1. Introduction
Epilepsy is a group of non-communicable neurological disorders that affects around 50 million people worldwide. Epilepsy is accompanied with structural and functional changes of neuroplasticity underlying seizures, neurodegeneration, and neural network rearrangement. Neuroplasticity is the basis of brain’s adaptation to changing conditions of the external and internal environment, and aberrant changes in plasticity are underlying various neurological and psychiatric diseases related to anxiety, depression, and cognitive dysfunctions, including epilepsy [
1,
2]. Increasing number of studies demonstrates that depressive disorders and epilepsy share common mechanisms [
3,
4] including dysfunction of hypothalamic-pituitary-adrenocortical (HPA) axis and neurotrophic factor systems, as well as neuroinflammation [
5,
6].
Neurotrophic factors are endogenous peptides or small proteins regulating the growth, proliferation, survival, migration, and differentiation of cells in the nervous system and can operate as potent molecular mediators of central synaptic plasticity. The central regulatory role of neurotrophic factors in neuroplasticity underlies their involvement in the pathogenesis of brain diseases including epilepsy and depression [
7,
8]. Functions of neurotrophic factors are realized in close interaction with other systems, in particular with the HPA axis and inflammatory system [
9]. Since these factors are believed to have neuroprotective and neurorestorative potential, their use in diagnostics, prognosis and treatment of neurological diseases is extensively studied and debated [
10,
11].
At present, the peptides of glial cell line-derived neurotrophic factor (GDNF) family and their receptors are regarded as one of the major neurotrophic network controlling multiple processes in the nervous system [
12]. These processes include development, maintenance and functioning of both different neurons and glial cells. In a healthy adult brain, GDNF is expressed in neurons, secreted in a paracrine mode and interacting with neuronal GDNF α1 (GFRα1) receptors. Acting through different signal transduction pathways, GDNF/GFRα1 complex conducts signals to nigro-striatal dopaminergic neurons, motor neurons, enteric neurons, sensory neurons, etc., supporting their survival [
13]. However, in an injured brain, GDNF expression occurs in glial cells as well. Importantly, in both activated astrocytes and microglia GDNF expression is induced by neuroinflammation. Thus, depending on the localization in the brain, the level and duration of glial cell activation, this disease-related GDNF overexpression can be either favorable (potentially adaptive) or harmful.
Our previous studies showed that neurotrophic factors in the lacrimal fluid (LF) may demonstrate changes much more pronounced than in BS. Particularly, in patients with focal epilepsy, CNTF levels were increased, while BDNF levels decreased both in the blood serum (BS) and LF, suggesting that high CNTF levels and low BDNF levels in the LF could be considered as non-invasive biomarkers of focal epilepsy [
14,
15]. Lacrimal GDNF levels were assessed in patients with bipolar disorder and major depressive disease (MDD) and it was shown that low GDNF concentration in LF could be a potential biomarker of depression [
16].
Taking into account potential involvement of GDNF in controllling neuronal networks of epileptic brain [
17], the aim of this study was to assess GDNF levels in LF and BS as well as to evaluate HPA and inflammatory indices in patients with focal epilepsy (PWFE), epilepsy and comorbid depression (PWFE+MDD) as compared with patients with depression (PWMDD) and healthy controls (HC). We also aimed to explore the potential of lacrimal GDNF as a biomarker of epilepsy.
2. Results
2.1. Characteristics of the Patients and the Healthy Control Groups
The demographic, clinical, and routine laboratory data of subjects in PWFE, PWFE+MDD, PWMDD and the HC groups, as well as the information about medical treatment, type and frequency of seizures of the patients, are shown in
Table 1. The groups studied did not significantly differ in age, gender, education level and most routine laboratory data. The hemogram showed few significant differences in groups of patients as compared with the HC group (
Table 1). Neutrophils were reduced in PWMDD, while monocytes were elevated in PWFE, compared to HC. PWFE+MDD and PWMDD showed augmented lymphocytes (%). PWMDD had higher prolactin levels compared to HC, most likely due to the higher percentage of patients in MDD group taking antipsychotics [
18].
It should be noted that according to the Beck II scale score patients with depression (PWFE+MDD and PWMDD) differed significantly from PWFE. According to the MMSE scale score PWFE+MDD had significantly lower cognitive level as compared to PWMDD.
Depending on the distribution, the data are presented as: mean ± SD (M±SD) or median with interquartile range (M [Q1; Q3]). Statistical significance (p<0.05): * compared to HC; ** compared to PWFE. Differences between groups with quantitative data were assessed by one-way ANOVA (with post hoc Tukey’s test) or Kruskal-Wallis test (with post hoc Dunn’s test). For qualitative data, Fisher’s exact test was used. MMSE – Mini-Mental-State Examination, BDI-II—depression inventory–II, TSH—thyroid-stimulating hormone, ACTH—adrenocorticotropic hormone, GDNF – glial cell line-derived neurotrophic factor, BDNF- brain-derived neurotrophic factor, TNF-α – tumor necrosis factor α.
