Submitted:
24 October 2023
Posted:
25 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and methods
Substances and Experimental Groups:
Bio-AgNP Synthesis
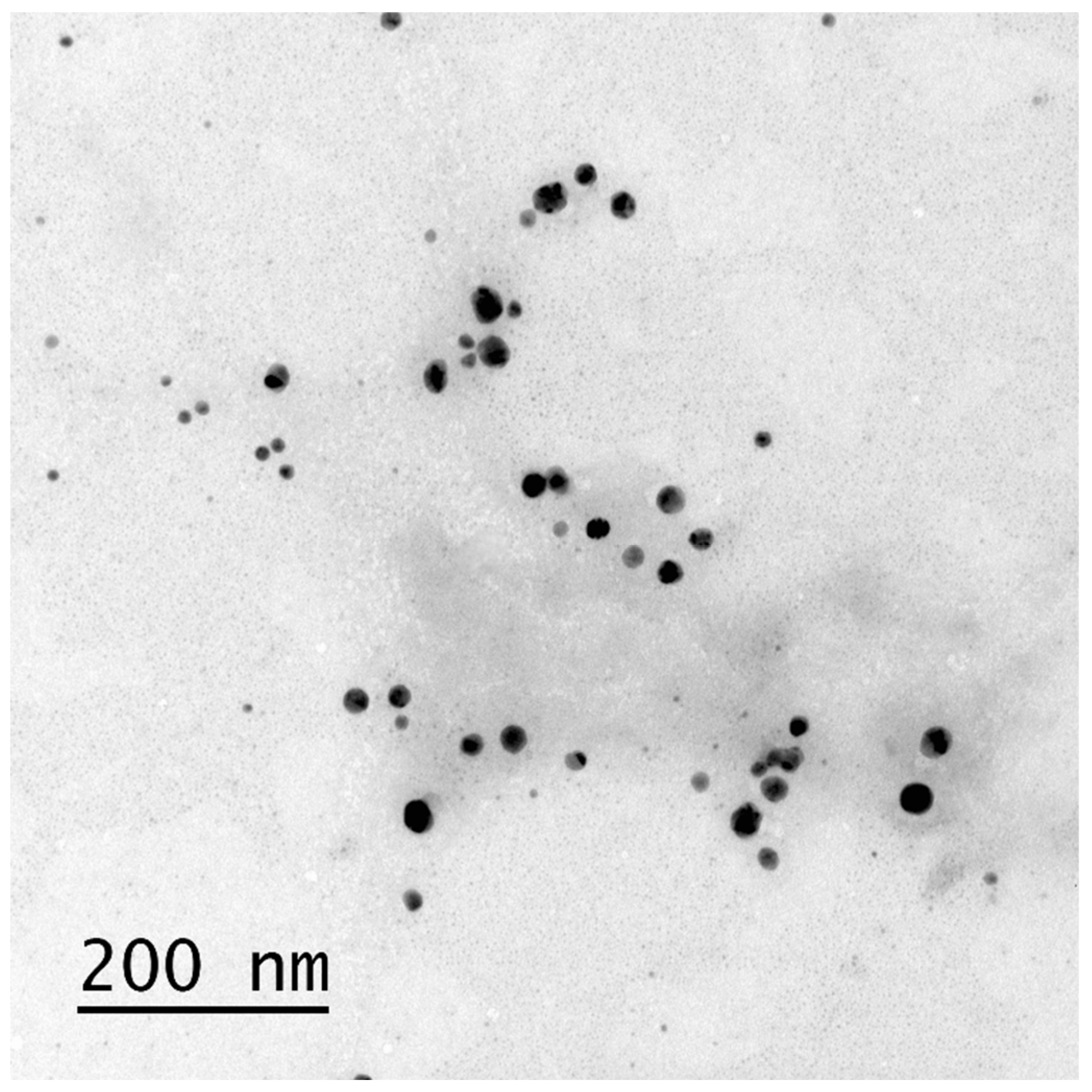
Bio-AgNP Characterization
Bio-AgNP and Simvastatin Antimicrobial Activity
Bacteria and growing conditions
Minimum inhibitory concentration (MIC) assay
Antimicrobial combination assay
Biofilm formation inhibition assay
Statistical Analyses
Results
Bio-AgNP Characterization
Minimum inhibitory concentration (MIC) and antimicrobial combination (FICI) assays
