1. Introduction
Geothermal energy is a potential source of sustainable and renewable energy. However, despite having the ability to provide clean and reliable power generation, geothermal energy has yet to reach its full potential. Because the performance and durability of various components of geothermal power plants are under constant threat of corrosion and scaling due to harsh environmental conditions such as high temperature, varied pH, humidity, silica, and acids [
1], these issues adversely affect the stability and efficiency of the power plant, leading to increased operation and maintenance (O&M) costs. Stainless steel and titanium are the most commonly used materials that are used in designing geothermal plants’ components. Stainless steel provides corrosion resistance, high-temperature sustainability, excellent mechanical strength, and durability. Titanium is also an excellent choice for geothermal heat exchangers due to its lightweight and outstanding corrosion resistance. Material selection depends on the geothermal fluid chemistry influenced by geographical location and heat exchanger configuration. In the long term, replacing components of the heat exchanger is expensive as compared to applying paints and coatings on the geothermal plant’s components. Coatings designed for geothermal power plant components, particularly geothermal heat exchangers, could be a feasible and economical solution, providing protection against extreme thermal gradients, aggressive fluids, and mechanical stress.. Furthermore, coatings, especially micro/nanostructured surfaces play a crucial role in improving the overall efficiency and reliability of geothermal energy generation [
2].
Thermal spray coatings have gained attention due to depositing high-quality coatings with tailored properties. Conventional powder-based thermal spray is widely accepted and employed in various industries but when it comes to depositing very small particles (<10 microns), poor injection due to low inertia of particles restricts the use of gas as a carrier . Therefore, liquid carrying submicron particles (suspension) or a solution of chemical precursors of the coating material, forming solid particles during flight are preferred so that coatings with nano- and micro-scale features can be obtained.[
3]. Besides, using liquid in the form of suspension or solution or both provides better flowability of the feedstock as well as enhanced control over coating microstructures [
4]. There is no specific microstructure that is suitable for each geothermal heat exchanger as the choice of microstructure is influenced by different factors including geothermal plant location (as the geothermal fluid chemistry and constituents vary with the location of the geothermal plant) and types of heat transfer (boilers, condensers, and evaporators). Geothermal heat exchangers’ components are exposed to high temperature and pressure, harsh chemicals and mechanical stresses. Therefore, microstructure of thermal spray coating needs to be engineered to resolve these issues, accordingly. For example, coatings with columnar microstructures are suitable for facilitating thermal cycling resistance but simultaneously, they might not be suitable for providing proper protection against corrosive environment due to the ingress of corrosive agents into the substrate. Similarly, coatings with vertically cracked microstructures could allow stress relief, decreasing the risk of coating delamination. But simultaneously, excessive cracking might compromise the structural integrity of the coating. Generally, dense coatings with controlled cracking or columnar structures are preferred in geothermal environment, providing better protection against corrosive agents along with the tolerance for thermal cycling. For better control over coating microstructure and properties, it is essential to understand the relationship between coating deposition/spray parameters and coating properties. The performance and characteristics of liquid feedstock-based thermal spray coatings are influenced by numerous key depositing parameters, for instance, feedstock type, feedstock flow rate, particle size, feedstock concentration, solvent type, plasma current and voltage, primary gases’ type and flow rate, stand-off distance, and substrate size, shape, and temperature [
5].
In this study, suspension plasma spray (SPS) was used to deposit TiO
2 coatings onto a carbon steel substrate. TiO2 coatings were chosen to protect the geothermal heat exchangers’ components against corrosion and erosion as geothermal fluids contain abrasive particles, and to provide high-temperature stability and chemical stability to geothermal heat exchanger components protecting them against extreme heat conditions. Besides there are many reports available mentioning that the use of TiO2 coatings in heat exchangers improving their thermal conductivity [
6]. These coatings are formed using an aqueous suspension of TiO
2 nanoparticles as the feedstock material. The aqueous suspension was chosen due to cost-effectiveness, environmental considerations, safety concerns and avoiding waste disposal issues. The effect of standoff distance (SOD; the distance between the injector nozzle and substrate surface) and feedstock flow rate (the rate at which the suspension feedstock is delivered to the spray gun) on coating microstructure was focused that is crucial for optimizing the coating process and attaining desirable coating performance. The SOD and feedstock flow rate influence the critical parameters of coating deposition, such as particle velocity, heat transfer, cooling rate, and adhesion. As soon as suspension feedstock is introduced into the system, it fragments into droplets due to having contact with the plasma plume, followed by solvent evaporation and coating material agglomeration because of high-enthalpy sintering and melting. Due to the acceleration toward the substrate, these feedstock droplets hit the substrate and form splats, developing coating through continuous stacking of splats [
7].
Comprehensive analyses of coatings deposited via systematically varied SOD, and the feedstock flow rate was done using scanning electron microscopy (SEM), roughness measurements, and tape adhesion test. The findings of this work will help in optimizing the SPS of TiO2 coatings on geothermal heat exchangers, enhancing their corrosion resistance, scaling resistance, and fouling resistance.
