1. Introduction
Antimicrobial photodynamic therapy (aPDT) is known to eliminate bacterial infectious agents using reactive oxygen species (ROS) generated by irradiating a photosensitizer (PS) with a light source, particularly laser, of the same excitation wavelength. In the field of dentistry, aPDT is currently being studied for use to treat periodontal diseases [
1,
2,
3], endodontics [
4,
5], and peri-implantitis [
3,
6,
7], as well as caries [
8,
9,
10,
11]. The clinical application of aPDT for caries treatment, particularly in the dentin, requires consideration of complex morphologies, such as dentinal tubules [
12,
13]. Therefore, in vitro studies of aPDT should be conducted under simulated clinical conditions. Using infected bovine dentin plates, Nagai et al. [
14] and Yoshii et al. [
15] demonstrated the bactericidal effect of aPDT against
Streptococcus mutans (
S. mutans) and
Lactobacillus acidophilus (
L. acidophilus), respectively.
Streptococcus sobrinus (
S. sobrinus) is widely recognized as a major cariogenic bacterium along with
S. mutans and
L. acidophilus [
16,
17,
18]. Okada et al. [
19] reported that schoolchildren carrying both
S. mutans and
S. sobrinus in their oral cavity had significantly higher incidence of caries in both permanent and primary teeth compared with those carrying only
S. mutans, suggesting that
S. sobrinus plays an important role in caries development and severity. In addition, Korona-Glowniak et al. [
20] reported the correlation between the dissemination rate of
S. sobrinus and the prevalence of dental caries. Therefore, it is important to evaluate the bactericidal effect of aPDT against
S. sobrinus. However, in vitro studies focusing on the above topic are scarce and are mostly conducted on agar medium or plastic plates; thus, a more clinically oriented evaluation of infected dentin is preferable.
aPDT is effective when using laser irradiation with a wavelength corresponding to the excitation wavelength of PS to which it is applied. Various combinations of PS and lasers have been investigated; however, no clear standards for laser irradiation time or power have been established yet, and different researchers used different irradiation protocols even if the PS and laser modes were the same. Furthermore, the optimal conditions for laser irradiation in aPDT have not yet been set up in experimental studies, and not all the parameters in different modes have been evaluated in review articles [
21,
22]. In addition, most of these studies involved only the parameters of irradiation time and power [
23]. Therefore, in the present study, several laser irradiation powers and times were set in aPDT, so that the amount of laser irradiation energy was identical.
This study aimed to compare the bactericidal effect of aPDT in S. sobrinus-infected dentin plates with various PSs and laser irradiation protocols, where the power and irradiation times varied, so that the amount of irradiation energy remained constant. The null hypothesis was that the aforementioned therapies have no difference in bactericidal effect.
2. Materials and Methods
2.1. Preparation of dentin plates
Dentin plates measuring approximately 3 × 3 × 1 mm were fabricated from the cervical parts of the crowns of extracted mandibular bovine teeth using IsoMet Low Speed (BUEHLER, Lake Bluff, IL. USA). A 40% phosphoric acid solution (K-etchant GEL, Kuraray Noritake Dental Inc., Tokyo, Japan) was applied to the dentin plate surface for 3 min for demineralization, followed by thorough rinsing with distilled water and ultrasonic cleaning using a machine (US Cleaner; As One, Osaka, Japan) for 5 min. Thus, preparation of the dentin plates enabled easy bacterial invasion and then sterilized by autoclave (2 atm, 121 ℃, 15 min).
2.2. Preparation of bacterial solution
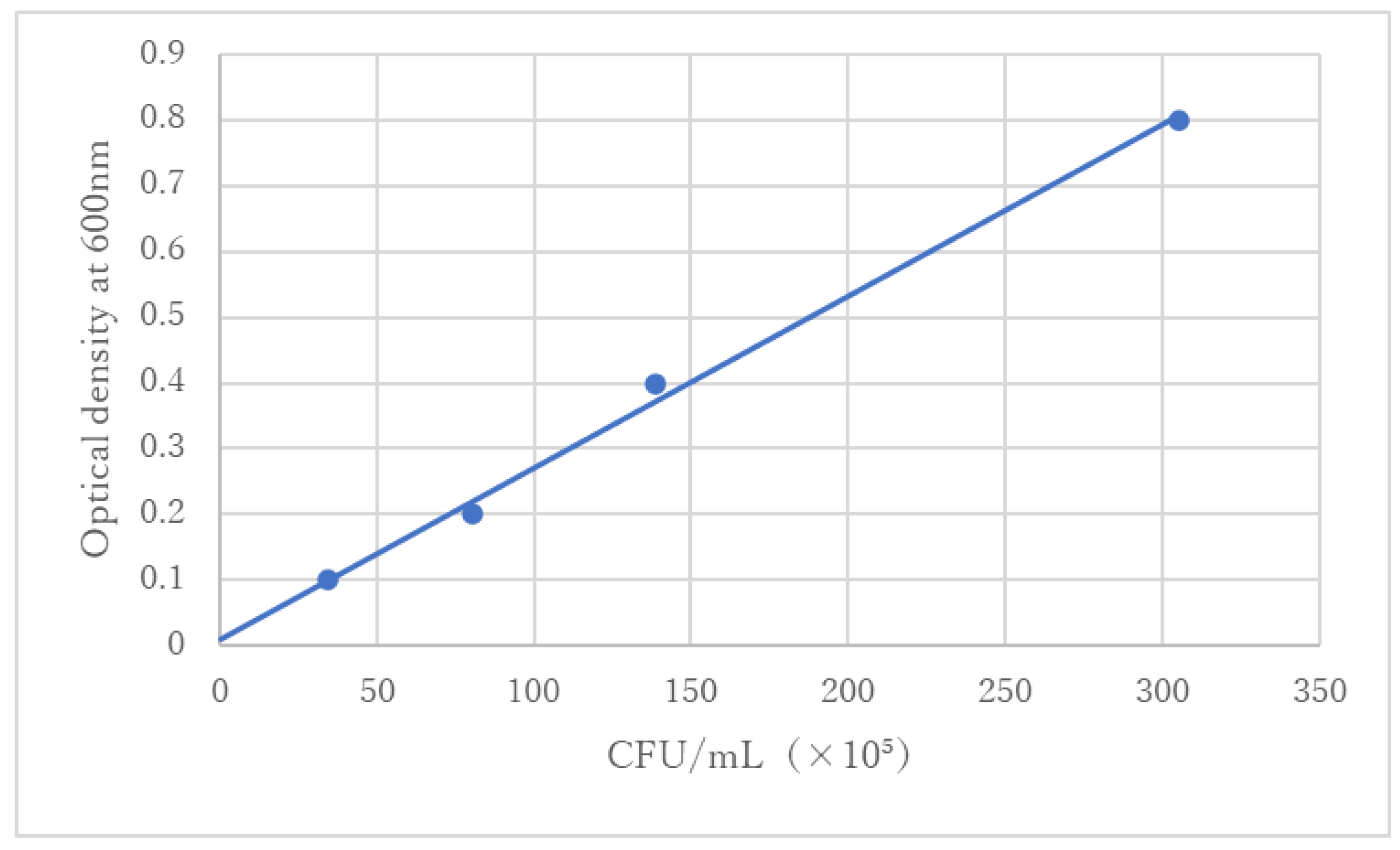
S. sobrinus (ATCC33478) specimens were immersed in sterile phosphate-buffered saline (PBS) (Fujifilm Wako Pure Chemical Industries Ltd., Tokyo, Japan), and the optical density of the obtained bacterial suspension liquid was adjusted to 0.3 at 600 nm based on the standard line (
Figure 1).