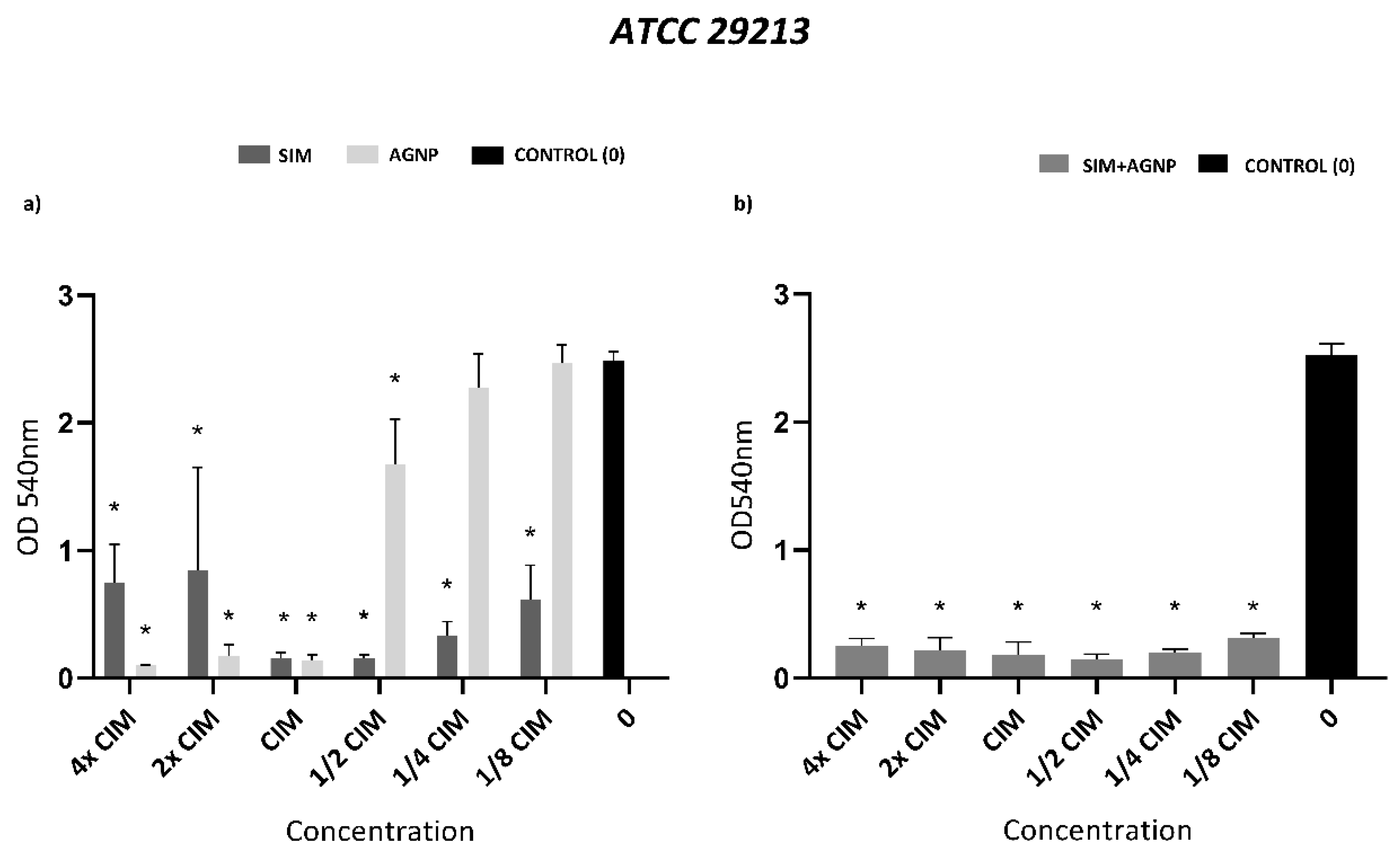
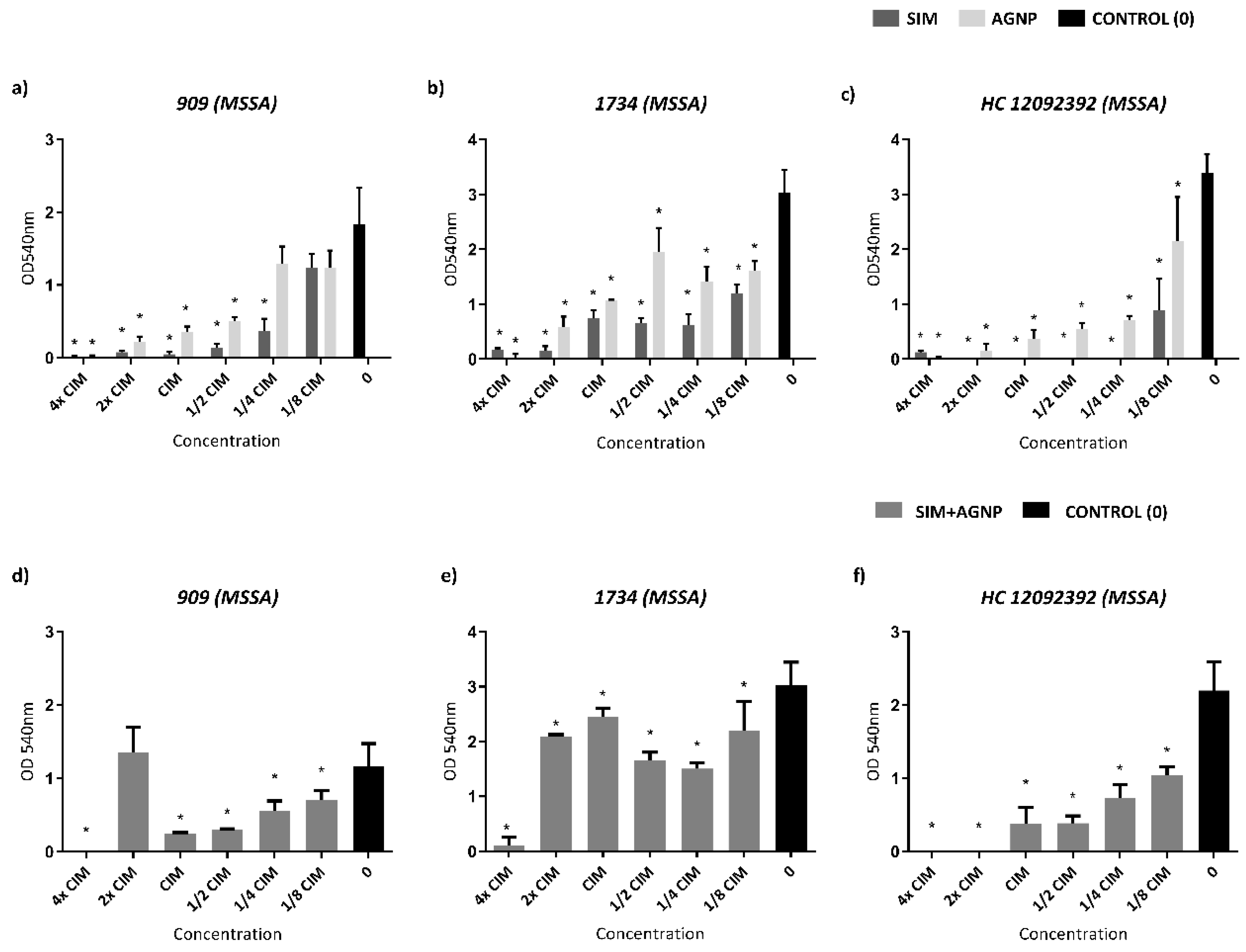
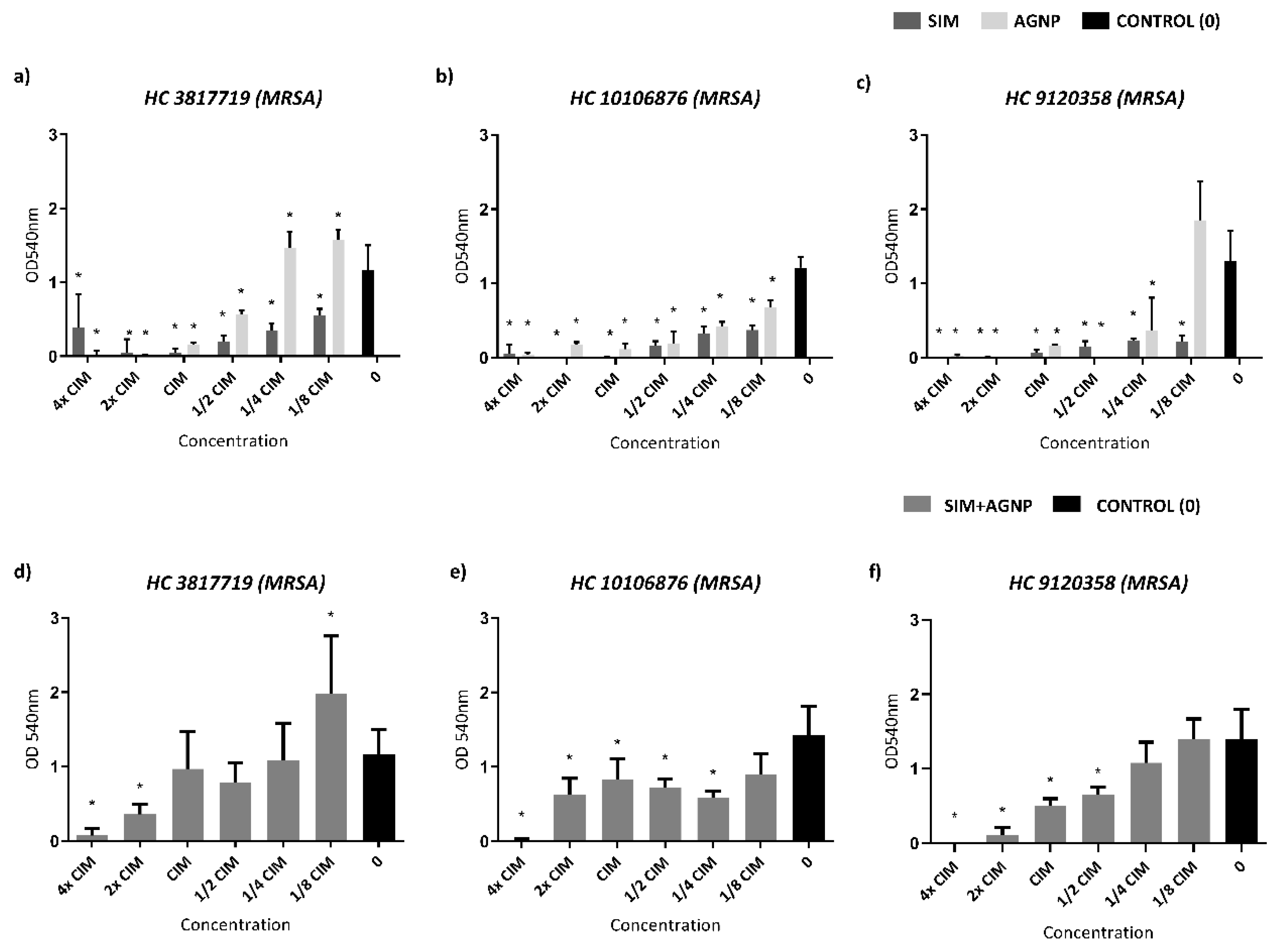
Biofilm formation inhibition assay
Discussion
Conclusion
Author Contributions
Funding
Ethical Approval and Consent to Participate
Consent to Publication
Data Availability Statement
Acknowledgements
Conflict of Interests
Abbreviations
| MRSA | methicillin-resistant Staphylococcus aureus |
| Bio-AgNP | Biologically synthesized silver nanoparticles |
| AgNP | Silver nanoparticles |
| EDS | Energy Dispersive X-ray Spectroscopy |
| TEM | Transmission Electron Microscopy |
| SIM | Simvastatin |
| FICI | Fractional Inhibitory Concentration Index |
| WHO | World Health Organization |
| PBP2a | Penicillin-binding protein 2a |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| HMG-CoA | 3-hydroxy-3-methylglutaryl-CoenzymeA reductase |
| LDL | Low density lipoprotein |
| DMSO | Dimethylsulfoxide |
| DLS | Dynamic light scattering |
| TSA | Tryptone Soy Agar |
| TSB | Tryptic Soy Broth |
| MIC | Minimum Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| MHB | Mueller Hinton Broth |
| OD | Optical density |
| TEM | Transmission Electron Microscopy |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| XRD | X-ray Diffraction Spectroscopy |
| ATCC | American Type Culture Collection |
References
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, Prevalence, and Management of MRSA Bacteremia across Patient Populations—A Review of Recent Developments in MRSA Management and Treatment. Crit Care 2017, 21, 211. [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect Dis 2018, 18, 318–327. [CrossRef]
- OMS, W.H.O. Global Report on Infection Prevention and Control; 2022; ISBN 9789240051164.