3. Results and discussion
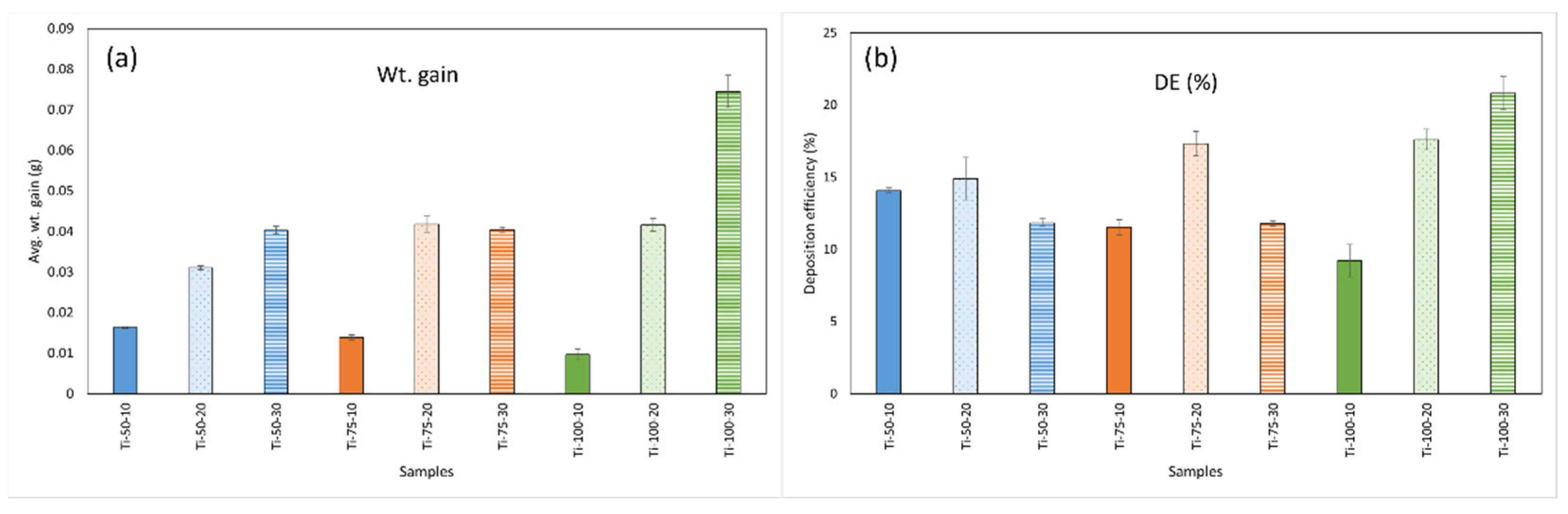
The average weight gain of all samples is presented in
Figure 1 (a). It can be seen from the figure that the weight increases along with the increased feedstock flowrate, which can be explained as when more feedstock material is inserted into the plasma flame, after fragmentation, bigger droplets are formed that travel in the core region of the plasma and do not deviate from the path [
12]. Hence, more material is deposited on the substrate, meaning more weight gain. In the case of SOD, the weight gain fluctuates with the feedstock flow rate. At a low feedstock flow rate (10 ml/min), the weight gain decreases with the increased SOD. This could be because, at a lower feedstock flow rate, smaller droplets with less material are formed, which, after evaporation of water, give rise to small-size agglomerates. Small droplets/particles tend to follow the gas trajectory and deviate from the central region, leading to less material deposition [
12]. Simultaneously, coatings deposited at higher SOD (100mm) with a higher flow rate (30 ml/min) show more weight gain due to the formation of larger droplets hitting the substrate.
The deposition efficiency (DE) of all samples is shown in
Figure 1 (b), and it was found that all samples show low DE. When spraying suspension feedstock, the solvent evaporates, and only solid content reaches the substrate. It should be noted that DE is the underestimated value due to the loss of feeding material during acceleration and deceleration and overspray of the torch robot [
7]. Although a larger substrate can help resolve overspray to some extent (for smaller substrate, due to being carried away with the air flow, sprayed particles tend to overshoot the edges and lost to the surrounding environment. While in the case of larger substrate, a greatest distance is travelled by sprayed particles before reaching the edges, retaining more material on the substrate), the loss of feedstock material during acceleration and deceleration is unavoidable. The comparison of DEs is still valid as these accuracies are standard for each sample. Low DE is also associated with the radial injection of the feedstock material because, sometimes, the small droplets cannot reach the core region of the plasma plume. Instead, they deflect from their path, leading to insufficient energy exchange, and hence, poor deposition occurs. It was observed that at a lower flow rate (10 ml/min), with the increased SOD, DE decreases. This can be elucidated by the fact that when a smaller amount of material is introduced into the plasma after fragmentation, it leads to the formation of smaller droplets. These smaller droplets may not efficiently reach the substrate as they tend to follow the gas trajectory. So, more material is deposited at a smaller SOD (50mm), where the substrate is comparatively closer to the gun, and the kinetic energy is higher than at longer SODs (100mm). While DE increases for a higher feedstock flow rate (30 ml/min) at longer SODs because, due to the injection of more feedstock material, larger droplets are formed that have more inertia and higher kinetic energy compared to the smaller droplets. Hence, more material is deposited.