2.3. Preparation of infected dentin plates
Dentin plates were placed one by one in the wells of a 24-well cell culture plate, and 20 µL of adjusted bacterial suspension was poured onto each of the dentin plates. The cell culture plate was centrifuged at 2,000 rpm for 10 min to allow bacterial invasion of dentinal tubules. Then, 1.0 mL of brain heart infusion (BHI) (Benton, Dickinson and Company Inc, Franklin Lakes, NJ, USA) was added to each well, and cell culture plate was incubated for 3 h at 37°C in a 10% CO2 environment using an incubator (SHAKING MIXER SHM-101, AGC TECHNO GLASS CO., LTD., Shizuoka, Japan). After incubation, BHI was removed from each well, and then 1.0 mL of PBS was added to the latter. The cell culture plate was shaken for 1 min to eliminate as many bacteria outside the dentinal tubules as possible. This procedure was repeated three times.
2.4. Laser irradiation modes
The semiconductor laser used in this study was the P2 Dental Laser System (Pioon Laser Technology Co, Wuhan, China). First, the laser handpiece used for aPDT was attached to a flexible arm, and the position of the irradiation light tip was adjusted, so that the irradiation distance was 10 mm. The wavelength, irradiation mode, and power of laser were set to 650 nm, CW, and 50 mW, 100 mW and 200 mW, respectively. The irradiation times for the powers of 50, 100, and 200 mW were set to 120, 60, and 30 s, respectively. Under all the conditions, the total energy doze and density were set to 6 J and 11.9 J/cm2, respectively.
2.5. Adjustment of PS
Brilliant blue (BB) (Tokyo Chemical Industries Co., Ltd., Tokyo, Japan), acid red (AR) (Tokyo Chemical Industries Co., Ltd., Tokyo, Japan), and methylene blue (MB) (Fujifilm Wako Pure Chemical Industries Ltd., Tokyo, Japan) were used as PS. Their concentrations were calibrated to 10 mg/mL via dilution with PBS. Then, PS solutions were sterilized via filtration with a 0.22-μm membrane filter (Merck Millipore Ltd., Tullagreen, Corrigtwahill, Co., Cork, IRL). Subsequently, the solutions were stored in the refrigerator at 4°C and brought into room temperature before use.
2.6. Experimental groups and aPDT procedure
Table 1 presents the code for each aPDT experimental group. Nine aPDT experimental groups were formed by setting the respective combined irradiation parameters (50 mW × 120 s, 100 mW × 60 s, and 200 mW × 30 s) and PS (BB, AR, and MB). The untreated experimental group was defined as the control (C). Power analysis was conducted using the G*power software (version 3.1.9.7; Franz Faul University, Kiel, Germany) with the effect size of 0.4 (Cohen's large-effect size) power of 0.8 and sample size of 110. As a result, the number of specimens in each group became 11.
Ten infected dentin plates were positioned in the wells of the 24-well cell culture plate, saturated with 200 µL of each PS, and then stored in a dark place for 5 min as pre-irradiation time. The plates were then irradiated with laser according to the irradiation parameters.
2.7. Evaluation of bactericidal effect
After performing aPDT, the PS solution on the surface of each dentin plate was washed out with PBS. The dentin plate was immersed in a microtube containing 1.0 mL of PBS and then subjected to ultrasonic cleaning using a machine (US Cleaner; As One, Osaka, Japan) for 3 min to detach the bacteria from the dentin plate. After taking out the dentin plate from the microtube, the PBS containing bacteria was vortexed for 1 min to prepare a bacterial suspension.
