Submitted:
10 November 2023
Posted:
10 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
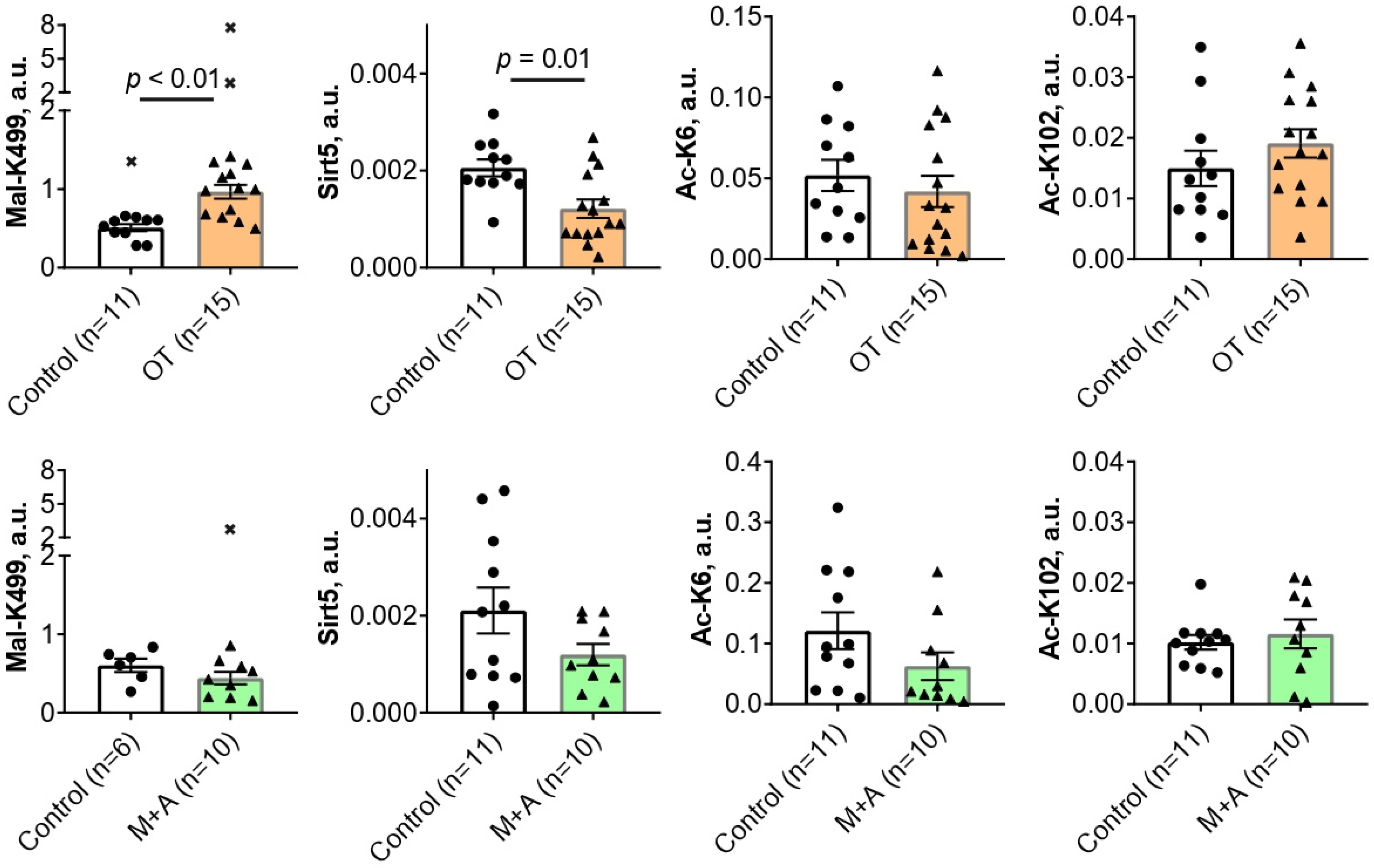
2.1. Effect of Inhibitors of Thiamine-Dependent Metabolism on TKT Acylations in the Rat Brain
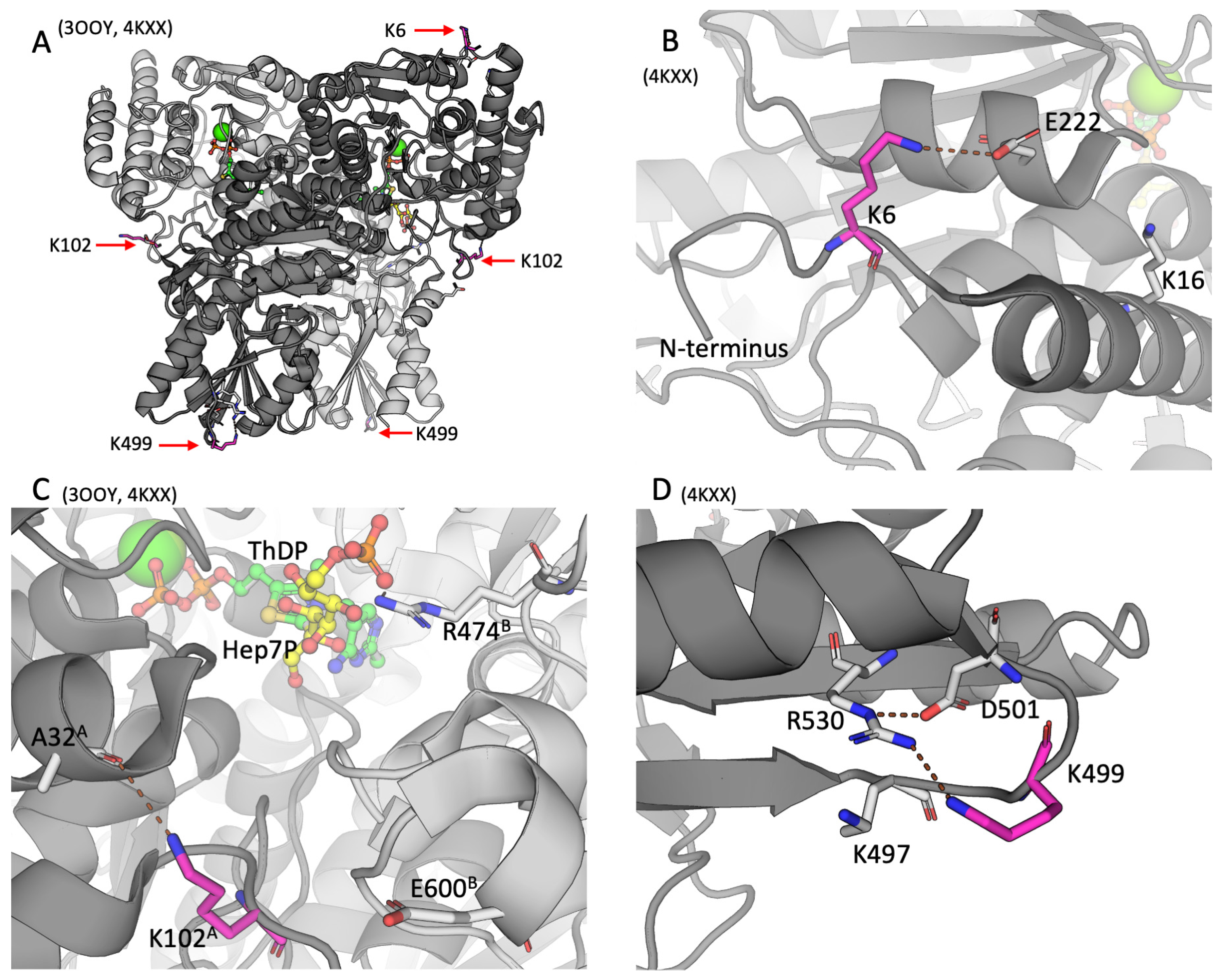
2.2. Positions of the Brain TKT Acylation Sites in the Resolved Structures of Mammalian TKT
2.3. Correlation Analysis of the Interplay between the Levels of TKT Expression, Activity, Acylations and Sirtuin 5
3. Discussion
3.1. The Brain TKT Regulation by Malonylation
3.2. The Brain TKT Regulation by Acetylation
4. Materials and Methods
4.1. Reagents
4.2. Animal Experiments
4.3. Homogenization of Rat Brain Tissue
4.4. TKT Activity Assay
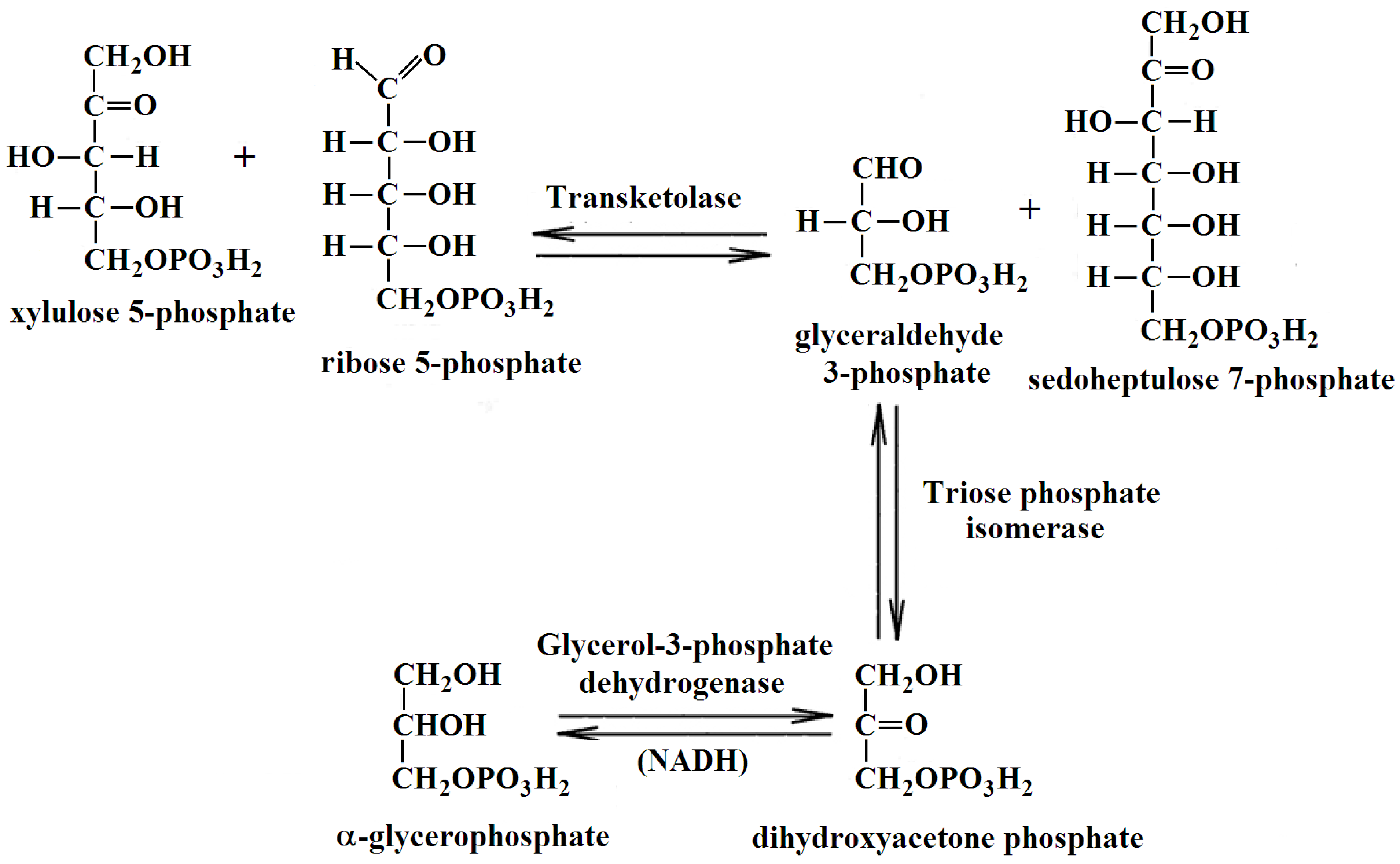
4.5. Analysis of Peptides by LC-MS/MS
4.6. Quantification of the TKT Acylations and Sirtuin 5 Protein Level
4.7. Structural Visualization
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
Abbreviations
| TKT | transketolase, |
| ThDP | thiamine diphosphate, |
| PPP | pentose phosphate pathway, |
| OT | oxythiamine, |
| M+A | metformin and amprolium, |
| MS | mass-spectrometry. |
References
- Lindqvist, Y.; Schneider, G.; Ermler, U.; Sundstrom, M. Three-dimensional structure of transketolase, a thiamine diphosphate dependent enzyme, at 2.5 A resolution. The EMBO journal 1992, 11, 2373–2379. [Google Scholar] [CrossRef]
- Blass, J.P.; Gibson, G.E. Abnormality of a thiamine-requiring enzyme in patients with Wernicke-Korsakoff syndrome. The New England journal of medicine 1977, 297, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.B.; Svoronos, S.; Ghazanfari, A.; Martin, P.R.; Fisher, A.; Roecklein, B.; Rodbard, D.; Staton, R.; Behar, D.; Berg, C.J.; et al. Transketolase abnormality in cultured fibroblasts from familial chronic alcoholic men and their male offspring. The Journal of clinical investigation 