Submitted:
23 October 2023
Posted:
25 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Results and Discussion
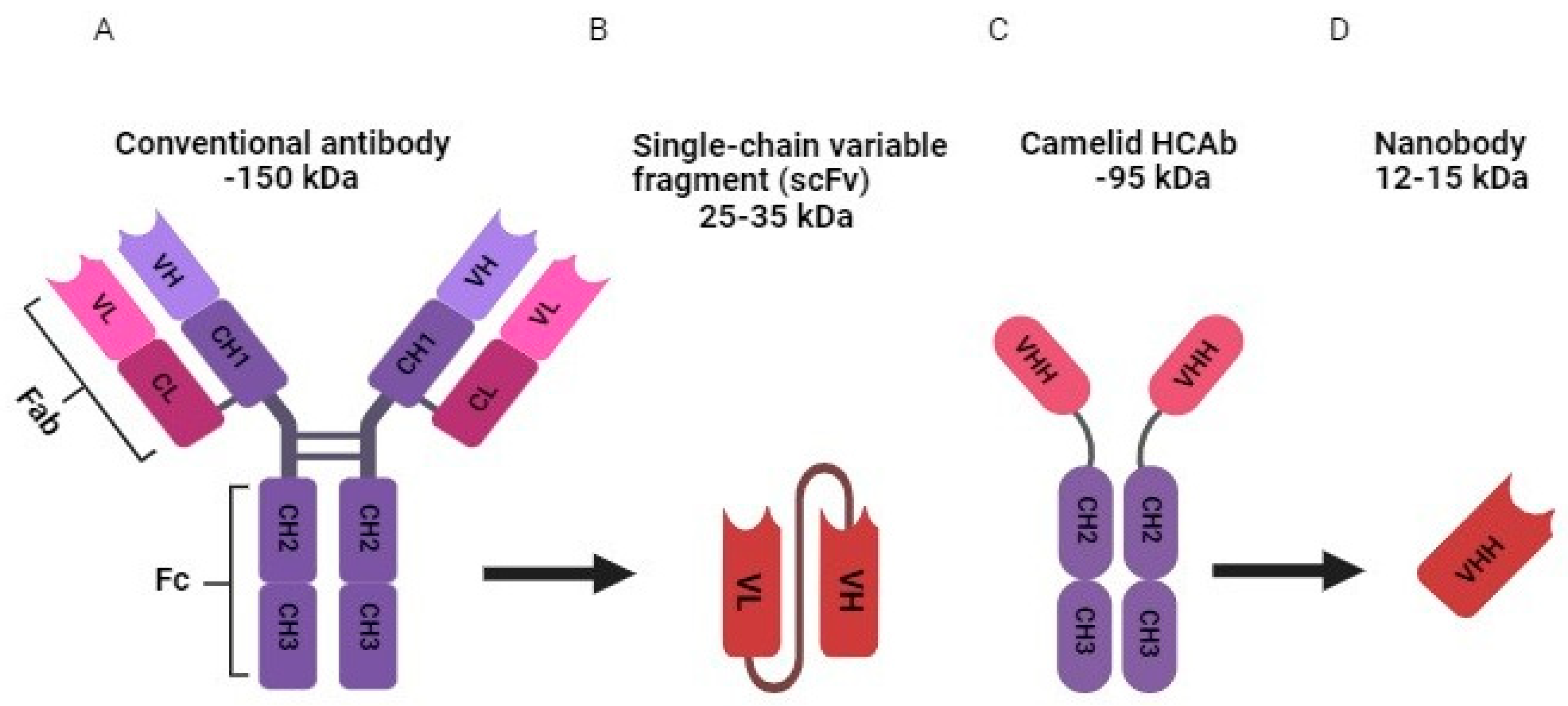
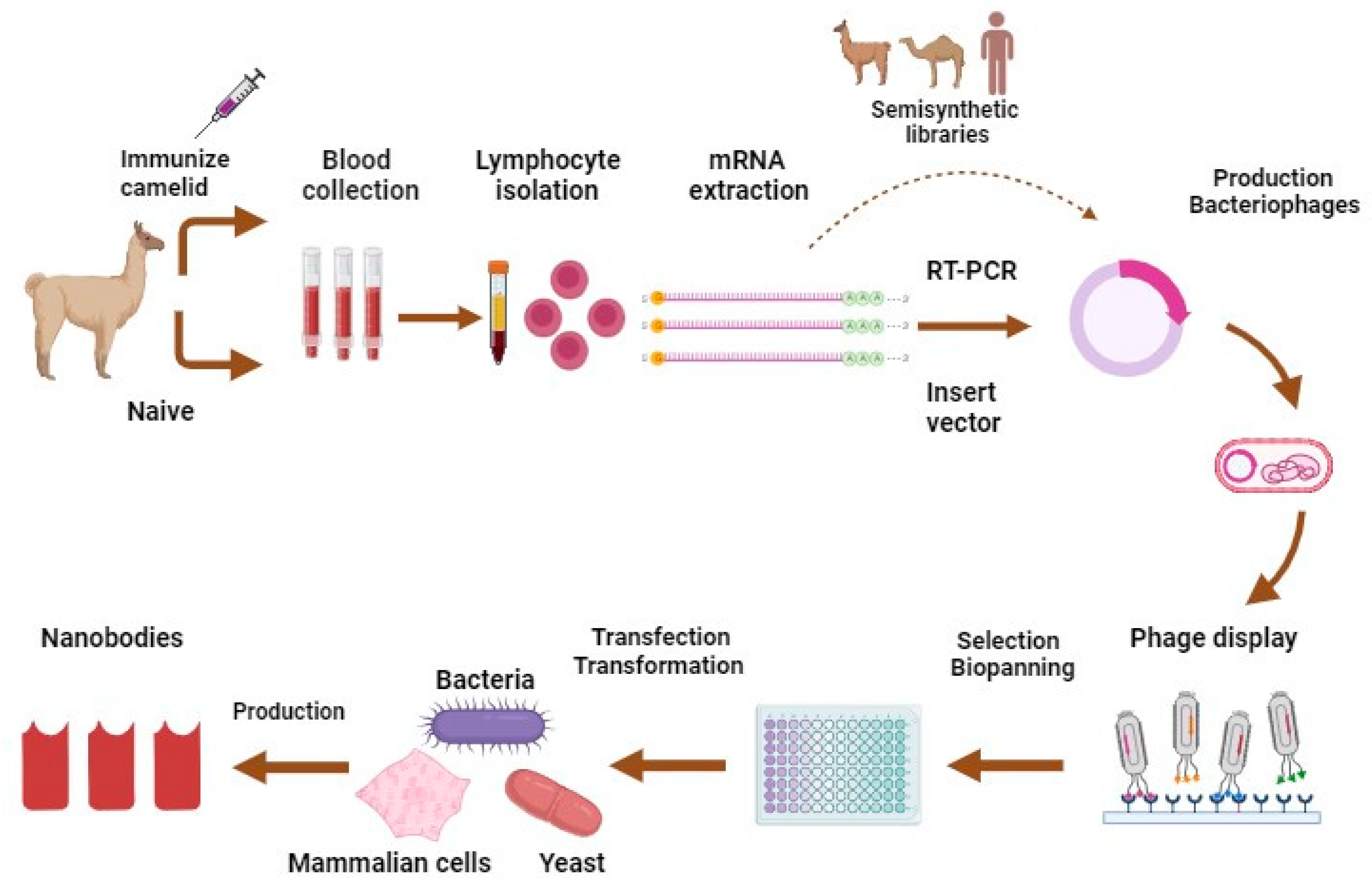
3.1. Nbs Characteristics and Production
3.2. Therapeutic Indications of Nbs
3.2.1. Nbs for Imaging
3.2.1.1. Nbs for Imaging in Cancer
3.2.1.2. Nbs for Imaging of Non-Malignant Diseases
3.2.2. Nbs for Diagnosis
3.2.2.1. Nbs for In Vitro Diagnosis
3.2.2.2. Nbs for In Vivo Diagnostic Imaging
3.2.3. Nbs for Oncology
3.2.4. Nbs for Infectious Diseases
3.2.5. Nbs for Metabolic Disorders
3.2.6. Nbs for Neurological Disorders
3.2.6.1. Nbs for Alzheimer’s Disease
3.2.6.2. Nbs for Parkinson's Disease
3.2.6.3. Nbs for Multiple Sclerosis
3.2.6.4. Nbs for Brain Tumor
3.2.6.5. Nbs for Infectious Disease Affecting CNS
3.2.6.6. Nbs for Other Neurological Disorders
3.2.7. Nbs for Other Disorders
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
| COVID-19 | Coronavirus disease-2019 |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme-linked immunosorbent assays |
| Fab | Fragment antigen binding |
| Fc | Fragment crystallizable |
| HCAb | Heavy-chain-only antibodies |
| HER | Human epidermal growth factor receptor |
| HIV | Human immunodeficiency viruses |
| Ig | Immunoglobulins |
| LFIA | Lateral flow immunoassays |
| Mab | Monoclonal antibody |
| MAbs | Monoclonal antibodies |
| Nb | Nanobody |
| Nbs | Nanobodies |