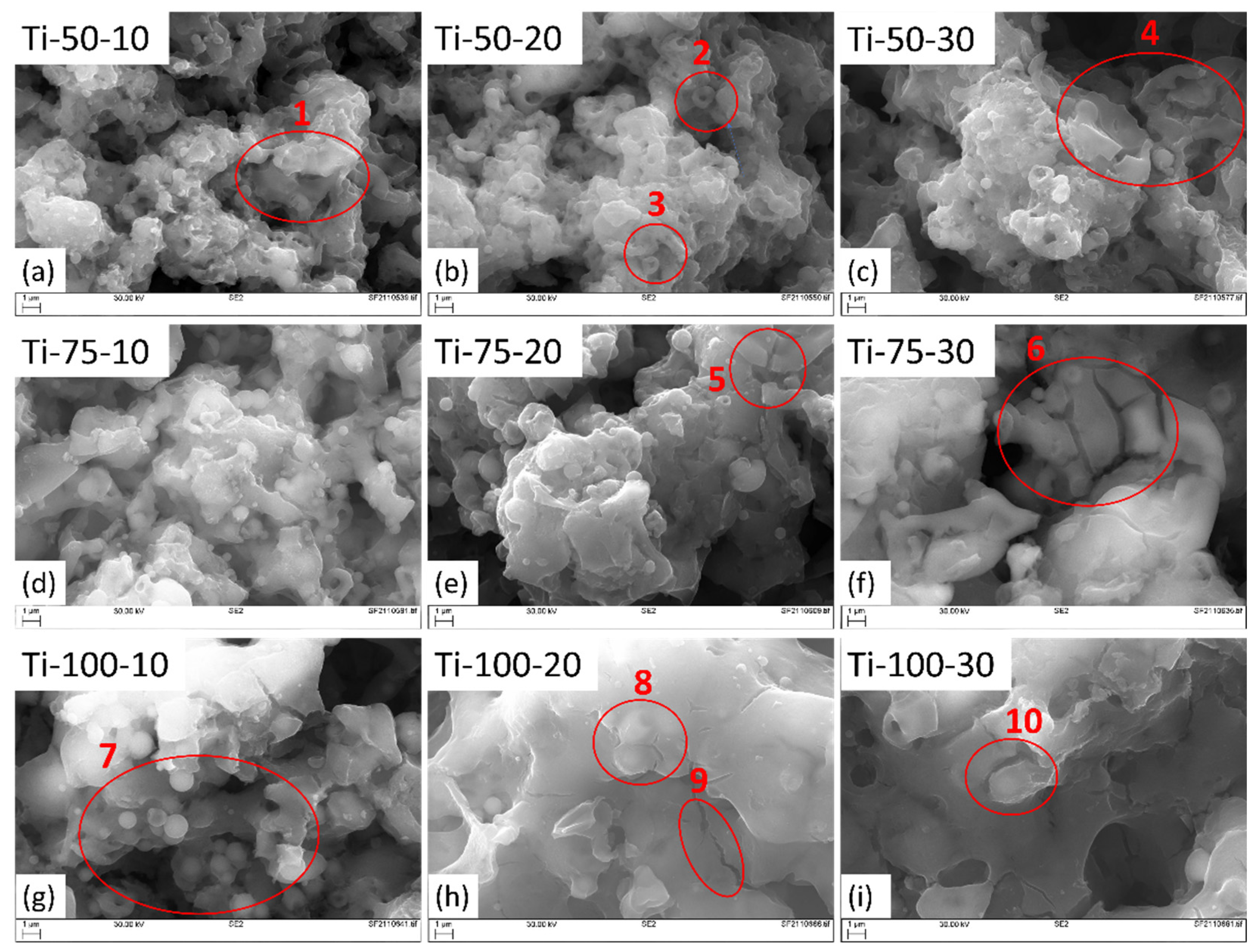
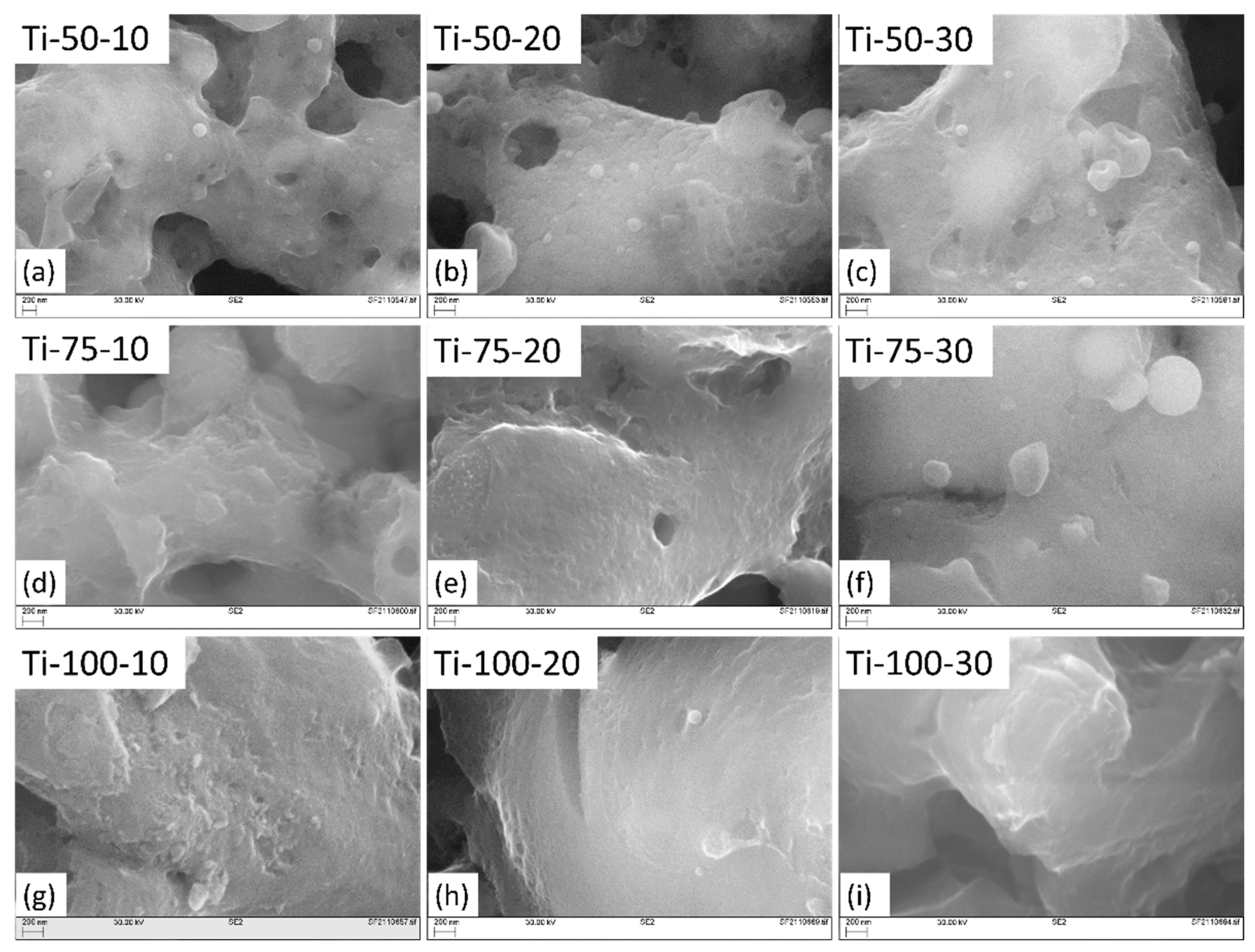
The SEM images of TiO
2 coatings at different magnifications are shown in and
Figure 3. All coatings show typical cauliflower-like topography due to the deposition of feedstock material in the asperities of the substrate after hitting it at shallow angles. This phenomenon is referred to as the “shadow effect,” and it becomes more pronounced when dealing with smaller droplets. These smaller droplets deviate from their intended path as they follow the gas trajectory, often depositing on substrate asperities while moving parallel to the substrate surface [
12].
It was found that fully-melted and thinner splats are formed at smaller SODs (; circle 1), while thicker splats with partially melted or re-solidified feedstock droplets (; circle 7) take place at longer SODs. This happens because, at smaller SOD, the thermal and kinetic energy of formed droplets are higher than the longer SODs. Hence, fully-melted droplets hit the substrate with a higher impact, spreading and covering the substrate properly. While at longer SODs, due to the insufficient thermal and kinetic energy transfer, rougher and thicker splats are formed. Also, at longer distances, nanomaterials, because of their high surface area, cool down very quickly and re-solidify even before hitting the substrate or sticking to the surface, giving rise to voids after being trapped between subsequent splats [
13].
With the increased feedstock flow rate, rougher splats formation, partial melting of feedstock droplets (; circles 2, 3, 8 & 9), and mud-like cracks formation (; 4, 5 & 6) also increase due to solvent evaporation. This can be explained by noting that when additional feedstock material is introduced, a greater volume of solvent (water) is also injected into the system. This additional solvent serves to cool down the flame. This leads to a comparatively lower heat transfer from the plasma to feedstock droplets. Hence, rougher and thicker splats and partial or no melting of TiO
2 droplets take place [
14]. When these partially molten or unmolten particles deposit on the substrate, there is a difference in their temperature, microstructures, mechanical properties and bonding characteristics, and the non-uniform distribution of these particles, could result in localized stress concentrations, potentially generating mud-crack formation in certain parts of the coating..
