1. Introduction
The cosmetics industry is growing globally and the discovery and use of new and innovative active principles is key for this sector [
1]. The global market value for natural cosmetics is forecasted to reach
$54.5 billion in 2027 [
2] and there has been an increase of 5% in the discovery of new biologically active compounds per year [
3]. Therefore, the marine environment can play an important role in cosmetics. The oceans host a huge biodiversity [
1,
3,
4], with more than 250,000 species described living in a multitude of habitats [
1], many of them producing active molecules that can be potentially used in new formulations [
1]. Moreover, and, unlike other terrestrial sources like plants, marine flora and fauna can not only produce unique biomolecules in terms of chemical structures and biological properties, but also be cultivated to produce significant amount of biomass [
5].
In this review, the different biological properties of marine-derived molecules are addressed, as well as the challenges and implications concerning the extraction of set compounds and new “blue biotechnology” breakthroughs and how they relate to a sustainable production of new and cutting-edge cosmetics.
This overview uses a compilation of the online available literature on the Google Scholar and Google platforms, as well as on PubMed database. The analysed literature was gathered between 1990 and 2023 and the keywords used included “cosmetics”, “marine”, “extracts”, “algae”, “biotechnology”. “sustainability” and “extraction”. Alone or in combination.
2. Main marine-derived cosmetic ingredients
2.1. Skin-whitening agents
Skin whitening products have been used across the world, with an especially significant market share in Asia [
6]. When skin cells are irradiated by UV radiation, damage of DNA is induced, with increase of cAMP (cyclic adenosine monophosphate) and, consequently, MITF (melanocyte inducing transcription factor), which results in the initiation of transcription of pigmentation genes, including melanin [
7]. Inhibiting the activity of tyrosinase, which catalyzes the rate-limiting step of skin pigmentation [
6], is the most common among the different strategies to promote hypopigmentation [
1,
6,
8]. Tyrosinase catalyzes, through a first reaction, the formation of DOPA (+++) and, in a second phase, the oxidation of DOPA to dopaquinone [
2].
Hydroquinone is used to lighten the dark patches of skin (also called hyperpigmentation, melasma, “liver spots,” “age spots” and “freckles”). Hydroquinone’s toxicity and adverse effects [
2,
9], such as contact dermatitis and exogenous ochronosis [
9], together with a growing tendency for sustainable and biological solutions, has motivated research for skin-whitening agents derived from marine products [
3]. Azelaic acid, kojic acid and phomaligol A are some of the already marketed examples of compounds that are from natural sources [
3,
10].
Brown algae are sources of skin-whitening agents [
2,
9], which are believed to be safer than conventional skin whiteners [
5]. Fucoidans (polysaccharides) and fucoxanthin (xanthophyll) possess tyrosinase inhibition activity, suppress tyrosinase-related protein 1 (TRP1) [
9], and reduce pigmentation [
11]. The oral consumption of fucoxanthin is able to repress COX-2 (cyclo-oxigenase 2), p75NTR (p75 neurotrophin receptor), EP1 and MC1R (melanocortin-1 receptor)-mRNA expression, reducing melanogenesis [
9]. Furthermore, phloroglucinol and its derived polymers phlorotannins [
9], such as 7-phloroeckol [
1,
2], fucophloroethol [
12], fucodiphloroethol [
12], fucotriphloroethol [
12] and dieckol [
4,
12], found exclusively in brown seaweeds [
9,
13], exhibit anti-tyrosinase activity [
1,
2,
5,
11]. Eckol and dieckol have reduced melanin synthesis in B16F10 cells (from a murine melanoma cell line) and dieckol was also reported as having an activity three times higher than kojic acid, with an IC
50 of 2.16 µg/mL inhibiting mushroom tyrosinase [
9]. Isolated from
E. stolonifera, a brown alga from Laminariaceae, dioxinodehydroeckol, another phlorotannin, downregulated melanogenic enzymes, such as tyrosinase, TRP1 and TRP2 (tyrosinase-related protein 2), in B16F10 mouse melanoma cells [
9].
SNA077, a crude extract of marine
Streptomyces sp., has a potential to act as a potent and effective whitening agent, as it showed to inhibit melanogenesis in the MNT-1 human melanoma cell line and in the mouse melanocyte cell lines Melan-a and B16, by downregulating melanogenic proteins in the cAMP/PKA (protein kinase A)/CREB (cAMP response element-binding protein) signaling pathway [
14].
Meroterpenoids isolated from
Sargassum serratifolium, like sargahydroquinoic acid, sargachromenol and sargaquinoic acid, also decrease melanogenesis in melanoma cells activated by α-MSH (α-melanocyte stimulating hormone), influencing CREB signaling pathways [
15].
Another important, less common, strategy to promote hypopigmentation is through preventing the maturation or intracellular trafficking of tyrosinase, inhibiting melanosomal transfer. An example is niacinamide, a vitamin B3 derivative, which is found in fish in general [
8].
2.2. Anti-aging activity
Skin aging is a complex process involving intrinsic and extrinsic mechanisms [
2,
16,
17,
18], resulting in macroscopical changes of the skin, such as xerosis, wrinkles, fine lines, laxity and vasculature prominences, and the appearance of benign neoplasms (e.g., seborrheic keratosis) [
2,
5,
17,
19].
The inevitable genetic and physiological changes that occur over time [
2,
12,
16,
17,
18], including ethnicity and hormonal and anatomical variations [
16], make up the intrinsic mechanism of skin aging, in which production of progerin increases [
2]. On the other hand, controllable and environmental variables, such as exposure to pollution, smoke, UV radiation and infections agents [
16,
18], are related to extrinsic skin aging [
2,
12,
16,
17,
18], in which DNA alteration and damage occurs [
2,
17]. A photoaged skin and a skin that has aged mostly due to intrinsic mechanisms are different morphologically and physiologically (
Table 1).
2.2.1. Photoaging and photo-protective activity
UV radiation, in particular UVA radiation because of its ability to penetrate the dermis [
16], is estimated to account for about 80 to 90% of the skin aging process [
16,
17]. Globally, exposure to sunlight is responsible for a series of biochemical and physiological outcomes, such as disruption of the extracellular matrix (ECM) turnover and of the dermal fibers network, DNA damage, formation of reactive oxygen species (ROS), increase in inflammatory mediators and activation of signaling pathways [
2,
16,
17,
18].
In a photo-aged skin, the disruption of the ECM turnover process, characterized by an upregulation of MMPs (collagenases, gelatinases and stromelysins-1) and a downregulation of TIMPs (tissue inhibitors of matrix metalloproteinases) in keratinocytes and fibroblasts [
17,
20], results in the deterioration of collagen, elastin and other ECM components [
16,
17,
18,
21]. Collagen fibrils, elastic fibers, glycoproteins and glycosaminoglycans (GAGs) are disorganized into a dermal-spreaded agglomeration [
18]. Together with an increased production of XPF (xeroderma pigmentosum factor) [
17], wrinkles appear in the aged skin.
UV radiation also damages the genetic material – through dimerization of pyrimidine (UVB) and formation of ROS or free radicals (UVA) [
16,
17]. Mitochondrial, peroxisomal, membranal and cytosolic ROS are particularly important in the extrinsic skin aging process [
18], since they influence numerous cellular processes, including the activation of MAP-K p38/JNK/ERK/AP-1 signaling pathway that leads to the already mentioned upregulation of MMPs [
16,
22] and, consequently, increased degradation of the ECM [
16]. Simultaneously, NF-kB and AP-1 play an important role in the balance between proliferation and apoptosis [
16]. Additionally, UV radiation interferes with enzymes that participate in the DNA repair process, as well with the immune system’s T cells and Langerhans cells, further damaging the skin in an indirect manner [
16].
MAAs (mycosporine-like amino acids, such as porphyra-35, porphyra-334 or shirorine) and scytonemin are examples of seaweed-derived UV filters [
5,
12,
15,
21,
23], which constitute alternatives to the other available UV filters, which use is controversial due to their environmental impact, sensitizing properties, and potential endocrine disrupting effect [
21]. MAAs absorb UV radiation in wavelengths ranging between 310 and 362 nm, presenting with a better protection efficiency when located outside the cell, whereas scytonemin has a maximum absorption at 386 nm [
21].
Erebusinone, a tryptophan derivative isolated from the Antarctic sponge
Isodictya erinacea with a similar chemical structure to 3-hydroxykynurenine, has possible photoprotective properties, absorbing UVA radiation with a peak absorbance of 370 nm [
24].
Isolated from cod eggs (
Gadus morhua L.), gadusol and gadusolate are structurally similar to mycosporines [
25], sharing some chemical and mechanistic features that make these compounds prone to be included in sunscreen formulations in the future [
21,
25]. However, studies still need to be done in order to determine the exact effectiveness of gadusol, especially what it comes to the SPF (Sun Protection Factor) [
21].
Marine bacteria, especially extremophiles, also produce photoprotective compounds. For instance,
Klebsiella aerogenes produces extracellular semiconductor particles of cadmium sulfide (CdS) in response to environmental stress, that absorb UV radiation [
22].
2.2.2. Inhibiton of MMPs (matrix metalloproteinases)
As stated before, MMPs, which include interstitial collagenases, gelatinases and stromelysin [
2], regulate, among other, the tissue remodeling process, the synthesis and secretion of cytokines and cell adhesion molecules and the degradation of components [
2,
26]. Suppressing and modulating MMPs activity in skin cells, either directly or via signaling pathways, is one of the strategies used nowadays in anti-aging formulations [
2].
Phlorotannin (from
Eisenia bicyclis, a brown seaweed) and phloroglucinol derivatives, such as eckol, dieckol, dioxinodehydroeckol and bieckol, are responsible for the inhibition of MMPs in human fibroblasts [
6,
12,
15]. Eckol and dieckol isolated from
E. stolonifera was able to inhibit MMP-1 expression in human dermal fibroblasts, by interfering with the expression of NF-κB and AP-1, that govern the transcription of MMP-1 [
2,
27]. The extract of
E. stolonifera seaweed showed an inhibitory effect of 76% and 66.7% on NF-κB and AP-1 gene reporter activity, respectively [
27].
Fucosterol, fucoidan and, to a lesser extent, fucoxanthin also present anti-MMP activity. Fucosterol, a phytosterol present in
Hizikia fusiformis (brown seaweed), modulates AP-1 and TGF-β1 signaling, leading to a less expressive activation of MMP-1 [
28]. In a similar manner, fucoidan, as other sulfated polysaccharides, not only inhibit the production of MMP-1 mRNA when fibroblasts are induced with UVB radiation, through ERK pathway, but also increase the expression of type I procollagen synthesis [
12,
15]. Also, by inhibition of the ERK/JNK pathway,
Streptomyces sp.’s derived sarmentosamide is another promising anti-aging agent that reduces the expression of UVB-induced MMP-1 in normal human dermal fibroblasts [
29]. Sargachromanol E, present in the extract of brown macroalgae
Sargassum horneri, activates tissue inhibitors of metalloproteinase 1 (TIMP-1) and 2 (TIMP-2), inhibiting MMPs expression, with a higher effect than retinoic acid [
2,
15].
Besides their potential as UV filters, the aforementioned MAAs also have anti-MMP activity, either by suppressing the expression of UVA-induced MMPs or by modulating the expression of procollagen C proteinase enhancer (PCOLCE) and elastin genes [
12].
2.2.3. Antioxidant activity
Oxidative stress, which is related to ROS production (superoxide anion, hydroxyl radical and hydrogen peroxide), plays an important role in the skin aging process [
2,
5,
18], especially in extrinsic aging, where UV radiation is the main factor [
18]. ROS cause damage to cellular components, including proteins, DNA and membrane lipids [
2,
5], the latter related to lipid peroxidation [
2], and activate numerous signaling pathways, such as MAPK/AP-1/NF-κB/TNF-α/IL-6-mediated inflammation-induced aging and p53/BAX/cleaved caspase-3/cytochrome c-mediated apoptosis-induced aging [
30]. The production of ROS also activates the production of MMPs [
30].
