1. Introduction
There has been significant debate about sugars intake and health among the global nutrition community. Research on added sugars is particularly challenging due to the complexity of accurately assessing and categorizing added sugars intake. This has yielded a highly heterogenous body of literature, complicating causal inference on added sugars intake and health outcomes.
While recommendations to limit intake of sugars in the diet are not new, the term and specific study of “added sugars” is a relatively recent development. Current authoritative guidance on sugars intakes range in their definitions of added sugars as well as the rationales and values for intake recommendations. Added sugars are defined by the U.S. Food and Drug Administration (FDA) as sugars that are either added during the processing or preparation of foods (such as sucrose or dextrose) or foods packaged as sweeteners (such as table sugar, syrups and honey), sugars from concentrated fruit or vegetable juices, excluding sugars naturally occurring in milk, fruits, and vegetables
[1], a definition similar to that of the European Food Safety Authority (EFSA)
[2]. The World Health Organization (WHO) does not recognize the term added sugars, instead using “free sugars”, which includes added sugars as defined by the FDA as well as fruit juices
[3]. Meanwhile, the United Kingdom uses the term “non-milk extrinsic sugars” (NMES), defined as sugars not contained within the cellular structure of food, except lactose in milk and dairy products
[4]. NMES differs from free sugars in that NMES also include half of the sugars from dried, stewed, or canned fruit, while free sugars do not account for the processing of fruit.
In recent years the public health nutrition community has highlighted added sugars as a target for nutritional intervention. The 2015 Dietary Guidelines for Americans (DGA) recommended that no more than 10% of an individual’s daily calories should come from added sugars
[5], which was carried forward in the 2020 Dietary Guidelines
[6]. This recommendation was based on food pattern modeling, designed to help individuals meet nutrient recommendations and stay within calorie needs, and not based on a threshold associated with adverse health outcomes
[5,6]. Similarly, in 2015 the World Health Organization (WHO) strongly recommended a global reduction in the intake of free sugars to less than 10% of total energy intake and offered a conditional recommendation to further reduce free sugars intake to less than 5% of total energy intake (TEI), based on very low quality evidence
[3]. These recommendations were made on the basis of data linking free sugars intake with risk of dental caries, not obesity or metabolic diseases.
In 2022, EFSA released a report detailing the outcomes of its objective to set an evidence-based tolerable upper intake level (UL) for dietary sugars
[2]. Following a comprehensive review of available evidence, EFSA was unable to identify a UL or a safe level of intake for added or free sugars but recommended that intake remain as low as possible. The panel’s inability to define a UL was due to numerous limitations of the data as well as the heterogeneity of the exposures of interest, health endpoints measured, and analytic approach. For example, EFSA’s report notes that it was possible to estimate added sugars intake from SSBs but not foods, and that the relationship between dietary sugars and health endpoints is highly dependent on isocaloric comparisons
[2].
Several distinct challenges make evidence-based reviews of added sugars difficult: The lack of a universally accepted definition for added sugars
[7,8], the difficulty of estimating exposures to added sugars
[2], the need to consider energy balance
[9], and potential differences between food and liquid sources of added sugars regarding their impact on health
[10]. To overcome such challenges, we used evidence mapping to 1) consolidate research used in dietary policy guidance and 2) characterize research on added sugars with a specific focus on food source (liquids vs mixed sources of foods and liquids), energy balance, and intake levels. The objective of this review was to produce an evidence map and publicly available database of the body of literature on dietary intake of added sugars and health outcomes to help guide future research and contribute to policy guidance.
2. Materials and Methods
Study selection and inclusion
The purpose of this evidence map was to capture and characterize studies on dietary added sugars from foods and/or beverages. Both observational and intervention studies assessing outcomes related to body composition, obesity, cardiovascular health, and diabetes mellitus were included.
To assess study eligibility, detailed inclusion and exclusion criteria were developed describing the populations, study designs, exposures, and outcomes eligible for inclusion in the database. Briefly, studies were included if they were primary literature, published in English, studies on dietary added sugars intake in humans, and measured body weight or composition, obesity, diabetes, or cardiovascular health as an outcome. Detailed inclusion/exclusion criteria are presented in
Table 1.
The search strategy employed both a primary literature search and extensive backwards citation screening. An electronic search for literature was conducted in PubMed on 12/10/2021 using the following search terms: “added sugar”[All Fields] OR "total sugar"[All Fields] OR "intrinsic sugar"[All Fields] OR "free sugar"[All Fields] OR "extrinsic sugar"[All Fields]) AND "humans"[MeSH Terms] AND 1990[EDAT]:2021[EDAT]. Search results were further limited to primary literature published in English. No outcome restrictions were set at this stage. A backwards citation search was then performed from two authoritative reports that included recommended intakes for added sugars
[2,11] to identify additional potentially relevant studies. Specifically, all references cited by the EFSA report and by Chapters 10 and 12 of the DGAC 2020 report were screened. To identify older literature using potentially different terminology, we performed a backwards citation search of the relevant chapters of the 2005 Institute of Medicine (IOM) report on dietary reference intakes
[12]. Title/abstract and full-text screening was performed using dual-review between 2-4 individuals, with conflicts resolved by a single senior reviewer and through team discussion when necessary.
Evidence synthesis
Data cleaning, transformation, descriptive analysis, and data visualization were performed in RStudio (Desktop version 2022.7.2.576,
http://www.rstudio.com/) and Tableau (Tableau Server Version 2022.2.0,
https://www.tableau.com/) to characterize the included studies by study design, population, exposure, outcomes, and total energy. Sources of added sugars were classified as liquids only vs. mixed (solids and liquids).
3. Results
Search results
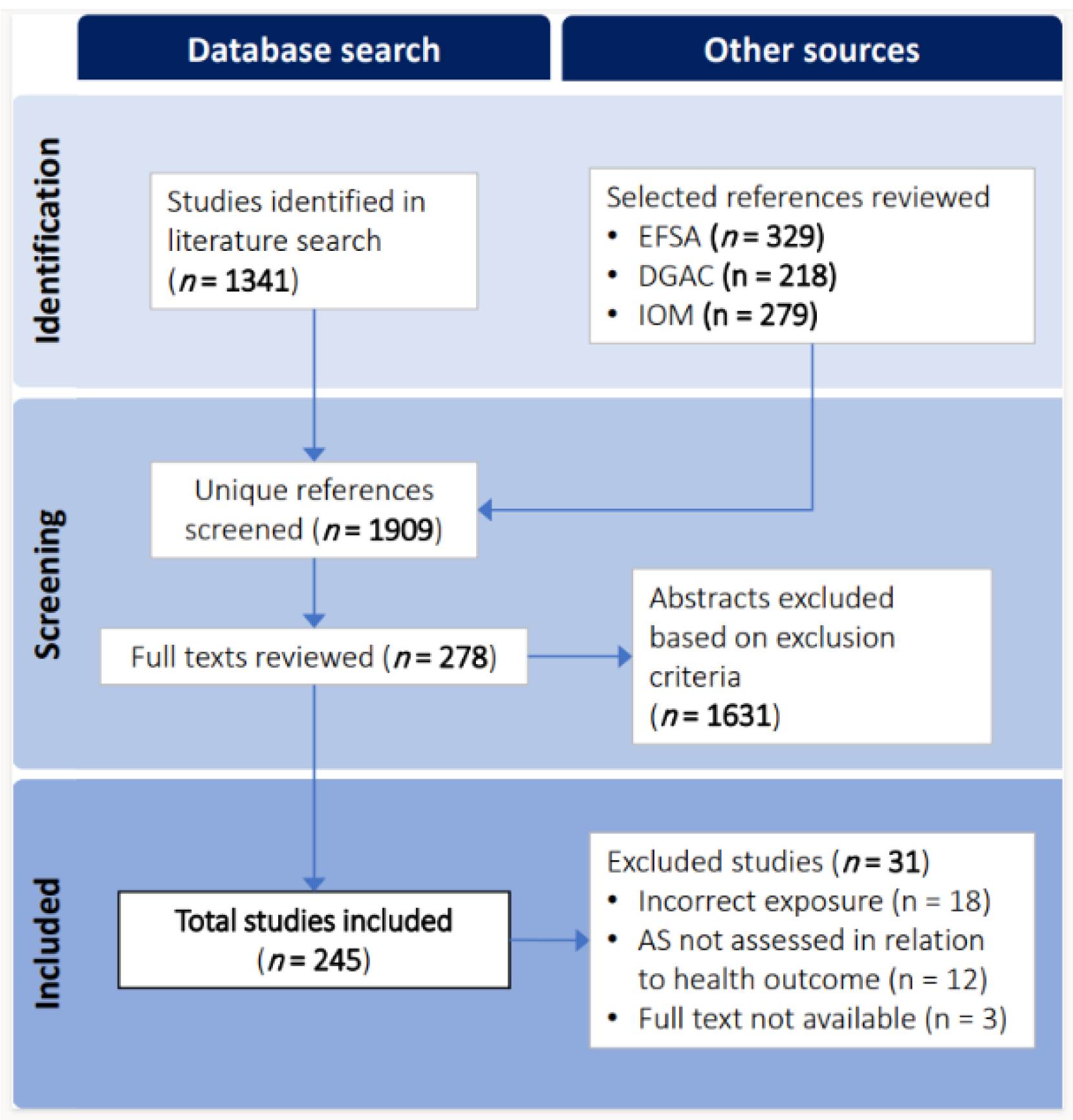
The initial MEDLINE literature search returned 1341 references which, combined with the references sourced from the relevant policy documents, yielded 1909 unique references screened at the title/abstract level. A total of 1631 references were excluded after title/abstract screening, leaving 278 full text articles that were assessed for eligibility. Having met all inclusion criteria, 245 publications were included in the final evidence map and database (
Figure 1).