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus Aureus. Nat Rev Dis Primers 2018, 4, 18033. [CrossRef]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus Aureus</i>. IUBMB Life 2014, 66, 572–577. [CrossRef]
- Kong, K.-F.; Schneper, L.; Mathee, K. Beta-Lactam Antibiotics: From Antibiosis to Resistance and Bacteriology. APMIS 2010, 118, 1–36. [CrossRef]
- Abd El-Hamid, M.I.; Y. El-Naenaeey, E.; M kandeel, T.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus Aureus. Antibiotics 2020, 9, 667. [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies To Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. ChemMedChem 2021, 16, 65–80. [CrossRef]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm Formation of Multidrug-Resistant MRSA Strains Isolated from Different Types of Human Infections. Pathogens 2021, 10, 970. [CrossRef]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in Vitro to in Vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I. Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules 2015, 20, 5286–5298. [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomedicine 2010, 6, 103–109. [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with Its Anticandidal and Antioxidant Effects. Front Microbiol 2017, 08. [CrossRef]
- Gimenez, I.F.; Anazetti, M.C.; Melo, P.S.; Haun, M.; De Azevedo, M.M.M.; Durán, N.; Alves, O.L. Cytotoxicity on V79 and HL60 Cell Lines by Thiolated-<I>Β</I>-Cyclodextrin-Au/Violacein Nanoparticles. J Biomed Nanotechnol 2005, 1, 352–358. [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J Nanobiotechnology 2018, 16, 14. [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.; Esposito, E. Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J Nanobiotechnology 2005, 3, 8. [CrossRef]
- Figueiredo, E.; Ribeiro, J.; Nishio, E.; Scandorieiro, S.; Costa, A.; Cardozo, V.; de Oliveira, A.; Durán, N.; Panagio, L.; Kobayashi, R.; et al. New Approach For Simvastatin As An Antibacterial: Synergistic Effect With Bio-Synthesized Silver Nanoparticles Against Multidrug-Resistant Bacteria</P>. Int J Nanomedicine 2019, Volume 14, 7975–7985. [CrossRef]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of Non-Antibiotic Drugs as an Alternative to Microbial Resistance: A Systematic Review. Int J Antimicrob Agents 2021, 58, 106380. [CrossRef]
- Annamanedi, M.; Varma, G.Y.N.; Anuradha, K.; Kalle, A.M. Celecoxib Enhances the Efficacy of Low-Dose Antibiotic Treatment against Polymicrobial Sepsis in Mice and Clinical Isolates of ESKAPE Pathogens. Front Microbiol 2017, 8. [CrossRef]
- de S. Machado, C.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Coelho, S.S.; Foletto, V.S.; Rampelotto, R.F.; Lorenzoni, V.V.; de L. Marion, S.; Hörner, R. In Vitro Evaluation of the Antibacterial Activity of Amitriptyline and Its Synergistic Effect with Ciprofloxacin, Sulfamethoxazole–Trimethoprim, and Colistin as an Alternative in Drug Repositioning. Medicinal Chemistry Research 2020, 29, 166–177. [CrossRef]
- Sankar, S.; Thangamalai, R.; Padmanaban, S.; Kannan, P.; R, S.M.; S, A.C. In-Vitro Synergistic Antibacterial Effect of Atorvastatin and Ampicillin against Resistant Staphylococcus Spp and E.Coli Isolated from Bovine Mastitis. 2019. [CrossRef]
- Ko, H.H.T.; Lareu, R.R.; Dix, B.R.; Hughes, J.D. Statins: Antimicrobial Resistance Breakers or Makers? PeerJ 2017, 5, e3952. [CrossRef]
- Pose, E.; Trebicka, J.; Mookerjee, R.P.; Angeli, P.; Ginès, P. Statins: Old Drugs as New Therapy for Liver Diseases? J Hepatol 2019, 70, 194–202. [CrossRef]