2.2.3.1. Carotenoids
Some of the most known antioxidants are carotenoids, such as β-carotene, astaxanthin, lycopene, torulene and torularhodin [
1,
31]. All have an excellent scavenging activity and an important capacity to inhibit and prevent the formation of ROS [
1,
31], thus being used to prevent UV-induced damage [
30].
Astaxanthin is widely distributed among crustaceans (shrimps, crawfish, crabs, and lobsters) and microalgae, showing stronger antioxidant properties than β-carotene [
12]. It scavenges ROS [
1,
12,
31], inhibits lipid peroxidation [
32] and the activation of the NF-jB transcription factor [
12] and blocks the production of pro-inflammatory cytokines, COX-2 and nitric oxide [
12,
32]. According to different authors [
15,
22,
33], algal extracts containing astaxanthin caused significant changes in SOD (superoxide dismutase) and GSH (glutathione), as well as a protective effect on UVA-induced DNA damage in UV-A irradiated human skin fibroblasts, human melanocytes and human intestinal Caco-2 cells. Extracts from
Haematococcus pluvialis (microalgae, species of Chlorophyta from the family
Haematococcaceae), the richest source of astaxanthin [
7,
21], show improvements in the skin’s macroscopic appearance, regarding wrinkle and texture, displaying a relevant protection against photooxidative damage [
12]. Isorenieratene, renieratene and renierapurpurin are other less known carotenoids isolated from marine sponges, including those from orders Poecilosclerida and Axinellida, that show similar oxygen scavenging and lipid peroxidation inhibitory activities as those of astaxanthin [
5]. Another important pigment with established antioxidant activity, β-carotene, is also in use nowadays, specially the one isolated from microalga
Dunaliella salina, as provitamin A [
1].
2.2.3.2. MAAs, fucoxanthin and pyropheophytin A
Aside from the already mentioned anti-MMPs and photo-absorbing activities, MAAs scavenge ROS, such as superoxide anion, and prevent lipid peroxidation [
5]. Notably, mycosporine-glycine offers a fast protection against oxidative stress, even prior to the intervention of endogenous antioxidant enzymes [
24]. The same goes for sargachromanol E – it already has a well established anti-MMPs activity, but research shows suppression of UVA-induced intracellular formation of ROS and inhibition of lipid peroxidation [
12].
Isolated from the acetone extract of the brown algae
Hijika fusiformis, fucoxanthin also showed potent antioxidant activity against the DPPH free radical [
12].
Cahyana et al. [
34] detected a strong antioxidant activity of both acidic and neutral fractions of the extract of
Eisenia bicyclis, an edible brown alga, through the ferric thiocyanate method, identifying pyropheophytin A as one of the contributors. This newly isolated compound showed a higher antioxidant activity than α-tocopherol [
22].
2.2.3.3. Polysaccharides and oligosaccharides
Regarding marine-derived polysaccharides, fucoidans, carrageenan and ulvans also present antioxidant properties. For example, superoxide and hydroxyl radical scavenging activity was observed in fucoidan isolated from
Laminaria japonica, while that from
Fucus vesiculosus exhibited some ferric reducing antioxidant power (FRAP) [
35].
Isolated from red algae, carrageenan has an established use in cosmetic products as a stabilizer, emulsifier and moisturizer [
36]. However, it also shows an interesting antioxidant activity, with κ-carrageenan exhibiting the highest DPPH reducing capability and, consequently, the highest ROS scavenging potential among the different carrageenan isomers, with a percentage removal of free radicals of 31.42% versus only 23.37% of vitamin E [
35,
36]. This ROS scavenging potential was measured using DPPH assay, in HaCaT cells [
35].
Extracted from the green algae
Ulva pertusa’s cell wall, sulfated heteropolysaccharides called ulvans have important biological properties, such as antioxidant activity and the ability to chelate ferrous ions. The higher the sulfate content in ulvans, the higher was the antioxidant activity [
35].
Lastly, agarose-derived oligosaccharides (AOSs), obtained from chemical and enzymatic hydrolysis of agar, showed some antioxidative potency, especially regarding the radical scavenging capacity in DPPH assays. Agarohexose showed the best results, reducing 50% of oxidants generated by hydrogen peroxide at 1 mg/mL, but agarobiose, agarotetrose and agarohexaose also demonstrated antioxidant properties. In particular, agarohexaose can protect against ROS-associated in vitro cell damage [
35].
2.2.4. Anti-inflammatory and wound healing ingredients
Exposure to sunlight leads not only to microvascular changes and transendothelial migration of leukocytes [
2], but also to the activation of proinflammatory genes, resulting in an inflammation cascade that triggers ROS production [
2,
12], which activate COX-2 and PGE2 [
12]. In parallel, a transcription factor that regulated higher oxidative stress called NFjB is activated by ROS, which stimulates the expression of proinflammatory cytokines such as TNF-α, IL-1a, IL-1b, IL-6, IL-8, IL-10, iNOS (inducible nitric oxide synthase) and COX-2 [
2,
12]. These molecules are produced in keratinocytes and are regulated by NF-κB, a transcription factor that regulates, among others, telomerase gene expression, inflammation and angiogenic activity [
2].
2.2.4.1. Polysaccharides and oligosaccharides
Different sulphated polysaccharides, including fucoidan, have shown in vivo anti-inflammatory activity in different studies [
36]. Fucoidan stands out as the most frequently cited compound for these properties, making it our primary focus. Purified from
Fucus vesiculosus, fucoidan reduces the production of pro-inflammatory molecules, such as nitric oxide (NO), prostaglandin E2 (PGE2), IL-1β and TNF-α, and disrupts the MAPK and AKT signaling pathways in BV2 microglial cell line, as shown by the western blotting applied [
36]. Fucoidan from the brown algae
Turbinaria ornate, reduced the levels of inflammatory biochemical markers in cotton-pelled induced granulomas in rats, such as cathepsin D, myeloperoxidase (MPO) and C-reactive protein (CRP), somewhat comparable to dexamethasone [
36], following a reduction of leukocytes’ recruitment to the site of inflammation [
36].
Fucoidan can be presented in two main isoforms: low molecular weight fucoidan, hereinafter referred to as LMF, and high molecular weight fucoidan (HMF). LMF has a better bioavailability in tissues in dermal wounds created on the dorsal back of rats [
5]. As stated by the study conducted by Park et al. [
37],
Undaria pinnatafida’s LMF is expected to act as a “wound-healing accelerator” [
36,
37], due to its anti-inflammation and angiogenesis activities [
5]. In this study, LMF enhanced the wound healing process, not only by reducing the recruitment of leukocytes, but also by promoting re-epithelization [
37]. The use of fucoidan in topical cosmetic after-sun formulations [
11] and in preventive and therapeutic agents against atopic dermatitis, through inhibition of several chemokines [
35], is also justified by the widely described wound healing and anti-inflammatory properties.
2.2.4.2. Phlorotannins
Dieckol isolated from
Ecklonia cava (a marine brown algae) suppressed, in a dose-dependent way and with no cytotoxicity, the production of nitric oxide (NO) and prostaglandin E2 (PGE2), but also of other pro-inflammatory cytokines (IL-1β and TNF-α) [
38]. The inhibition of NF-κB and p38-MAPK signaling pathways and, consequently, the inhibition of ROS production is another mechanism in which dieckol can prevent inflammation [
38]. Other phloroglucinol derivatives, including phlorofucofuroeckol A and B, which downregulate iNOS and PGE2, inhibiting NO production, and diphlorethohydroxycarmalol, which also downregulates iNOS, COX-2 and NF-κβ, have also been described as anti-inflammatory compounds [
2].
2.1.4.3. Coral-derived pseudopterosins
Corals are also a source of cosmetically interesting compounds. The most relevant ones are
Pseudopterogorgia elisabethae-derived pseudopterosins A, B, C and D, a group of diterpene glycosides that possess various biological activities including anti-inflammatory, analgesic, antibacterial and anti-acne [
5]. These glycosides are already being commercialized as Resilience
® by Estée Lauder [
5]. Pseudopterosin A is the most studied compound. Its anti-inflammatory properties differ from those described so far, as it inhibits phagosome formation and triggers the G-receptor-mediated release of intracellular calcium ions [
5].
2.1.4.4. Sea cucumber-derived fatty acids
Sea cucumbers are known in folk medicine for the treatment of wound healing, being used to accelerate the wound contraction rate [
5]. Some of the most common bioactive compounds in sea cucumbers include saponins, collagen, vitamins A, B1, B2 and B3, amino acids, bioactive peptides, minerals, fatty acids and gelatin [
5].
Stichopus hermanni-based hydrogel has numerous advantages, mostly due to the immobilization of biological active compounds for a longer period in the matrix, which creates a controlled release system that easily interacts with the wounds and facilitates the healing process at a later stage. This hydrogel, which was created by incorporating sea cucumber in a standard hydrogel formulation using an electron beam irradiation technique, showed enhanced histological reorganization and modulation of the inflammatory responses, with a significant reduction of pro-inflammatory cytokines (such as IL-1α, IL-1β and IL-6), in deep partial skin thickness burn wound in rats [
39].
Globally, studies have been corroborating that aqueous extracts of sea cucumbers are much more cosmetically interesting than organic extracts, because of their content in fatty acids and antioxidants, the latter playing an important role in controlling ROS production at wound sites [
5]. For example, decosahexaenoic acid (DHA) from
Stichopus chloronotus has been linked to the stimulation of pro-inflammatory cytokine production at wound sites, leading to a stimulation of the migration and proliferation of skin cells and to a breakdown of ECM proteins [
5]. Moreover, the major fatty acids in sea cucumbers, both DHA and EPA (eicosapentaenoic acid), also stimulate the production of resolvins (inhibiting IL-1β) and protectins (inhibiting TNF-α and IL-1β) via COX-2 and 5-LOX (5-lipoxygenase) pathways [
5]. Saponins present in the extracts of sea cucumbers have also been linked to the prevention of TNF-α production by NF-κB [
5].
The improvement of the levels of TNF-α after the incorporation of sea cucumber extracts into Carbopol
® gel base in diabetic foot ulcer patients, as described by Haryanto et al. [
40], is a practical example of the benefits of these marine organisms in wound healing and inflammation.
2.2.5. Collagen
Collagen, the main structural protein in the ECM, plays a structural role in supporting the formation, tensile strength and flexibility of joints [
41]. The different types of collagen, namely types I, II, III, V and XI, organize themselves into fibrils that allow for support and resistance to mechanical stress in connective tissues [
41].
One of the common sources of collagen is bovine and porcine skin. However, a series of bovine spongiform encephalopathy cases, as well as religious issues, limit its use [
41,
42]. Therefore, marine collagen arises as an important alternative, commonly isolated from fish, jellyfish and sponges [
41]. Aside from accelerating wound healing, through increased vascularization and epidermal growth, and regenerating bone [
41], marine collagen has been showing anti-aging properties, through reduction of wrinkles and improvement of skin elasticity, structure, and appearance [
41]. Furthermore, marine-derived collagen has also shown ROS-scavenging activity, with antioxidant properties [
43].
Collagen derivatives, namely marine collagen peptides (MCPs), have also shown advantages regarding skin and bone repair [
43]. These MCPs are obtained via enzymatic digestion of collagen [
43,
44], using trypsin for example [
44]. MCPs are considered anti-aging compounds, since they promote photoprotection and immunomodulation, as well as improvement of premature senescence of the skin cells [
43]. In particular, Pozzolini et al. [
44] suggests that MCHs (marine collagen hydrolysates) derived from the marine sponge
Chondrosia reniformis can be used both in drug and cosmetic formulations for damaged or photoaged skin repair, due to its capacity to stimulate cell growth.