Study characteristics
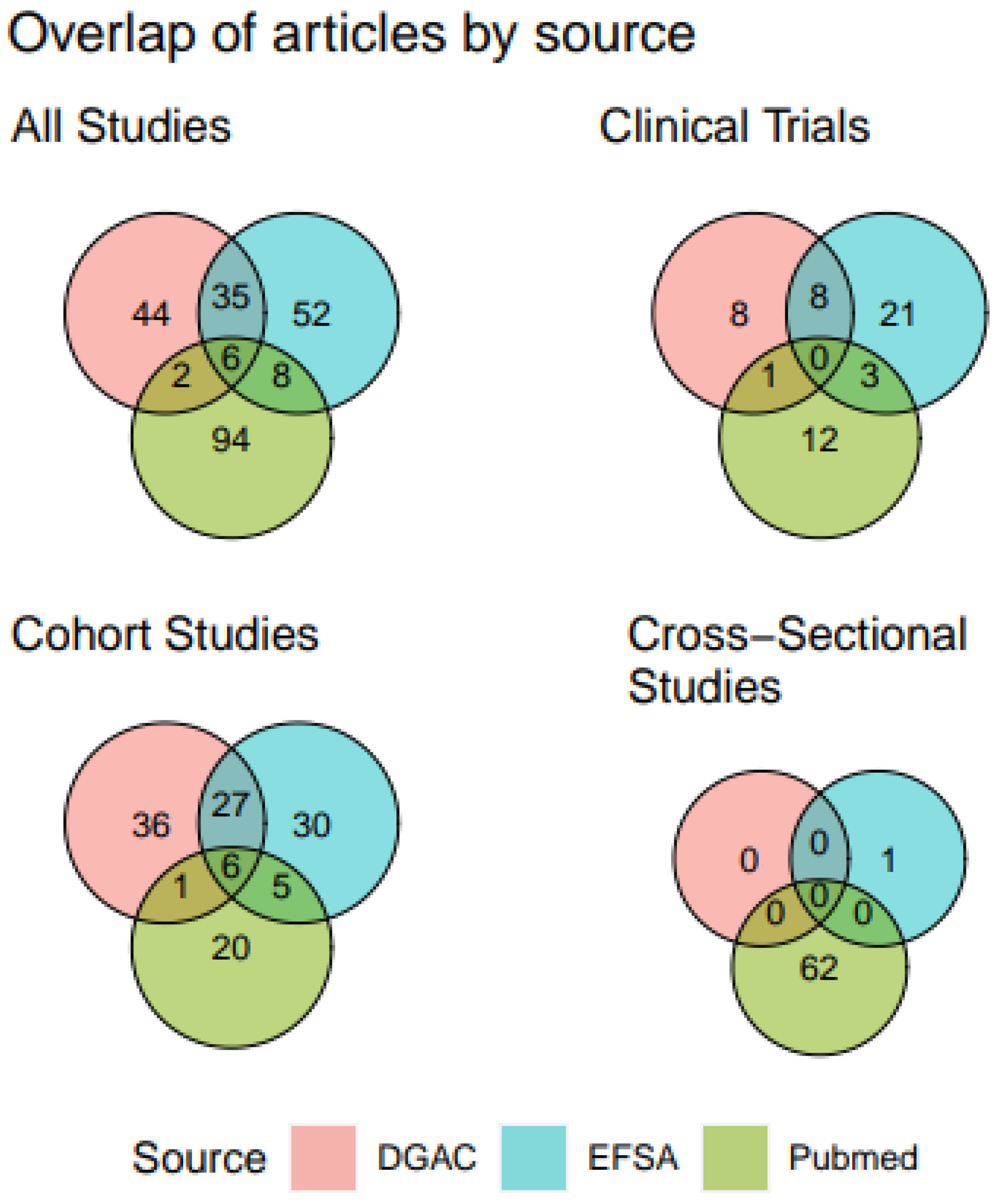
A total of 245 unique publications met criteria for analysis. Publications were examined according to source (DGAC 2020, EFSA 2022, and primary literature search) (
Figure 2). Only 4 unique publications were identified from the IOM report, thus they are not shown in
Figure 2. Six publications (<3% of all articles, all prospective cohorts) were common to all three major sources, whereas 35 publications were cited by both EFSA and DGAC 2020. Overall, references from the PubMed literature search had a low degree of overlap with the two reports. The PubMed search returned the only cross-sectional studies (as these were purposefully excluded by the other three authoritative reports) and fewer cohort studies. The EFSA and DGAC reports overlapped in 27/125 articles describing cohorts and cited an additional 30-36 unique articles on cohorts each. The EFSA report comprised the most comprehensive source of clinical trials.
Among the 245 included articles, 191 (78%) were observational and 54 (22%) were interventions (
Table 2). More than half (56%) described liquid-only sources of added sugars. Most articles reported on studies of adults (65%, ages 18-64) and a third of children (34%, ages 3-11), with 91% describing participants as healthy or not specifying a specific disease status at baseline. Weight status at baseline included mixed weights for 208 (85%) of the articles, with few exclusively recruiting/analyzing normal, overweight, or obese populations. Most studies included multiple primary outcomes, with body composition (36%), cardiovascular health (32%), body weight (22%), and diabetes (22%) being the most frequently reported. Of all included articles, only 13% reported a standardized measure of dietary quality (
e.g., DASH score, HEI-2015, etc.), thus data are not shown here. Of note, there were multiple articles published using data from the same cohorts and surveys. Thus, the number of articles reported in
Table 2 is greater than the number of unique interventions, cohorts, and surveys conducted. This information can be viewed in the database provided in the supplementary material.