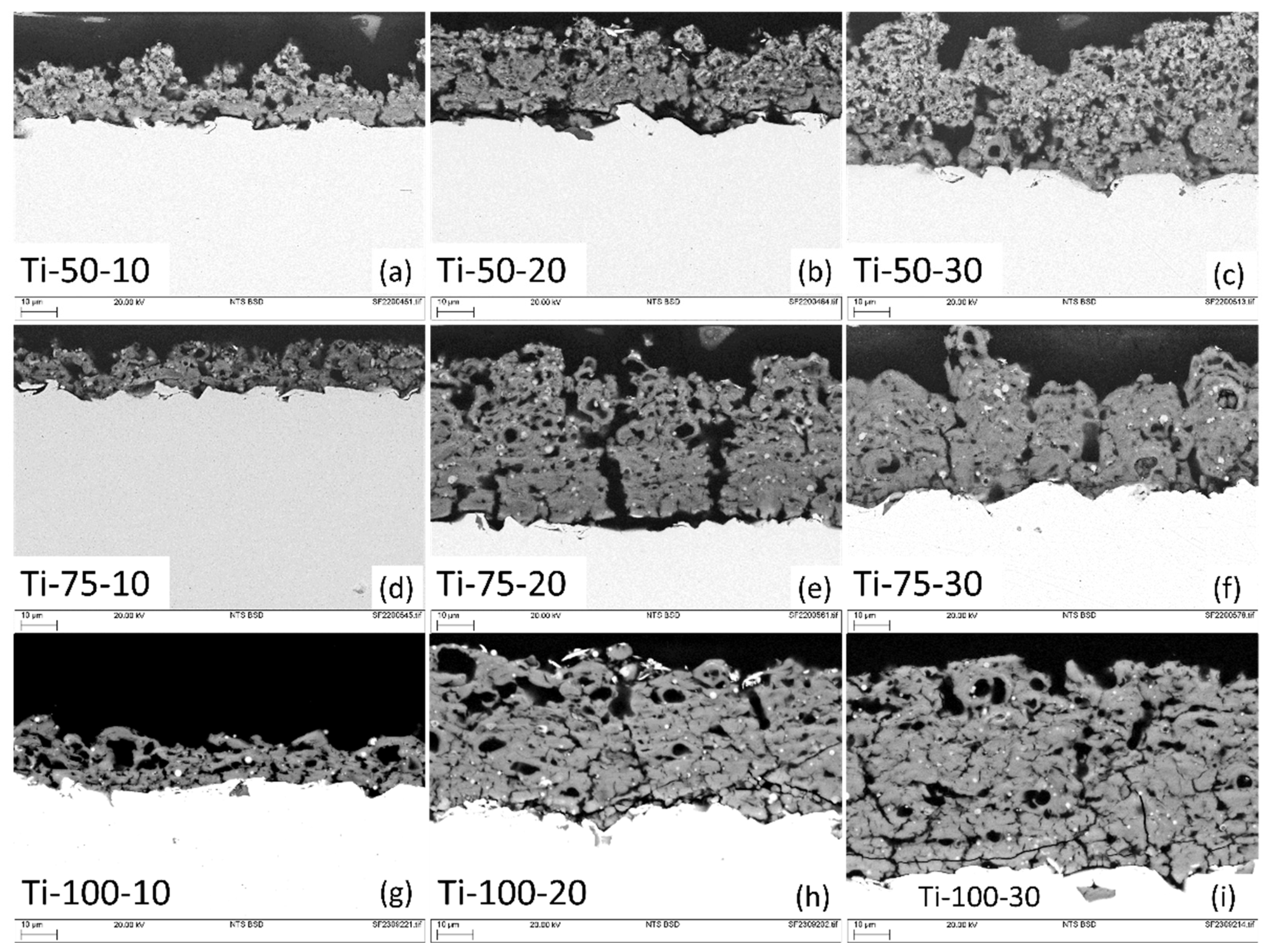
Cross-sectional back-scattered SEM images of developed coatings are shown in
Figure 4. Since the substrate was pretreated with alumina grit blasting before coating deposition for better adhesion between coating and substrate, the formation of columnar structures is more favourable than vertical cracks or smooth coatings [
15]. This happens because when particle droplets travel toward the substrate perpendicularly, only big particles possessing a high moment of inertia hit the substrate perpendicularly. Smaller particles easily get influenced by the drag force of the plasma trajectory and deviate from their original direction, impacting the substrate at a shallow angle, and deposit on the nearest asperities, forming columnar features (shadowing effect). After subsequent deposition and vertical and horizontal growth, cone-shaped structures are developed, where inter-columnar voids separate these cones/columns, and the head of columns gives rise to a typical cauliflower-like morphology [
16].
Coatings deposited using 10ml/min do not exhibit column formation prominently due to the inefficient coverage of the substrate (thinner coatings). Simultaneously, coatings deposited at 50mm SOD show sharper peaks in cross-sections because of the high impact and kinetic energy of the particles, which hit the substrate with high accelerated force, depositing well-spread splat over the substrate’s asperities. As the coating gets thicker, column formation and their separation (inter-columnar voids) can be observed clearly (
Figure 4 (e & f)) [
16]. At longer SOD, due to inefficient kinetic and thermal energy exchange, thicker splats are formed that give rise to wide and comparatively round-shaped peaks. Also, it was found that with the increased feedstock flow rate, partially melted, un-melted, and agglomerated/sintered feedstock particles were observed more prominently, leading to more porous coating with vertical cracks formation (
Figure 4 (c)). The coatings at 50 mm SOD, especially deposited using 20 and 30 ml/min feedstock flow rate, exhibited the presence of more partially melted or un-melted feedstock particles and more porosity as compared to the coatings developed at 75 mm SOD and 20 and 30 ml/min feedstock flow rate (
Figure 4 e&f). This can be explained as, at a shorter distance, feedstock droplets do not get sufficient time for solvent evaporation, agglomeration, sintering, and melting, consequently giving rise to more porous coatings [
17]. While at longer SOD, sometimes, melted material cools down even before hitting the substrate and, rather than forming a splat, adheres to the previously deposited splats.