1987, 79, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.R.; McCool, B.A.; Singleton, C.K. Molecular genetics of transketolase in the pathogenesis of the Wernicke-Korsakoff syndrome. Metabolic brain disease 1995, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ou, T.; Yang, W.; Li, W.; Lu, Y.; Dong, Z.; Zhu, H.; Sun, X.; Dong, Z.; Weng, X.; Chang, S.; et al. SIRT5 deficiency enhances the proliferative and therapeutic capacities of adipose-derived mesenchymal stem cells via metabolic switching. Clin Transl Med 2020, 10, e172. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Chen, Y.; Wang, Y.Q.; Tao, E.W.; Tan, J.; Liu, Q.Q.; Li, C.M.; Tong, X.M.; Gao, Q.Y.; Hong, J.; et al. Sirtuin5 protects colorectal cancer from DNA damage by keeping nucleotide availability. Nature communications 2022, 13, 6121. [Google Scholar] [CrossRef]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.; Gibson, B.W.; et al. SIRT5 Regulates both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Molecular cell 2015, 59, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.J.; Meier, S.; Tyanova, S.; Terrigno, M.; Wang, C.; Punt, A.M.; Mientjes, E.J.; Vautheny, A.; Distel, B.; Kremer, T.; et al. A cross-species spatiotemporal proteomic analysis identifies UBE3A-dependent signaling pathways and targets. Mol Psychiatry 2022, 27, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Pinson, A.; Xing, L.; Namba, T.; Kalebic, N.; Peters, J.; Oegema, C.E.; Traikov, S.; Reppe, K.; Riesenberg, S.; Maricic, T.; et al. Human TKTL1 implies greater neurogenesis in frontal neocortex of modern humans than Neanderthals. Science 2022, 377, eabl6422. [Google Scholar] [CrossRef]
- Bunik, V.I.; Tylicki, A.; Lukashev, N.V. Thiamin diphosphate-dependent enzymes: from enzymology to metabolic regulation, drug design and disease models. The FEBS journal 2013, 280, 6412–6442. [Google Scholar] [CrossRef]
