 614-616. [CrossRef]
- Suh, W.M.; Park, S.B.; Lee, S.; Kim, H.H.; Suk, K.; Son, J.H.; Kwon, T.K; et al. K,; et al. Suppression of mast-cell-mediated allergic inflammation by Lindera obtusiloba. Experimental Biology and Medicine. 2011, 236.2, 240–246. [Google Scholar] [CrossRef]
- Park, K.J.; Park, S.H.; Kim, J.K. Anti-wrinkle activity of Lindera obtusiloba extract. J Soc Cosmet Sci Korea. 2009, 35, 317–323. [Google Scholar]
- Bang, C.Y.; Won, E.K.; Park, K.W.; Lee, G.W.; Choung, S.Y. Antioxidant activites and whitening effect from Lindera obtusiloba BL. Extract. Yakhak Hoeji. 2008, 52.5, 355–360. [Google Scholar]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and antithrombotic effects of the extract of Lindera obtusiloba leaves. Biomol Ther, 2016, 24.6, 659–664. [CrossRef]
- Lee, J.O.; Oak, M.H.; Jung, S.H.; Park, D.H.; Auger, C. An ethanolic extract of Lindera obtusiloba stems causes NO-mediated endothelium dependent relaxations in rat aortic rings and prevents angiotensin II-induced hypertension and endothelial dysfunction in rats. Naunyn Schmiedebergs Arch Pharmacol. 2011, 383, 635–645. [Google Scholar] [CrossRef]
- Seo, K.H.; Baek, M.Y.; Lee, D.Y.; Cho, J.G. Isolation of Flavonoids and Lignans from the Stem Wood of Lindera Obtusioba Blume. J Appl Biol Chem. 2011, 54.3, 178–183. [Google Scholar] [CrossRef]
- Kwon, D.J.; Kim, J.K.; Bae, Y.S. Essential oils from leaves and twigs of Lindera obtusiloba. J Korean For Soc. 2007, 96.1, 65–69. [Google Scholar]
- Lee, K.Y.; Kim, S.H.; Jeong, E.J.; Park, J.H.; Kim, S.H. Kim, Y.C. Sung, S.H. New Secoisolariciresinol Derivatives from Lindera obtusiloba Stems and Their Neuroprotective Activities. Planta Med. 2010, 76.03, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Ruehl, M.; Erben, U.; Kim, K.; Freise, C.; Dagdelen, T.; Eisele, S.; Trowitzsch-Kienast, W.; Zeitz, M.; Jia, J.; Stickel, F., Somasundaram, R. Extracts of Lindera obtusiloba induce antifibrotic effects in hepatic stellate cells via suppression of a TGF-betamediated profibrotic gene expression pattern. J Nutr Biochem. 2009, 20.8, 597–606.
- Choi, H.G.; Choi, Y.H.; Kim, J.H.; Kim, H.H.; Kim, S.H.; Kim, J.A. A new neolignan and lignans from the stems of Lindera obtusiloba Blume and their anti-allergic inflammatory effects. Arch Pharm Res. 2014, 37, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Freise, C.; Erben, U.; Neuman, U.; Kim, K.; Zeitz, M.; Somasundaram, R.; Ruehl, M. An active extract of Lindera obtusiloba inhibits adipogenesis via sustained Wnt signaling and exerts anti-inflammatory effects in the 3T3-L1 preadipocytes. J Nutr Biochem. 2011, 21.12, 1170–1177. [Google Scholar] [CrossRef]
- Mark, D.T.; Belinda, N.; Tara, H.; Daniel. J.P. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochimica et Biophysica Acta. 2014, 1843.11, 2563-2582.
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37.9, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical science. 2012, 122.4, 143–159. [Google Scholar] [CrossRef]
- Jang, H.J.; Lim, H.J.; Park, E.J.; Lee, S.J.; Lee, S.; Lee, S.W.; Rho, M.C. STAT3-inhibitory activity of sesquiterpenoids and diterpenoids from Curcuma phaeocaulis. Bioorganic Chemistry. 2019, 93, 103267. [Google Scholar] [CrossRef]
- Lee, S.J.; Jang, H.J.; Kim, Y.; Oh, H.M.; Lee, S.; Jung, K.; Rho, M.C. Inhibitory effects of IL-6-induced STAT3 activation of bio-active compounds derived from Salvia plebeia R. Br. Process Biochemistry. 2016, 51.12, 2222–2229. [Google Scholar] [CrossRef]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. The Journal of Nutrition. 2006, 136.4, 906–912. [Google Scholar] [CrossRef]
- Chang, C.M.; Chen, T.H.; Huang, Y.H.; Lin, J.J. Cytotoxic lignan ester from cinnamomum osmophloeum. Planta Med. 2009, 76.6, 613–619. [Google Scholar] [CrossRef]
- Sadhu, S.K.; Khatun, A.; Phattanawasin, P.; Ohtsuki, T.; Ishibashi, M. Lignan glycosides and flavonoids from Saraca asoca with antioxidant activity. J Nat Med. 2007, 61, 480–482. [Google Scholar] [CrossRef]
- Rahman, M.A.; Katayama, T.; Suzuki, T.; Nakagawa, T. Stereochemistry and biosynthesis of (+)-lyoniresinol, a syringyl tetrahydronaphthalene lignan in Lyonia ovalifolia var. elliptica I: isolation and stereochemistry of syringyl lignans and predicted precursors to (+)-lyoniresinol from wood. Journal of wood science. 2007, 53.2, 161–167. [Google Scholar] [CrossRef]
- Chen, T.H.; Huang, Y.H.; Lin, J.J.; Liau, B.C.; Wang, S.Y.; Wu, Y.C.; Jong, T.T. Cytotoxic lignan esters from Cinnamomum osmophloeum, Planta medica. 2010, 76.6, 613-619. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