Publication trends
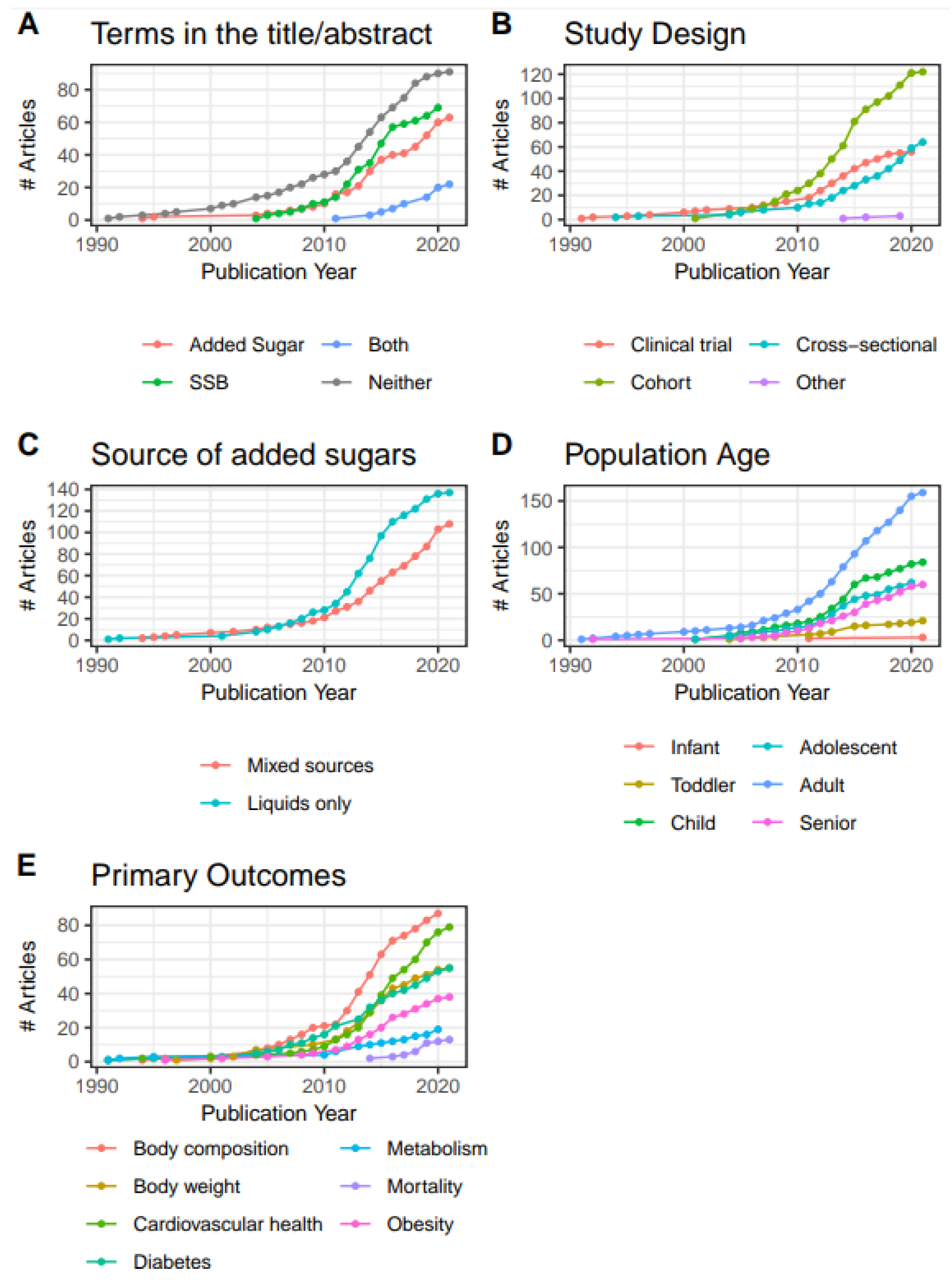
Published articles on added sugars have increased steadily since about 2010 (
Figure 3A). Most articles on the topic of added sugars do not use the phrases “added sugars” or “sugar-sweetened beverage” in titles and abstracts, with few using both (in either their singular or plural forms). Usage of the term “added sugars” first appeared in 1994, as it related to the scope of this evidence map. The present evidence map is dominated by cohort study reports, with the number of clinical trials plateauing from 2018-2020, and a persistent increase in cross-sectional trials observed starting in 2011 (
Figure 3B). Beginning in 2007, articles reporting on studies of added sugars from liquid-only sources eclipsed that of articles on mixed sources (
Figure 3C). From 1990 to 2021, publication of articles examining adult populations have been the most abundant, followed by children and adolescents (
Figure 3D).
Starting in 2005 publications reporting on added sugars and body composition as a primary outcome began increasing and then entered a period of steep growth beginning in 2010 that has been sustained through 2020 (
Figure 3E). Diabetes was the second most published topic from 2007 until 2014, when articles on cardiovascular health rapidly increased and eclipsed those on diabetes and body weight. Articles specifically assessing obesity as a primary outcome are below articles whose primary outcomes were body weight or composition, but not necessarily obesity. Otherwise, there were few articles that measured outcomes outside of our primary focus, such as general metabolism and mortality.
Intake of added sugars
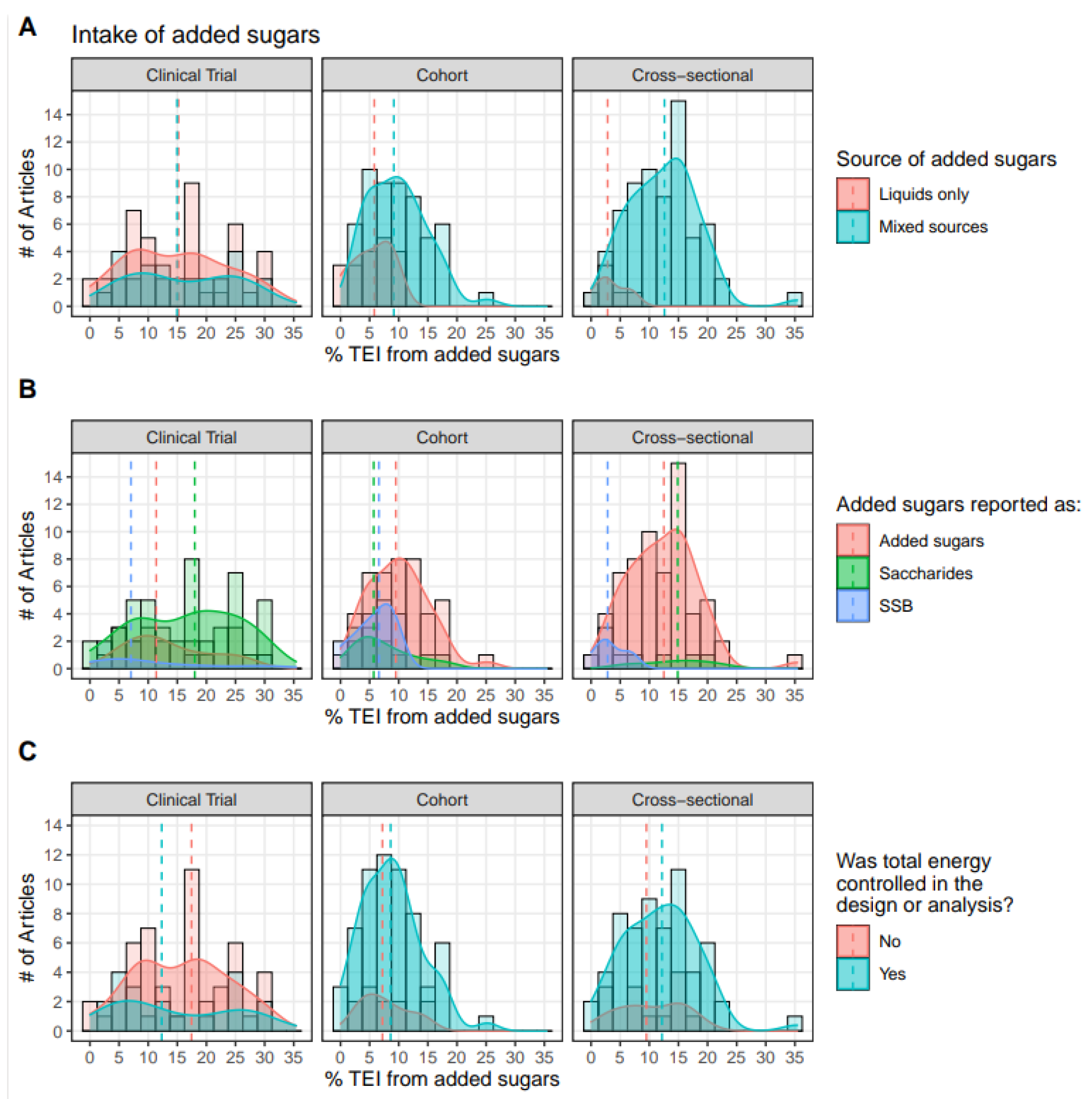
Of all 245 articles, 109 (45%) reported added sugars intake on a % TEI basis; these are described in
Figure 4. Added sugars were classified as coming from liquid sources (
e.g., SSB, experimental sugar solutions, sweetened dairy) or from mixed sources (
e.g., granola bars, sweetened yogurt, biscuits, cereal products, bread, jellybeans, SSB and candy, sweet desserts, fruit drinks, and foods not specified). Clinical trials measured intake levels across a wide range from 0-30% TEI, regardless of the source. Conversely, cohorts and cross-sectional studies tended to report intake of levels <20%, with intakes of liquid sources at levels <10%, whereas studies reporting intake of mixed sources reported intakes primarily up to 20% TEI (
Figure 4A).
Intakes were classified by source of added sugars according to whether they directly represented added sugars, distinct saccharides (fructose, sucrose, glucose), or SSB. Cohorts and cross-sectionals tended to provide the totality of energy from added sugars (
Figure 4B). When added sugars were not reported in observational trials, SSB and then specific saccharides were usually provided. As both SSB and saccharides represent a fraction of the total added sugars, exposures to added sugars from SSB and saccharides appear across a smaller and narrower range than that of total added sugars. The study of specific saccharides was rich across all intake levels in clinical trials, nearly absent in cross-sectional trials, and modestly available from cohort studies.