- Zhang, F.; Masania, J.; Anwar, A.; Xue, M.; Zehnder, D.; Kanji, H.; Rabbani, N.; Thornalley, P.J. The uremic toxin oxythiamine causes functional thiamine deficiency in end-stage renal disease by inhibiting transketolase activity. Kidney international 2016, 90, 396–403. [Google Scholar] [CrossRef]
- Golan, O.; Dyer, R.; Sinclair, G.; Blydt-Hansen, T. Investigating oxythiamine levels in children undergoing kidney transplantation and the risk of immediate post-operative metabolic and hemodynamic decompensation. Pediatr Nephrol 2021, 36, 987–993. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Bunik, V.I. Mechanisms of Non-coenzyme Action of Thiamine: Protein Targets and Medical Significance. Biochemistry. Biokhimiia 2019, 84, 829–850. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.F. General discussion of antithiamin compounds and thiamin antagonists. Annals of the New York Academy of Sciences 1982, 378, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, T.; Hawsah, M.A.; Alojayri, G.; Mares, M.M.; Aljawdah, H.M.A.; Maodaa, S.N.; Al-Shaebi, E.M.; Dkhil, M.A.; Thagfan, F.A.; Al-Quraishy, S.; et al. In vivo anticoccidial, antioxidant, and anti-inflammatory activities of avocado fruit, Persea americana (Lauraceae), against Eimeria papillata infection. Parasitol Int 2023, 95, 102741. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A. Analysis of the Protein Binding Sites for Thiamin and Its Derivatives to Elucidate the Molecular Mechanisms of the Noncoenzyme Action of Thiamin (Vitamin B1). Studies in Natural Products Chemistry 2017, 53, 375–429. [Google Scholar] [CrossRef]
- Ludtke, S.; Neumann, P.; Erixon, K.M.; Leeper, F.; Kluger, R.; Ficner, R.; Tittmann, K. Sub-angstrom-resolution crystallography reveals physical distortions that enhance reactivity of a covalent enzymatic intermediate. Nat Chem 2013, 5, 762–767. [Google Scholar] [CrossRef]