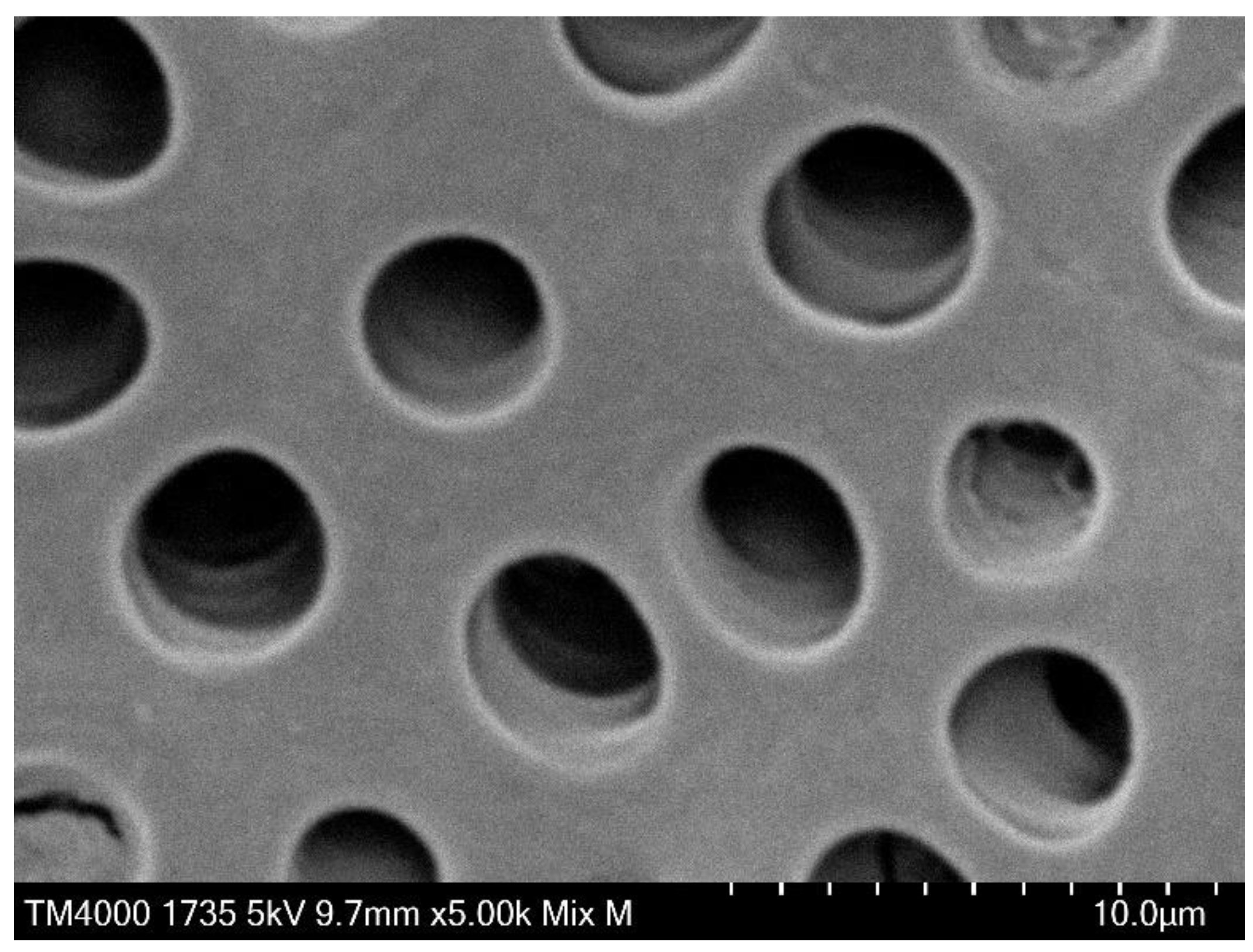
Separation of the bacteria from the dentin plate was confirmed via Scanning electron microscopy (SEM) observation in a preliminary experiment. In the experiment, the infected dentin plates were immersed in 2.5% glutaraldehyde, fixed for 1 h at 4 ℃, immersed in 70% ethanol overnight, and then subjected to stepwise dehydration (80%, 90%, and twice of 99%) in ethanol for 1 h per step. After the indicated process, they were degasified in a vacuum desiccator for 24 h, and then platinum for SEM observation was deposited on their surfaces. The surface of the dentin plate was observed under high magnification at an acceleration voltage of 5 to 15 kV to confirm bacteria detachment using TM4000 PLUS Miniscope (HITACHI High-Technologies Corp., Tokyo, Japan). Comparison of the SEM images before (
Figure 2) and after (
Figure 3) treatment for bacterial detachment showed that the bacteria were sufficiently separated and transferred from the dentin plate.
To evaluate the bactericidal effect of aPDT, adenosine triphosphate (ATP) assay and serial step dilution colony count were performed. ATP assay was performed as follows. First, ATP was extracted from the bacterial suspension by mixing 0.1-mL ATP extraction reagent (Toyo B-net Co., Tokyo, Japan) with 0.1-mL bacterial suspension and leaving the mixture for 10 s. After adding 0.1 mL of Kinshiro® ATP Luminescence Kit version 2 (Toyo B-net Co., Tokyo, Japan) to 0.1 mL of the ATP extract, luminescence (relative light unit [RLU]) was evaluated using Lumat3 LB 9508 (Berthold Technologies, Bad Wildbad, Germany). Measurements were performed 5 s after reaction with the reagent, and the RLU for 30 s was estimated. The luminescence of each ATP extract was measured twice, and the average of the obtained measurement value was calculated. For the colony count, 0.1 mL of the one-tenth diluted bacterial suspension was seeded on BHI agar medium and incubated for 48 h at 37°C in a 10% CO2 environment. After incubation, the number of colonies on the agar medium was counted, and the colony-forming unit (CFU) per mL was calculated.
2.8. Evaluation of infected dentin plate surface after aPDT
Specimens were prepared for SEM observation of infected dentin plate surface following aPDT. The specimen preparation and method for SEM observation were described above.
2.9. Statistical analysis
The Levene test was conducted to assess the equality of variance for the obtained data. Based on the results, either one-way analysis of variance or a Kruskal-Wallis test was chosen for comparative analysis between the experimental groups using BellCurve version 4.04 for Excel (Social Survey Research Information Co.).
3. Results
3.1. Results of ATP and colony-count assays
Because both the ATP and colony-count assays did not show equal data variances, the Kruskal-Wallis and Steel-Dwass post hoc tests were used to examine continuous variables in the groups (significance level p < 0.05).