2.2. Neurotrophic Factors
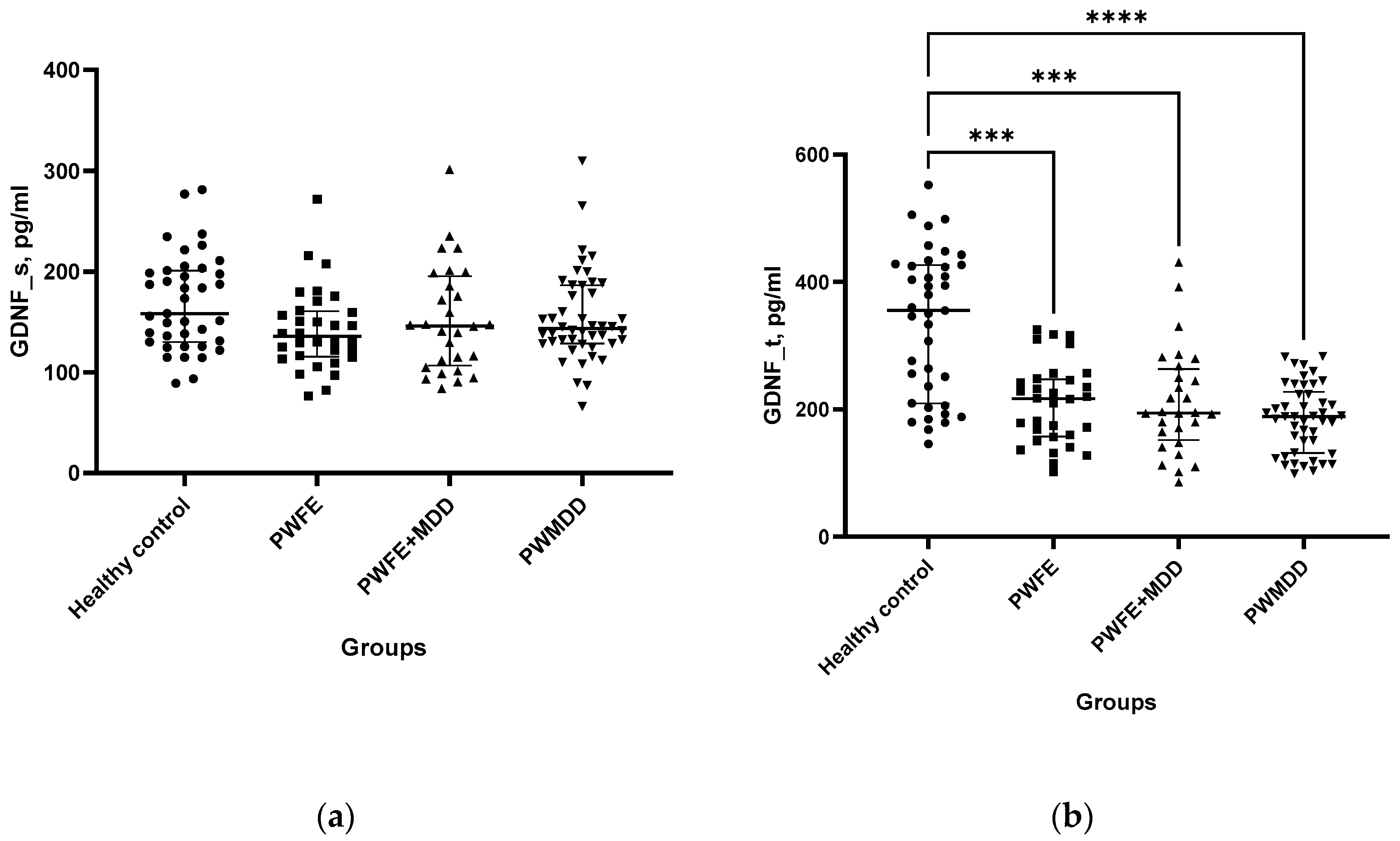
GDNF levels in BS did not significantly differ between patients and healthy controls (
Figure 1a). However, GDNF concentration in LF was significantly lower in patients as compared to HC group (
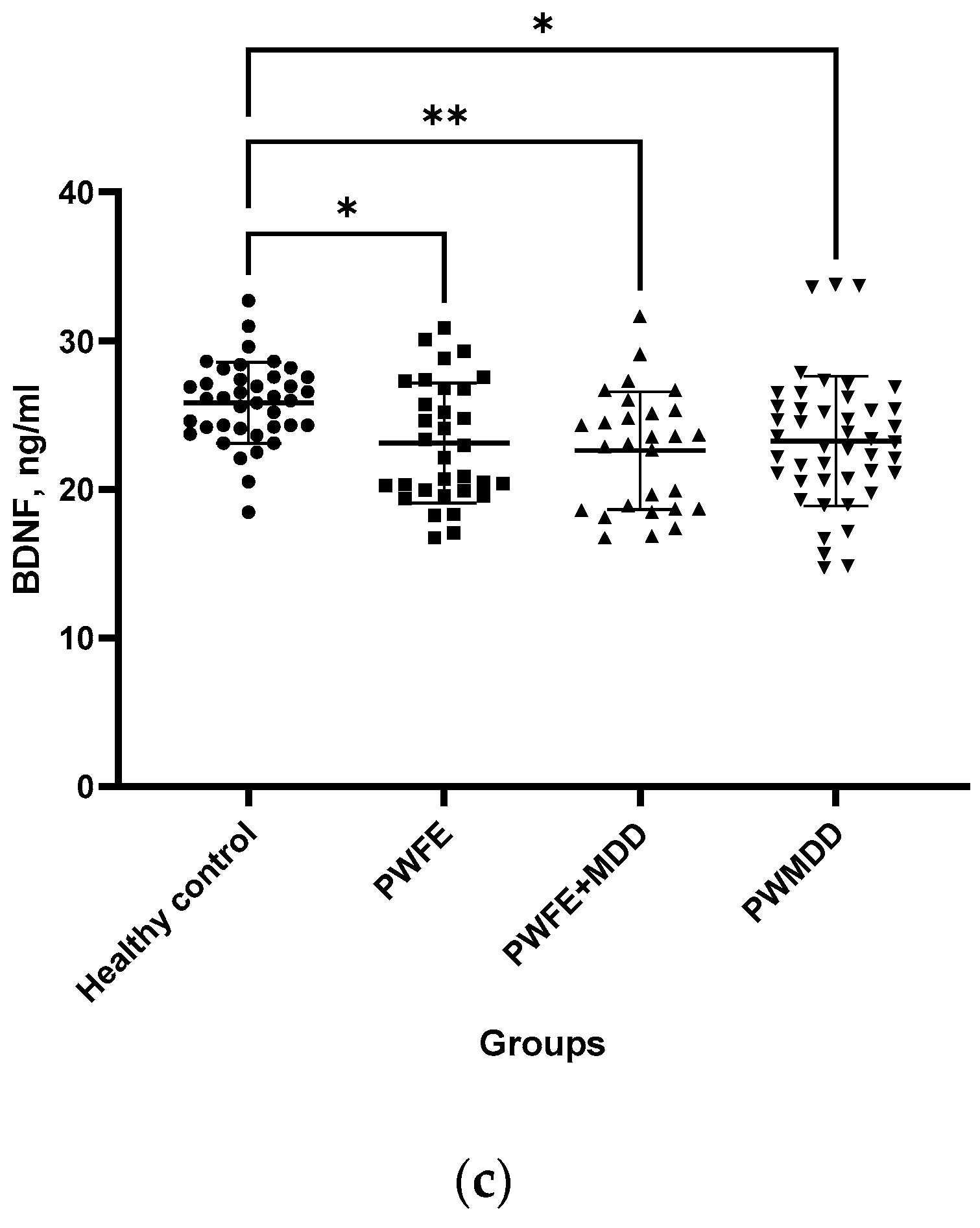
Figure 1b). BDNF levels also were significantly lower in BS of PWFE, PWFE+MDD and PWMDD compared to HC (
Figure 1c).