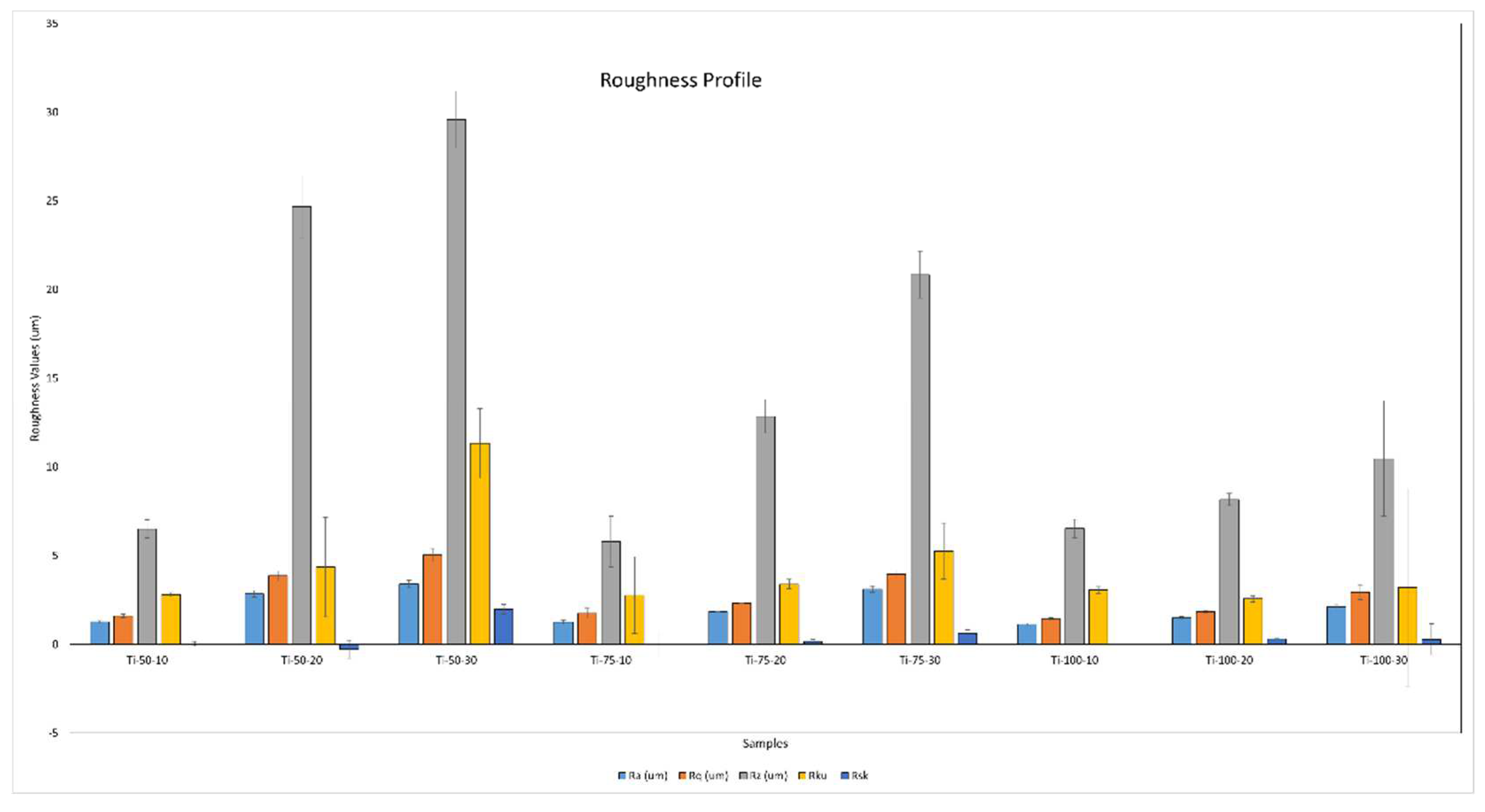
The spray parameters are critical in influencing the particle impact energy, coating microstructures, and coating roughness. The R
a (average roughness), R
q (root mean square roughness), R
z (maximum height roughness), R
ku (kurtosis: sharpness of the surface’s peaks and valleys), and R
sk (skewness: asymmetry of the surface) values are shown in
Figure 5. It was found that with increased feedstock flow rate, the R
a, R
q, R
z, and R
sk increase because at a higher feedstock flow rate, particle accumulation, over-deposition, and insufficient particle flattening take place, resulting in a coating with higher porosity and rougher surface texture. With the feedstock flow rate, R
ku was increased but only for 50 mm SOD. In comparison, for 75mm and 100mm SODs, R
ku was almost similar, which means at 50mm SOD, due to the comparatively high impact of particles, sharper peaks and valleys are formed on the substrate’s asperities. For Ti-50-20, the R
sk value was negative, indicating that there are more valleys or depressions on the surface than peaks.
The roughness values were almost similar for the coatings deposited at different SODs using a low feedstock flow rate, i.e., 10 ml/min. In comparison, this trend changes at higher 20 and 30 ml/min, resulting in a decreased roughness with the increased SOD. This can be elucidated by the fact that, at lower feedstock flow rates, the formation of flattened splats with extensive substrate coverage occurs. This results in a well-adhered coating due to the adequate melting of the coating material. Also, it can be concluded that at higher SODs, the sharpness of peaks/columns decreases because the insufficient kinetic and thermal energy exchange gives rise to thicker (disc-like) splats, forming columns with globular heads after subsequent deposition [
18,
19].