- Zavileyskiy, L.G.; Aleshin, V.A.; Kaehne, T.; Karlina, I.S.; Artiukhov, A.V.; Maslova, M.V.; Graf, A.V.; Bunik, V.I. The Brain Protein Acylation System Responds to Seizures in the Rat Model of PTZ-Induced Epilepsy. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Graf, A.V.; Kazantsev, A.V.; Boyko, A.I.; Aleshin, V.A.; Ksenofontov, A.L.; Bunik, V.I. Increasing Inhibition of the Rat Brain 2-Oxoglutarate Dehydrogenase Decreases Glutathione Redox State, Elevating Anxiety and Perturbing Stress Adaptation. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Zhou, X.; Krishnan, S.; Karlsson, A.; Bunik, V.I. Interplay Between Thiamine and p53/p21 Axes Affects Antiproliferative Action of Cisplatin in Lung Adenocarcinoma Cells by Changing Metabolism of 2-Oxoglutarate/Glutamate. Frontiers in Genetics 2021, 12. [Google Scholar] [CrossRef]
- Nikkola, M.; Lindqvist, Y.; Schneider, G. Refined structure of transketolase from Saccharomyces cerevisiae at 2.0 A resolution. Journal of molecular biology 1994, 238, 387–404. [Google Scholar] [CrossRef]
- Solovjeva, O.N. Isolation and Properties of Noncovalent Complex of Transketolase with RNA. Biochemistry (Moscow) 2002, 67, 667–671. [Google Scholar] [CrossRef]
- Tikhomirova, N.K.; Merchan, A.Y.; Kochetov, G.A. A new form of baker’s yeast transketolase. An enzyme-RNA complex. FEBS letters 1990, 274, 27–29. [Google Scholar] [CrossRef]
- Kochetov, G.A.; Nikitushkina, L.I.; Chernov, N.N. A complex of functionally-bound enzymes: transketolase and glyceraldehydephosphate dehydrogenase. Biochemical and biophysical research communications 1970, 40, 873–879. [Google Scholar] [CrossRef]