2.3. Anti-acne activity
Acne vulgaris is the most common skin disease, characterized by the chronic inflammation of the pilosebaceous unit [
5,
45]. It is a multifactorial disorder, in which hormonal, microbiological and immunological mechanisms can be taken into account, and exacerbated sebum production, hyperkeratinization of the follicles and bacterial proliferation are some of the main factors that contribute to acne’s severity and progression [
9,
45]. Microcomedones, the primary type of acne lesions, result from the occlusion of the pilosebaceous ducts due to follicle blockage and accumulation of sebum. These can be closed, resulting in white heads, or open, making up black heads [
45].
Regarding bacterial proliferation,
Propionibacterium acnes and
Staphylococcus epidermidis are the main microbiological targets, because they stimulate an inflammatory environment through the release of ROS and cytokines and the activation of TLR (Toll-like receptors) both in early-stage and late-stage acne inflammation [
5,
46].
P. acnes also releases lipases that digest the excess skin oil and sebum, resulting in local inflammation [
5].
However, defensins, immunocompetent cells, peptidases, PPARs and pro-inflammatory neuropeptides also play an important role in acne-derived inflammation [
46]. Sargafuran, derived from
Sargassum macrocarpum, has antibacterial activity against
P. acnes, with a minimum inhibitory concentration (MIC) of 15 µg/mL [
5], which may be useful in new skincare cosmetics to prevent acne [
2,
47]. On the other hand,
E. bicyclis-derived phlorotannins (a brown alga from the family
Lessoniaceae) also present with an effective inhibitory activity against
P. acnes,
Staphylococcus aureus and
S. epidermidis. The latter is also inhibited by carrageenan (from red algae) with a MIC of 0.325 mg/mL and by sulfated galactan also from red algae [
15].
Diterpenes, originated from soft corals, including cembrene diterpenoids, display several biological properties of interest to the cosmetic industry. Wei Chen et al. [
48] demonstrated that sinulariolides from
S. flexibilis, such as SC-2, SC-7 and sinularin (SC-9), inhibit keratinocyte over-proliferation and anti-NO production properties. SC-9 also inhibited sebum secretion.
Sinularia flexibilis is a species of soft coral in the family
Alcyoniidae. Overall, these compounds show great potential to be integrated in anti-acne formulations.
Brominated compounds, isolated from Rhodophyta species (red algae), constitute a wide group of anti-acne molecules. Ranging from simple compounds like bromophenols or bromoform, to more complex molecules, such as organobromine compounds, these exhibit anti-bacterial activity against
P. acnes and
S. epidermidis.
Symphyocladia latiuscula, a red algae (Rhodomelaceae), contains high amounts of bromophenols, which are toxic to some bacteria, showing ability to inhibit
C. acnes growth, with a MIC of 0.21 mg/mL.
Symphyocladia latiuscula (Harvey) Yamada is predominantly distributed along the coasts of Korea, Japan and northern China [
45].
Osmundaria serrata’s lanosol ethyl ether, another brominated phenol, is highly bacteriostatic and mildly bactericidal, with a MIC of 0.08 mg/mL [
45]. Apart from lanosol, brominated nonterpenoid metabolites such as acetogenins, bromoform, brominated monoterpenes and indoles also present with anti-bacterial activity. Finally,
Asparagopsis armata’s organobromine compounds also have shown an important inhibition in a
P. acnes culture [
45].
2.4. Formulation promoters and facilitators
So far, we have been discussing the different uses of marine-derived products as cosmetic active substances. However, the marine environment also provides interesting excipients and ingredients that facilitate and promote more cosmetically appealing formulations for consumers. Excipients are also indispensable for the product’s long-term stability and microbial resistance to contamination [
4].
2.4.1. Sea water
There is a very limited number of cosmetics, specifically powders, lipsticks and nail polishes that do not include water in their formulations. Given that fresh water is a limited resource, sea water can be an interesting alternative, since it is a well-known source of minerals, including chlorides, magnesium, sodium, calcium, potassium, bromides, sulfates and bicarbonate, with benefits in inflammatory skin disorders, such as atopic dermatitis [
21].
2.4.2. Polysaccharides as gel forming agents and viscosity controllers
Polysaccharides and oligosaccharides include carrageenans, alginates, agar, laminarin, fucoidan, xylans and mannans. The cosmetic properties of different marine-derived polysaccharides and the species where these compounds can be found are depicted in
Table 2.
Alginates and its derived salts can be obtained from brown marine macroalgae, with their cosmetic properties (
Table 2) being linked to physical properties, biocompatibility and biodegradability [
21]. Their role as viscosity controllers requires the presence of a divalent cation, normally Ca
2+ [
21]. Furthermore, the application of alginates in microencapsulating materials for new cosmetic formulations, including those involving a controlled release, is also important, especially at a pH higher than 3-4 for better stability [
4,
21]. At a low pH, these polysaccharides also solidify and stabilize emulsions in a highly efficient manner [
11].
Carrageenans are already widely used in cosmetics, such as creams, shampoos, sticks, sprays, and foams [
4,
11], with gel-forming, emulsifying, thickening and stabilizing properties, besides those described in
Table 2 [
4]. These can form single and double helices, which make up their gelling capacity [
4], depending on the concentration, temperature, presence of other solutes and the type of carrageenan used [
21].
Agar, mainly formed by agarose and agaropectin, is found in the cell wall of red macroalgae and acts as a gelling, emulsifying and suspending agent [
4]. The use of agar as an excipient has been suggested in creams, lotions, deodorants and anti-aging and anti-acne formulations [
11].
Agarose, a natural polymer of galactose also extracted from red seaweed, is a candidate for an emulsifier and thickener used in cosmetics. However, due to its strong hydrophilic and gel properties, algae-derived agarose needs to be chemically modified in order to gain hydrophobicity, reducing its gel strength and giving it amphiphilicity [
50]. In an attempt to better design surfactant-free cosmetics (SFCs), Xiao et al. [
50] used agarose stearate-carbomer940 as a stabilizer and rheology modifier. The final results showed SFCs with good appearance and sensation, with a satisfactory gel-like behavior and a rheological behavior similar to commercial cosmetic creams.
Finally, fucoidan, extracted from brown macroalgae, can be used as a drug carrier for controlled release systems in skin formulations, as a wall component of several pharmaceutical forms, such as microparticles, nanoparticles, hydrogels and nanocapsules [
4].
2.4.3. Carotenoids, chlorophylls and phycobilins as hair dyes
Natural colorants and dyes benefit from a better reputation and acceptance by consumers than the artificial ones, due to the latter’s negative impact on human health and environment [
4]. Macroalgae and cyanobacteria are sources of colorant molecules, such as carotenoids, chlorophylls and phycobilins, with a lower side effects profile and a variety of colors covered – blue, yellow, orange and red [
4,
21]. Phycobillins, chemically tetrapyrroles, constitute the main photosynthetic accessory pigments from some algae of
Glaucophyte,
Crystophyte and
Rhodophyte groups and are covalently bound to proteins, forming phycobiliproteins [
21].
However, the potential use of these compounds comes with some formulation challenges. Chlorophylls and carotenoids are lipophilic molecules, requiring organic solvents for their extraction (
e.g., methanol or DMS), which are incompatible with cosmetics [
4]. On the other hand, water can be used in extracting phycobiliproteins, as they are polar molecules [
4], but it will ultimately alter the organoleptic properties of some formulations, as water should not be used in make-up [
21].
2.4.4. Marine biosurfactants
Marine biosurfactants are byproducts of marine microorganisms’ metabolism. In bacteria, for example, these molecules are produced so that they can use substrates that are not water-soluble [
36]. These amphiphilic molecules, containing both hydrophilic and hydrophobic domains, allow for an easier solubilisation of hydrophobic substances in water, by reducing the interfacial tension. Biosurfactants have low critical micelle concentrations (CMC), which allows using lower concentrations of these compounds when compared to chemically produced surfactants [
36,
51].
Biosurfactants can be classified in high molecular weight (HMW) biosurfactants or bioemulsifiers and in low molecular weight (LMW) biosurfactants, the latter including fatty acids and lipoaminoacids [
51] Especifically, sophorolipids (in
Paracoccus, e.g.,) and rhamnolipids (in
Pseudomonas aeruginosa, e.g.,) have emulsifying and solubilizing properties, among others, justifying their potential use in cosmetic formulations [
51]. Advantages in the use of marine biosurfactants include lower irritancy to the skin, when compared to their synthetic counterparts [
13], and their low eco-toxicity, showing an acceptable environmental impact [
22].
2.4.5. Flavonoids as preservatives
Polyphenols like flavonoids, found in several species of algae, have a significant antibacterial activity.
Gracilaria dendroides (macroalgae), for example, contains high concentrations of rutin, quercetin and kaempferol (10.5 mg/kg, 7.5 mg/kg and 15.2 mg/kg, respectively), which are able to successfully inhibit
E. coli,
P. aeruginosa,
S. aureus and
E. faecalis. These compounds have been extracted using using ethanol, chloroform, petroleum ether and water and the anti-bacterial activity of each compound has been determined using the agar diffusion technique, with ampicillin, oxacillin and ceftazidime as controls [
21].
3. Extraction of marine-derived cosmetic ingredients: challenges and implications
Globally, extracts can be obtained through several unit operations, including upstream processing (preparation for cultivation), cultivation in photobioreactors and downstream processing, in which cell harvesting, rehydration, extraction and ultrafiltration are included [
52].
Conventional methods of extraction include infusion, percolation, Soxhlet extraction, maceration, steam distillation, among others. Advanced, alternative or contemporary extraction methods include supercritical fluid extraction (SFE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE) and electro-technologies. An overview of the existing methods of extraction, some of which will be mentioned in this paper, will be briefly approached in Supplementary Material.
3.3. Challenges regarding the extraction and utilization of cosmetic ingredients
Despite all the benefits and potentialities that come with the utilization of marine-derived ingredients in cosmetics, it is important to consider the different biological, technical and supply challenges, as well as problems regarding contamination and reproducibility of extracts obtained from raw materials.
3.3.1. Biological and technical challenges
It is undeniable that marine biodiversity is both an advantage and a disadvantage for the cosmetic industry and manufacturers – on the one hand, there is a considerable number of compounds that can be explored in cosmetic formulations, but identifying and exploring this diversity in a sustainable and safe manner is yet challenging [
60].
Obtaining marine raw materials remains very difficult, still relying on robotic and engineering advances regarding the access to the ocean, especially the deepest locations. Thus, most studies are conducted in shallow coastal waters, due to difficulties in reaching deeper spots (normally more than 30 m), leading to gaps in biodiversity and chemo-diversity knowledge. ROVs (remotely operated vehicles) are an interesting solution to this problem, but are very expensive and unattractive in developing countries from the tropical and subtropical regions where these natural products are most likely unknown [
60].
Access to biodiversity on natural resources is now under the Convention of Biological Diversity (CBD), in which “the potential role of access and benefit-sharing to contribute to the conservation and sustainable use of biological diversity” is acknowledged [
61]. The use of marine products for biological purposes implies a correct taxonomic identification and classification – an incorrect taxonomic identification and classification can, on the one hand, compromise the entire cosmetic discovery project and lead to problems in the reproducibility of the extracts obtained. Nowadays, this is considered a challenge for the use of marine-derived products, both for the lack of taxonomic knowledge and for the large number of undiscovered and undescribed species [
60].