- Stoll, L.L.; McCormick, M.L.; Denning, G.M.; Weintraub, N.L. Antioxidant Effects of Statins. Drugs of Today 2004, 40, 975. [CrossRef]
- Tsubaki, M.; Fujiwara, D.; Takeda, T.; Kino, T.; Tomonari, Y.; Itoh, T.; Imano, M.; Satou, T.; Sakaguchi, K.; Nishida, S. The Sensitivity of Head and Neck Carcinoma Cells to Statins Is Related to the Expression of Their Ras Expression Status, and Statin-Induced Apoptosis Is Mediated via Suppression of the Ras/ERK and Ras/MTOR Pathways. Clin Exp Pharmacol Physiol 2017, 44, 222–234. [CrossRef]
- Hsu, C.; Brahmandam, A.; Brownson, K.E.; Huynh, N.; Reynolds, J.; Lee, A.I.; Fares, W.H.; Ochoa Chaar, C.I. Statin Therapy Associated with Improved Thrombus Resolution in Patients with Deep Vein Thrombosis. J Vasc Surg Venous Lymphat Disord 2019, 7, 169-175.e4. [CrossRef]
- Li, G.; Zhao, J.; Li, B.; Zhang, X.; Ma, J.; Ma, X.; Liu, J. The Anti-Inflammatory Effects of Statins on Patients with Rheumatoid Arthritis: A Systemic Review and Meta-Analysis of 15 Randomized Controlled Trials. Autoimmun Rev 2018, 17, 215–225. [CrossRef]
- Sheridan, A.; Wheeler-Jones, C.P.D.; Gage, M.C. The Immunomodulatory Effects of Statins on Macrophages. Immuno 2022, 2, 317–343. [CrossRef]
- Jerwood, S.; Cohen, J. Unexpected Antimicrobial Effect of Statins. Journal of Antimicrobial Chemotherapy 2007, 61, 362–364. [CrossRef]
- Graziano, T.S.; Cuzzullin, M.C.; Franco, G.C.; Schwartz-Filho, H.O.; de Andrade, E.D.; Groppo, F.C.; Cogo-Müller, K. Statins and Antimicrobial Effects: Simvastatin as a Potential Drug against Staphylococcus Aureus Biofilm. PLoS ONE 2015, 10, e0128098. [CrossRef]
- de Carvalho, R.D.P.; Casarin, R.C.V.; de Lima, P.O.; Cogo-Müller, K. Statins with Potential to Control Periodontitis: From Biological Mechanisms to Clinical Studies. J Oral Biosci 2021, 63, 232–244. [CrossRef]
- Parolina de Carvalho, R.D.; de Andrade Moreno, J.; Roque, S.M.; Chan, D.C.H.; Torrez, W.B.; Stipp, R.N.; Bueno-Silva, B.; de Lima, P.O.; Cogo-Müller, K. Statins and Oral Biofilm: Simvastatin as a Promising Drug to Control Periodontal Dysbiosis. Oral Dis 2022. [CrossRef]
- Sun, T.; Huang, J.; Zhang, W.; Zheng, X.; Wang, H.; Liu, J.; Leng, H.; Yuan, W.; Song, C. Simvastatin-Hydroxyapatite Coatings Prevent Biofilm Formation and Improve Bone Formation in Implant-Associated Infections. Bioact Mater 2023, 21, 44–56. [CrossRef]
- Ansari, M.; Khan, H.; Khan, A.; Cameotra, S.; Alzohairy, M. Anti-Biofilm Efficacy of Silver Nanoparticles against MRSA and MRSE Isolated from Wounds in a Tertiary Care Hospital. Indian J Med Microbiol 2015, 33, 101–109. [CrossRef]
- Tarusha, L.; Paoletti, S.; Travan, A.; Marsich, E. Alginate Membranes Loaded with Hyaluronic Acid and Silver Nanoparticles to Foster Tissue Healing and to Control Bacterial Contamination of Non-Healing Wounds. J Mater Sci Mater Med 2018, 29, 22. [CrossRef]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiology and Molecular Biology Reviews 2014, 78, 510–543. [CrossRef]
- Cluzet, V.C.; Gerber, J.S.; Nachamkin, I.; Metlay, J.P.; Zaoutis, T.E.; Davis, M.F.; Julian, K.G.; Royer, D.; Linkin, D.R.; Coffin, S.E.; et al. Duration of Colonization and Determinants of Earlier Clearance of Colonization With Methicillin-Resistant Staphylococcus Aureus. Clinical Infectious Diseases 2015, 60, 1489–1496. [CrossRef]
- Kumar, S.A.; Ansary, A.A.; Ahmad, A.; Khan, M.I. Extracellular Biosynthesis of CdSe Quantum Dots by the Fungus, <I>Fusarium Oxysporum</I> J Biomed Nanotechnol 2007, 3, 190–194. [CrossRef]
- CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute 2018, 11th ed.