Intake levels were grouped according to whether or not total energy was controlled for in the study (
Figure 4c). There did not seem to be a clear relationship between energy intake from added sugars and whether total energy was controlled for. Few clinical trials, but most cohorts and cross-sectionals, controlled for total energy intake. Intake levels were slightly lower in cohort studies that did not control for energy intake compared to those that did. Energy intake from added sugars was measured at similar levels between cross-sectional studies regardless of control for energy intake.
Health outcomes
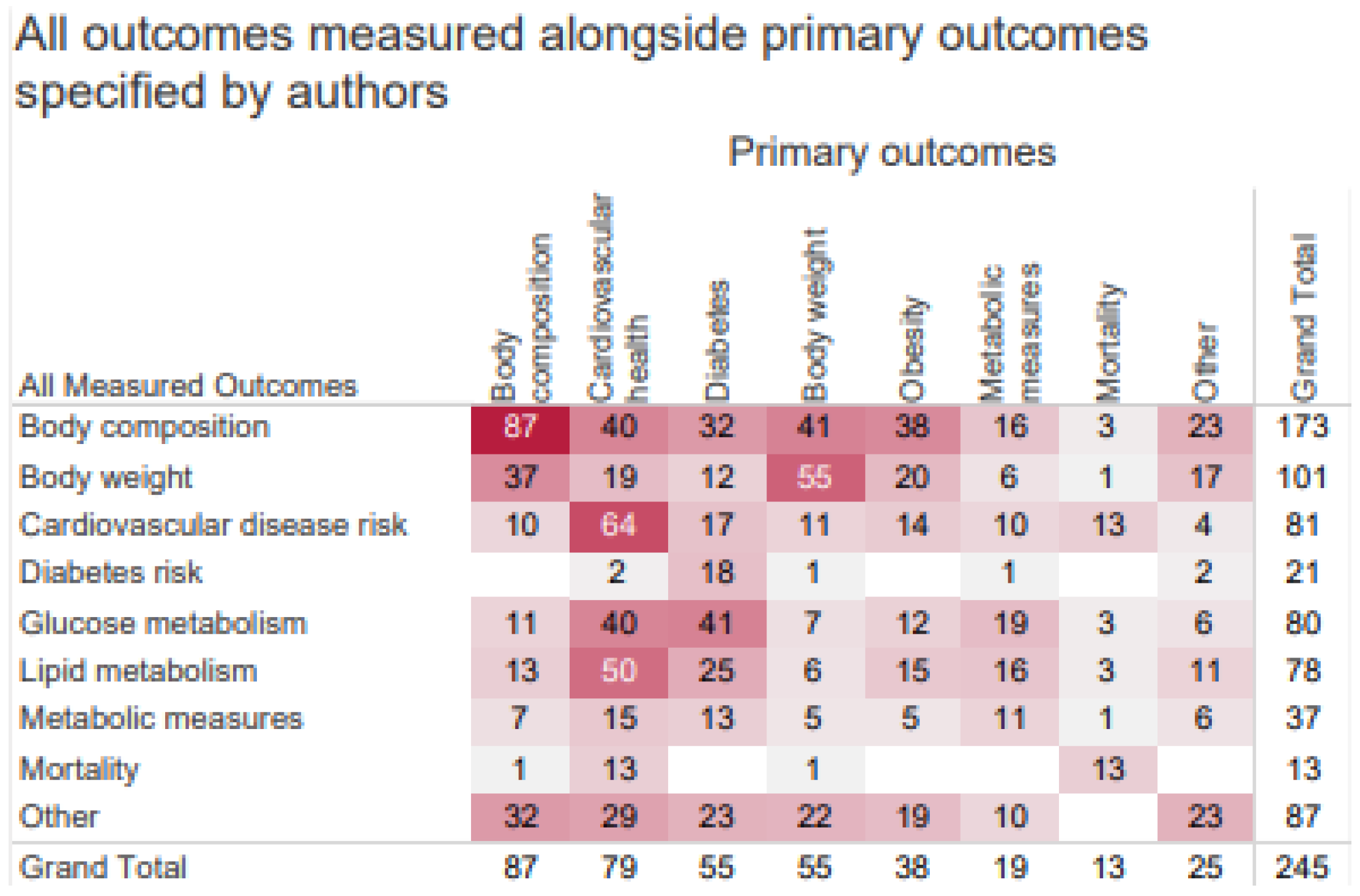
To account for differences in the reporting of a priori primary outcomes and the measured outcome variables,
Figure 5 demonstrates the relationship between the two. Across all articles, body composition, cardiovascular health, diabetes, and body weight were the most studied primary outcomes and measured outcomes, given our search criteria. Overall, there was high overlap between articles reporting outcomes related to metabolic health, cardiovascular health, and DM. Body composition overlapped with many other outcome indicators because BMI is a commonly measured outcome. Articles with a primary focus on cardiovascular health frequently measured both glucose and lipid metabolism, whereas articles whose primary focus was diabetes tended to focus on glucose metabolism.
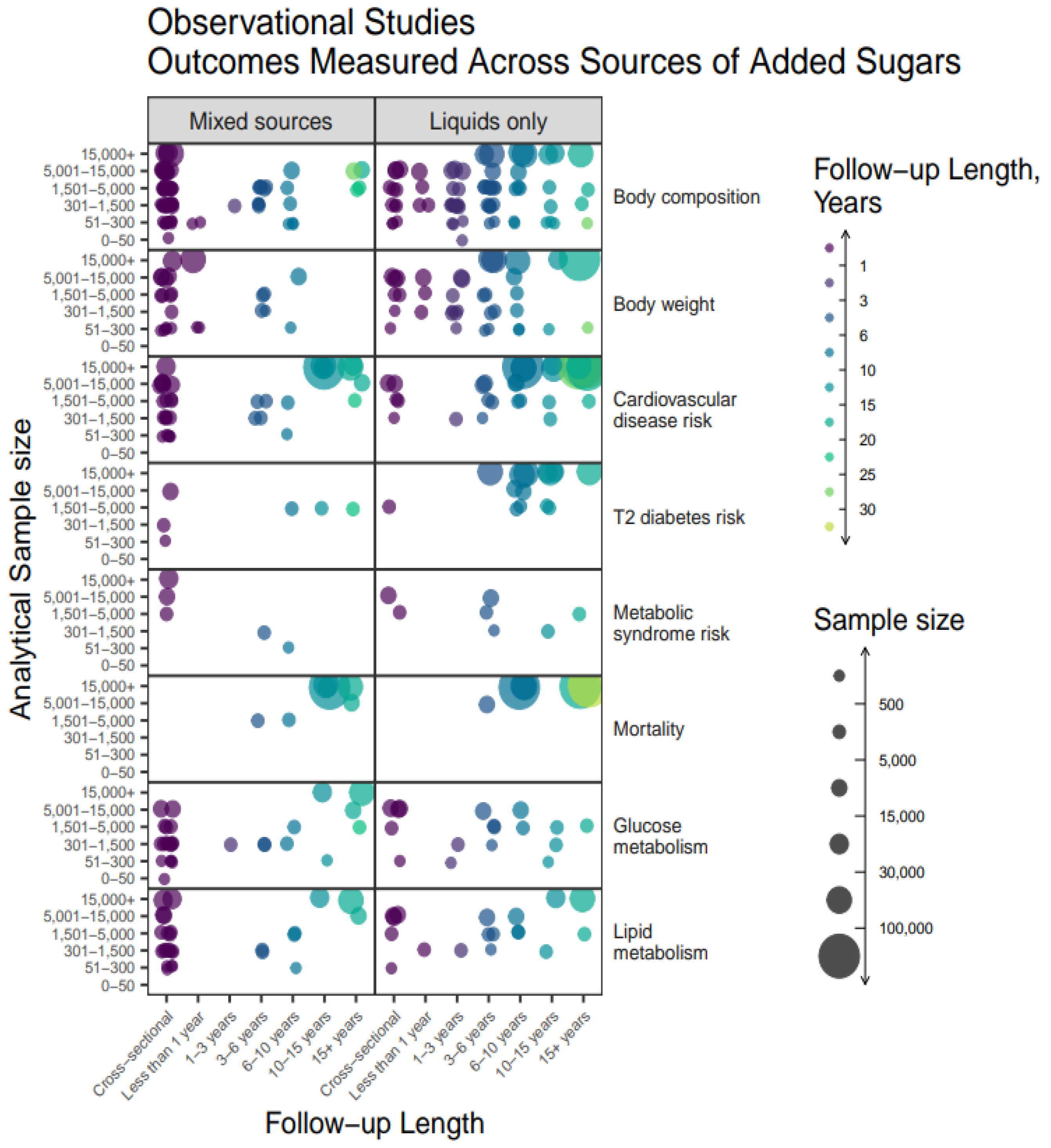
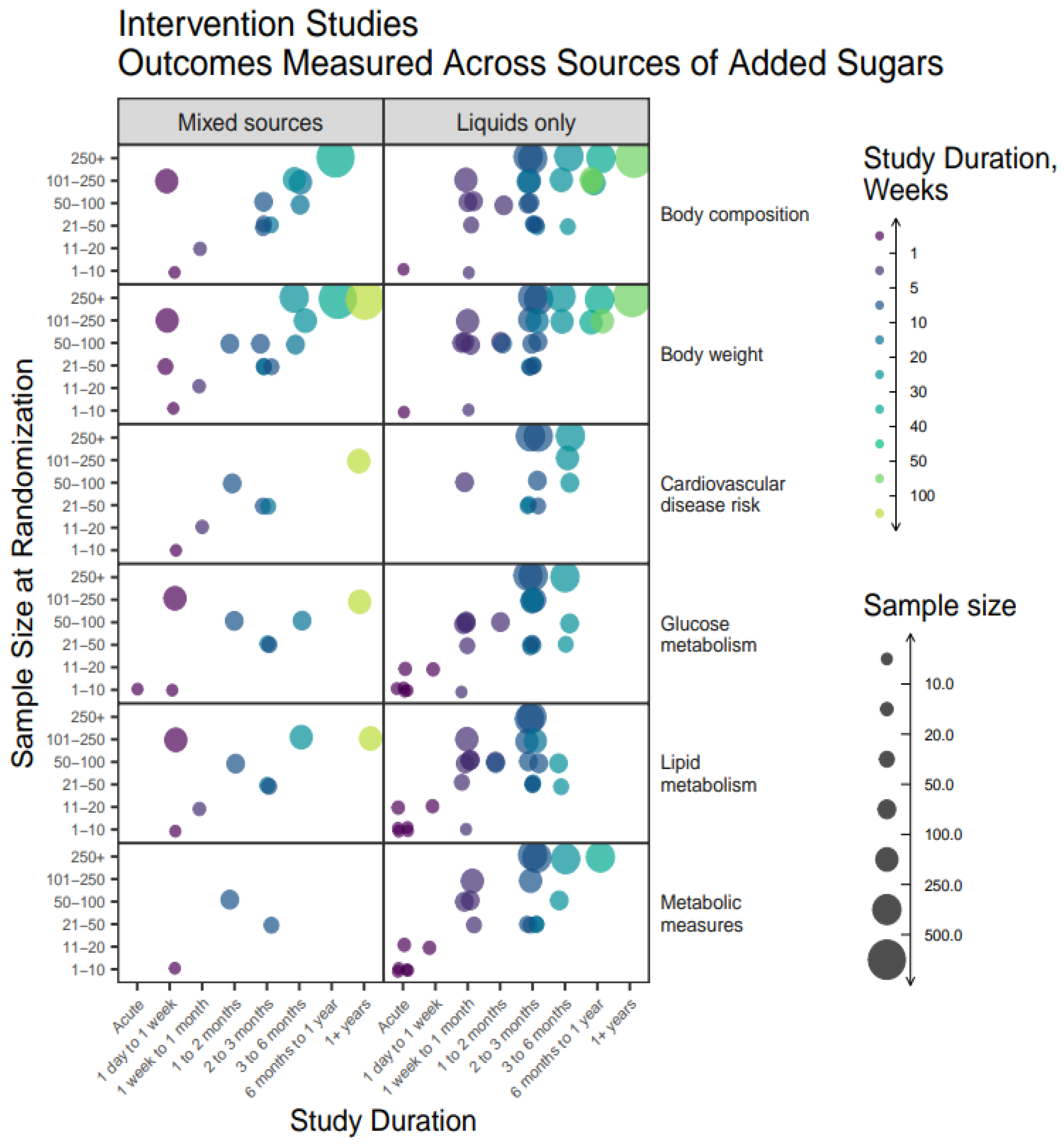
The relationship between measured outcomes, source of added sugars, sample size, and study duration are shown in
Figure 6 and
Figure 7. Cohorts most frequently followed-up between 3 and 15 years, with samples sizes at a wide range between 300-15,000 (
Figure 6). Overall, the outcomes measured were similar between articles on liquids only or mixed sources. Across all variables measured, articles on liquids only tended to have larger sample sizes and longer durations as compared to mixed sources articles. Observational studies with the largest sample sizes and longest durations were those measuring CVD and mortality outcomes.
A high proportion of intervention studies included liquid only sources of added sugars, and these studies tended to have larger sample sizes and shorter durations compared to studies on mixed sources (
Figure 7). For all outcomes, acute studies were conducted more frequently on liquid-only sources. The most common intervention duration was 1 week to 1 month, or 2-3 months in studies on liquid sources, with more variability in duration for studies on mixed sources. Otherwise, studies on mixed sources measured similar variables but in fewer numbers.
Total energy intake and diet energy balance
Total energy intake was controlled for either in the study design or analysis of 27% of all clinical trials, compared to 76% of cohort studies and 63% of cross-sectional studies (