2.3. Cortisol and TNF-α
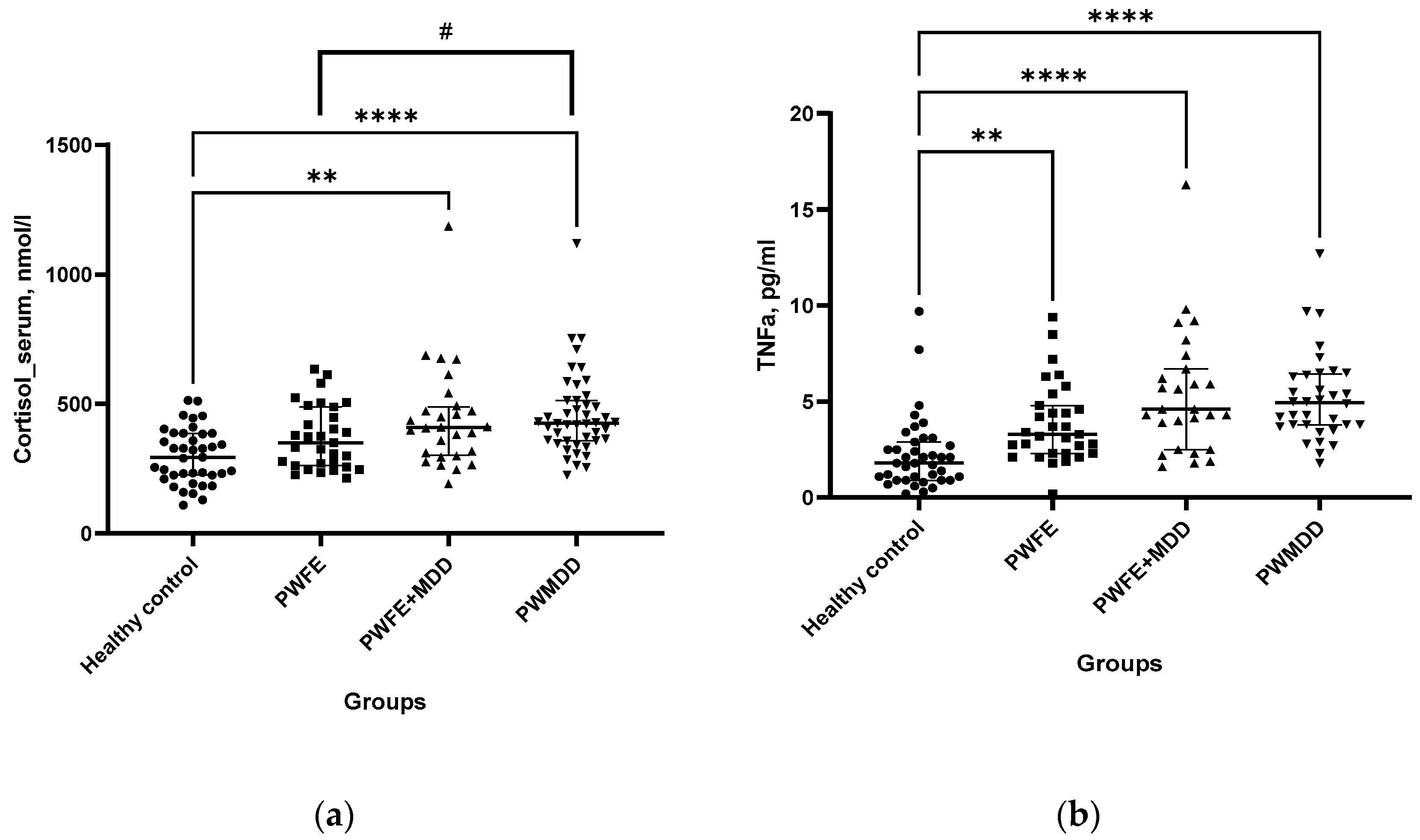
Cortisol levels in BS of PWFE+MDD and PWMDD were augmented as compared with the HC group (
Figure 2a). When groups of patients were compared, in PWMDD serum cortisol concentrations were insignificantly higher than in PWFE (trend, p < 0.09). Concentrations of TNF-α in BS of all patient groups were augmented as compared with the HC (
Figure 2b).
2.4. Effects of Age, Gender, Medical Treatment, Etiology of Epilepsy, Type and Frequency of Seizures on Lacrimal and Serum GDNF, Serum BDNF, Cortisol, and TNF-α Levels
2.4.1. Age
The levels of lacrimal and serum GDNF, BDNF, cortisol, TNF-α did not depend on age of persons participating in the study(
Table 2).
2.4.2. Gender
No significant differences were found in lacrimal GDNF, serum GDNF, BDNF, cortisol and TNF-α levels between men and women (P = 0.8, 0.84, 0.72, 0.99, 0.63, respectively).
2.4.3. Medical Treatment
Serum GDNF levels were higher in PWFE taking valproates as mono- or polytherapy (median=148.2pg/mL) as compared with PWFE not taking medications (median=115.8 pg/mL) (p=0.005). Other antiepileptic or antidepressant medications did not significantly influence GDNF in BS. GDNF level in LF was not influenced by either drug used.
Serum BDNF levels were higher in PWFE+MDD taking valproates as mono- or polytherapy (median=23.71 ng/mL) as compared to PWFE+MDD not taking medications (median=20.3 ng/mL) (p=0.03).