3.3.2. Sustainable supply
As their terrestrial counterparts, marine organisms contain a high biological and biochemical variability, particularly when considering the quantity and variety of metabolites generated based on the specific environmental conditions in which they grown [
60]. Ensuring a sustainable source can be quite demanding when it comes to harnessing natural products, either because the desired compound exists in minimal quantities within the raw material, which can be influenced by external factors, or because isolating these compounds poses significant challenges. Hence, large quantities of raw material are necessary [
60]. Efforts have been made to address the issue of sustainable supply, such as optimization of molecular biology and aquaculture technologies, the latter including for example IMTA (Integrated Multi Trophic Aquaculture) and RAS (Recirculating Aquaculture Systems), yet it remains a persistent and significant challenge [
60,
62].
Therefore, the development of synthetic or hemisynthetic analogues has been applied. However, due to the intricate nature of naturally-derived molecules, which often feature multiple stereocenters, and the complexities involved in the purification processes, executing this approach becomes intricate and challenging. The need for virtual screening of molecules and the misassignment of natural products are also key points [
60].
3.3.3. Reproducibility of extracts and challenges in the scale-up process
Reproducibility of extracts and the expansion of cultivations from a laboratory scale to an industrial unit are some of the most important challenges industries face when dealing with natural products in general.
Conventional extraction methods show low reproducibility, because of the lack of automation. Therefore, using advanced methods of extraction, such as supercritical fluid extraction (SFE) or electro-technologies, can be beneficial, both due to better reproducibility between extract batches and due to sustainability [
52].
As previously mentioned, natural products alter their metabolite levels and growth rates in response to environmental conditions as a protective mechanism [
63]. Different factors, including temperature, light availability, oxygen levels, mixing parameters, nutrient levels, risk of contamination or infestation, biomass film formation and loss of bioreactor’s transparency are some well-known key factors that influence macro and microalgal growth [
64]. For example, the protein levels of the macroalgae
P. palmata harvested in the French Atlantic coast, fluctuated annually, ranging from 9% to 25%, with a peak in May [
54]. Strategies to harmonize metabolites’ production in algae has been used due to rising research on this topic. For example, red light photons have shown to accelerate cell cycle and green light has shown to increase lipid production [
63].
The scale-up process is crucial in process development [
64] and is usually linked to significant productivity losses, since optimal conditions determined in a laboratory scale are drastically different from those at an industrial scale [
63,
64]. Real time monitorization of temperature and pH of each photobioreactor, in the specific case of microalgae, is an important approach to standardize and optimize the quality of the produced biomass. Management of the cooling system and harvest during the semi-continuous phase on a daily basis are also strategies to overcome set challenges, allowing for different growth conditions, whether optimal or to increase the production of certain metabolites, and bigger consistency between batches [
63,
64]. Before the full implementation of the commercial plant, data obtained from the scaling-up stage must be subjected to rigorous analysis and consideration and the economical aspects of the process should be considered [
64].
3.3.4. Potential contamination of raw material and extracts
The contamination of raw materials and, consequently, of the obtained extracts is a significant concern when incorporating natural ingredients into cosmetics formulation and production. We will delve into two specific types of contamination, namely biological and chemical, in the following sections.
3.3.4.1. Biological contamination
Biological contamination can be divided into two groups, namely a cell-growth affecting contamination and a protein accumulation-affecting contamination. The latter is related to the contaminant’s ability to reduce or consume the molecules produced by the organism of interest. Sources of biological contamination include for example aquatic and air pollution, and the accumulation of nutrients, salt and other organisms in the blind angles of bioreactors used for microalgae cultivation [
65].
In order to reduce these problems, optimization of growth conditions and control and the application of standardized procedures can ensure a constant and repeatable level of contaminants-free, microbiologically-pure components in each raw product unit [
55]. The choice of media is also fundamental for this purpose. An artificial media can, for instance, substitute supplemented municipal domestic wastewater in a culture of algae, reducing the probability of water-related contamination. When wastewater is effectively used in the growth media, pH control can prevent grazers’ contamination (such as zooplankton) [
64]. The use of detergents or phenols can also be an alternative for bacterial contamination [
64].
3.3.4.2. Chemical contamination
Arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), nickel (Ni) and zinc (Zn) are the most found heavy metals in the water environment, with significant environmental and evolutionary toxicity [
21]. Algae, especially macroalgae, absorb heavy metals from marine water, through a bioaccumulation process [
21,
66]. Chang et al. [
66] demonstrated the presence of arsenic (As, 3.9 ppm), iron (Fe, 14.9 ppm) and zinc (Zn, 3.0 ppm) in
Eucheuma cottonii (a red macroalgae) collected in a coastal area of Malaysia. In a study developed with seaweeds from the Venice lagoon, a group of investigators found high contamination levels of lead in
Ulva sp. and, to a lesser extent, in
Gracilaria sp., whereas
Cystoseira sp. was highly contaminated with arsenic [
67].
Besides heavy metals, iodine (I) is also present in ocean-bound algae and other marine products [
52]. However, both heavy metals and iodine are prohibited in cosmetics according to the EU regulation on cosmetic products [
68]. An important note should be taken on Article 17 of the European Union regulation on cosmetic products – it states that “the non-intended presence of a small quantity of a prohibited substance, stemming from impurities of natural or synthetic ingredients, the manufacturing process, storage, migration from packaging, which is technically unavoidable in good manufacturing practice, shall be permitted provided that such presence is in conformity with Article 3” [
68].
Although the presence of heavy metals in marine products is consensual, there is still a lack of information regarding their presence in cosmetic products. However, these chemical elements are expected to be found in their extracts, which will later be incorporated in cosmetic products. In a study by Grillo et.al. [
69], the heavy metal content in the extracts of two different species collected in the Venice lagoon was determined prior to incorporation in a cosmetic formulation –
Sargassum muticum (Heterokontophyta brown algae) and
Ulva lactuca (green algae). These extracts were obtained through MAE (microwave-assisted extraction) in a hydroalcoholic solution (70% EtOH/30% water). Arsenic (As) was not found in any samples, but nickel (Ni), chromium (Cr), lead (Pb), cadmium (Cd) and cobalt (Co) were found in the algal extracts. Particularly in
S. muticum, the levels of nickel and chromium exceeded the legal limits imposed by the already mentioned Article 17 [
69]. In order to reduce the contamination of the final product in levels exceeding the imposed legal limits, the authors suggest a different harvesting location for
S. muticum [
69].
Periodically, and due to oil spills for example, contamination of seawater by VOCs (volatile organic compounds) such as various alkanes and benzene compounds are also possible, and they have already been found in
Cystoseira corniculata and
Jania rubens [
21].
3.4. Public policy and regulatory framework
The use of marine-derived products in cosmetic products still needs further development regarding its regulatory framework, even though its use is increasingly common in the cosmetic industry.
CosIng, EU’s cosmetic ingredient database [
70], along with Regulation 1223/2009/EC [
68], contains all legal requirements and restrictions on each substance. There are several allowed marine-derived cosmetic ingredients in this database, such as fucoidan and carrageenan [
70,
71]. In the EU, a clear science-based regulatory environment is established, where cosmetic claims need to be supported by scientific and adequate evidence and address certain determined criteria [
71]. ISO standard guidelines, such as ISO 16128-1:2016, regarding natural and organic cosmetic ingredients, and ISO 16128-2:2017, regarding the quality criteria for ingredients and products, are also important tools for the cosmetic industry, even though they don’t have legal and regulatory power [
71].
Besides EU’s regulatory documents and ISO guidelines, CEN, the European Committee for Standardization, elaborated several technical reports (TR) about the use of algae in cosmetics – CEN/TR 454 [
73] and CEN/TR 17611 [
74]. The latter will be further discussed below. CEN/TR 454 (standards for algae) considers that algae-derived raw materials should be treated in the same way as plants-derived materials, addressing possible quality and contamination issues with specific standards [
73].
3.4.1. CEN/TR 17611 Algae and algae products – Specifications for cosmetic sector applications
This technical report was formulated in January 2021 in response to a request from the European Commission for CEN to create a standardized guideline on this subject. In this technical report, several important aspects are mentioned regarding product characteristics, product information documents, traceability, sustainable development and labelling [
74].
Regarding purity, for example, the presence of GMO (Genetically Modified Organisms) material and non-organic material in algae and algae products is considered as impurity, that can be determined with macroscopical/microscopical characterization and other identification tests. All powdered materials should be analyzed through microscopical characterization [
74].
Heavy metal, physical (e.g., plastic fragments) and microbiological contamination are some of the addressed subjects in this technical report. Attention for long-term safety aspects are needed, especially regarding local toxicity on skin and eye irritation and sensitization. Dioxins, PAHs (polycyclic aromatic hydrocarbons) and other xenobiotics are also some of the discussed contaminants [
74].
4. Sustainability and marine biotechnology in the cosmetic industry
4.1. Ensuring sustainability in marine-derived cosmetics: a life-cycle approach
Approximately 7 billion tonnes of the 9.2 billion tonnes of plastic manufactured between 1950 and 2017 were transformed into plastic waste, eventually ending up in landfills or being discarded, with the potential to disrupt habitats and jeopardize ecosystems [
75]. While sustainability is currently gaining popularity as a trendy topic, it remains crucial for the well-being of our planet’s environment to address this issue. This is due to the significant ecological, economic, and social impact that the cosmetic industry has, whether it pertains to packaging, production methods, or the implications for the population. The demand for marine natural products in skincare is substantial, and it is imperative to guarantee their sustainable and eco-friendly utilization [
60].
4.1.1. Fundamentals of sustainability and its application to the cosmetic industry
According to the UN’s 1987 Brundtland Report on Environment and Development, sustainability features a balanced consideration of three dimensions: social, economic, and environmental [
76,
77,
78,
79]. Sustainable development is thus defined as the
“development that meets the needs of the present without compromising the ability of future generations to meet their own needs.” [
79]
Innovation and technology are considered important drivers of sustainability and it can concern products or processes [
76,
79]. The rise of ‘eco-friendly’ cosmetics and a special concern with the use of clean technologies and the rational use of natural resources has led to the use of life-cycle approaches (LCA) [
77,
78,
82]. A LCA comprises three steps [
76]: the definition of goal, scope and process system boundaries; the construction of a life-cycle inventory, with inputs and outputs of relevance throughout the entire product’s life cycle; and impact assessment of each life cycle phase, including areas for improvement. LCA covers the product’s full life cycle process, in a holistic way and where the different stages are interdependent, initiating in the extraction of raw materials and including the production, formulation and recycling steps [
77,
78,
81].
It is important to take into consideration that there are no materials that can be considered 100% sustainable – the environmental impact of using certain materials occurs throughout the whole cosmetics supply chain [
83].
4.2.“. Blue biotechnology” and sustainability
4.2.1. Biotechnology, marine biotechnology and “blue biotechnology”
OECD defines biotechnology as the “application of science and technology to living organisms, as well as parts, products and models thereof, to alter living or non-living materials for the production of knowledge, goods and services.” On the other hand, marine biotechnology is defined as “[encompassing] efforts that involve marine bio-resources, as either the source or the target of biotechnology applications” [
85]. Biotechnology can be considered an “umbrella term” [
78,
85], as it includes other sectors, such as “red biotechnology” (or medical, health and pharmaceutical biotechnology), “green biotechnology” (related to agriculture), “yellow biotechnology” (or environmental biotechnology) and “white biotechnology” (related to industry) [
85].