Table 3). Sixty-nine percent of all clinical trials neither controlled for TEI nor specified the diet energy balance of the participants. Across all study designs, 96% did not specify diet energy balance. Finally, participants’ TEI was reported by 59% of clinical trials, 70% of cohort studies, and 69% of cross-sectional studies. Details regarding TEI were also examined by form of added sugars (liquids only vs. mixed), demonstrating that 29% of mixed-source studies did not report TEI, compared to 36% of the liquids only studies (data not shown). The vast majority of studies on liquids and mixed sources did not specify diet energy balance (98% vs. 93%, respectively).
4. Discussion
We used scoping review and evidence mapping techniques to characterize the current state of and identify gaps in the literature on added sugars. We found the literature is dominated by prospective cohorts and cross-sectional studies, those reporting intakes of liquid sources of added sugars, adults and children, and measurement of body composition and metabolic-related parameters. Like many other assessments
[1,2,12], we found significant heterogeneity in study design, subject population, and the exposures reported. Our assessment investigated not only the primary outcomes assessed, but all outcome measures collected in each study, in addition to extracting the quantitative intake of added sugars reported, and how energy balance was treated in the study design. We found that publications on this topic started to rise significantly in the late 2000s, with peak publication rates between 2010-2015.
Heterogeneity in terminology disrupts the ability to derive scientific conclusions
One of the striking findings from this evidence map was the distinct gap between literature used for policy guidance and that which fit our inclusion criteria. While the 2020 DGAC report and guidance by EFSA have different scopes and inclusion criteria, both reports identified over 30 different articles on cohort studies that the other report did not include in their analysis (
Figure 2). Similarly, EFSA identified 21 clinical trials that were absent from the 2020 DGAC report. Our own literature search identified a completely different set of studies than either document, largely due to our inclusion of cross-sectional studies. The difficulty of performing a systematic review on this topic is highlighted by the fact that many studies that are relevant to the topic use neither the terms “added sugars” nor “sugar-sweetened beverage” (or its abbreviation to SSB) in titles and abstracts (
Figure 3A), exacerbating the difficulty of finding applicable literature.
This assessment is not the first to identify numerous challenges to the interpretation of the research due to high heterogeneity. The 2020 DGAC report
[11] cites limitations including a lack of standardization of reporting exposures such as variability in categories of intake, treatment of continuous variables, and the lack of non-linear dose curve assessments. The 2020 DGAC report states that very few RCTs were available for review as the interventions available were ineffective in changing added sugars intake, and many studies were unable to separate behavioral from nutritional effects. Such limitations likely contributed to the DGAC finding insufficient evidence to determine the relationship between added sugars and risk of CVD.