Serum TNF-α levels were lower in PWFE not taking medications (median=4.9 pg/mL) (p=0.03) (median=3.1pg/mL) as compared with PWFE taking sodium channel blockers as mono- or polytherapy.
2.4.4. Type or Frequency of Seizures
Seizure type or seizure frequency did not influence BDNF, cortisol, TNF-a, GDNF levels in BS and in LF. Comparison of groups with different frequency of seizures using Kruskal-Wallis test did not reveal any significant effect of the seizure frequency either on scale scores (for MMSE p=0.14; for Beck II p=0.17) or the levels of lacrimal or serum GDNF, BDNF, cortisol, TNF-a (p= 0.46; 0.36; 0.81; 0.11; 0.42, respectively).
2.4.5. Etiology of Focal Epilepsy
Levels of lacrimal and GDNF, serum BDNF, and cortisol were not significantly different between the groups of patients with focal epilepsy of different etiology. Serum TNF-α levels were higher in patients with symptomatic focal epilepsy (SFE) associated with traumatic brain injury, stroke, or other cerebrovascular disorders as compared to patients with SFE related to other cause (
Table 3).
Depending on the distribution, the data are presented as: mean ± SD (M±SD) or median with interquartile range (M [Q1; Q3]). Statistical significance (p<0.05): * compared to SFE (multiple cause), ** compared to SFE (after epi surgery). Differences between groups with quantitative data were assessed by one-way ANOVA (with post hoc Tukey’s test) or Kruskal-Wallis test (with post hoc Dunn’s test).). For qualitative data, Fisher’s exact test was used. SFE – symptomatic focal epilepsy; CFE – cryptogenic focal epilepsy
2.4.6. Correlation analysis
GDNF concentrations in LF and BS showed significant correlation in the HC group only (R=0.43; p-value 0.01; Spearman rank correlation). In this group, serum GDNF concentration negatively correlated with TNF-α level (R=-0.33; p-value 0.04; Spearman rank correlation). No significant correlations between LF and BS indices could be found in either group of patients.
2.5. Combinations of Biochemical Indices - Predictors for the Probability Assessment of Focal Epilepsy, Depression or Focal Epilepsy with Depression
In order to find a combination of biochemical parameters capable of predicting the probability of developing focal epilepsy, MDD and their comorbidity, three models were generated using the logistic regression method.
GDNF level in LF (GDNF_t, pg/ml); GDNF level in BS (GDNF_s, pg/ml); BDNF level in BS, ng/ml; TNF-a level in BS, pg/ml; cortisol level in BS, nmol/l; Gender (Sex) and age (Age) as predictors (independent variables) were used to build the models. The table for building models contained: for focal epilepsy - 71 observations and 8 columns; for MDD – 73 observations and 8 columns; for epilepsy comorbid MDD – 66 observations and 8 columns. All datasets were randomly divided into 80% training and 20% test data (as a general rule of thumb). The models were selected using the Maximum Likelihood Estimation (MLE) technique. The models converged correctly, without errors.
2.5.1. The model for Focal Epilepsy
The coefficients in the summary table of the model show that only variables such as GDNF_t and BDNF have a statistically significant effect on the occurrence of focal epilepsy (
Figure S1a,b). The odds ratio is presented in logarithmic form. Pseudo R squared values indicate that the model explains 31.4 – 47% of the data (
Figure S1c). Likelihood ratio test is 24.69 with a p-value less than 0.05 that points to good predictive power of the selected model (
Figure S1c). Graphs (
Figure S1e,f) show that increasing the level of GDNF in LF and BDNF reduces the likelihood of developing epilepsy.
According to the selected model, reducing GDNF level in LF by 10 pg/ml increases the probability of focal epilepsy by 9.8 times, reducing BDNF level in BS by 10 ng/ml increases the probability of focal epilepsy by 7.6 times (
Figure S1d).
When compiling a classification table with the participation of tested data from 14 participants (20% of the entire sample) in this model with a cut-off point of 0.5, the precision (how good our model is when the prediction is positive) of the model is 100%, the recall (how good our model is at correctly predicting positive classes) is 90% and F1 score (integrates precision and recall into a single metric to gain a better understanding of model performance) of the model is 95%. The accuracy of selected model with a cut-off point of 0.5 for the binary classification is 85.7%. A cut-off point of 0.68 is required for 100% accuracy (
Figure S1g).
2.5.2. The Model for MDD
The coefficients in the summary table of the model show that variables such as GDNF_t, cortisol and TNF-a have a statistically significant effect on the occurrence of MDD (
Figure S2a,b). The odds are presented in the form of logarithms. (
Figure S2d).
Pseudo R squared values indicate that the model explains 64.7 – 78.8% of the data (
Figure S2c). Likelihood ratio test is 51.27 with a p-value less than 0.05 that points to good predictive power of the selected model (
Figure S2c). Graphs (
Figure S2e,f,g) show that increasing the level of GDNF in LF and decreasing the levels of cortisol and TNF-α reduces the likelihood of developing depression.
According to the model selected reducing GDNF level in LF by 10 pg/ml increases the probability of MDD by 9.8 times, increasing cortisol level in BS by 10 nmol/L elevates the probability of MDD by 10.1 times, increasing TNF-α level in BS by 1pg/ml elevates the probability of MDD by 1.8 times (
Figure S2d).
When compiling a classification table with the participation of tested data from 15 participants (20% of the entire sample) in this model with a cut-off point of 0.5, the precision of the model is 85.7%, the recall was 100% and F1 score of the model is 92.3%. The accuracy of selected model with a cut-off point of 0.5 for the binary classification is 93.3%. A cut-off point of 0.35 is required for 100% accuracy (
Figure S2h).
2.5.3. The model for Focal Epilepsy with MDD
The coefficients in the summary table of the model show that variables such as GDNF_t, BDNF and TNF-α have a statistically significant effect on the occurrence of epilepsy with MDD development (
Figure S3a,b). The odds are presented in the form of logarithms. (
Figure S2c).
Pseudo R squared values indicate that the model explains 57.3 – 72.9% of the data (
Figure S3c). Likelihood ratio test is 41.57 with a p-value less than 0.05 that points to good predictive power of the selected model (
Figure S3c). Graphs (
Figure S3e) show that increasing the level of GDNF in LF and BDNF as well as a decrease of TNF-α level reduces the likelihood of developing comorbid depression and epilepsy.