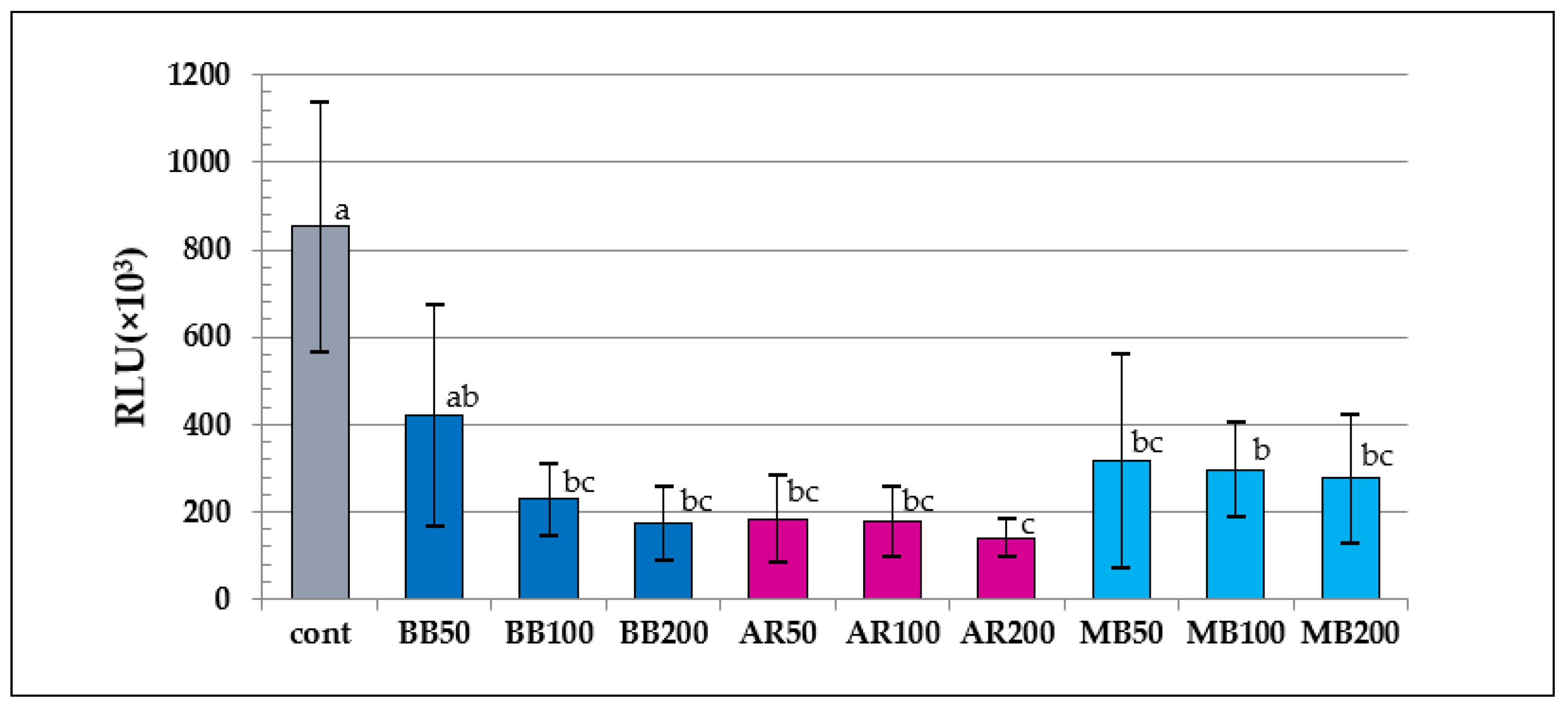
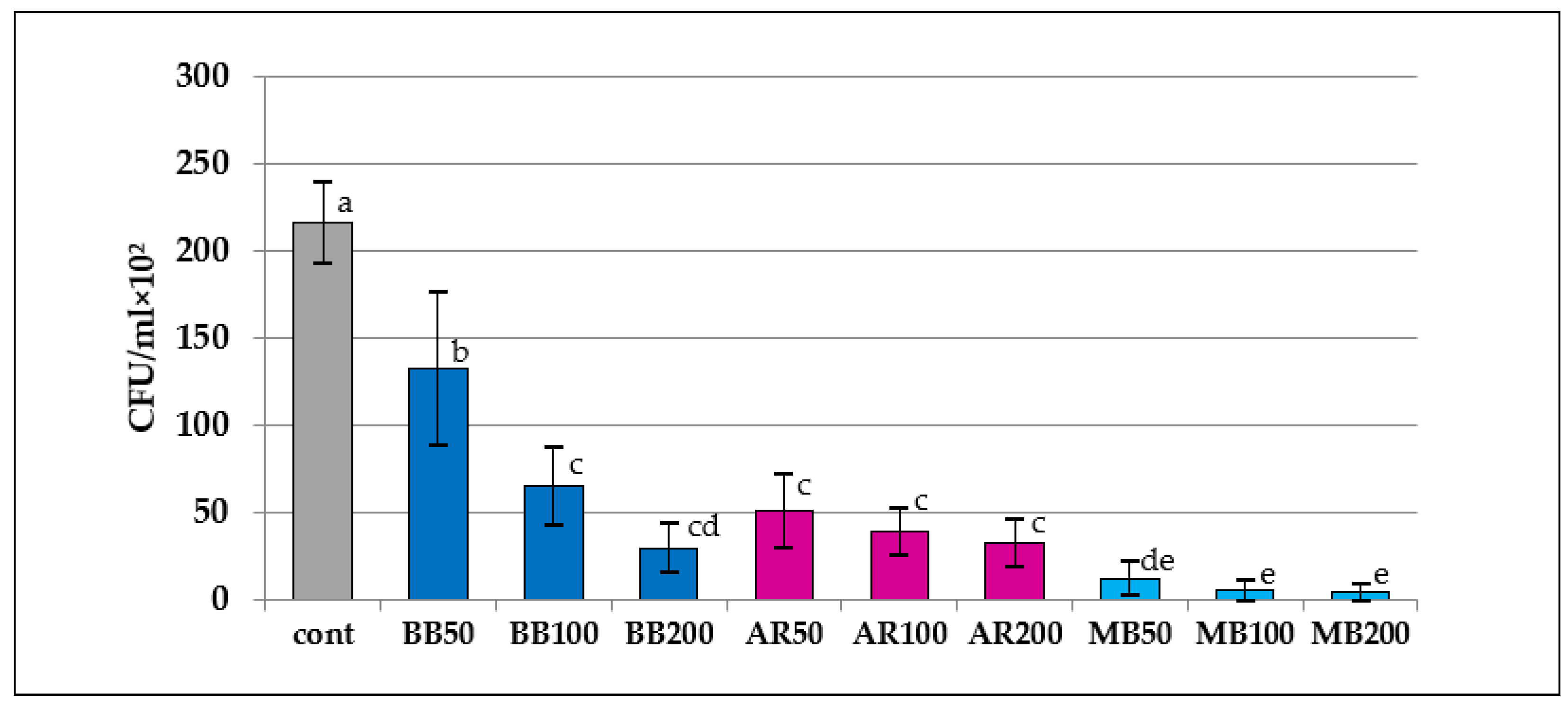
The results of the ATP assay indicated that the RLU values of the aPDT groups except for BB50 were significantly lower than that of the control (p < 0.05). In the comparative analysis of the RLU values of the aPDT groups, the value of AR200 was significantly lower than that of BB50 or MB100 (p < 0.04); however, no differences were observed among the other aPDT groups (p > 0.11). The results of the colony-count assay indicated that the CFU values of all the aPDT groups were significantly lower than those in the control. In terms of the CFU values in the aPDT groups, the MB groups had significantly lower values compared with the BB except for BB200, and AR, regardless of irradiation conditions (p < 0.01), whereas the values for BB50 were significantly higher than those for BB100 and BB200 (p < 0.03); however, the AR and MB groups did not differ by irradiation modes (p > 0.37).