Research on added sugars disproportionately emphasizes liquid sources
Emerging evidence supports the hypothesis that liquid sources of added sugars impact diet quality and health differently than solid sources
[13,14]. For example, a series of systematic reviews and meta-analyses have demonstrated that the food source alters whether or not fructose intake affects adiposity
[15], body weight
[16], glycemic control
[9], NAFLD
[17], and fasting blood uric acid
[18]. Our results show there is more literature on liquid sources of added sugars (
Figure 3C), that observational studies on liquid sources tend to report lower exposures to added sugars compared to studies on mixed sources of added sugars (
Figure 4A), and there are a greater number of large and lengthy studies on added sugars from liquid sources than mixed (
Figure 6 and
Figure 7). Thus, available data for policy guidance is biased towards that of liquid sources. This is evidenced by the 2022 EFSA report which described estimating intake of sugars from SSBs and 100% fruit juices, but not other foods due to the large heterogeneity in reporting of sugars
[2].
The overrepresentation of liquid sources of added sugars in the literature conflicts with estimates of exposure to added sugars. The 2020 DGAC report identified that 70% of added sugars intake came from the following 5 NHANES food categories for ages two and older: sweetened beverages (24%), desserts and sweet snacks (19%), coffee and tea (with their additions) (11%), candy and sugars (9%), breakfast cereals and bars (7%)"
[11]. The 2022 EFSA report notes that the food groups contributing the most to added sugars intake were first “sugars and confectionary”, followed by beverages and bakery wares
[2]. Despite global agreement that food sources of added sugars contribute substantially to added sugars intake, there is a staggering lack of available evidence describing the impact of added sugars from food sources on health outcomes of interest.
We speculate that a major reason for such over-representation of research on added sugars from liquid sources stems from the ease of studying SSB. We found it easier to identify the amount of energy from added sugars in studies on liquid sources than those including food sources because liquid sources of added sugars tend to contain fewer or no other nutrients that contribute energy. Thus, when articles report the amount of energy consumed from soft drinks, the energy can reasonably be assumed to come only from added sugars. This is either more complex or not possible when assessing studies on mixed sources of added sugars. For example, Attuquayefio
et al., describe an intervention using a breakfast meal high in saturated fat and added sugars
[19]. However, their diet tables only describe “sugar” and do not differentiate between “added sugars” and “total sugars”. While this semantic difference may seem small, the lack of specificity in language inhibits the ability to separate the effects of added sugars from total sugars intake or from liquid and mixed sources.
There is a greater need for consideration of energy intake and balance
Several reports have highlighted the need to control for energy intake in the study of dietary sugars. For example, a meta-analysis by Choo
et al. describes how fructose from sugar-sweetened beverages raises fasting glucose but only when added on top of the background diet, as a hypercaloric comparison
[9]. Te Morenga
et al., also concluded that "isoenergetic exchange of sugars with other carbohydrates was not associated with weight change”
[20]. Indeed, the 2022 EFSA report describes finding no evidence from prospective cohorts (PCs) that isocaloric exchange of added sugars with other macronutrients is related to any chronic disease they reviewed
[2]. However, there is still evidence that an overall positive relationship exists between added and free sugars intake and the risk of obesity and dyslipidemia
[2]. Better understanding this relationship is key to making appropriate targets for added sugars intake, reformulation, and improved public health outcomes.
Knowing energy balance is a crucial component of understanding the relationship between added sugars and health, it was further surprising to see that clinical trials frequently did not control for energy intake (
Figure 3C). While some clinical trials used free-feeding or ad libitum designs purposefully and did not use energy intake as a statistical covariate
[21,22,23,24,25,26,27,28], this choice did not always appear purposeful. Beyond controlling for energy intake, 23 of the 56 clinical trials in this evidence map did not report total energy intake at all. On the other hand, despite cross-sectional studies suffering from a lack of experimental control, they exhibit much higher rates of measuring added sugars (
Figure 3B), reporting TEI, and adjusting for TEI in their statistical models (
Table 3). Although 95 of 125 cohorts reported controlling for TEI and 88 of 125 reported the actual TEI, only 1% of cohorts reported the energy balance of their subjects. For example, Jensen
et al., reported TEI, estimated total energy expenditure (TEE), and reported the TEI:TEE ratio, demonstrating that children in their study consumed slightly less than they expended
[29]. Although they did not use the TEI:TEE ratio in their modelling and note inclusion of TEI in their models did not affect estimates overall, this allowed the authors to better isolate the root differences between groups and measure under/over-reporting intake.
While 150 of 245 studies (61%) we assessed controlled for total energy intake (experimentally or statistically), only 15 of 245 (6%) studies we assessed clearly and directly reported the energy balance of their subjects. As most studies collected all the data necessary to calculate energy requirements and total energy intake, analyzing, controlling for, and reporting energy balance represents a simple and cost-effective solution that nearly all future studies could take advantage of to further the scientific field.
Research gaps and opportunities
The results from this evidence map clearly show a bias towards liquid sources of added sugars. Additionally, certain populations are underrepresented in this body of literature – namely, infants, toddlers, seniors, pregnant and lactating women, and obese and diabetic individuals. Fortunately, most other limitations are not due to irreversible choices in study design or population recruitment. Rather, improvements in the statistical analysis and reporting on added sugars would alleviate numerous concerns. Studies could be strengthened by analyzing and reporting nutrient intakes as an outcome or over time. Energy balance should always be analyzed and reported when the data are available to do so, and when appropriate, both energy balance and nutrient intakes should be included as covariates in multivariate statistical models.
Inconsistencies in terminology significantly impact researchers’ ability to both locate relevant studies on added sugars and draw appropriate inferences from study results. For example, the consistent application of standardized terms for added sugars and sugar-sweetened beverages would allow for improved clarity on the precise exposure being measured and facilitate greater consistency in comparison across studies. Improvements can also be made in the specification of reporting total vs added sugars intake, as well as replacing the use of generic terms such as “sugar intake,” by more descriptive terms such as “total sugars intake” or “total added sugars intake,” for example. Finally, all nutritional science research could benefit from making nutrient intake data publicly available for secondary analysis.
Strengths and limitations
This evidence mapping exercise offers numerous strengths. The scraping of references from leading policy documents on added sugars intake ensured that the resultant body of literature included studies deemed important by global policymakers. The data extraction process was strengthened by the systematic dual-extraction approach, increasing the data reliability. Notably, the comprehensive data extraction approach yielded important information not gathered in previous reviews on added sugars intake, included the handling of TEI, and reported added sugars concentrations.
While this review included numerous systematic elements, it was limited by a weak literature search strategy. Defining appropriate search terms was challenging as there are no MeSH terms for added sugars. While MeSH terms do exist for sugar-sweetened beverages and dietary sugars, an overwhelming amount of research returned using these terms did not assess added sugars, or they described the exposure to added sugars using vague terminology. Additionally, the inclusion of specific health outcomes in the search terms along with added sugars terms yielded far fewer relevant articles than expected. Thus, we used a combination of phrase searching and leveraged backwards citation screening to identify relevant research. Overall, a stronger literature search strategy would have strengthened this evidence map by capturing a more comprehensive set of relevant publications. Another notable limitation of this evidence map is that data were not extracted at the comparator level, and therefore studies reporting multiple added sugars sources were not fully represented. For example, we prioritized extracting intake data on total dietary added sugars, rather than extracting all possible exposures that contributed to added sugars intake (e.g., SSB, sucrose, bakery products, etc.)
5. Conclusions
This scoping review and evidence map provides valuable insights into the literature on dietary added sugars and select health outcomes. The identified publications reflect a significant increase in research on added sugars since 2010, with a notable emphasis on body composition and cardiovascular health in adults, particularly focusing on liquid sources of added sugars. Broad overlap in outcomes measured in studies primarily focused on diabetes, cardiovascular health, and body composition suggest the body of evidence is substantially large, highlighting the opportunity to mine exposure-endpoint associations from adjacent domains. However, our analysis revealed substantial heterogeneity across various methodological aspects, including study designs, exposures, outcomes, terminology, and reporting of dietary intake data. The limited reporting of energy balance and energy intake in the studies raises concerns about potential confounding factors and the comprehensive understanding of the effects of added sugars. By addressing these gaps and improving the quality of research in this domain, the state of knowledge on added sugars will be enhanced and contribute to more informed policies and dietary recommendations for public health. The research described in this publicly available database may be used by the scientific community to alleviate the burden of heterogeneity and assist with finding in-scope research for future reviews and meta-analyses.
Author Contributions
Conceptualization: SAF, TLP, PCG, MOS; Methodology: SAF, JAP, TLP; Investigation: SAF, JAP, TLP; Data curation: SAF, TLP, JAP; Validation: All authors; Visualization: SAF, JAP; Formal analysis: SAF, JAP; Funding acquisition: SAF; Project administration: JAP, TLP; Supervision: SAF; Writing – original draft: SAF, JAP; Writing – review & editing: SAF, JAP, TLP, PCG, MOS. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sugar Association Inc.