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. Journal of Antimicrobial Chemotherapy 2003, 52, 1–1. [CrossRef]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J Clin Microbiol 2014, 52, 4124–4128. [CrossRef]
- Wu, W.-S.; Chen, C.-C.; Chuang, Y.-C.; Su, B.-A.; Chiu, Y.-H.; Hsu, H.-J.; Ko, W.-C.; Tang, H.-J. Efficacy of Combination Oral Antimicrobial Agents against Biofilm-Embedded Methicillin-Resistant Staphylococcus Aureus. J Microbiol Immunol Infect 2013, 46, 89–95. [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green Synthesis of Silver Nanoparticles Induced by the Fungus Penicillium Citrinum. Tropical Journal of Pharmaceutical Research 2013, 12. [CrossRef]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of Silver Nanoparticles by Phoma Glomerata and Its Combined Effect against Escherichia Coli , Pseudomonas Aeruginosa and Staphylococcus Aureus. Lett Appl Microbiol 2009, 48, 173–179. [CrossRef]
- Soni, N.; Prakash, S. Synthesis of Gold Nanoparticles by the Fungus Aspergillus Niger and Its Efficacy against Mosquito Larvae. Reports in Parasitology 2012, 1. [CrossRef]
- Vala, A.K.; Shah, S.; Patel, R.N. Biogenesis of Silver Nanoparticles by Marine- Derived Fungus Aspergillus Flavus from Bhavnagar Coast, Gulf of Khambhat, India. Journal of Marine Biology & Oceanography 2014, 03. [CrossRef]
- Raj, S.; Chand Mali, S.; Trivedi, R. Green Synthesis and Characterization of Silver Nanoparticles Using Enicostemma Axillare (Lam.) Leaf Extract. Biochem Biophys Res Commun 2018, 503, 2814–2819. [CrossRef]
- Baharara, J.; Ramezani, T.; Hosseini, N.; Mousavi, M. Silver Nanoparticles Synthesized Coating with Zataria Multiflora Leaves Extract Induced Apoptosis in HeLa Cells Through P53 Activation. Iran J Pharm Res 2018, 17, 627–639.
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver Nanoparticles in Dentistry. Dental Materials 2017, 33, 1110–1126. [CrossRef]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.; Nakamura, C. V.; de Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and Additive Effect of Oregano Essential Oil and Biological Silver Nanoparticles against Multidrug-Resistant Bacterial Strains. Front Microbiol 2016, 7. [CrossRef]
- Saeki, E.K.; Yamada, A.Y.; de Araujo, L.A.; Anversa, L.; Garcia, D. de O.; de Souza, R.L.B.; Martins, H.M.; Kobayashi, R.K.T.; Nakazato, G. Subinhibitory Concentrations of Biogenic Silver Nanoparticles Affect Motility and Biofilm Formation in Pseudomonas Aeruginosa. Front Cell Infect Microbiol 2021, 11. [CrossRef]
- Habash, M.B.; Goodyear, M.C.; Park, A.J.; Surette, M.D.; Vis, E.C.; Harris, R.J.; Khursigara, C.M. Potentiation of Tobramycin by Silver Nanoparticles against Pseudomonas Aeruginosa Biofilms. Antimicrob Agents Chemother 2017, 61. [CrossRef]