Figure 4.
Results of the ATP assay. RLU, relative light units. Different letters indicate a statistically significant difference (p < 0.05).
Figure 4.
Results of the ATP assay. RLU, relative light units. Different letters indicate a statistically significant difference (p < 0.05).
Figure 5.
Results of the colony-count assay. CFU, colony-forming units. Different letters indicate a statistically significant difference (p < 0.05).
Figure 5.
Results of the colony-count assay. CFU, colony-forming units. Different letters indicate a statistically significant difference (p < 0.05).
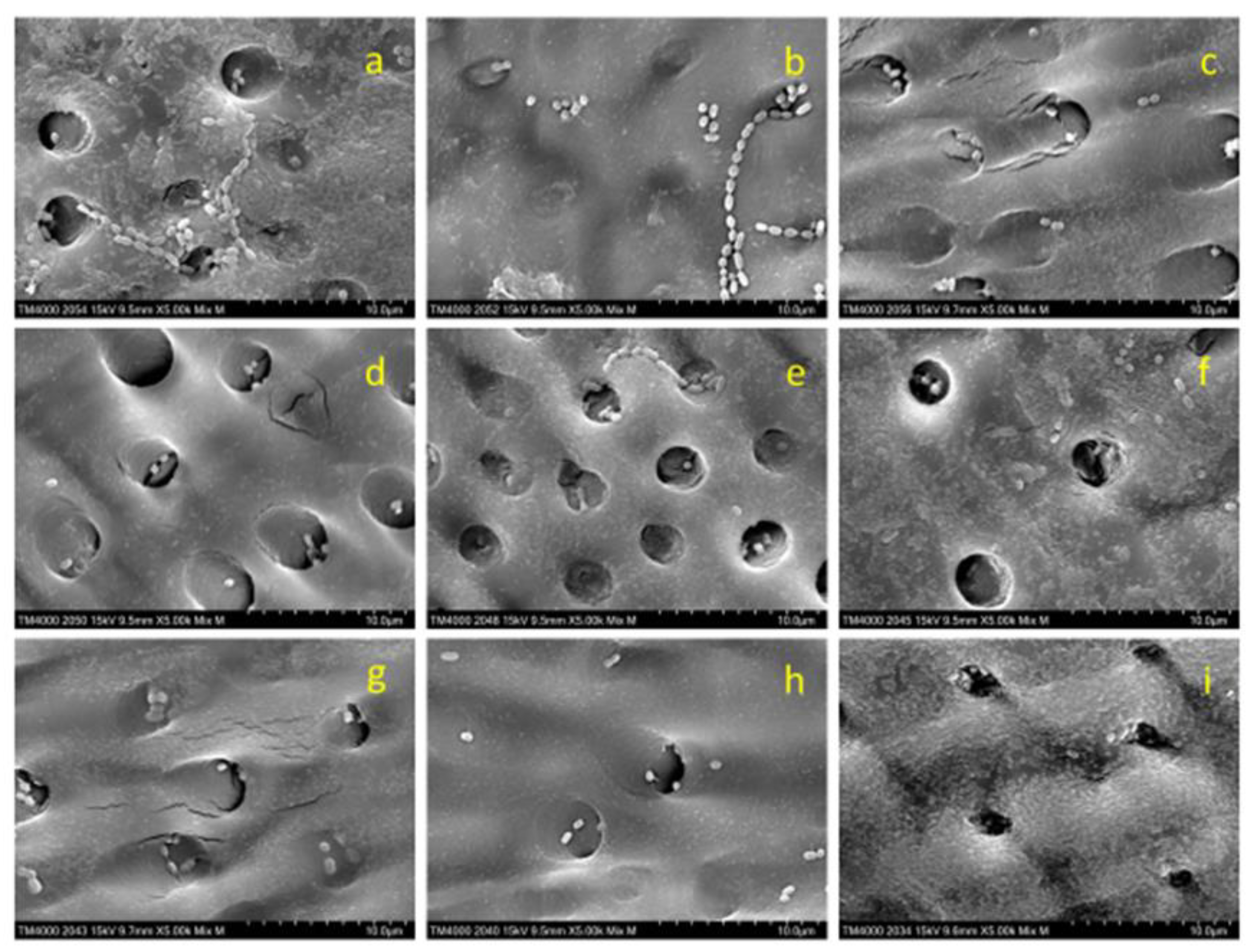
3.2. Scanning electron microscope observations
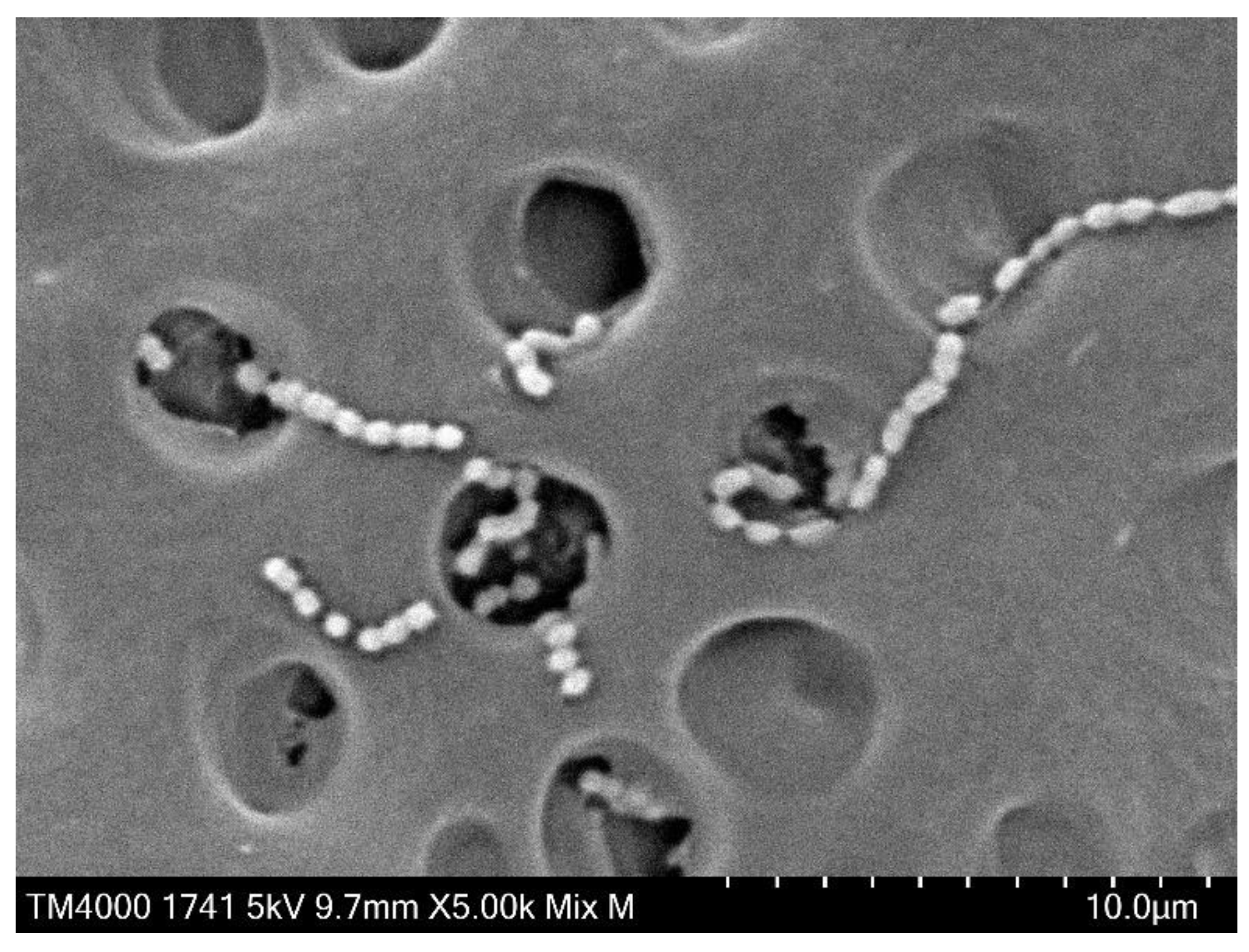
Figure 6 presents SEM images (×5,000) of the dentin plates after each aPDT was performed, and the PS solution was washed out. Compared with the infected dentin plates assessed before aPDT (
Figure 2), a higher number of
S. sobrinus with broken chains was observed on those evaluated after aPDT application (
Figure 6).
S. sobrinus with chains was observed relatively more frequently in BB50 and BB100 (
Figure 6a and Figure 6b), accounting for only about 10% of all specimens in BB200, AR50, AR100, and AR200 (
Figure 6c,
Figure 6d,
Figure 6e and
Figure 6f) and almost 0% in the MB group (
Figure 6g,
Figure 6h, and
Figure 6i).
4. Discussion
The results of this study indicated that aPDT using a semiconductor laser with a 650 nm wavelength in combination with BB, AR, or MB exhibited high bactericidal efficacy against S. sobrinus infection. Furthermore, when the irradiation power and time were regulated to keep the irradiation energy constant, the antimicrobial effect obtained with higher power and shorter irradiation time tended to be higher than that observed with lower power and longer irradiation time, although it was not significant under the set conditions in this experiment, and this tendency was more pronounced for BB. This resulted in the rejection of the null hypothesis.
PS is activated when light with a compatible wavelength is absorbed and firstly gets an excited singlet form and then transitions to a triplet. The PS at this stage produces various ROS, including free oxygen, under the mechanism called types 1 and 2 [