| PCR | Polymerase chain reaction |
| PET | Positron emission tomography |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| scFvs | Single-chain variable fragments |
| SPECT | Single photon emission computed tomography |
| TNF | Tumor necrosis factor |
| VHHs | Variable domain of heavy chain of HCAb |
References
- Megha, K.; Mohanan, P. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2020, 169, 28–38. [CrossRef]
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30. [CrossRef]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies. Antibodies 2019, 8, 43. [CrossRef]
- Rajpal, A.; Strop, P.; Yeung, Y.A.; Chaparro-Riggers, J.; Pons, J. Introduction: Antibody Structure and Function. 2013.
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [CrossRef]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A comprehensive comparison between camelid nanobodies and single chain variable fragments. Biomark. Res. 2021, 9, 1–20. [CrossRef]
- Liu, S.; Liu, X. IgG N-glycans. Advances in Clinical Chemistry 2021, 105, 1-47.
- Jenner, E. On the Origin of the Vaccine Inoculation. The Medical and physical journal 1801, 5, 505–508.
- Rees, A. Antibodies: A History of Their Discovery and Properties. In Introduction to Antibody Engineering, Rüker, F., Wozniak-Knopp, G., Eds. Springer International Publishing: Cham, 2021; 10.1007/978-3-030-54630-4_2pp. 5-39.
- Packer, D. The history of the antibody as a tool. Acta Histochem. 2021, 123, 151710. [CrossRef]