- Wood, T.; Muzariri, C.C.; Malaba, L. Complex formation between transketolase, transaldolase, and glyceraldehyde phosphate dehydrogenase. International Journal of Biochemistry 1985, 17, 1109–1115. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Yong, H.; Xu, J.; Qu, P.; Qiao, S.; Hou, P.; Li, Z.; Chu, S.; Zheng, J.; et al. Transketolase promotes colorectal cancer metastasis through regulating AKT phosphorylation. Cell Death Dis 2022, 13, 99. [Google Scholar] [CrossRef]
- Sevostyanova, I.; Solovjeva, O.; Selivanov, V.; Kochetov, G. Half-of-the-sites reactivity of transketolase from Saccharomyces cerevisiae. Biochemical and biophysical research communications 2009, 379, 851–854. [Google Scholar] [CrossRef]
- Kovina, M.V.; Kochetov, G.A. Cooperativity and flexibility of active sites in homodimeric transketolase. FEBS letters 1998, 440, 81–84. [Google Scholar] [CrossRef]
- Zecha, J.; Gabriel, W.; Spallek, R.; Chang, Y.C.; Mergner, J.; Wilhelm, M.; Bassermann, F.; Kuster, B. Linking post-translational modifications and protein turnover by site-resolved protein turnover profiling. Nature communications 2022, 13, 165. [Google Scholar] [CrossRef]
- Jeffery, C.J. An enzyme in the test tube, and a transcription factor in the cell: Moonlighting proteins and cellular factors that affect their behavior. Protein science: A publication of the Protein Society 2019, 28, 1233–1238. [Google Scholar] [CrossRef]
- Domain, F.; Bina, X.R.; Levy, S.B. Transketolase A, an enzyme in central metabolism, derepresses the marRAB multiple antibiotic resistance operon of Escherichia coli by interaction with MarR. Molecular microbiology 2007, 66, 383–394. [Google Scholar] [CrossRef]
- Ahn, C.S.; Kim, J.G.; Shin, M.H.; Lee, Y.A.; Kong, Y. Comparison of Secretome Profile of Pathogenic and Non-Pathogenic Entamoeba histolytica. Proteomics 2018, 18, e1700341. [Google Scholar] [CrossRef]