24,
25,
26]. Although singlet oxygen exerts high bactericidal effect due to extremely strong oxidative capacity, its diffusion depth is very shallow (10–55 nm) due to extremely short expression time (10–320 ns) [
27,
28]. Hence, aPDT may be effective at a peripheral area near PS, whereas its effect may be diminished at a more distant area. Considering these characteristics of aPDT, penetration of PS into caries lesions is an important factor for the elimination of infectious agents from caries lesions by aPDT.
The tendency of bactericidal effect demonstrated by particular colony count was different from that shown by ATP assay depending on the type of PS used in each experimental group. For the bactericidal effects of aPDT with BB and AR, the results of colony count were almost consistent with those of ATP assay, whereas the results of the former with MB were quite different from those of the latter. Nagai et al. investigated the bactericidal effect of aPDT involving MB and laser (650 nm) irradiation with the power of 100 mW for 60 s for S. mutans and demonstrated a 90% reduction in CFU value against a 70%–75% decrease in RLU value compared with the control [
14]. In our study, the bactericidal effect of a similar aPDT for
S. sobrinus exhibited approximately 97% reduction of CFU value and approximately 65% of that of RLU, which is almost consistent with the report by Nagai et al. In the ATP assay, the amount of ATP extracted from each bacterium, even
Streptococci, is measured as an RLU value. Meanwhile, in a colony-count assay, chained bacteria are counted as a single colony, and the CFU value is calculated. Therefore, the number of viable cells by colony count may be lower than that detected by ATP assay. Furthermore, even after rinsing PS with PBS from the dentin plate surface after aPDT treatment, the remains of BB and AR were slightly observed on the dentin plate surface. The BB and AR pigments remaining on the sample surface diffused into the bacterial extract solution, resulting in a slight coloration of the solution. It could be assumed that this coloration masked the luminescence of the viable bacteria, and thus, the RLU values were lower than the actual ones.