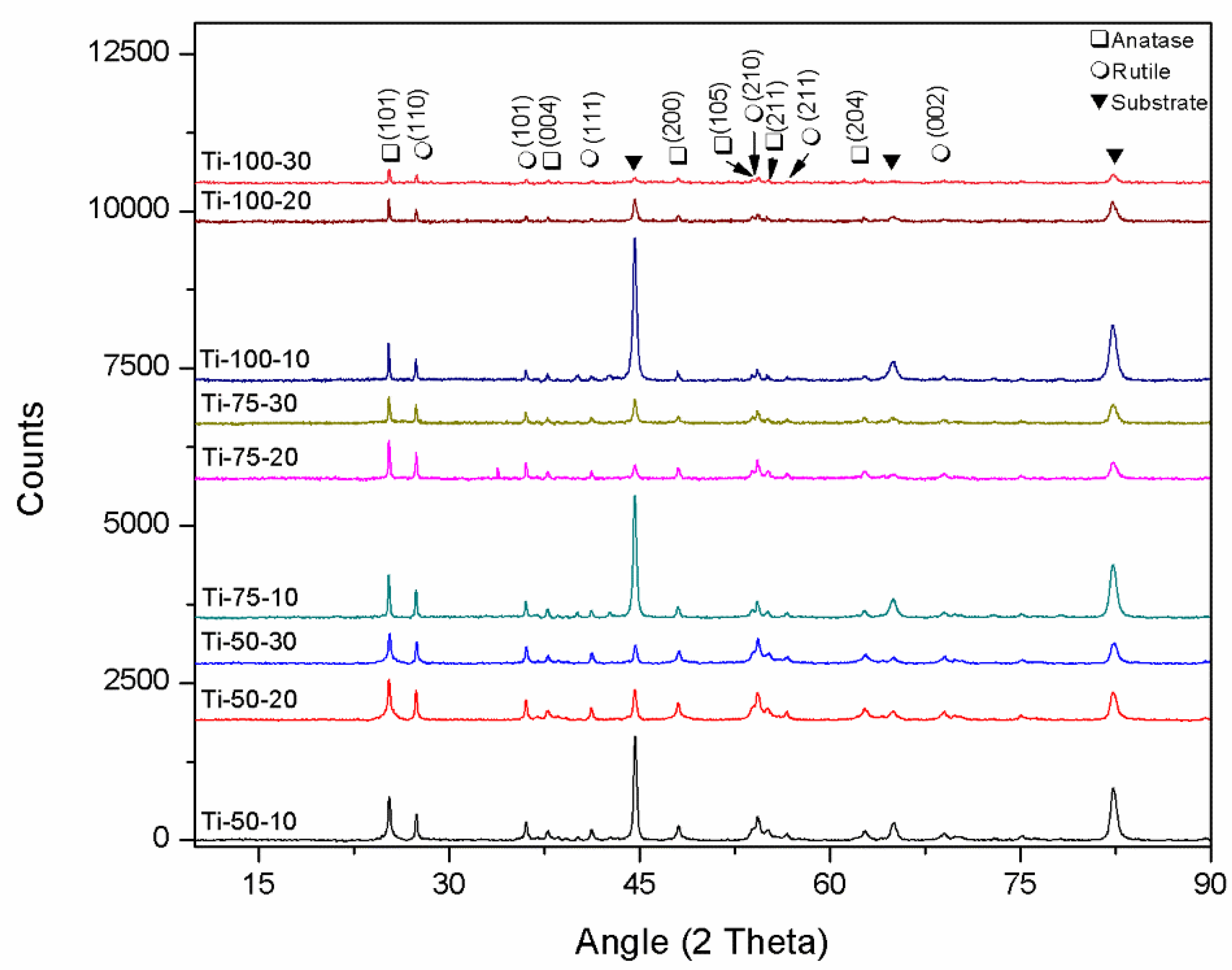
The crystalline structure of TiO
2 coatings deposited at different spray conditions was analysed by comparing XRD data, shown in
Figure 6. All coatings were found to show two main phases of TiO
2, i.e., anatase and rutile, and characteristic peaks related to the substrate. The peak intensity was highest for the coatings developed using a low feedstock flow rate because of low coating thickness. As the feedstock flow rate increases, the peak intensity of the substrate’s characteristic peaks decreases. Various spray parameters, including SOD and feedstock flow rate, affect the phase formation and crystallite size of SPS coatings [
18,
19,
20]. The crystallite size of coatings was calculated using the Scherrer equation to anatase (101) and rutile (110) peaks (
Table 2). Regarding the anatase crystallite size, a reduction was noted with higher feedstock flow rates and a decrease in stand-off distance (SOD). This can be clarified by understanding that at longer spray distances, there is more time in-flight before reaching the substrate. This extended travel favours the formation of larger agglomerates, allowing for sufficient sintering of the material and leading to an increase in crystallite size [
20]. At a higher feedstock flow rate, insufficient sintering or re-solidification due to the insertion of more solvent and reduced flame enthalpy leads to reduced crystallite size. For the rutile phase, it was observed that coatings deposited using 20ml/min exhibited larger crystallites. The possible explanation behind this could be that at 20ml/min, more solid content is present in the droplets, which, after efficient melting, agglomeration, and sintering, form larger crystallites as compared to the coatings deposited using 30ml/min, where comparatively insufficient melting occurs. Although no significant difference was observed in the anatase content of all deposited coatings, a slight increase was found in the anatase content with the increased SOD (
Table 2). With the increased SOD, agglomerates have more time to re-solidify during flight, forming anatase phase proportions due to homogeneous nucleation. At the same time, agglomerates that solidify on the substrate (in the case of shorter SODs) can form anatase or rutile, depending on various factors, including agglomerate size. Usually, for agglomerates solidifying on the substrate, rutile phase formation is favoured due to the lower Gibbs free energy. However, if the agglomerate size is in the submicron range, metastable anatase phase formation takes place due to the fast solidification, suppressing the heterogeneous nucleation [
20,
21,
22].