- Grundel, A.; Pfeiffer, M.; Jacobs, E.; Dumke, R. Network of Surface-Displayed Glycolytic Enzymes in Mycoplasma pneumoniae and Their Interactions with Human Plasminogen. Infect Immun 2015, 84, 666–676. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, Z.; Hua, S.; Yang, W.; Chen, Y.; Huang, F.; Fan, Y.; Tong, L.; Xu, T.; Tong, X.; et al. Nuclear Tkt promotes ischemic heart failure via the cleaved Parp1/Aif axis. Basic Res Cardiol 2022, 117, 18. [Google Scholar] [CrossRef]
- Gubler, C.J.; Johnson, L.R.; Wittorf, J.H. [22] Yeast transketolase (sedoheptulose-7-phosphate:d-glyceraldehyde-3-phosphate dihydroxyacetonetransferase, EC 2.2.1.1) assay of thiamine diphosphate. In Vitamins and Coenzymes, 1970; pp. 120-125. [CrossRef]
- Brin, M. Effects of thiamine deficiency and of oxythiamine on rat tissue transketolase. The Journal of nutrition 1962, 78, 179–183. [Google Scholar] [CrossRef]
- Moraes, J.O.; Rodrigues, S.D.C.; Pereira, L.M.; Medeiros, R.C.N.; de Cordova, C.A.S.; de Cordova, F.M. Amprolium exposure alters mice behavior and metabolism in vivo. Animal Model Exp Med 2018, 1, 272–281. [Google Scholar] [CrossRef]
- Oliveira, W.H.; Nunes, A.K.; Franca, M.E.; Santos, L.A.; Los, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain research 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Kalariya, N.M.; Shoeb, M.; Ansari, N.H.; Srivastava, S.K.; Ramana, K.V. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Investigative ophthalmology & visual science 2012, 53, 3431–3440. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration. Journal of neurochemistry 2020, 153, 80–102. [Google Scholar] [CrossRef]
- de la Haba, G.; Leder, I.G.; Racker, E. Crystalline Transketolase from Bakers’ Yeast: Isolation and Properties. Journal of Biological Chemistry 1955, 214, 409–426. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Graf, A.V.; Bunik, V.I. Daytime Dependence of the Activity of the Rat Brain Pyruvate Dehydrogenase Corresponds to the Mitochondrial Sirtuin 3 Level and Acetylation of Brain Proteins, All Regulated by Thiamine Administration Decreasing Phosphorylation of PDHA Ser293. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC bioinformatics 2006, 7, 123. [Google Scholar] [CrossRef]