The results of the colony-count assay indicated that aPDT with MB had a more significant bactericidal effect compared with that with BB or AR. These outcomes may be attributed to the differences in the penetration of each PS into bacteria. The cell walls of Gram-positive bacteria have a porous structure; therefore, the molecules with the weights of 30,000 to 60,000 Da are permeable into the bacteria [
29]. The PS used in this study can penetrate bacterial cell wall. However, it has been reported that only molecules with a weight of 700 Da or less can enter the cell membrane and cytoplasm [
30]. The molecular weights of BB, AR, and MB, which were used in this study, were 825.97, 691.86, and 373.90, respectively. As the molecular weight of MB is almost half that of BB or AR, MB may penetrate bacterial cell walls more deeply than BB or AR. Hence, it was speculated that MB penetrated not only the cell wall but also the cell membrane and cytoplasm of
S. sobrinus, and that the ROS generated by laser irradiation might have destroyed the bacterial cell resulting in irreversible damage in the bacteria. It has been reported that the number of viable gram-positive bacteria (
Staphylococcus aureus) decreases with elevated MB concentration when the bacteria in various concentrations were immersed in MB solution for 60 min in the dark [
30]. This report indicated that MB may have possessed bactericidal efficacy by itself; however, no study has suggested these properties of BB and AR. This difference in the bactericidal efficacy of PS itself is probably the reason for the significant difference in the CFU values measured after 48 h between the experimental groups.
The results of our study indicated that short-time laser irradiation at a high power tended to exert a more significant bactericidal effect than long-time laser irradiation at a low power, and this tendency was more pronounced in BB. Mark et al. measured the amount of ROS generated over time during laser irradiation in PDT, where the irradiation power and time varied, so that the amount of irradiation energy remained constant. They reported that the final total amount of ROS was higher with long-time irradiation at a low power whereas the ROS generation rate tended to be higher with short-time irradiation at a high power [
31]. Yamamoto et al. also evaluated the amount of ROS during PDT and reported a similar tendency [
32]. Although these are reports on PDT-targeting cells, the use of ROS is identical to aPDT targeting bacteria, which was performed in our study. Therefore, as the maximum concentration of ROS is more important for the assessment of bactericidal effect than its total amount, and a threshold may exist for destroying bacteria with cell walls, the experimental results indicated that the high-power short-time irradiation tended to have stronger bactericidal effect when the energy level was constant. The results of the present study can potentially reduce the burden on patients in clinical application, as high-power short-time irradiation leads to a shorter time for aPDT.
SEM observation of dentin plate surface showed that more S. sobrinus with broken chains were detected after aPDT and that the number of S. sobrinus specimens with broken chains tended to increase with increasing laser power in BB and AR. This phenomenon of broken chains may be induced by cell wall injury as Streptococci are connected to each other by their cell walls. Hence, higher concentrations of ROS generated by laser irradiation with high power at a short time are expected to be more effective in breaking bacterial cell walls. The applied aPDT with MB showed fewer residual chains regardless of laser power. These results may be attributed to the efficient penetration of MB into bacterial cell wall and the sufficient effect of aPDT involving low-power laser irradiation.
In the present study, aPDT with MB exhibited a significant bactericidal effect, which is consistent with previous reports. However, some studies showed that aPDT with MB exerted adverse effects associated with gingival fibroblasts and osteoblasts [
33,
34]. Meanwhile, the safety of BB and AR may be higher than that of MB, as they are used as dyes for caries detection. Further studies are warranted to determine the conditions for laser irradiation of aPDT with a more significant bactericidal effect and to confirm the toxicity of aPDT to neighboring tissues such as gingiva and pulp.
5. Conclusions
aPDT performed with a semiconductor laser at a 650-nm wavelength in combination with BB, AR, and MB exerted a noticeable bactericidal effect against S. sobrinus. In particular, PDT with MB exhibited significantly higher bactericidal efficacy in the colony-count assay. The results of aPDT with various irradiation time and power to keep irradiation energy constant indicated no significant differences in the AR and MB groups in terms of conditions, whereas BB showed a more significant bactericidal effect when irradiated for a shorter time at a higher irradiation power.
Author Contributions
Yohei Yamaguchi; Conceptualization, Methodology, Investigation, Writing – original draft. Daiki Yoshii; Methodology, Supervision. Hiroaki Katsuragi; Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. Koichi Shinkai; Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics review
Ethics Review was not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from corresponding author.