According to the selected model reducing GDNF level in LF by 10 pg/ml increases the probability of epilepsy with MDD by 9.9 times, reducing BDNF level in BS by 10 ng/ml elevates the probability of epilepsy with MDD by 5.9 times, increasing TNF-a level in BS by 1pg/ml elevates the probability of epilepsy with MDD by 1.8 times (
Figure S3d).
When compiling a classification table with the participation of tested data from 13 participants (20% of the entire sample) of this model with a cut-off point of 0.5, the precision of the model is 90%, the recall is 100% and F1 score of the model is 94.7%. The accuracy of the selected model with a cut-off point of 0.5 for the binary classification is 92.3%. A cut-off point of 0.75 is required for 92.3% accuracy (
Figure S3h).
3. Discussion
3.1. Neurotrophic Factors in Epilepsy
Several lines of evidence suggest that neurotrophic factors are intimately involved in the development of acquired epileptic syndromes, though they can have contrasting effects [
19]. Yet the neurotrophic and neuroprotective properties of these factors can be potentially used to treat epilepsy, e.g., by inhibiting BDNF-TrkB signaling and reinforcing the NPY system [
7,
20]. Though not deeply explored yet, therapeutic potential of GDNF for hippocampus-related neurological disorders (including epilepsy) is regarded as fairly high [
17]. The involvement of GDNF in the pathogenesis of epilepsy has been studied using animal models. In particular, in rat models of epilepsy, GDNF delivered by various routes had beneficial effect suppressing seizures and/or reducing their frequency [
11,
21,
22]. All the more surprising that the information on GDNF levels in BS or plasma of PWFE is scarce.
In the present study, GDNF was assessed in BS; and it did not differ in PWFE, PWMDD and PWFE+MDD as compared to healthy controls. This corresponds to the results of the single study related to the comparison of GDNF content in blood plasma of healthy individuals and patients with epilepsy (including focal and generalized epilepsies): no difference between patients with epilepsy and controls has been found [
23]. Importantly, in our study, GDNF in LF was significantly reduced in PWFE, PWFE+MDD and PWMDD. Our recent study found GDNF changes in LF of patients with bipolar disorder and MDD [
16] similar to those in PWFE and in PWFE+MDD in the present study. However, in the present study, depression did not influence GDNF content either in LF or BS. It can be suggested that PWFE+MDD did not show much lower GDNF level in LF because the potential for further decrease has been exhausted. Thus, a reduction of GDNF level in LF might be one of potential biomarkers of both depression and epilepsy.
Other neurotrophic factors show different alterations in LF of epilepsy patients. We showed BDNF decrease both in BS and LF of epilepsy patients [
15], while, on the contrary, CNTF in these media was increased [
14]. In present study, serum BDNF was reduces in all groups of patients as compared with HC. Overall, these data confirm the results of other studies which suggest an involvement of BDNF in the pathogenesis of epilepsy [
7] and depression [
24] and our previous data [
25].
An interesting finding is a significant increase of serum GDNF and BDNF in PWFE taking valproates. This is in line with several studies, which showed that valproates significantly increase GDNF and BDNF expression in rat C6 glioma cells [
26] and astrocytes [
27,
28]. Similarly, we have previously shown that PWFE receiving valproates as mono- or polytherapy had higher BDNF level in BS [
15]. McGonigal et.al. [
29] also found the effects of valproates on serum BDNF levels in patients with epilepsy. Effects of valproates are known to be mediated by epigenetic mechanisms, including histone deacetylases, and BDNF and GDNF modulation by valproates are pivotal to orientate neurons toward a neuroprotective status and promote dendritic spines organization [
30].
3.2. Relationship between Neurotrophic Factors, HPA axis and Inflammation
The HPA axis as well as inflammation is involved in the pathophysiology of many neurological and neuropsychiatric disorders. Glucocorticoid hormones ensure the coordinated functioning of crucial mechanisms of hippocampal plasticity: neurogenesis, glutamatergic neurotransmission, microglia and astrocytes, systems of neurotrophic factors, neuroinflammation, etc. [
6]. Regulatory mechanisms are miscellaneous and include the direct action of glucocorticoids through their receptors and effects of HPA axis on numerous interactions between various systems and components.
The results of many clinical and animal studies confirm that disturbed neurotrophic factor systems, especially BDNF, and inflammation are two important risk factors in the pathogenesis of depression [
9,
30,
31]. Elevated levels of inflammatory mediators may reduce expression of BDNF, while BDNF play a negative regulatory role in neuroinflammation. TNF-α is one of most extensively investigated mediators in the studies on inflammatory factors in human epilepsy [
33] and depression [
34]. In the present study, concentrations of TNF-α in BS of all patient groups were augmented as compared to HC, and this was accompanied by a significant decrease in GDNF levels in LF and BDNF levels in BS. These data confirm the concept that impaired immunoregulatory mechanisms may induce systemic neuroinflammation and decrease of trophic support.
In present study, higher basal cortisol levels were found in PWFE+MDD and PWMDD as compared with respective HC, confirming many previous reports. No significant increase was found in cortisol level in PWFE. Cano-López and González-Bono have analyzed the data of 38 studies on cortisol levels in adults with epilepsy and found higher basal cortisol levels in PWE as compared with respective controls only in 45% of studies [
35]. Taking into consideration that epilepsy may be regarded as a model of chronic stress [
5], the lack of pronounced cortisol activation in PWFE may be due to deeper exhaustion of HPA axis in some patients with epilepsy. In our previous study it was shown that in PWMDD serum and cortisol concentrations were significantly higher than in PWFE [
25]. When such groups of patients were compared in the present study, cortisol level in PWMDD serum was insignificantly higher than in PWFE, though showing a statistical trend trend (p< 0.09). However, similarly to previous data, changes in levels of GDNF in LF and BDNF and cortisol in BS, assessed in the present study, did not depend on the etiology of epilepsy and were related to epilepsy in general, independently of its etiology [
25].