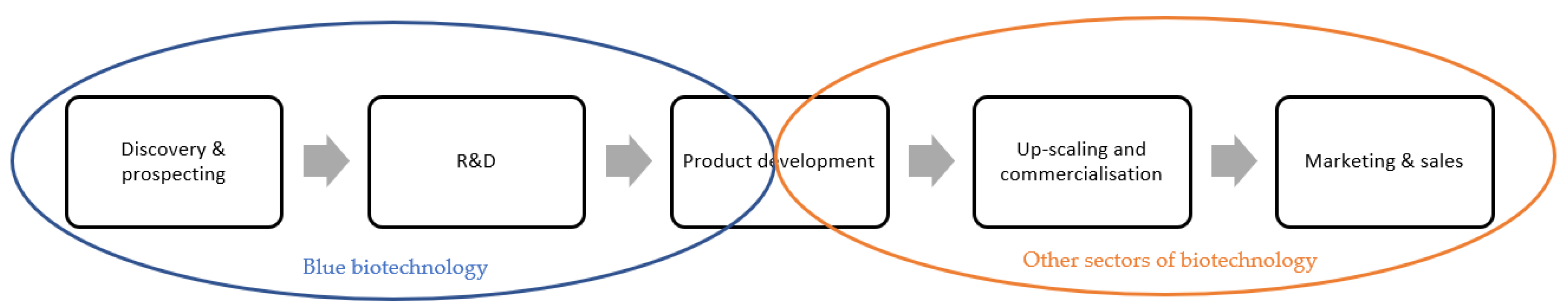
“Blue biotechnology” is related to the use of marine resources as source materials, which is a unique case among the other biotechnology sectors. Therefore, “blue biotechnology” is only applied to the first part of the development pipeline, including sampling, discovery and bioprospecting, research & development (R&D) and initial product development phases [
85]. As represented in
Figure 1, “blue biotechnology” ends in the early stages of product development; the subsequent stages are then inserted in the other sectors of biotechnology [
85].
4.2.2. Biorefinaries in the sustainability assessment of “blue biotechnology” processes
The EU Green Deal, which aims for climate neutrality by 2050, together with the consumers’ preferences with eco-cosmetics, have produced an important environmental awareness among the cosmetics industry [
82]. Using a life cycle approach and considering that “blue biotechnology” is only applied to the initial steps of a cosmetic’s life cycle, it is important to assess the different biotechnological processes regarding their sustainability.
Pagels et al. [
82] conducted an environmental assessment of sea-harvested
Fucus vesiculosus processing and compared it with the antioxidant profiles of vitamin C and green tea extracts, employing a LCA. Their study demonstrated that utilizing seaweed harvested from the sea entails significantly less human intervention and a notably higher growth rate, all without the need for terrestrial fertilizers, as compared to land-based plants. Furthermore, seaweeds have a similar environmental load when compared to ascorbic acid, even presenting an advantage regarding marine eutrophication and water consumption [
64,
82].
To reduce seaweed waste and maximize its diverse applications, the concept of a biorefinery can be applied. A biorefinery involves integrating various biomass conversion processes to generate both energy and value-added products, making it possible to establish such a facility. These processes align with a cascade transformation approach that is nearly zero-waste, simultaneously enhancing the efficiency of biomass conversion [
86].
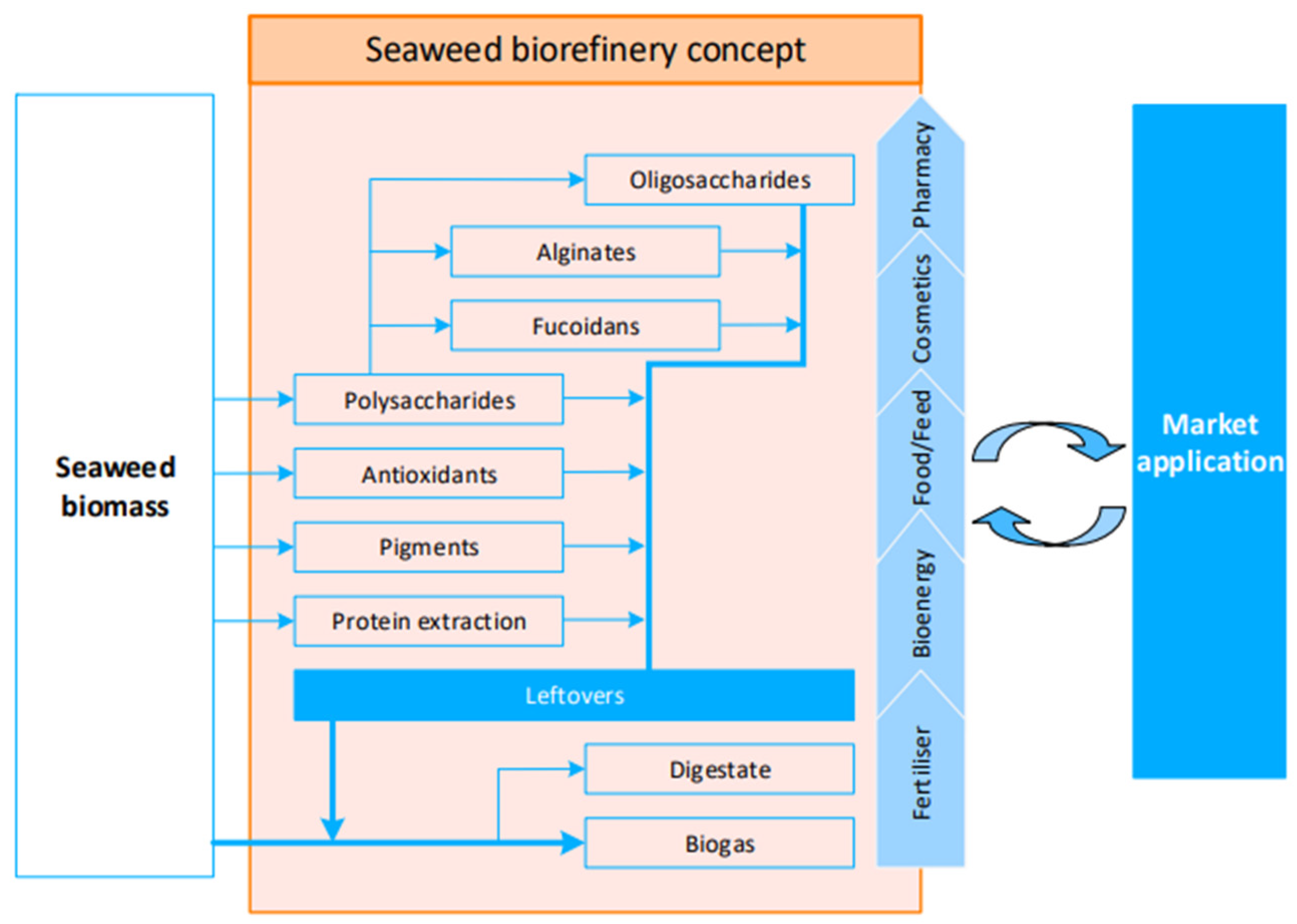
Figure 2 contains a seaweed biorefinary presented by Balina et.al. [
86].
A seaweed biorefinery concept can be used to mainly address the use of seaweed species whose overgrowth is causing ecological damages and disruptions of the coastal environments, due to eutrophication [
86]. Pagels et al. [
82] also introduced a biorefinery model suitable for cosmetics, where an initial extract is created for cosmetic applications, and any residual material is employed either as a fertilizer (acting as a biostimulant and biopesticide) or in the generation of biogas.
Besides antioxidants, the production of astaxanthin from
Haematococcus pluvialis can also have remarkable benefits, whether economical, social and environmental. As demonstrated by Pérez-López et al. [
78], most of the environmental impact was associated with the cultivation stage, especially due to the production of electricity for artificial illumination and air supply. When sunlight was used, instead of artificial illumination, this environmental impact was significantly reduced, but with a decrease in biomass productivity [
87]. This process was economically favorable, with high profitability and low payback time, even considering less favorable conditions and a considerable level of uncertainty [
78].
4.2.3.“. Blue biotechnology”-based applications to the cosmetic industry
4.2.3.1. Marine viruses as a source of ceramides
Despite being not that obvious, marine viruses outnumber any living organism in the sea and have an enormous potential in biotechnology, including in the cosmetic industry [
88].
English researchers from the Plymouth Marine Laboratory and the Sanger Institute in Cambridge discovered that EhV-86, a marine virus, contains a cluster of at least 7 genes that encodes several components of sphingolipid biosynthesis after the virus’ genome had been sequenced. These components then lead to the formation of ceramides, which have been increasingly used in the cosmetics industry as active ingredients due to their skin protection and hydration properties [
88]. The use of ceramides from marine sources have several advantages, because, similarly to collagen, the animal sources used nowadays have a risk of contamination with pathogenic agents such as bacteria or prions (BSE, for example) [
88].
4.2.3.2. Marine-derived biosurfactants
Surfactants and active surface agents are one of the fundamental components of a cosmetic formulation, due to their cleansing, emulsification, foaming, solubilization and conditioning properties [
36]. While this paper has previously discussed marine biosurfactants, we will now explore various sustainability-related advantages associated with their usage, along with providing some illustrative examples.
Artificial surfactants like sodium lauryl sulfate (SLS) and sodium laureth sulfate (SLES) are produced from petroleum-derived sources, leading to concerns about their impact on bioaccumulation, biodegradability, and biocompatibility. These concerns extend to both the environment and human health, with a particular emphasis on the latter due to multiple studies indicating that SLS and SLES can induce skin damage and irritation [
36]. A biosurfactant obtained from
Nocardiospsis VITSISB (a marine actinobacteria) allowed for a more pH-optimum toothpaste formulation than the one formulated with SLS. Liposan and Yansan, biosurfactants from
Yarrowia lipolytica, are also other examples. Yansan, specifically, has a higher emulsification activity and its stability ranges from pH 3.0 and 9.0 [
36].
4.2.3.3. Immobilized lipases from Antarctic fungi and yeasts
Lipases are enzymes that naturally catalyze the hydrolysis of carboxylic ester bonds in hydrophobic compounds, exhibiting compatibility with fatty, non-aqueous media and emulsions found in cosmetics, as well as possessing a wide range of substrate acceptance [
88]. In cosmetic products, lipases can be used both as active ingredients or as biocatalysts in the synthesis of specific cosmetic ingredients [
89]. Examples of lipase applications as active ingredients include facial cleansing, anti-cellulitis treatments, and body slimming products. These molecules are responsible for a mild skin peeling, as they affect the stratum corneum’s keratinocytes and break down fat deposits [
89].
Using their hydrolytic, esterifying and acylating properties, lipases can also be used as biocatalysts of numerous cosmetic ingredients, as part of a “green chemistry” strategy. Enzymes like lipases are a part of this strategy due to their selectivity and stability and do not usually involve the use of organic solvents for their isolation, which can be environmentally hazardous. Furthermore, enzymatic reactions reduce energy consumption, by-product formation (which is itself biodegradable) and the time for heating, with both environmental and economic advantages and a reduction in the production costs [
89].
Marine Antarctic fungi and yeasts were isolated to discover low temperature active lipolytic enzymes. Enzymes like lipases were isolated from the phylum Basidiomycota, from sea urchin-derived
Cryptococcus laurentii and from
Palmaria decipiens and
Geomyces sp. [
81].
4.3. Invasive species as a source of compounds of interest
Climate change, a prominent concern of the 21st century, is manifested through alterations in the behavior, abundance, diversity, and distribution of marine species [
90]. These changes, coupled with environmental shifts in the receiving ecosystems, create favorable conditions for the emergence of invasive alien species (IAS) or nonindigenous marine species (NIMS) [
90,
91]. Typically, these invasive species achieve successful invasions due to characteristics such as rapid growth rates, vegetative propagation, innovative growth strategies, high levels of sexual reproduction and broad environmental tolerance [
91].
To address these species sustainably, one effective approach is to highlight their potential value in developing new products, including cosmetics [
90]. Following this, we will introduce examples of invasive species possessing cosmetic appeal, which present opportunities for sustainable utilization, thereby minimizing their impact on ecosystems.