Data Availability Statement
Acknowledgments
The authors wish to thank Dr. Flora Wang for her review and commentary on drafts of the manuscript.
Conflicts of Interest
Author SAF has ownership in Traverse Science. TLP and SAF are employees of Traverse Science. JAP consulted for Traverse Science. MOS and PCG are employees of Sugar Association Inc.
References
- U.S. Food & Drug Administration Added Sugars on the New Nutrition Facts Label Available online:. Available online: https://www.fda.gov/food/new-nutrition-facts-label/added-sugars-new-nutrition-facts-label (accessed on 1 August 2023).
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Bohn, T.; Castenmiller, J.; Henauw, S. de; Hirsch-Ernst, K.; Knutsen, H.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Dietary Sugars. EFSA Journal 2022, 20. [CrossRef]
-
World Health Organization Guideline: Sugars Intake for Adults and Children; WHO Press: Geneva, Switzerland, 2015.
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A Review of Total & Added Sugar Intakes and Dietary Sources in Europe. Nutr J 2017, 16, 6. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture 2015-2020 Dietary Guidelines for Americans; 8th ed.; 2015.
- U.S. Department of Agriculture and U.S. Department of Health and Human Services Dietary Guidelines for Americans, 2020-2025; 9th ed.; 2020.
- Erickson, J.; Slavin, J. Are Restrictive Guidelines for Added Sugars Science Based? Nutr J 2015, 14, 124. [Google Scholar] [CrossRef]
- Hess, J.; Latulippe, M.E.; Ayoob, K.; Slavin, J. The Confusing World of Dietary Sugars: Definitions, Intakes, Food Sources and International Dietary Recommendations. Food Funct 2012, 3, 477–486. [Google Scholar] [CrossRef]
- Choo, V.L.; Viguiliouk, E.; Mejia, S.B.; Cozma, A.I.; Khan, T.A.; Ha, V.; Wolever, T.M.S.; Leiter, L.A.; Vuksan, V.; Kendall, C.W.C.; et al. Food Sources of Fructose-Containing Sugars and Glycaemic Control: Systematic Review and Meta-Analysis of Controlled Intervention Studies. Bmj 2018, 363, k4644. [Google Scholar] [CrossRef]
- Yan, R.R.; Chan, C.B.; Louie, J.C.Y. Current WHO Recommendation to Reduce Free Sugar Intake from All Sources to below 10% of Daily Energy Intake for Supporting Overall Health Is Not Well Supported by Available Evidence. Am J Clin Nutrition 2022, 116, 15–39. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, 2020.
- Medicine, I. of Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, 2005. [Google Scholar]
- Sundborn, G.; Thornley, S.; Merriman, T.R.; Lang, B.; King, C.; Lanaspa, M.A.; Johnson, R.J. Are Liquid Sugars Different from Solid Sugar in Their Ability to Cause Metabolic Syndrome? Obesity 2019, 27, 879–887. [Google Scholar] [CrossRef]
- Wang, J.; Shang, L.; Light, K.; O’Loughlin, J.; Paradis, G.; Gray-Donald, K. Associations between Added Sugar (Solid vs. Liquid) Intakes, Diet Quality, and Adiposity Indicators in Canadian Children. Appl Physiology Nutrition Metabolism 2015, 40, 835–841. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Cheung, A.; Ayoub-Charette, S.; Ahmed, A.; Lee, D.; Au-Yeung, F.; Qi, X.; Back, S.; McGlynn, N.; Ha, V.; et al. Important Food Sources of Fructose-Containing Sugars and Adiposity: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Am J Clin Nutrition 2023, 117, 741–765. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; Souza, R.J. de; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Buono, M.D.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of Fructose on Body Weight in Controlled Feeding Trials: A Systematic Review and Meta-Analysis. Ann Intern Med 2012, 156, 291. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Sievenpiper, J.L.; Souza, R.J. de; Cozma, A.I.; Mirrahimi, A.; Carleton, A.J.; Ha, V.; Buono, M.D.; Jenkins, A.L.; Leiter, L.A.; et al. Effect of Fructose on Markers of Non-Alcoholic Fatty Liver Disease (NAFLD): A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Eur J Clin Nutr 2014, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Ayoub-Charette, S.; Liu, Q.; Khan, T.A.; Au-Yeung, F.; Mejia, S.B.; Souza, R.J. de; Wolever, T.M.; Leiter, L.A.; Kendall, C.; Sievenpiper, J.L. Important Food Sources of Fructose-Containing Sugars and Incident Gout: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Bmj Open 2019, 9, e024171. [Google Scholar] [CrossRef] [PubMed]
- Attuquayefio, T.; Stevenson, R.J.; Oaten, M.J.; Francis, H.M. A Four-Day Western-Style Dietary Intervention Causes Reductions in Hippocampal-Dependent Learning and Memory and Interoceptive Sensitivity. Plos One 2017, 12, e0172645. [Google Scholar] [CrossRef] [PubMed]
- Morenga, L.T.; Mallard, S.; Mann, J. Dietary Sugars and Body Weight: Systematic Review and Meta-Analyses of Randomised Controlled Trials and Cohort Studies. Bmj Br Medical J 2013, 346, e7492. [Google Scholar] [CrossRef]
- Rasad, H.; Entezari, M.H.; Ghadiri, E.; Mahaki, B.; Pahlavani, N. The Effect of Honey Consumption Compared with Sucrose on Lipid Profile in Young Healthy Subjects (Randomized Clinical Trial). Clin Nutrition Espen 2018, 26, 8–12. [Google Scholar] [CrossRef]
- Angelopoulos, T.J.; Lowndes, J.; Sinnett, S.; Rippe, J.M. Fructose Containing Sugars at Normal Levels of Consumption Do Not Effect Adversely Components of the Metabolic Syndrome and Risk Factors for Cardiovascular Disease. Nutrients 2016, 8, 179. [Google Scholar] [CrossRef]
- DiMeglio, D.; Mattes, R. Liquid versus Solid Carbohydrate: Effects on Food Intake and Body Weight. Int J Obesity 2000, 24, 794–800. [Google Scholar] [CrossRef]
- Yu, Z.; Lowndes, J.; Rippe, J. High-Fructose Corn Syrup and Sucrose Have Equivalent Effects on Energy-Regulating Hormones at Normal Human Consumption Levels. Nutr Res 2013, 33, 1043–1052. [Google Scholar] [CrossRef]
- Aeberli, I.; Hochuli, M.; Gerber, P.A.; Sze, L.; Murer, S.B.; Tappy, L.; Spinas, G.A.; Berneis, K. Moderate Amounts of Fructose Consumption Impair Insulin Sensitivity in Healthy Young Men. Diabetes Care 2013, 36, 150–156. [Google Scholar] [CrossRef]
- Angelopoulos, T.J.; Lowndes, J.; Sinnett, S.; Rippe, J.M. Fructose Containing Sugars Do Not Raise Blood Pressure or Uric Acid at Normal Levels of Human Consumption. J Clin Hypertens 2015, 17, 87–94. [Google Scholar] [CrossRef]
- Reid, M.; Hammersley, R.; Hill, A.J.; Skidmore, P. Long-Term Dietary Compensation for Added Sugar: Effects of Supplementary Sucrose Drinks over a 4-Week Period. Brit J Nutr 2007, 97, 193–203. [Google Scholar] [CrossRef]
- Campos, V.; Despland, C.; Brandejsky, V.; Kreis, R.; Schneiter, P.; Chiolero, A.; Boesch, C.; Tappy, L. Sugar- and Artificially Sweetened Beverages and Intrahepatic Fat: A Randomized Controlled Trial. Obesity 2015, 23, 2335–2339. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.W.; Nielsen, B.M.; Husby, I.; Bugge, A.; El-Naaman, B.; Andersen, L.B.; Trolle, E.; Heitmann, B.L. Association between Sweet Drink Intake and Adiposity in Danish Children Participating in a Long-term Intervention Study. Pediatr Obes 2013, 8, 259–270. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Literature search and selection flow diagram.
Figure 1.
Literature search and selection flow diagram.
Figure 2.
Venn diagram of citation overlap for all references included in the final database. For visual purposes, “Cohort Studies” includes both case-control and case-cohort studies. References from the IOM were not included in this overlap as they contributed only 5 unique studies.
Figure 2.
Venn diagram of citation overlap for all references included in the final database. For visual purposes, “Cohort Studies” includes both case-control and case-cohort studies. References from the IOM were not included in this overlap as they contributed only 5 unique studies.
Figure 3.