In order to find a combination of biochemical parameters capable of predicting the probability of developing focal epilepsy, MDD and their comorbid state,we have created three models using the logistic regression method. To generate the models, we used the cortisol level, representing the functioning of HPA axis, TNF-α level reflecting the activation of inflammatory processes and concentrations of neurotrophic factors (GDNF in LF and BDNF in BS), reasonably assuming the involvement of all these systems in the pathophysiology of both epilepsy and depression. We have shown that the probability of developing focal epilepsy can be estimated with moderate predictive power using GDNF in LF and BDNF in BS, the probability of developing MDD with high predictive power can be estimated using GDNF in LF, cortisol in BS and TNF-α in BS, while the probability of developing epilepsy with MDD can be estimated with high predictive power using GDNF in LF, TNF-α in BS and BDNF in BS. Comparison of combinations of biochemical predictors for focal epilepsy, depression and their comorbid state, forecasting the likehood of their development, suggests that pathophysiological processes in PWFE, PWMDD and PWFE+MDD include both identical and specific changes. Thus, for the prognosis of focal epilepsy most important was the reduction of neurotrophic factors, for MDD – a decrease in neurotrophic factors accompanied by an increase in cortisol and TNF-α, for epilepsy with depression – a reduction of neurotrophic factors accompanied by an increase in TNF-α.
4. Materials and Methods
4.1. Methods
4.1.1. Subjects
A group of 60 consecutive patients over 18 years old diagnosed with focal epilepsy (PWFE, n = 32), with focal epilepsy and comorbid MDD (PWFE+MDD, n=28) and a comparison group of patients of similar age and gender proportion with MDD (PWMDD, n = 46) were recruited at the Moscow Research and Clinical Center for Neuropsychiatry between October 2020 to August 2021. As well, 39 generally healthy volunteers of similar age and gender without signs of mental disorder, both at the time of including into the study and in their medical records, were enrolled as healthy controls.
Inclusion criteria for the group with epilepsy were: focal epilepsy, thoroughly diagnosed through consensus by at least two experienced neurologists according to the criteria for epilepsy, as based upon the International League Against Epilepsy (ILAE) classification [
36,
37]. All PWFE underwent electroencephalography (EEG) and magnetic resonance imaging (MRI) of the brain. Subjects were excluded from the study if they had no records of seizure frequency, generalized, combined or epilepsy of unknown origin, significant psychiatric comorbidity (excluding depression), history of psychogenic nonepileptic seizures, presence of serious somatic, neurological or systemic disorders. All patients were examined by an experienced psychiatrist to diagnose depression and exclude other psychiatric comorbidities.
Inclusion criteria for the group with MDD were: the diagnosis of current depressive episode, age 18 years and above, the ability to provide an informed consent and comply with the study protocol. The exclusion criteria were cognitive impairment (score less than or equal to 24 on the Mini-Mental State Examination (MMSE) [
38], current or past psychotic disorders, alcohol or substance use disorders, manic/hypomanic symptoms/episodes, severe concomitant somatic (e.g., diabetes mellitus, autoimmune or oncological diseases) or neurological (e.g., Alzheimer’s and Parkinson’s diseases) disorders. Persons with initial or mild manifestations of somatic diseases, such as essential hypertension, ischemic heart disease, or cardiac arrhythmias, were not excluded. A mental disorder diagnosis was established by a psychiatrist using a Mini-International Neuropsychiatric Interview (MINI v 7.0.2).
The patients were not treatment naïve and received appropriate medications (treatment as usual) prescribed by an experienced psychiatrist. Pharmacotherapy of patients with epilepsy, in addition to antipsychotics, antidepressants, tranquilizers and valproates included sodium channel blockers (eslicarbazepine, fenitoin, lamotrigine, lacosamide, oxcarbazepine, ocarbamazepine), GABA inhibitors (benzobarbital, diazepam, fenazepam, phenobarbital, clonazepam), neurotransmitter release inhibitors (pregabaline, gabapentine, levetiracetame, brivaracetame, ethosuximide), zonisamide, topiramate.
The Russian version of the Beck depression inventory—II (BDI-II) was used to evaluate the severity of depression [
39].
All patients signed an informed consent form prior to participating in the study. This study adhered to the tenets of the Declaration of Helsinki and had local ethics committee approval (#42, 23.08.2019) with informed consent obtained from all subjects.
4.1.2. Samples
Biochemical and hormonal indices were measured in blood serum obtained from fasting morning venous blood. Samples were collected in Gel/Clotting activator S-Monovette tubes and centrifuged at 2000× g for 10 min at 8 ◦C on an Allegra X-30R Centrifuge (Beckman Coulter, Brea, CA, USA).
Stimulated LF (secreted by the lacrimal gland in response to a mechanical stimulation of the cornea) was sampled by a pipette at a volume of 100–200 μL from the lower conjunctival fornix of one randomly selected eye. Samples were stored at −80 °C in polypropylene tubes (Sarstedt GmbH, Nümbrecht, Germany) and analyzed within 3 months from sampling. Upon thawing, samples were centrifuged at 4000 x g for 15 minutes at 4 °C to ensure a complete debris removal. Based upon previously described methods [
40], an acid-treatment procedure was implemented to allow the quantification of total GDNF levels in biological samples.
4.1.3. Assessment of Biochemical Indices and Hormones
Concentrations of GDNF were measured in biological fluids using Human GDNF ELISA Kit (Ray Biotech, Norcross, GA) according to the manufacturer’s instructions. The sensitivity of the assay (minimum quantifiable value) was 4.0 pg/mL. All measured values were in the validated assay range. Sample volume permitting, two replicates were used. The concentrations of brain derived neurotrophic factor (BDNF) were determined by enzyme-linked immunosorbent assay (ELISA) in blood serum using corresponding Quantikine ELISA test systems (R&D Systems, Minneapolis, MN, USA). Cortisol, thyroid stimulating hormone (TSH), were measured in blood serum via competitive enzyme immunoassay using applicable kits (Beckman Coulter, USA) and an ACCESS® 2 immunoassay system (Beckman Coulter, USA). The concentration of tumor necrosis factor-α (TNF-α) was determined by ELISA with corresponding Human high sensitivity ELISA kits (eBioscience, Bender MedSystems GmbH, Vienna, Austria). Adrenocorticotropic hormone (ACTH) was assessed using enzyme immunoassay kits from Biomerica (Irvine, CA, USA). GDNF, BDNF, TNF-α and ACTH levels were measured on an automated enzyme immunoassay analyzer (ChemWell 2910, Awareness Technologies Inc., Palm City, FL, USA). Routine biochemical parameters and ions were determined in blood serum on a biochemical automated analyzer Beckman Coulter AU 680 (Beckman Coulter, Brea, CA, USA) using corresponding kits (Beckman Coulter, USA). Complete blood count with differential white blood cell count (CBC with diff) and hemogram were performed on an automated analyzer LH-500 (Beckman Coulter, USA).