4.3.1. Sargassum spp.
Sargassum spp. constitute the most abundant group of brown algae, which present a unique mechanism of photon absorption during photosynthesis and a considerable amount of carotenoids, such as fucoxanthin [
92].
The largest concentration of Sargassum in the world is present in the Gulf of Mexico and the Sargasso Sea, containing mostly
S. natans and
S. fuitans. However, excessive amounts of Sargassum on beaches have been reported due to excessive blooming [
93], together with the appareance of a new area of algal masses – the Great Atlantic “Sargasso” Belt – spreading across 8850 km in the Atlantic Ocean [
92]. This accumulation can block sunlight penetration, originating anoxic conditions, loss of nutrients and the production of toxic gases, with subsequent death of species [
92].
Fucoxanthin is the main pigment in Sargassum [
92], with skin-whitening properties, due to the inhibition of tyrosinase activity and the suppression of TRP1 [
9]. Furthermore, meroterpenoids found in
S. serratifolium, with skin-whitening activity [
15], and sargachromanol E in
S. horneri, that activates TIMP-1 and TIMP-2 [
2,
15], are also some of the already mentioned Sargassum-derived compounds.
Especifically, according to Susano et.al., the extract of
S. muticum, that occurs in several European coastlines in high amounts, brought presumptively through
Crassostrea gigas shipments, has anti-aging, anti-acne and anti-UV radiation properties, allowing for the maintenance of skin’s microbiome homeostasis [
90]. Therefore, using this brown macroalgae in the cosmetic industry will not only allow for a better mitigation of this spreading species, but also the retrieval of several cosmetically-important active ingredients [
90].
4.3.2. Ulva lactuca and ulvans
Ulva lactuca is a macroalgae from the phylum
Chlorophyta. Due to its dual reproductive capacity, whether through sexual reproduction or fragmentation of the thallus (asexual reproduction), it can rapidly spread out, covering the water surface and exhibiting an invasive pattern [
93]. Therefore,
Ulva blooms occur frequently, invading beaches and damaging marine ecosystems. These blooms have been observed worldwide, including Europe, Asia, America and Australia – in Europe, Brittany’s north coasts have the biggest
Ulva blooms. As a consequence of
Ulva biodegradation, acidic vapors can be released, leading to the death of animals [
93].
Green macroalgae (with
Ulva lactuca as an example) are rich in sulphated heteropolysaccharides called ulvans, which contribute to the cell wall’s strength [
93]. Ulvans can take part in the encapsulation of active ingredients, as shown by Selvasudha et.al. [
94], in which a combination of ulvan and sodium alginate in a 1:1 proportion resulted in the development of curcumin-loaded microbeads with a 99.2% encapsulation efficiency, significant curcumin release and a good safety and skin tolerance. Furthermore, the presence of phenolic, chlorophyll and carotenoids [
93], as well as ulvans themselves [
35], is also important, due to their radical scavenging activity (antioxidant) [
35,
93].
4.3.3. Undaria pinnatifida
Undaria pinnatifida is a brown seaweed from Japan, Korea and China, which has dispersed through other parts of the globe including Europe and North and America. It is considered one of the most widespread seaweed species in the world, with an invasive behavior [
95]. By outcompeting native species,
U. pinnatifida can negatively impact the biodiversity of certain ecosystems [
95]. However, this macroalgae is rich in fucoidans, a polysaccharide found in brown seaweeds, and phlorotannins, that are reported to be the most concentrated type of phenolics in
U. pinnatifida [
95].
As discussed before in section 1, fucoidans from
U. pinnatifida, especially LMF (low molecular weight fucoidans), act as “wound-healing accelerators” [
36,
37], promoting re-epithelization [
37] and angiogenesis and reducing inflammation [
5]. Furthermore, fucoidan, extracted from brown macroalgae, in which
U. pinnatifida is included, can be used a drug carrier for controlled release systems in skin formulations [
4].
4.4. Marine waste products as a source of compounds of interest
Fishing, farming and processing fish and other sea products are huge generators of leftovers and waste [
96], which are normally disposed of directly into the environment without any treatment [
96,
97]. According to the Food and Agriculture Organization of the United Nations (FAO), make up the annual discards from world fisheries are approximately 20 million tons, including processing leftovers, by-products and unwanted species [
96]. Most of the marine discards have been seen as low-commercially appealing products, but the utilization of this sea waste not only allows for new important cosmeceutical ingredients, but also endorses a zero-waste strategy, pursuing the Sustainable Development Goals (SDGs) of the UN [
96].
4.4.1. Chitin and chitosan
Chitosan is a cationic polysaccharide produced through the deacetylation of chitin, a polysaccharide found in the exoskeleton of crustaceans, through an alkalization process at high temperatures. Chitosan has medical and pharmaceutical applications, with constraints due to its molecular weight and viscosity [
96]. Not only chitosan is biocompatible, biodegradable and non-toxic, but also has antibacterial and antifungal properties [
97].
Crab and shrimp shells are the most known sources of chitin [
96]. In cosmetics, chitosan has been used as a carrier and stabilizer in sunscreen preparations, in the form of nanoparticles, with good storage and color stability in stability studies [
96]. These chitosan-based nanoparticles can also be used as carriers of other cosmetic ingredients, such as anti-aging molecules [
97]. Furthermore, a good cosmetic mask has been developed using a chitosan film. This mask showed good flexibility, good water retention characteristics and is compatible with other active ingredients [
96].
4.4.2. Collagen
Collagen is one of the most used active ingredients in cosmetics and it is biodegradable and biocompatible [
98]. It is a structural protein, being found in the various connective tissues of the human body [
98]. While animal-derived collagen is the most commonly selected source of collagen, religious beliefs, ethnicity, and the existence of various diseases (such as BSE or bovine spongiform encephalopathy) can pose disadvantages to its use [
98]. Therefore, marine-derived collagen (and its derivatives) can be an effective and sustainable option, especially when derived from waste [
96,
98].
When comparing mammal-derived collagen to fish-derived collagen, there is a difference in their effectiveness on the skin. Fish collagen contains lower levels of proline and hydroxyproline, leading to reduced stability and cross-linking compatibility [
98]. Moreover, the human body faces challenges in absorbing peptides with high molecular weights. Therefore, marine hydrolyzed collagen is expected to be more effectively absorbed by human skin [
96]. Fishbone, skin, scale, and swim bladders are rich in a collagen matrix, especially type I collagen [
97]. Collagen has the capacity to efficiently absorb water, resulting in effective moisturizing effects without causing skin irritation [
96,
98].
Guan et al. [
99] studied the cosmeceutical properties of silver carp skin collagen (SCSC). They found that SCSC exhibited excellent foaming and emulsifying properties, along with superior water absorption capacity and oil absorption capacity when compared to proteins sourced from terrestrial origins.
4.4.3. Natural calcium phosphates
Calcium phosphates (CaP) occur naturally in the bones and teeth of vertebrates [
96,
100].
Fisheries’ byproducts, like fish bones, serve as a source of these calcium phosphates (CaPs), including hydroxyapatite, which has demonstrated effective absorption of the entire range of UV radiation [
100], which is of particular interest in the formulation of broad-spectrum sunscreens [
96]. Moreover, in a study conducted by Righi et al. [
100], it was determined that the utilization of natural calcium phosphates offers greater environmental benefits when compared to the use of zinc oxide nanoparticles, particularly concerning issues related to eutrophication and ecotoxicity. Furthermore, these nanoparticles do not exhibit adverse effects on the four aquatic species tested (
Dunaliella tertiolecta,
Tigriopus fulvus,
Corophium insidiosum and
Gammarus aequicauda) [
100].
5. Conclusions
Years of research and development have underscored the vast potential of marine-derived ingredients in the cosmetic industry. This potential is attributed to the multitude of beneficial biological properties inherent in these products and their eco-friendly, sustainable nature. In the present day, there is a growing trend towards sustainable cosmetics and natural ingredients, driven by mounting concerns regarding the environmental and human health impacts of cosmetics. In this review, we have not only highlighted the benefits and positive aspects of incorporating marine-derived ingredients but also delved into the challenges and implications associated with their utilization. Anti-aging, anti-tyrosinase, anti-inflammation, wound healing – these are some of the properties we can find in marine species, ranging from the most obvious algae to the less known sea cucumbers, without forgetting fish and crustaceans. Looking ahead, it is essential to enhance the extraction methods. This is imperative not only to mitigate the potential risks of biological and chemical contamination, which can be addressed through various conventional and advanced techniques, but also to address the relatively high sustainability burden stemming from the substantial volume of raw material required to obtain these compounds and also from the use of hazardous solvents in the extraction phases. This review also delved into the existing regulatory framework governing marine-derived cosmetics. The Regulation 1223/2009/EC, coupled with ISO guidelines and CEN technical reports, constitutes the primary regulatory documents concerning the incorporation of marine-derived ingredients into cosmetics. With the growing adoption of these ingredients, there is a pressing need for an update to the regulatory and normative framework in the coming years. Another challenge pertains to the prevalence of iodine in most marine species, given that it is among the prohibited substances in cosmetic products.
Finally, solutions rooted in “blue biotechnology” hold the potential to offer more sustainable alternatives for cosmetics. One of the primary advantages of these scientific advancements is their reduced environmental and societal impact. Exploring invasive species to mitigate their adverse effects and recycling oceanic waste and byproducts represent innovative approaches to minimize the environmental footprint of cosmetics while delivering novel and cutting-edge products to consumers. Furthermore, a deeper comprehension of sustainability principles applied throughout the cosmetic product lifecycle is essential, particularly to minimize the associated carbon footprint and greenhouse gas emissions. The utilization of marine-derived cosmetic ingredients isn’t a concept we envision solely for the future; it is already a reality in the present. To address the challenges associated with this domain, the cosmetic industry must rely on innovation, creative thinking, and technology, with a particular focus on incorporating “blue biotechnology” solutions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.C., L.C. and C.P.R.; methodology, J.C., L.C. and C.P.R.; formal analysis and investigation, J.C.; writing—original draft preparation, J.C.; writing—review and editing, L.C. and C.P.R.; supervision, L.C. and C.P.R.; funding acquisition, C.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge Fundação para a Ciência e a Tecnologia (FCT) for financial support through Projects UIDB/00645/2020, UIDB/04138/2020 and UIDP/04138/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guillerme, J. B., Couteau, C., & Coiffard, L. (2017). Applications for marine resources in cosmetics. In Cosmetics (Vol. 4, Issue 3). MDPI AG. [CrossRef]
- Thiyagarasaiyar, K., Goh, B. H., Jeon, Y. J., & Yow, Y. Y. (2020). Algae metabolites in cosmeceutical: An overview of current applications and challenges. In Marine Drugs (Vol. 18, Issue 6). MDPI AG. [CrossRef]
- Corinaldesi, C., Barone, G., Marcellini, F., Dell’Anno, A., & Danovaro, R. (2017). Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. In Marine Drugs (Vol. 15, Issue 4). MDPI AG. [CrossRef]
- Lourenço-Lopes, C., Fraga-Corral, M., Jimenez-Lopez, C., Pereira, A. G., Garcia-Oliveira, P., Carpena, M., Prieto, M. A., & Simal-Gandara, J. (2020). Metabolites from macroalgae and its applications in the cosmetic industry: A circular economy approach. In Resources (Vol. 9, Issue 9). MDPI AG. [CrossRef]
- Alves, A., Sousa, E., Sousa, E., Kijjoa, A., Pinto, M., & Pinto, M. (2020). Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. In Molecules (Vol. 25, Issue 11). MDPI. [CrossRef]
- Thomas, N. V., & Kim, S. K. (2013). Beneficial effects of marine algal compounds in cosmeceuticals. In Marine Drugs (Vol. 11, Issue 1, pp. 146–164). MDPI AG. [CrossRef]
- Hawryluk, E. B., Oztan, A., & Fisher, D. E. (2014). Effects of Ultraviolet Exposure Behaviors on Skin Pigmentation and Melanoma. [CrossRef]
- Smit, N., Vicanova, J., & Pavel, S. (2009). The hunt for natural skin whitening agents. In International Journal of Molecular Sciences (Vol. 10, Issue 12, pp. 5326–5349). [CrossRef]
- Azam, M. S., Choi, J., Lee, M. S., & Kim, H. R. (2017). Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. In Marine Drugs (Vol. 15, Issue 10). MDPI AG. [CrossRef]
- Li, X., Jeong, J. H., Lee, K. T., Rho, J. R., Choi, H. D., Kang, J. S., & Son, B. W. (2003). γ-Pyrone derivatives, kojic acid methyl ethers from a marine-derived fungus Alternaria sp. Archives of Pharmacal Research, 26(7), 532–534. [CrossRef]
- Halasariya, H. S., Yadav, V. K., Yadav, K. K., Tirth, V., Algahtani, A., Islam, S., Gupta, N., & Jeon, B. H. (2021). Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. In Molecules (Vol. 26, Issue 17). MDPI. [CrossRef]
- Berthon, J. Y., Nachat-Kappes, R., Bey, M., Cadoret, J. P., Renimel, I., & Filaire, E. (2017). Marine algae as attractive source to skin care. In Free Radical Research (Vol. 51, Issue 6, pp. 555–567). Taylor and Francis Ltd. [CrossRef]
- Mateos, R., Pérez-Correa, J. R., & Domínguez, H. (2020). Bioactive properties of marine phenolics. In Marine Drugs (Vol. 18, Issue 10). MDPI AG. [CrossRef]
- Lim, S. J., Jung, D. W., Hillman, P. F., Nam, S. J., & Lee, C. S. (2022). SNA077, an Extract of Marine Streptomyces sp., Inhibits Melanogenesis by Downregulating Melanogenic Proteins via Inactivation of cAMP/PKA/CREB Signaling. International Journal of Molecular Sciences, 23(23). [CrossRef]
- Jesumani, V., Du, H., Aslam, M., Pei, P., & Huang, N. (2019). Potential use of seaweed bioactive compounds in skincare—a review. In Marine Drugs (Vol. 17, Issue 12). MDPI AG. [CrossRef]
- Farage, M. A., Miller, K. W., Elsner, P., & Maibach, H. I. (2008). Intrinsic and extrinsic factors in skin ageing: A review. In International Journal of Cosmetic Science (Vol. 30, Issue 2, pp. 87–95). [CrossRef]
- N. Puizina-Ivi (2008). Skin aging. In Acta Dermatoven APA (Vol. 17, No 2, pp. 47-54).