- Davies, D.R.; Chacko, S. Antibody structure. Accounts Chem. Res. 1993, 26, 421–427. [CrossRef]
- Lemieux, R.U.; Spohr, U. How Emil Fischer was led to the lock and key concept for enzyme specificity. Adv Carbohydr Chem Biochem 1994, 50, 1-20.
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O.; Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Fundamentals and history of ELISA: the evolution of the immunoassays until invention of ELISA. Enzyme-linked Immunosorbent Assay (ELISA) From A to Z 2018, 1-18.
- Grillo-López, A.J. The first antibody therapy for cancer: a personal experience. Expert Rev. Anticancer. Ther. 2013, 13, 399–406. [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [CrossRef]
- Mahmuda, A.; Bande, F.; Al-Zihiry, K.J.K.; Abdulhaleem, N.; Majid, R.A.; Hamat, R.A.; Abdullah, W.O.; Unyah, Z. Monoclonal antibodies: A review of therapeutic applications and future prospects. Trop. J. Pharm. Res. 2017, 16, 713–722. [CrossRef]
- Castelli, M.S.; McGonigle, P.; Hornby, P.J. The pharmacology and therapeutic applications of monoclonal antibodies. Pharmacol. Res. Perspect. 2019, 7, e00535. [CrossRef]
- Erstad, B.L.; Davis, L.E. Fixed Versus Body-Sized-Based Dosing of Monoclonal Antibodies. Ann. Pharmacother. 2023, 58, 91–95. [CrossRef]
- Gupta, S.K.; Chaudhary, P. Therapeutic human monoclonal antibodies. In Biomedical Translational Research: Drug Design and Discovery, Springer: 2022; pp. 401-418.