Acknowledgments
I would like to thank M. Mikami for the useful discussions and technical assistance with the experiments.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Kitanaka, Y.; Takeuchi, Y.; Hiratsuka, K.; Aung, N.; Sakamaki, Y.; Nemoto, T.; Meinzer, W.; Izumi, Y.; Iwata, T.; Aoki, A. The effect of antimicrobial photodynamic therapy using yellow-green LED and rose bengal on Porphyromonas gingivalis. Photodiagn. Photodyn. Ther. 2020, 32, 102033. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Nuernberg, M.A.A.; Rodrigues, J.V.S.; Belizário, L.C.G.; Batista, J.A.; Duque, C.; Garcia, V.G.; Theodoro, L.H. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: a randomized controlled clinical study. Photodiagn. Photodyn. Ther. 2021, 35, 102451. [Google Scholar] [CrossRef]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: an American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Hamblin, M.R. Aguinaldo S. Garcez, Michael R. Hamblin, methylene blue and hydrogen peroxide for photodynamic inactivation in root canal-a new protocol for use in endodontics. Eur. Endod. J. 2017, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Leelanarathiwat, K.; Katsuta, Y.; Katsuragi, H.; Watanabe, F. Antibacterial activity of blue high-power light-emitting diode-activated flavin mononucleotide against Staphylococcus aureus biofilm on a sandblasted and etched surface. Photodiagn. Photodyn. Ther. 2020, 31, 101855. [Google Scholar] [CrossRef]
- Zhao, T.; Song, J.; Ping, Y.; Li, M. The application of antimicrobial photodynamic therapy (apdt) in the treatment of peri-implantitis. Comput. Math. Methods Med. 2022, 2022, 3547398. [Google Scholar] [CrossRef]
- ‘Nemezio, M.A.; de Souza Farias, S.S.; Borsatto, M.C.; Aires, C.P.; Corona, S.A.M. Effect of methylene blue-induced photodynamic therapy on a Streptococcus mutans biofilm model. Photodiagn. Photodyn. Ther. 2017, 20, 234–237. [Google Scholar] [CrossRef] [PubMed]
- ‘Araújo, P.V.; Correia-Silva, Jde. F.; Gomez, R.S.; Massara, Mde L.; Cortes, M.E.; Poletto, L.T. Antimicrobial effect of photodynamic therapy in carious lesions in vivo, using culture and real-time PCR methods. Photodiagn. Photodyn. Ther. 2015, 12, 401–407. [Google Scholar] [CrossRef]
- Melo, M.A.; Rolim, J.P.; Passos, V.F.; Lima, R.A.; Zanin, I.C.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagn. Photodyn. Ther. 2015, 12, 581–586. [Google Scholar] [CrossRef]
- Melo, M.A.; Rolim, J.P.; Passos, V.F.; Lima, R.A.; Zanin, I.C.; Codes, B.M.; Rocha, S.S.; Rodrigues, L.K. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagn. Photodyn. Ther. 2015, 12, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M. Regional variation in root dentinal tubule infection by Streptococcus gordonii. J. Endod. 1996, 22, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Suzuki, A.; Katsuragi, H.; Shinkai, K. Effect of antimicrobial photodynamic therapy (aPDT) on the sterilization of infected dentin in vitro. Odontology. 2018, 106, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, D.; Katsuragi, H.; Shinkai, K. Bactericidal effect of antimicrobial photodynamic therapy (aPDT) on dentin plate infected with Lactobacillus acidophilus. Odontology. 2021, 109, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Conrads, G.; de Soet, J.J.; Song, L.; Henne, K.; Sztajer, H.; Wagner-Döbler, I.; Zeng, A.P. Comparing the cariogenic species Streptococcus sobrinus and S. mutans on whole genome level. J. Oral Microbiol. 2014, 6, 26189. [Google Scholar] [CrossRef] [PubMed]
- Hirose, H.; Hirose, K.; Isogai, E.; Miura, H.; Ueda, I. Close association between streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 1993, 27, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Abe, A.; Kishi, K.; Ohara-Nemoto, Y.; Kimura, S.; Yonemitsu, M. Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Community Dent. Oral Epidemiol. 2009, 37, 241–249. [Google Scholar] [CrossRef]
- Okada, M.; Kawamura, M.; Oda, Y.; Yasuda, R.; Kojima, T.; Kurihara, H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int. J. Paediatr. Dent. 2012, 22, 342–348. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Skawinska-Bednarczyk, A.; Wrobel, R.; Pietrak, J.; Tkacz-Ciebiera, I.; Maslanko-Switala, M.; Krawczyk, D.; Bakiera, A.; Borek, A.; Malm, A.; Mielnik-Blaszczak, M. Streptococcus sobrinus as a predominant oral bacteria related to the occurrence of dental caries in polish children at 12 years old. Int. J. Environ. Res. Public Health. 2022, 19, 15005. [Google Scholar] [CrossRef]
- Mylona, V.; Anagnostaki, E.; Parker, S.; Cronshaw, M.; Lynch, E.; Grootveld, M. Laser-assisted aPDT protocols in randomized controlled clinical trials in dentistry: a systematic review. Dent. J. (Basel). 2020, 8, 107. [Google Scholar] [CrossRef]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: a systematic review. Photodiagn. Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef] [PubMed]
- Cabral, F.V.; Souza, T. H.; Sellera, F. H.; Fontes, A.; Ribeiro, M. S. Strengthening collaborations at the Biology-Physics interface: trends in antimicrobial photodynamic therapy. Biophys Rev. 2023, 15, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.C.M.; Regis, W.F.M.; Rodrigues, L.K.A. Scientific evidence in antimicrobial photodynamic therapy: an alternative approach for reducing cariogenic bacteria. Photodiagn. Photodyn. Ther. 2019, 26, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, K.; Nakamura, K.; Ikai, H.; Kanno, T.; Kohno, M.; Sasaki, K.; Niwano, Y. Bactericidal action of photogenerated singlet oxygen from photosensitizers used in plaque disclosing agents. PLOS ONE. 2012, 7, e37871. [Google Scholar] [CrossRef]
- Jori, G.; Camerin, M.M.; Guidorin, L.S.; Coppellotti, O. Antimicrobial photodynamic therapy: basic principles. In photodynamic inactivation of microbial pathogens. Medical and Environmental Applications. Hamblin MR, Jori G, Eds. 1; RSC: Cambridge, UK, 2011; pp. 1–18. [Google Scholar]
- Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.W.; Matczyszyn, K. Biogenic gold nanoparticles decrease methylene blue photobleaching and enhance antimicrobial photodynamic therapy. Molecules. 2021, 26, 623. [Google Scholar] [CrossRef]
- Niedre, M.J.; Secord, A.J.; Patterson, M.S.; Wilson, B.C. In vitro tests of the validity of singlet oxygen luminescence measurements as a dose metric in photodynamic therapy. Cancer Res. 2003, 63, 7986–7994. [Google Scholar]
- Yamamoto, J.; Yamamoto, S.; Hirano, T.; Li, S.; Koide, M.; Kohno, E.; Okada, M.; Inenaga, C.; Tokuyama, T.; Yokota, N.; Terakawa, S.; Namba, H. Monitoring of singlet oxygen is useful for predicting the photodynamic effects in the treatment for experimental glioma. Clin. Cancer Res. 2006, 12, 7132–7139. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, S.; Xiao, S.; Ding, Y. Enhanced wound healing and osteogenic potential of photodynamic therapy on human gingival fibroblasts. Photodiagn. Photodyn. Ther. 2020, 32, 101967. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial photodynamic activity and cytocompatibility of Au25(Capt)18 clusters photoexcited by blue LED light irradiation. Int. J. Nanomedicine. 2017, 12, 2703–2716. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).