- Rinnerthaler, M., Bischof, J., Streubel, M. K., Trost, A., & Richter, K. (2015). Oxidative stress in aging human skin. In Biomolecules (Vol. 5, Issue 2, pp. 545–589). MDPI AG. [CrossRef]
- McCallion, R., & Po, A. L. W. (1993). Dry and photo–aged skin: and manifestations management. In Journal of Clinical Pharmacy and Therapeutics (Vol. 18, Issue 1, pp. 15–32). [CrossRef]
- Yalcinkaya, E., Celik, M., & Bugan, B. (2014). Extracellular matrix turnover: a balance between MMPs and their inhibitors. Arquivos Brasileiros de Cardiologia, 102(5), 519–520. [CrossRef]
- Couteau, C., & Coiffard, L. (2020). Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. In Marine Drugs (Vol. 18, Issue 6). MDPI AG. [CrossRef]
- Pallela, R., Na-Young, Y., & Kim, S. K. (2010). Anti-photoaging and photoprotective compounds derived from marine organisms. In Marine Drugs (Vol. 8, Issue 4, pp. 1189–1202). MDPI AG. [CrossRef]
- Ryu, J., Park, S. J., Kim, I. H., Choi, Y. H., & Nam, T. J. (2014). Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. International Journal of Molecular Medicine, 34(3), 796–803. [CrossRef]
- Núñez-Pons, L., Avila, C., Romano, G., Verde, C., & Giordano, D. (2018). UV-protective compounds in marine organisms from the southern ocean. In Marine Drugs (Vol. 16, Issue 9). MDPI AG. [CrossRef]
- Osantos, R., Churio, ] M Sandra, & Ampedro, D. (n.d.). Computational Exploration of the Photoprotective Potential of Gadusol. [CrossRef]
- Zhang, C., & Kim, S. K. (2009). Matrix metalloproteinase inhibitors (MMPIs) from marine natural products: The current situation and future prospects. In Marine Drugs (Vol. 7, Issue 2, pp. 71–84). MDPI AG. [CrossRef]
- Joe, M.-J., Kim, S.-N., Choi, H.-Y., Shin, W.-S., Park, G.-M., Kang, D.-W., & Kim, Y. K. (2006). The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. In Biol. Pharm. Bull (Vol. 29, Issue 8).
- Hwang, E., Park, S. Y., Sun, Z. wang, Shin, H. S., Lee, D. G., & Yi, T. H. (2014). The Protective Effects of Fucosterol Against Skin Damage in UVB-Irradiated Human Dermal Fibroblasts. Marine Biotechnology, 16(3), 361–370. [CrossRef]
- Lee, E. S., Lee, E. Y., Yoon, J., Hong, A., Nam, S. J., & Ko, J. (2020). Sarmentosamide, an anti-aging compound from a marine-derived streptomyces sp. APmarine042. Marine Drugs, 18(9). [CrossRef]
- Xu, H., Zheng, Y.-W., Liu, Q., Liu, L.-P., Luo, F.-L., Zhou, H.-C., Isoda, H., Ohkohchi, N., & Li, Y.-M. (2018). Reactive Oxygen Species in Skin Repair, Regeneration, Aging, and Inflammation. In Reactive Oxygen Species (ROS) in Living Cells. InTech. [CrossRef]
- Vitale, G. A., Coppola, D., Esposito, F. P., Buonocore, C., Ausuri, J., Tortorella, E., & de Pascale, D. (2020). Antioxidant molecules from marine fungi: Methodologies and perspectives. In Antioxidants (Vol. 9, Issue 12, pp. 1–35). MDPI. [CrossRef]
- Šimat, V., Rathod, N. B., Čagalj, M., Hamed, I., & Mekinić, I. G. (2022). Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. In Marine Drugs (Vol. 20, Issue 3). MDPI. [CrossRef]
- ons, N. M., & O’Brien, N. M. (2002). Modulatory effects of an algal extract containing astaxanthin on UVA-irradiated cells in culture. Journal of Dermatological Science, 30(1), 73–84. [CrossRef]
- Herry Cahyana, A., Shuto, Y., & Kinoshita, Y. (1992). Pyropheophytin a as an Antioxidative Substance from the Marine Alga, Arame (Eisenia bicyclis). Bioscience, Biotechnology, and Biochemistry, 56(10), 1533–1535. 10. [CrossRef]
- Kim, J. H., Lee, J. E., Kim, K. H., & Kang, N. J. (2018). Beneficial effects of marine algae-derived carbohydrates for skin health. In Marine Drugs (Vol. 16, Issue 11). MDPI AG. [CrossRef]
- Anestopoulos, I., Kiousi, D. E., Klavaris, A., Maijo, M., Serpico, A., Suarez, A., Sanchez, G., Salek, K., Chasapi, S. A., Zompra, A. A., Galanis, A., Spyroulias, G. A., Gombau, L., Euston, S. R., Pappa, A., & Panayiotidis, M. I. (2020). Marine-derived surface active agents: Health- promoting properties and blue biotechnology-based applications. In Biomolecules (Vol. 10, Issue 6, pp. 1–28). MDPI AG. [CrossRef]
- Park, J. H., Choi, S. H., Park, S. J., Lee, Y. J., Park, J. H., Song, P. H., Cho, C. M., Ku, S. K., & Song, C. H. (2017). Promoting wound healing using low molecular weight fucoidan in a full-thickness dermal excision rat model. Marine Drugs, 15(4). [CrossRef]
- Jung, W.-K., Heo, S.-J., Jeon, Y.-J., Lee, C.-M., Park, Y.-M., Byun, H.-G., Choi, Y. H., Park, S.-G., & Choi, I.-W. (2009). Inhibitory Effects and Molecular Mechanism of Dieckol Isolated from Marine Brown Alga on COX-2 and iNOS in Microglial Cells. Journal of Agricultural and Food Chemistry, 57(10), 4439–4446. [CrossRef]
- Zohdi, R. M., Zakaria, Z. A. B., Yusof, N., Mustapha, N. M., & Abdullah, M. N. H. (2011). Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 98B(1), 30–37. [CrossRef]
- Ogai, K., Nakagami, G., Oe, M., Nakatani, T., Okuwa, M., Sanada, H., & Sugama, J. (2017). A prospective observational study using sea cucumber and honey as topical therapy for diabetic foot ulcers in Indonesia. Journal of Wellness and Health Care (Vol. 41, pp. 41-56).
- Geahchan, S., Baharlouei, P., & Rahman, M. A. (2022). Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. In Marine Drugs (Vol. 20, Issue 1). MDPI. [CrossRef]
- Venkatesan, J., Anil, S., Kim, S. K., & Shim, M. S. (2017). Marine fish proteins and peptides for cosmeceuticals: A review. In Marine Drugs (Vol. 15, Issue 5). MDPI AG. [CrossRef]
- de Luca, C., Mikhal’Chik, E. v., Suprun, M. v., Papacharalambous, M., Truhanov, A. I., & Korkina, L. G. (2016). Skin antiageing and systemic Redox effects of supplementation with marine collagen peptides and plant-derived antioxidants: A single-blind case-control clinical study. Oxidative Medicine and Cellular Longevity, 2016. [CrossRef]
- Pozzolini, M., Millo, E., Oliveri, C., Mirata, S., Salis, A., Damonte, G., Arkel, M., & Scarfì, S. (2018). Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Marine Drugs, 16(12). [CrossRef]
- Januário, A. P., Félix, R., Félix, C., Reboleira, J., Valentão, P., & Lemos, M. F. L. (2021). Red seaweed-derived compounds as a potential new approach for acne vulgaris care. In Pharmaceutics (Vol. 13, Issue 11). MDPI. [CrossRef]
- Tanghetti, E. A. (2013). The Role of Inflammation in the Pathology of Acne. In J Clin Aesthet Dermatol (Vol. 6, Issue 9).