- Behl, A.; Wani, Z.A.; Das, N.N.; Parmar, V.S.; Len, C.; Malhotra, S.; Chhillar, A.K. Monoclonal antibodies in breast cancer: A critical appraisal. Crit. Rev. Oncol. 2023, 183, 103915. [CrossRef]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 2022, 7, 1–28. [CrossRef]
- Suzuki, M.; Kato, C.; Kato, A. Therapeutic antibodies: their mechanisms of action and the pathological findings they induce in toxicity studies. J. Toxicol. Pathol. 2015, 28, 133–139. [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [CrossRef]
- Navabi, P.; Ganjalikhany, M.R.; Jafari, S.; Dehbashi, M.; Ganjalikhani-Hakemi, M. Designing and generating a single-chain fragment variable (scFv) antibody against IL2Rα (CD25): An in silico and in vitro study. Iranian Journal of Basic Medical Sciences 2021, 24, 360–368. [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448.
- Streltsov, V.A.; Carmichael, J.A.; Nuttall, S.D. Structure of a shark IgNAR antibody variable domain and modeling of an early-developmental isotype. Protein Sci. 2005, 14, 2901–2909. [CrossRef]
- Pillay, T.S.; Muyldermans, S. Application of single-domain antibodies (“nanobodies”) to laboratory diagnosis. Ann Lab Med 2021, 41, 549-558.
- Siontorou, C.G. Nanobodies as novel agents for disease diagnosis and therapy. Int. J. Nanomed. 2013, 8, 4215–27. [CrossRef]
- Duggan, S. Caplacizumab: First Global Approval. Drugs 2018, 78, 1639–1642. [CrossRef]
- Keam, S.J. Ozoralizumab: First Approval. Drugs 2022, 83, 87–92. [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [CrossRef]
- Abbady, A.; Al-Daoude, A.; Al-Mariri, A.; Zarkawi, M.; Muyldermans, S. Chaperonin GroEL a Brucella immunodominant antigen identified using Nanobody and MALDI-TOF-MS technologies. Veter- Immunol. Immunopathol. 2012, 146, 254–263. [CrossRef]
- Wang, W.; Yuan, J.; Jiang, C. Applications of nanobodies in plant science and biotechnology. Plant Mol. Biol. 2020, 105, 43–53. [CrossRef]
- Tang, H.; Gao, Y.; Han, J. Application Progress of the Single Domain Antibody in Medicine. Int. J. Mol. Sci. 2023, 24, 4176. [CrossRef]
- Jovcevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [CrossRef]
- Beghein, E.; Gettemans, J. Nanobody Technology: A Versatile Toolkit for Microscopic Imaging, Protein–Protein Interaction Analysis, and Protein Function Exploration. Front. Immunol. 2017, 8, 771. [CrossRef]
- Singh, S.; Murillo, G.; Richner, J.; Singh, S.P.; Berleth, E.; Kumar, V.; Mehta, R.; Ramiya, V.; Parihar, A.S. A Broad-Based Characterization of a Cell-Penetrating, Single Domain Camelid Bi-Specific Antibody Monomer That Targets STAT3 and KRAS Dependent Cancers. Int. J. Mol. Sci. 2022, 23, 7565. [CrossRef]
- Muyldermans, S. A guide to: Generation and design of nanobodies. FEBS J. 2021, 288, 2084–2102. [CrossRef]
- Contreras, M.A.; Serrano-Rivero, Y.; González-Pose, A.; Salazar-Uribe, J.; Rubio-Carrasquilla, M.; Soares-Alves, M.; Parra, N.C.; Camacho-Casanova, F.; Sánchez-Ramos, O.; Moreno, E. Design and Construction of a Synthetic Nanobody Library: Testing Its Potential with a Single Selection Round Strategy. Molecules 2023, 28, 3708. [CrossRef]
- de Marco, A. Recombinant expression of nanobodies and nanobody-derived immunoreagents. Protein expression and purification 2020, 172, 105645.
- Jin, B.-k.; Odongo, S.; Radwanska, M.; Magez, S. Nanobodies: a review of generation, diagnostics and therapeutics. International Journal of Molecular Sciences 2023, 24, 5994.