4.1.4. Statistical Analysis
Statistical analysis was performed using STATISTICA 10.0 (StatSoft Inc., Tulsa, OK USA) and GraphPad Prism version 9.4.1. software (GraphPad Software, Inc., San Diego, CA, USA) and in the R programming environment on the RStudio version 2023.06 platform. 2 (2009-2023, Posit Software, PBC) using libraries: ggplot2, ROCR, dplyr, tidyr, MASS, caret, margins. The normality of distribution was determined using the Shapiro–Wilk and Kolmogorov-Smirnov tests. Fisher’s exact test was used to compare qualitative data. To compare quantitative data between several unrelated groups depending on the distribution, either the ANOVA test with post hoc analysis using Tukey’s test or the Kruskel-Wallis test with post hoc analysis using Dunn’s test were applied. Correlation analysis was carried out using the Spearman rank correlation test. The data in the graphs and in the tables are presented as: mean with SD, median with interquartile range or as percents. Differences were considered significant at p < 0.05. A backward logistic regression model was used. The significance level for each variable’s entry to the model was set at 0.05. A logistic regression model involves some independent (predictor) variables (nominal or continuous) that may be used to predict a dependent (outcome) binominal variable.
5. Conclusions
Our results reveal a high predictive value of assessing levels of lacrimal GDNF in epilepsy, depression and epilepsy with depression opposite to serum GDNF levels. This result support the use of LF as a promising source of disease biomarkers [
41], LF analysis being a way for opening a window into the brain. Epilepsy as a stress-associated disorder shares many vital links of depression pathogenesis, HPA axis disturbances, inflammatory alterations and trophic support decrease [
5]. Models obtained using logistic regression in this study suggest that changes in these systems in PWFE, PWMDD and PWFE+MDD include both similar processes, potentially important for comorbidity, and specific mechanisms for either epilepsy or depression. The results obtained confirm the involvement of HPA axis, the system of neurotrophic factors (GDNF, BDNF) and the inflammation (TNF-α) in the pathogenesis of epilepsy and depression. The models created in this study can be further developed and used both to predict the course of emotional disorders in patients with epilepsy, and to form the basis for personalized approaches to their therapy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org: Figure S1. Predictors of focal epilepsy development; Figure S2. Predictors of depression development; Figure S3. Predictors of development of comorbid depression and epilepsy.
Author Contributions
Conceptualization, A.A.S. and N.V.G.; methodology, T.A.D.; software, M.Y.Z.; validation, T.A.D., F.K.R. and S.B.P.; formal analysis, M.Y.Z and T.A.D. investigation, F.K.R. and S.B.P.; resources, A.B.G.; data curation, A.A.S. and N.V.G.; writing—original draft preparation, T.A.D. and M.Y.Z; writing—review and editing, A.A.S. and N.V.G.; supervision, A.B.G. and N.V.G.; project administration, T.A.D.; funding acquisition, N.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Moscow Center for Healthcare Innovations, Grant #2412-55/22.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Moscow Research and Clinical Centre for Neuropsychiatry (protocol #42, 23.08.2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data generated in the present study ar available upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gulyaeva, N.V. Molecular Mechanisms of Neuroplasticity: An Expanding Universe. Biochemistry (Mosc) 2017, 82, 237–242. [Google Scholar] [CrossRef]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry 2020, 25, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. Psychiatric comorbidities in new onset epilepsy: Should they be always investigated? Seizure 2017, 49, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Vinti, V.; Dell’Isola, G.B.; Tascini, G.; Mencaroni, E.; Cara, G.D.; Striano, P.; Verrotti, A. Temporal Lobe Epilepsy and Psychiatric Comorbidity. Front. Neurol. 2021, 12, 775781. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Stress-Associated Molecular and Cellular Hippocampal Mechanisms Common for Epilepsy and Comorbid Depressive Disorders. Biochemistry (Mosc). 2021, 86, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Glucocorticoids Orchestrate Adult Hippocampal Plasticity: Growth Points and Translational Aspects. Biochemistry (Mosc) 2023, 88, 565–589. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; Lucaccioni, L.; Fugetto, F.; Predieri, B.; Berardi, A.; Ferrari, F. Brain-derived neurotrophic factor and epilepsy: A systematic review. Neuropeptides 2018, 72, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N.V. Interplay between Brain BDNF and Glutamatergic Systems: A Brief State of the Evidence and Association with the Pathogenesis of Depression. Biochemistry (Mosc). 2017, 82, 301–307. [Google Scholar] [CrossRef]
- Paolone, G.; Falcicchia, C.; Lovisari, F.; Kokaia, M.; Bell, W.J.; Fradet, T.; Barbieri, M.; Wahlberg, L.U.; Emerich, D.F.; Simonato, M. Long-Term, Targeted Delivery of GDNF from Encapsulated Cells Is Neuroprotective and Reduces Seizures in the Pilocarpine Model of Epilepsy. J. Neurosci. 2019, 39, 2144–2156. [Google Scholar] [CrossRef]
- Mikroulis, A.; Waloschková, E.; Bengzon, J.; Woldbye, D.; Pinborg, L.H.; Jespersen, B.; Avila, A.S.; Laszlo, Z.I.; Henstridge, C.; Ledri, M.; Kokaia, M. GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway. Int. J. Mol. Sci. 2022, 23, 13190. [Google Scholar] [CrossRef]