| A. Perturbations in thiamine metabolism | |||||||
|---|---|---|---|---|---|---|---|
| \ M+A OT \ |
Mal-K499 | Ac-K6 | Ac-K102 | TKT-expr | Act -ThDP | Act +ThDP | Sirt5 |
| Mal-K499 | 1.00 | -0.14 | -0.15 | 0.20 | -0.07 | -0.22 | -0.19 |
| 0.00 | 0.66 | 0.62 | 0.51 | 0.80 | 0.43 | 0.53 | |
| Ac-K6 | -0.22 | 1.00 | -0.08 | 0.22 | -0.35 | -0.38 | 0.76 |
| 0.31 | 0.00 | 0.74 | 0.35 | 0.12 | 0.09 | 0.00 | |
| Ac-K102 | 0.03 | 0.13 | 1.00 | 0.26 | -0.45 | -0.41 | -0.11 |
| 0.88 | 0.54 | 0.00 | 0.25 | 0.04 | 0.07 | 0.65 | |
| TKT-expr | -0.08 | -0.04 | 0.12 | 1.00 | -0.45 | -0.47 | 0.59 |
| 0.73 | 0.84 | 0.55 | 0.00 | 0.04 | 0.03 | 0.01 | |
| Act -ThDP | -0.52 | -0.47 | -0.10 | 0.15 | 1.00 | 0.95 | -0.26 |
| 0.01 | 0.02 | 0.62 | 0.47 | 0.00 | 0.00 | 0.26 | |
| Act +ThDP | -0.40 | -0.50 | -0.20 | -0.08 | 0.97 | 1.00 | -0.30 |
| 0.07 | 0.01 | 0.35 | 0.72 | 0.00 | 0.00 | 0.19 | |
| Sirt5 | -0.16 | 0.60 | -0.18 | 0.51 | -0.21 | -0.30 | 1.00 |
| 0.47 | 0.00 | 0.38 | 0.01 | 0.31 | 0.15 | 0.00 | |
| B. Control state | |||||||
| Mal-K499 | Ac-K6 | Ac-K102 | TKT-expr | Act -ThDP | Act +ThDP | Sirt5 | |
| Mal-K499 | 1.00 | -0.12 | -0.26 | 0.32 | -0.31 | -0.36 | 0.18 |
| 0.00 | 0.68 | 0.34 | 0.24 | 0.23 | 0.19 | 0.52 | |
| Ac-K6 | 1.00 | -0.36 | -0.20 | -0.16 | -0.25 | 0.61 | |
| 0.00 | 0.10 | 0.37 | 0.49 | 0.28 | 0.00 | ||
| Ac-K102 | 1.00 | 0.04 | 0.07 | 0.02 | -0.52 | ||
| 0.00 | 0.88 | 0.76 | 0.94 | 0.01 | |||
| TKT-expr | 1.00 | 0.07 | -0.10 | 0.40 | |||
| 0.00 | 0.76 | 0.67 | 0.06 | ||||
| Act -ThDP | 1.00 | 0.93 | -0.10 | ||||
| 0.00 | 0.00 | 0.67 | |||||
| Act +ThDP | 1.00 | -0.14 | |||||
| 0.00 | 0.53 | ||||||
| Sirt5 | 1.00 | ||||||
| 0.00 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