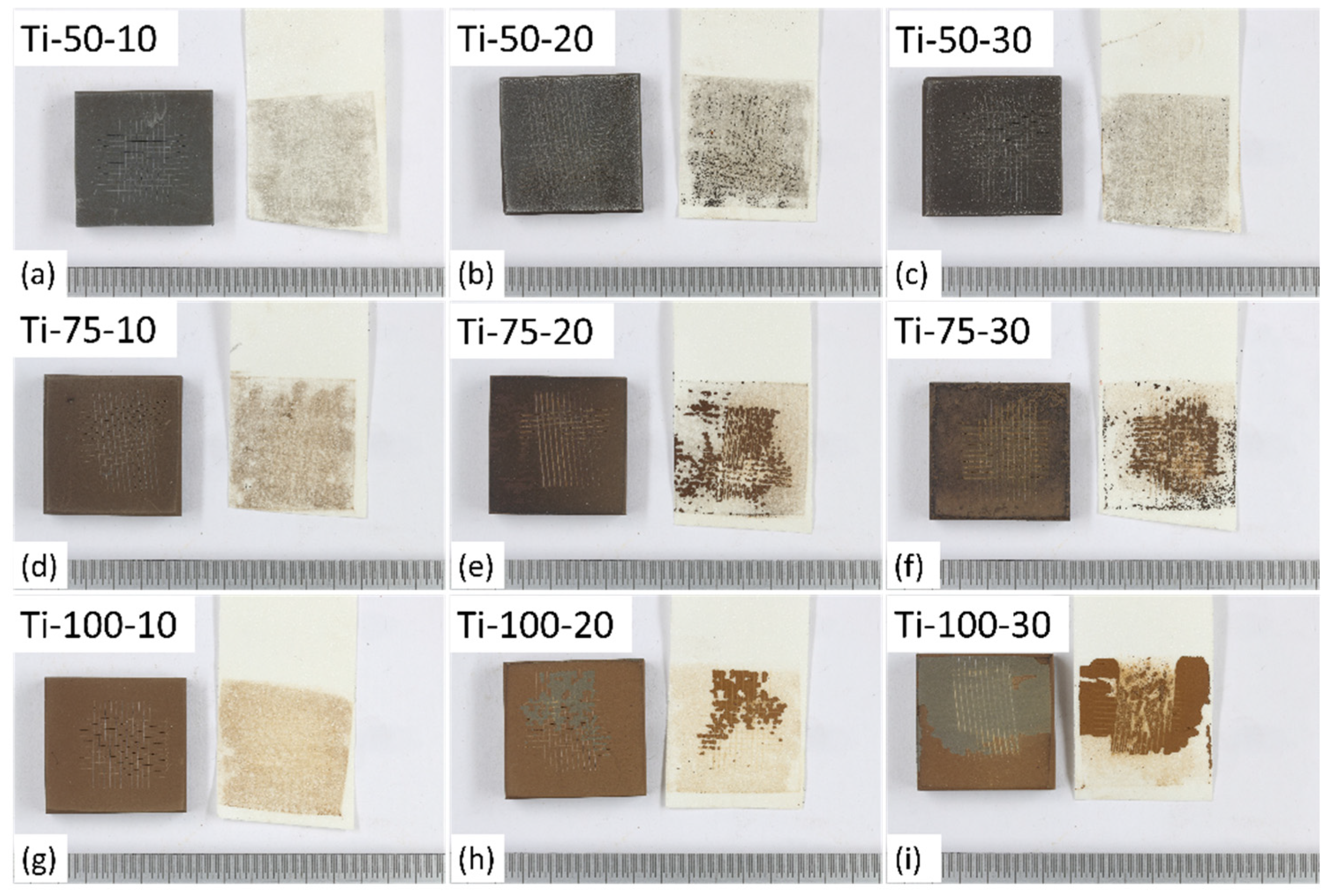
The macrographs of different coatings after performing tape adhesion tests (shown in
Figure 7) show that the coatings deposited at a lower SOD, i.e., 50mm, were well adhered to the substrate due to high impact and comparatively better thermal energy transfer at low SOD between torch and feedstock particles. Also, coatings deposited using a low feedstock flow rate, i.e., 10 ml/min, showed good adhesion. When higher feedstock flow rates are used, along with more material, more solvent (water) is introduced, hence improper melting of particles due to cooling down of the plasma flame [
7]. Therefore, Ti-75-20, Ti-75-30, Ti-100-20, and Ti-100-30 showed a high degree of delamination compared to others.