- Harmand, T.J.; Islam, A.; Pishesha, N.; Ploegh, H.L. Nanobodies as in vivo, non-invasive, imaging agents. RSC Chemical Biology 2021, 2, 685-701.
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: a promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 1–13. [CrossRef]
- Küppers, J.; Kürpig, S.; Bundschuh, R.A.; Essler, M.; Lütje, S. Radiolabeling Strategies of Nanobodies for Imaging Applications. Diagnostics 2021, 11, 1530. [CrossRef]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D'Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. 18F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [CrossRef]
- Warnders, F.J.; van Scheltinga, A.G.T.; Knuehl, C.; van Roy, M.; de Vries, E.F.; Kosterink, J.G.; de Vries, E.G.; Lub-de Hooge, M.N. Human epidermal growth factor receptor 3–specific tumor uptake and biodistribution of 89Zr-MSB0010853 visualized by real-time and noninvasive PET imaging. Journal of Nuclear Medicine 2017, 58, 1210-1215.
- Jailkhani, N.; Ingram, J.R.; Rashidian, M.; Rickelt, S.; Tian, C.; Mak, H.; Jiang, Z.; Ploegh, H.L.; Hynes, R.O. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl. Acad. Sci. USA 2019, 116, 14181–14190. [CrossRef]
- Bridoux, J.; Broos, K.; Lecocq, Q.; Debie, P.; Martin, C.; Ballet, S.; Raes, G.; Neyt, S.; Vanhove, C.; Breckpot, K.; et al. Anti-Human PD-L1 Nanobody for Immuno-PET Imaging: Validation of a Conjugation Strategy for Clinical Translation. Biomolecules 2020, 10, 1388. [CrossRef]
- Li, D.; Cheng, S.; Zou, S.; Zhu, D.; Zhu, T.; Wang, P.; Zhu, X. Immuno-PET Imaging of 89Zr Labeled Anti-PD-L1 Domain Antibody. Mol. Pharm. 2018, 15, 1674–1681. [CrossRef]
- Li, D.; Zou, S.; Cheng, S.; Song, S.; Wang, P.; Zhu, X. Monitoring the Response of PD-L1 Expression to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Nonsmall-Cell Lung Cancer Xenografts by Immuno-PET Imaging. Mol. Pharm. 2019, 16, 3469–3476. [CrossRef]
- Berland, L.; Kim, L.; Abousaway, O.; Mines, A.; Mishra, S.; Clark, L.; Hofman, P.; Rashidian, M. Nanobodies for medical imaging: about ready for prime time? Biomolecules 2021, 11, 637.
- Wang, W.; Hou, X.; Yang, X.; Liu, A.; Tang, Z.; Mo, F.; Yin, S.; Lu, X. Highly sensitive detection of CTLA-4-positive T-cell subgroups based on nanobody and fluorescent carbon quantum dots. Oncology Letters 2019, 18, 109-116.