Cumulative growth of publications (n=245) from 1990 – 2021, by (A) terms used in the title/abstract, (B) study design, (C) source of added sugars, (D) included age group, and (E) primary outcomes. Infant, <12 months old; Toddler, 12 months to <3 years old; Child, 3-11 years old; Adolescent, 12-17 years old; Adult, 18-64 years old; Senior, 65 years and older.
Figure 3.
Cumulative growth of publications (n=245) from 1990 – 2021, by (A) terms used in the title/abstract, (B) study design, (C) source of added sugars, (D) included age group, and (E) primary outcomes. Infant, <12 months old; Toddler, 12 months to <3 years old; Child, 3-11 years old; Adolescent, 12-17 years old; Adult, 18-64 years old; Senior, 65 years and older.
Figure 4.
Distribution of reported added sugars intake (% TEI) by study types for (A) source, (B) form, and (C) energy control. Note that this figure represents a subset of the included studies, as only those that quantified added sugars intake at the % TEI level are represented (111/247 studies). These intakes are means, medians, or quartiles for all participats or stratified by participant characteristics (e.g., sex, BMI category, etc.). The dashed line represents the median.
Figure 4.
Distribution of reported added sugars intake (% TEI) by study types for (A) source, (B) form, and (C) energy control. Note that this figure represents a subset of the included studies, as only those that quantified added sugars intake at the % TEI level are represented (111/247 studies). These intakes are means, medians, or quartiles for all participats or stratified by participant characteristics (e.g., sex, BMI category, etc.). The dashed line represents the median.
Figure 5.
Heat map of all measured outcomes (rows) compared to primary outcomes (columns). “Other” outcomes include risk of metabolic syndrome, inflammatory markers, appetite, dietary intake, and other unrelated outcomes.
Figure 5.
Heat map of all measured outcomes (rows) compared to primary outcomes (columns). “Other” outcomes include risk of metabolic syndrome, inflammatory markers, appetite, dietary intake, and other unrelated outcomes.
Figure 6.
Heat map bubble plot of outcome categories, study duration, and sample size by source of added sugars among observational studies.
Figure 6.
Heat map bubble plot of outcome categories, study duration, and sample size by source of added sugars among observational studies.
Figure 7.
Heat map bubble plot of outcome categories, study duration, and sample size by source of added sugars among intervention studies.
Figure 7.
Heat map bubble plot of outcome categories, study duration, and sample size by source of added sugars among intervention studies.
Table 1.
Study eligibility criteria .
Table 1.
Study eligibility criteria .
| Inclusion Criteria |
Exclusion Criteria |
|
Population: Humans of any health status; pregnant women were included if the outcome was measured at the maternal level. |
Population: Pregnant women where outcomes were measured at the infant- or dyad-level. |
|
Study Design: All types of intervention and observational study designs (primary literature). |
Study Design: Ecological studies; narrative reviews; systematic reviews; meta-analyses. |
|
Exposure: Oral intake of: Added sugars, free sugars, extrinsic sugars, or SSB. SSB were defined broadly to include all kinds of sweetened beverages and oral sugar solutions (e.g., sweet tea, lemonade, sports drinks, energy drinks, fruit drinks, sweetened/flavored milks, experimentally created glucose solutions, etc.). |
Exposure: Parenteral or enteral nutrition; studies only reporting total or intrinsic sugars; sugar used as an analgesic in infants; label, marketing, or educational studies on consumer perception of sugar; dietary pattern studies where added sugars intake wasn’t directly assessed in relation to a health outcome; whole fruit intake; intervention/exposure groups that did not differ significantly by sugar content/intake but by another nutrient (e.g., fiber); studies assessing the effects of policy/tax changes on added sugars intake. |
|
Outcome: Body weight, body composition, obesity, diabetes, and cardiovascular health; any intermediate biomarkers for these diseases; cause-specific mortality due to these diseases. |
Analysis: Studies that did not statistically assess the association between added sugars intake and a prespecified health outcome; studies that only analyzed added sugars intake as a confounder/covariate. |
|
Timeline: Studies from 1990-2021. |
Language: Non-English publications. |
Table 2.
Summary of study design and population characteristics of studies included in the evidence map and database, stratified by source of added sugars (n=245) .
Table 2.
Summary of study design and population characteristics of studies included in the evidence map and database, stratified by source of added sugars (n=245) .
| Characteristic |
Grand Total |
Source of Added Sugars |
| Liquids Only |
Mixed |
| n (% of total, column-wise) |
245 |
137 |
108 |
| Study design |
|
|
|
| Parallel-arm trial |
40 (16%) |
30 (22%) |
10 (9%) |
| Crossover trial |
14 (6%) |
7 (5%) |
7 (6%) |
| Cohort |
122 (50%) |
85 (62%) |
37 (34%) |
| Cross-sectional |
64 (26%) |
13 (9%) |
51 (47%) |
| Other |
5 (2%) |
2 (1%) |
3 (1%) |
|
Age group1,2
|
|
|
|
| Infant |
3 (1%) |
1 (1%) |
2 (2%) |
| Toddler |
21 (9%) |
13 (9%) |
8 (7%) |
| Child |
84 (34%) |
50 (36%) |
34 (31%) |
| Adolescent |
62 (25%) |
29 (21%) |
33 (31%) |
| Adult |
159 (65%) |
87 (64%) |
72 (67%) |
| Senior |
60 (24%) |
29 (21%) |
31 (29%) |
|
Baseline health status1
|
|
|
|
| Healthy |
223 (91%) |
130 (95%) |
93 (86%) |
| Diabetes |
11 (4%) |
2 (1%) |
8 (8%) |
| Cardiovascular disease |
7 (3%) |
4 (3%) |
3 (3%) |
| Other health condition |
4 (2%) |
1 (1%) |
4 (3%) |
| Baseline weight status |
|
|
|
| Exclusively normal weight |
9 (4%) |
5 (4%) |
4 (4%) |
| Exclusively overweight or obese |
28 (11%) |
17 (12%) |
11 (10%) |
| Mixed status3 |
208 (85%) |
115 (84%) |
93 (86%) |
|
Primary Outcomes1,3
|
|
|
|
| Body composition |
87 (36%) |
50 (36%) |
37 (34%) |
| Body weight |
55 (22%) |
32 (23%) |
23 (21%) |
| Cardiovascular health |
79 (32%) |
44 (32%) |
35 (32%) |
| Diabetes mellitus |
55 (22%) |
34 (25%) |
21 (19%) |
| Metabolic measures |
19 (8%) |
9 (7%) |
10 (9%) |
| Mortality |
13 (5%) |
6 (4%) |
7 (6%) |
| Obesity |
38 (16%) |
23 (17%) |
15 (14%) |
| Other |
25 (10%) |
9 (7%) |
16 (15%) |
|
1Not mutually exclusive categories. |
|
2Age categories were defined as follows: Infant – < 12 months; Toddler – 12 months to < 3 years; Child – 3-11 years; Adolescent – 12-17 years; Adult – 18-64 years; Senior – 65+ years. |
|
3Primary outcomes represent domains of interest as expressed by the authors but does not represent all measured outcomes. |
Table 3.
Diet energy balance and total energy intake design characteristics, by study design (n=245).
Table 3.
Diet energy balance and total energy intake design characteristics, by study design (n=245).
| Characteristic |
Clinical Trial (n=56) |
Cohort (n=125) |
Cross-sectional (n=64) |
| TEI controlled1 |
TEI not controlled1 |
TEI controlled1 |
TEI not controlled1 |
TEI controlled1 |
TEI not controlled1 |
| n (% within column) |
15 (27%) |
41 (73%) |
95 (76%) |
30 (24%) |
40 (63%) |
24 (38%) |
| Diet Energy Balance2 |
|
|
|
|
|
|
| Positive |
0 |
0 |
1 (1%) |
0 |
0 |
0 |
| Neutral |
6 (40%) |
4 (10%) |
1 (1%) |
0 |
1 (3%) |
0 |
| Negative |
2 (13%) |
0 |
1 (1%) |
0 |
0 |
0 |
| Unspecified |
9 (60%) |
39 (95%) |
93 (98%) |
30 (100%) |
39 (98%) |
24 (100%) |
| Reported TEI |
11 (73%) |
22 (54%) |
79 (83%) |
9 (30%) |
32 (80%) |
12 (50%) |
|
1Studies were recorded as having controlled for total energy intake if participants were matched on TEI, investigators prescribed a diet with known TEI, or if TEI was statistically adjusted for in the analysis. |
|
2Not mutually exclusive categories. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).