- Villa, F.; Remelli, W.; Forlani, F.; Gambino, M.; Landini, P.; Cappitelli, F. Effects of Chronic Sub-Lethal Oxidative Stress on Biofilm Formation by Azotobacter Vinelandii. Biofouling 2012, 28, 823–833. [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int J Nanomedicine 2017, Volume 12, 1227–1249. [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med Cell Longev 2019, 2019, 1–13. [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal Activity of Silver Nanoparticles and Simvastatin against Toxigenic Species of Aspergillus. Int J Food Microbiol 2019, 291, 79–86. [CrossRef]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized Silver Nanoparticles Enhance Contact Killing and Show Highest Efficacy: Elucidation of the Mechanism of Bactericidal Action of Silver. Nanoscale 2013, 5, 7328. [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.-B. Antimicrobial Surface Functionalization of Plastic Catheters by Silver Nanoparticles. Journal of Antimicrobial Chemotherapy 2008, 61, 869–876. [CrossRef]
- Seymour, C. Audit of Catheter-Associated UTI Using Silver Alloy-Coated Foley Catheters. British Journal of Nursing 2006, 15, 598–603. [CrossRef]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial Coatings on Haemodialysis Catheters by Photochemical Deposition of Silver Nanoparticles. J Mater Sci Mater Med 2011, 22, 2005–2012. [CrossRef]
- Mussin, J.; Robles-Botero, V.; Casañas-Pimentel, R.; Rojas, F.; Angiolella, L.; San Martín-Martínez, E.; Giusiano, G. Antimicrobial and Cytotoxic Activity of Green Synthesis Silver Nanoparticles Targeting Skin and Soft Tissue Infectious Agents. Sci Rep 2021, 11, 14566. [CrossRef]
- Orta-García, S.T.; Plascencia-Villa, G.; Ochoa-Martínez, A.C.; Ruiz-Vera, T.; Pérez-Vázquez, F.J.; Velázquez-Salazar, J.J.; Yacamán, M.J.; Navarro-Contreras, H.R.; Pérez-Maldonado, I.N. Analysis of Cytotoxic Effects of Silver Nanoclusters on Human Peripheral Blood Mononuclear Cells ‘ in Vitro .’ Journal of Applied Toxicology 2015, 35, 1189–1199. [CrossRef]
- Alves, M.F.; Paschoal, A.C.C.; Klimeck, T.D.F.; Kuligovski, C.; Marcon, B.H.; de Aguiar, A.M.; Murray, P.G. Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium Thermophilum—Sustainable Nanotechnology. Journal of Fungi 2022, 8, 605. [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall AgNP-Impregnated Biocompatible Hydrogel with Highly Effective Biofilm Elimination Properties. ACS Appl Mater Interfaces 2020, 12, 41011–41025. [CrossRef]
| Strains | MIC | Association MIC | FICI index | |
|---|---|---|---|---|
| SIM (µM) | Bio-AgNP (μM) | SIM + Bio-AgNP | ||
| S. aureus ATCC 29213 | 74.66 | 31.88 | 74.66 + 31.88 | 0.32 |
| S. aureus ATCC 43300 | 149.32 | 63.75 | 149.32 + 63.75 | 2.0 |
| S. aureus ATCC 33591 | 149.32 | 31.88 | 149.32 + 31.88 | 2.0 |
| S. aureus ATCC 6538 | 74.66 | 31.88 | 74.66 + 31.88 | 2.0 |
| S. aureus HC 3817719 (MRSA) | 74.66 | 187.5 | 74.66 + 187.5 | 2.0 |
| S. aureus HC 10106876 (MRSA) | 74.66 | 187.5 | 74.66 + 187.5 | 2.0 |
| S. aureus HC 9120358 (MRSA) | 74.66 | 187.5 | 74.66 + 187.5 | 2.0 |
| S. aureus HC 12092392 (MSSA) | 74.66 | 93.75 | 74.66 + 93.7 | 2.0 |
| S. aureus HC 985444 (MSSA) | 74.66 | 93.75 | 74.66 + 93.75 | 2.0 |
| S. aureus 909 (MSSA) | 149.32 | 187.5 | 149.32 + 187.5 | 2.0 |
| S. aureus 1734 (MSSA) | 74.66 | 93.75 | 74.66 + 93.75 | 2.0 |
| S. aureus 1744 (MSSA) | 74.66 | 187.5 | 74.66 + 187.5 | 2.0 |
| S. aureus 1641 (MSSA) | 74.66 | 187.5 | 74.66 + 187.5 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