- Ibáñez, C.F.; Andressoo, J.O. Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol. Dis. 2017, 97(Pt B), 80–89. [Google Scholar] [CrossRef]
- Duarte Azevedo, M.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Shpak, A.; Guekht, A.; Druzhkova, T.; Rider, F.; Gudkova, A.; Gulyaeva, N. Increased ciliary neurotrophic factor in blood serum and lacrimal fluid as a potential biomarkers of focal epilepsy. Neurol. Sci. 2022, 43, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Shpak, A.A.; Guekht, A.B.; Druzhkova, T.A.; Rider, F.K.; Gulyaeva, N.V. Brain-derived neurotrophic factor in blood serum and lacrimal fluid of patients with focal epilepsy. Epilepsy Res. 2021, 176, 106707. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, M.S.; Guekht, A.B.; Druzhkova, T.A.; Gulyaeva, N.V.; Shpak, A.A. Glial cell line-derived neurotrophic factor (GDNF) in blood serum and lacrimal fluid of patients with a current depressive episode. J. Affect. Disord. 2022, 318, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Chiavellini, P.; Canatelli-Mallat, M.; Lehmann, M.; Goya, R.G.; Morel, G.R. Therapeutic potential of glial cell line-derived neurotrophic factor and cell reprogramming for hippocampal-related neurological disorders. Neural Regen. Res. 2022, 17, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, L.; Romosan, A.M.; Papava, I.; Bredicean, C.A.; Dumitrascu, V.; Ursoniu, S.; Romosan, R.S. Prolactin response to antipsychotics: An inpatient study. PLoS One. 2020, 15, e0228648. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Tongiorgi, E.; Kokaia, M. Angels and demons: neurotrophic factors and epilepsy. Trends Pharmacol. Sci. 2006, 27, 631–638. [Google Scholar] [CrossRef]
- Nowroozi, A.; Salehi, M.A.; Mohammadi, S. Brain-derived neurotrophic factor in patients with epilepsy: A systematic review and meta-analysis. Epilepsy Res. 2021, 178, 106794. [Google Scholar] [CrossRef]
- Martin, D.; Miller, G.; Rosendahl, M.; Russell, D.A. Potent inhibitory effects of glial derived neurotrophic factor against kainic acid mediated seizures in the rat. Brain Res. 1995, 683, 172–178. [Google Scholar] [CrossRef]
- Kanter-Schlifke, I.; Georgievska, B.; Kirik, D.; Kokaia, M. Seizure suppression by GDNF gene therapy in animal models of epilepsy. Mol.Ther. 2007, 15, 1106–1113. [Google Scholar] [CrossRef]
- Alvim, M.K.M.; Morita-Sherman, M.E.; Yasuda, C.L.; Rocha, N.P.; Vieira, L.; Pimentel-Silva, L.R.; Nogueira, M.H.; Barbosa, R.; Watanabe, N.; Coan, A.C.; Lopes-Cendes, I.; Teixeira, A.L.; Cendes, F. Inflammatory and neurotrophic factor plasma levels are related to epilepsy independently of etiology. Epilepsia 2021, 62, 2385–2394. [Google Scholar] [CrossRef]
- Miyanishi, H.; Nitta, A. A Role of BDNF in the Depression Pathogenesis and a Potential Target as Antidepressant: The Modulator of Stress Sensitivity “Shati/Nat8l-BDNF System” in the Dorsal Striatum. Pharmaceuticals (Basel). 2021, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Druzhkova, T.A.; Yakovlev, A.A.; Rider, F.K.; Zinchuk, M.S.; Guekht, A.B.; Gulyaeva, N.V. Elevated Serum Cortisol Levels in Patients with Focal Epilepsy, Depression, and Comorbid Epilepsy and Depression. Int. J. Mol. Sci. 2022, 23, 10414. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.M.; Gallant, M.; Niles, L.P. Novel targets for valproic acid: up-regulation of melatonin receptors and neurotrophic factors in C6 glioma cells. J.Neurochem. 2005, 95, 1227–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Zhang, J.P.; Kwok-Yan Shum, D.; Chan, Y.S. Localization of nerve growthfactor, neurotrophin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/Bl mice. J. Comp. Neurol. 2006, 497, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, P.S.; Dallas, S.; Wilson, B.; Block, M.L.; Wang, C.C.; Kinyamu, H.; Lu, N.; Gao, X.; Leng, Y.; Chuang, D.M.; Zhang, W.; Lu, R.B.; Hong, J.S. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 2008, 11, 1123–34. [Google Scholar] [CrossRef] [PubMed]
- McGonigal, A.; Becker, C.; Fath, J.; Hammam, K.; Baumstarck, K.; Fernandes, S.; Giusiano, B.; Dufau, S.; Rheims, S.; Maillard, L.; Biraben, A.; Benoliel, J.J.; Bernard, C.; Bartolomei, F. BDNF as potential biomarker of epilepsy severity and psychiatric comorbidity: pitfalls in the clinical population. Epilepsy Res. 2023, 195, 107200. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr. Neuropharmacol. 2019, 17, 926–946. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Ravizza, T. Inflammation and epilepsy. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2012; Volume 107, pp. 163–175. ISBN 9780444528988. [Google Scholar]
- de Vries, E.E.; Munckhof, B.V.D.; Braun, K.P.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Inflammatory mediators in human epilepsy: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Berk, M.; Maes, M. Translational evidence for the Inflammatory Response System (IRS)/Compensatory Immune Response System (CIRS) and neuroprogression theory of major depression. Prog. Neuro-Psychopharmacol, 2021. [Google Scholar]
- Cano-López, I.; Gonzalez-Bono, E. Cortisol levels and seizures in adults with epilepsy: A systematic review. Neurosci. Biobehav. Rev. 2019, 103, 216–229. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for The Beck Depression Inventory, 2nd ed.; (BDI-II); Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Okragly, A.J.; Haak-Frendscho, M. An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp. Neurol. 1997, 145, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Vavilina, Ia. S.; Shpak, A. A.; Druzhkova, T. A.; Guekht, A. B.; Gulyaeva, N. V. Shedding Valuable Tears: Tear Fluid as a Promising Source of Disease Biomarkers. Neurochem. J. 2023, 17, 702–714. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).