- Kamei, Y., Sueyoshi, M., Hayashi, K. I., Terada, R., & Nozaki, H. (2009). The novel anti-Propionibacterium acnes compound, Sargafuran, found in the marine brown alga Sargassum macrocarpum. Journal of Antibiotics, 62(5), 259–263. [CrossRef]
- Chen, L. W., Chung, H. L., Wang, C. C., Su, J. H., Chen, Y. J., & Lee, C. J. (2020). Anti-acne effects of cembrene diterpenoids from the cultured soft coral sinularia flexibilis. Marine Drugs, 18(10). [CrossRef]
- Quitério, E., Grosso, C., Ferraz, R., Delerue-Matos, C., & Soares, C. (2022). A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. In Marine Drugs (Vol. 20, Issue 11). MDPI. [CrossRef]
- Xiao, Q., Chen, G., Zhang, Y. H., Chen, F. Q., Weng, H. F., & Xiao, A. F. (2021). Agarose stearate-carbomer940 as stabilizer and rheology modifier for surfactant-free cosmetic formulations. Marine Drugs, 19(6). [CrossRef]
- Kubicki, S., Bollinger, A., Katzke, N., Jaeger, K. E., Loeschcke, A., & Thies, S. (2019). Marine biosurfactants: biosynthesis, structural diversity and biotechnological applications. In Marine Drugs (Vol. 17, Issue 7). MDPI AG. [CrossRef]
- Chojnacka, K., & Kim, S.-K. (2015). Introduction of Marine Algae Extracts. [CrossRef]
- Matos, G. S., Pereira, S. G., Genisheva, Z. A., Gomes, A. M., Teixeira, J. A., Rocha, C. M. R., Alexandre, C., Manuel, J., Saraiva, A., & Pintado, M. (2021). foods Advances in Extraction Methods to Recover Added-Value Compounds from Seaweeds: Sustainability and Functionality. [CrossRef]
- Bleakley, S., & Hayes, M. (2017). Algal proteins: Extraction, application, and challenges concerning production. Foods, 6(5), 1–34. [CrossRef]
- Łęska, B., Messyasz, B., & Schroeder, G. (2018). Application of Algae Biomass and Algae Extracts in Cosmetic Formulations. In Algae Biomass: Characteristics and Applications (pp. 89–101). Springer International Publishing. [CrossRef]
- Lourenço-Lopes, C., Garcia-Oliveira, P., Carpena, M., Fraga-Corral, M., Jimenez-Lopez, C., Pereira, A. G., Prieto, M. A., & Simal-Gandara, J. (2020). Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. In Foods (Vol. 9, Issue 8). MDPI AG. [CrossRef]
- Michalak, I., & Chojnacka, K. (2014). Algal extracts: Technology and advances. Engineering in Life Sciences, 14(6), 581–591. [CrossRef]
- Nadar, S. S., Rao, P., & Rathod, V. K. (2018). Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Research International, 108, 309–330. [CrossRef]
- Gupta, A. K., Seth, K., Maheshwari, K., Baroliya, P. K., Meena, M., Kumar, A., Vinayak, V., & Harish. (2021). Biosynthesis and extraction of high-value carotenoid from algae. In Frontiers in Bioscience – Landmark (Vol. 26, Issue 6, pp. 171–190). Frontiers in Bioscience. [CrossRef]
- Martins, A., Vieira, H., Gaspar, H., & Santos, S. (2014). Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. In Marine Drugs (Vol. 12, Issue 2, pp. 1066–1101). MDPI AG. [CrossRef]
- United Nations (2011). Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising From Their Utilization to the Convention on Biological Diversity. Avaliable in https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf.
- Pangestuti, R., Siahaan, E., & Kim, S.-K. (2018). Photoprotective Substances Derived from Marine Algae. Marine Drugs, 16(11), 399. 11. [CrossRef]
- M Power, D. et.al. (2020), Handbook for Microalgae-Based Novel High Added-Value Products for Cosmetic and Aquaculture. Under the Algae 4a-b Project.
- da Silva, T. L., & Reis, A. (2015). Scale-up Problems for the Large Scale Production of Algae. In Algal Biorefinery: An Integrated Approach (pp. 125–149). Springer International Publishing. [CrossRef]
- Zhu, Z., Jiang, J., & Fa, Y. (2020). Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules, 25(22), 5220. 22. [CrossRef]
- Chang, V. S., & Teo, *. (2016). Evaluation of heavy metal, antioxidant and anti-tyrosinase activities of red seaweed (Eucheuma cottonii). In Journal homepage. 1, Jalan Menara Gading, UCSI Height (Vol. 23, Issue 6).
- Caliceti, M., Argese, E., Sfriso, A., & Pavoni, B. (2002). Heavy metal contamination in the seaweeds of the Venice lagoon. Chemosphere, 47(4), 443–45. 4. [CrossRef]
- Regulation (EC) N° 1223/2009 on cosmetic products. Available in https://eur-lex.europa.eu/legal-content/EN.
- Grillo, G., Tabasso, S., Solarino, R., Cravotto, G., Toson, C., Ghedini, E., Menegazzo, F., & Signoretto, M. (2021). From seaweeds to cosmeceutics: A multidisciplinar approach. Sustainability (Switzerland), 13(23). [CrossRef]
- CosIng – Cosmetic Ingredient Database. Available in https://ec.europa.eu/growth/tools-databases/cosing.
- Lähteenmäki-Uutela, A., Rahikainen, M., Camarena-Gómez, M. T., Piiparinen, J., Spilling, K., & Yang, B. (2021). European Union legislation on macroalgae products. Aquaculture International, 29(2), 487–509. [CrossRef]
- European Sub-Working Group on Claims. (2017) Technical Document on Cosmetic Claims Agreed (2017). Available in https://ec.europa.eu/docsroom/documents/24847.
- European Commitee for Standardization (CEN) Technical Report 454 (CEN/TR 454) Algae and algae products (2021).
- European Commitee for Standardization (CEN) Technical Report 17611 (CEN/TR 17611) Algae and algae products – Specifications for cosmetic sector applications. (2021).
- UN Environment Programme – Plastic pollution. Available in https://www.unep.org/plastic-pollution.
- Cosmetics Europe. Good Sustainability Practice (GSP) for the Cosmetics Industry. Available in https://www.cosmeticseurope.eu/files/4214/6521/4452/GSP_Brochure.
- Tolnay, A., Koris, A., & Magda, R. (2019). Sustainable Development of Cosmetic Products in the Frame of the Laboratory Market. Visegrad Journal on Bioeconomy and Sustainable Development, 7(2), 62–66. [CrossRef]
- Pérez-López, P., Feijoo,G., & Moreira, M.T. (2018). Sustainability Assessment of Blue Biotechnology Processes: Addressing Environmental, Social and Economic Dimensions. In Designing Sustainable Technologies, Products and Policies (pp. 475–486).
- World Commission on Environment and Development (1987). Brundtland Report of the World Commission on Environment and Development: Our Common Future.
- Pereira De Carvalho, A., & Barbieri, J. C. (2012). Innovation and Sustainability in the Supply Chain of a Cosmetics Company: a Case Study. In J. Technol. Manag. Innov. 2012 (Vol. 7, Issue 2). http://www.jotmi.org.
- Murray, P. M., Moane, S., Collins, C., Beletskaya, T., Thomas, O. P., Duarte, A. W. F., Nobre, F. S., Owoyemi, I. O., Pagnocca, F. C., Sette, L. D., McHugh, E., Causse, E., Pérez-López, P., Feijoo, G., Moreira, M. T., Rubiolo, J., Leirós, M., Botana, L. M., Pinteus, S., … Walsh, D. J. (2013). Sustainable production of biologically active molecules of marine based origin. New Biotechnology, 30(6), 839–850. 6. [CrossRef]
- Pagels, F., Arias, A., Guerreiro, A., Guedes, A. C., & Moreira, M. T. (2022). Seaweed Cosmetics under the Spotlight of Sustainability. Phycology, 2(4), 374–383. [CrossRef]
- Bom, S., MRibeiro, H. M., & Marto, J. (2020). Sustainability calculator: A tool to assess sustainability in cosmetic products. Sustainability (Switzerland), 12(4). [CrossRef]
- University of Connecticut, Institute of the Environment, Office of Sustainability. Sustainable Beauty. Available in https://sustainability.uconn.edu/2020/09/11/sustainable-beauty/#:~:text=The%20bottles%2C%20tubes%2C%20and%20containers,70%25%20ends%20up%20in%20landfills.
- Collins, J., Broggiato, A., & Vanagt, T. (2022). Blue Biotechnology. In Building Industries at Sea - ‘Blue Growth’ and the New Maritime Economy (pp. 39–71). River Publishers. [CrossRef]
- Balina, K., Romagnoli, F., & Blumberga, D. (2017). Seaweed biorefinery concept for sustainable use of marine resources. In Energy Procedia, 128, 504–511. [CrossRef]
- Pérez-López P, González-García S, Jeffryes C, Agathos S.N, McHugh E, Walsh D, Murray P, Moane S, Feijoo G, Moreira M.T, Life cycle assessment of the production of the red anti-oxidant carotenoid astaxanthin by microalgae: From lab to pilot scale, Journal of Cleaner Production, Vol. 64, 2014, pp 332–344.
- Sánchez-Paz, A., Muhlia-Almazan, A., Saborowski, R., García-Carreño, F., Sablok, G., & Mendoza-Cano, F. (2014). Marine Viruses: the Beneficial Side of a Threat. In Applied Biochemistry and Biotechnology (Vol. 174, Issue 7, pp. 2368–2379). Humana Press Inc. [CrossRef]
- Ansorge-Schumacher, M. B., & Thum, O. (2013). Immobilised lipases in the cosmetics industry. Chemical Society Reviews, 42(15), 6475–6490. [CrossRef]
- Susano, P., Silva, J., Alves, C., Martins, A., Pinteus, S., Gaspar, H., Goettert, M. I., & Pedrosa, R. (2022). Mitigating the negative impacts of marine invasive species – Sargassum muticum - a key seaweed for skincare products development. Algal Research, 62. [CrossRef]
- ndreakis, N., & Schaffelke, B. (2012). Invasive Marine Seaweeds: Pest or Prize? (pp. 235–262). [CrossRef]
- Lopresto, C. G., Paletta, R., Filippelli, P., Galluccio, L., de la Rosa, C., Amaro, E., Jáuregui-Haza, U., & de Frias, J. A. (2022). Sargassum Invasion in the Caribbean: An Opportunity for Coastal Communities to Produce Bioenergy Based on Biorefinery—An Overview. In Waste and Biomass Valorization (Vol. 13, Issue 6). [CrossRef]
- Dominguez, H., & Loret, E. P. (2019). Ulva lactuca, A Source of Troubles and Potential Riches. Marine Drugs, 17(6), 357. 6. [CrossRef]
- Selvasudha, N., Goswami, R., Tamil Mani Subi, M., Rajesh, S., Kishore, K., & Vasanthi, H. R. (2023). Seaweeds derived ulvan and alginate polysaccharides encapsulated microbeads–Alternate for plastic microbeads in exfoliating cosmetic products. Carbohydrate Polymer Technologies and Applications, 6, 100342. [CrossRef]
- Gan, A., & Baroutian, S. (2022). Subcritical water extraction for recovery of phenolics and fucoidan from New Zealand Wakame (Undaria pinnatifida) seaweed. The Journal of Supercritical Fluids, 190, 105732. [CrossRef]
- Siahaan, E. A., Agusman, Pangestuti, R., Shin, K. H., & Kim, S. K. (2022). Potential Cosmetic Active Ingredients Derived from Marine By-Products. In Marine Drugs (Vol. 20, Issue 12). MDPI. [CrossRef]
- Santos, V. P., Marques, N. S. S., Maia, P. C. S. V., de Lima, M. A. B., Franco, L. de O., & de Campos-Takaki, G. M. (2020). Seafood waste as attractive source of chitin and chitosan production and their applications. In International Journal of Molecular Sciences (Vol. 21, Issue 12, pp. 1–17). MDPI AG. [CrossRef]
- Oslan, S. N. H., Li, C. X., Shapawi, R., Mokhtar, R. A. M., Noordin, W. N. M., & Huda, N. (2022). Extraction and Characterization of Bioactive Fish By-Product Collagen as Promising for Potential Wound Healing Agent in Pharmaceutical Applications: Current Trend and Future Perspective. International Journal of Food Science, 2022. [CrossRef]
- Guan, Y., He, J., Chen, J., Li, Y., Zhang, X., Zheng, Y., & Jia, L. (2022). Valorization of Fish Processing By-Products: Microstructural, Rheological, Functional, and Properties of Silver Carp Skin Type I Collagen. Foods, 11(19). [CrossRef]
- Righi, S., Prato, E., Magnani, G., Lama, V., Biandolino, F., Parlapiano, I., Carella, F., Iafisco, M., & Adamiano, A. (2023). Calcium phosphates from fish bones in sunscreen: An LCA and toxicity study of an emerging material for circular economy. Science of The Total Environment, 862, 160751. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).