- Lecocq, Q.; Zeven, K.; De Vlaeminck, Y.; Martens, S.; Massa, S.; Goyvaerts, C.; Raes, G.; Keyaerts, M.; Breckpot, K.; Devoogdt, N. Noninvasive Imaging of the Immune Checkpoint LAG-3 Using Nanobodies, from Development to Pre-Clinical Use. Biomolecules 2019, 9, 548. [CrossRef]
- Lecocq, Q.; Awad, R.M.; De Vlaeminck, Y.; de Mey, W.; Ertveldt, T.; Goyvaerts, C.; Raes, G.; Thielemans, K.; Keyaerts, M.; Devoogdt, N.; et al. Single-Domain Antibody Nuclear Imaging Allows Noninvasive Quantification of LAG-3 Expression by Tumor-Infiltrating Leukocytes and Predicts Response of Immune Checkpoint Blockade. J. Nucl. Med. 2021, 62, 1638–1644. [CrossRef]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 2017, 214, 2243–2255. [CrossRef]
- Rashidian, M.; LaFleur, M.W.; Verschoor, V.L.; Dongre, A.; Zhang, Y.; Nguyen, T.H.; Kolifrath, S.; Aref, A.R.; Lau, C.J.; Paweletz, C.P.; et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc. Natl. Acad. Sci. 2019, 116, 16971–16980. [CrossRef]
- Zhao, H.; Wang, C.; Yang, Y.; Sun, Y.; Wei, W.; Wang, C.; Wan, L.; Zhu, C.; Li, L.; Huang, G.; et al. ImmunoPET imaging of human CD8+ T cells with novel 68Ga-labeled nanobody companion diagnostic agents. J. Nanobiotechnology 2021, 19, 1–11. [CrossRef]
- Gondry, O.; Xavier, C.; Raes, L.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Breckpot, K.; Lecocq, Q.; Decoster, L.; Fontaine, C.; et al. Phase I Study of [68Ga]Ga-Anti-CD206-sdAb for PET/CT Assessment of Protumorigenic Macrophage Presence in Solid Tumors (MMR Phase I). J. Nucl. Med. 2023, 64, 1378–1384. [CrossRef]
- Punjabi, M.; Xu, L.; Ochoa-Espinosa, A.; Kosareva, A.; Wolff, T.; Murtaja, A.; Broisat, A.; Devoogdt, N.; Kaufmann, B.A. Ultrasound molecular imaging of atherosclerosis with nanobodies: translatable microbubble targeting murine and human VCAM (vascular cell adhesion molecule) 1. Arteriosclerosis, Thrombosis, and Vascular Biology 2019, 39, 2520-2530.
- Iljina, M.; Hong, L.; Horrocks, M.H.; Ludtmann, M.H.; Choi, M.L.; Hughes, C.D.; Ruggeri, F.S.; Guilliams, T.; Buell, A.K.; Lee, J.-E.; et al. Nanobodies raised against monomeric ɑ-synuclein inhibit fibril formation and destabilize toxic oligomeric species. BMC Biol. 2017, 15, 57–57. [CrossRef]
- Gerdes, C.; Waal, N.; Offner, T.; Fornasiero, E.F.; Wender, N.; Verbarg, H.; Manzini, I.; Trenkwalder, C.; Mollenhauer, B.; Strohäker, T.; et al. A nanobody-based fluorescent reporter reveals human α-synuclein in the cell cytosol. Nat. Commun. 2020, 11, 1–13. [CrossRef]
- Demine, S.; Ribeiro, R.G.; Thevenet, J.; Marselli, L.; Marchetti, P.; Pattou, F.; Kerr-Conte, J.; Devoogdt, N.; Eizirik, D.L. A nanobody-based nuclear imaging tracer targeting dipeptidyl peptidase 6 to determine the mass of human beta cell grafts in mice. Diabetologia 2019, 63, 825–836. [CrossRef]
- Muyldermans, S. Applications of nanobodies. Annual review of animal biosciences 2021, 9, 401-421.
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays in biochemistry 2016, 60, 111-120.
- De Genst, E.; Silence, K.; Decanniere, K.; Conrath, K.; Loris, R.; Kinne, J.; Muyldermans, S.; Wyns, L. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. 2006, 103, 4586–4591. [CrossRef]
- Mohseni, A.; Molakarimi, M.; Taghdir, M.; Sajedi, R.H.; Hasannia, S. Exploring single-domain antibody thermostability by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2018, 37, 3686–3696. [CrossRef]
- Torres, J.E.P.; Goossens, J.; Ding, J.; Li, Z.; Lu, S.; Vertommen, D.; Naniima, P.; Chen, R.; Muyldermans, S.; Sterckx, Y.G.-J.; et al. Development of a Nanobody-based lateral flow assay to detect active Trypanosoma congolense infections. Sci. Rep. 2018, 8, 1–15. [CrossRef]
- Vincke, C.; Gutiérrez, C.; Wernery, U.; Devoogdt, N.; Hassanzadeh-ghassabeh, G.; Muyldermans, S. Generation of Single Domain Antibody Fragments Derived from Camelids and Generation of Manifold Constructs. In Antibody Engineering: Methods and Protocols; Second Edition 2012; Humana Press: Totowa, NJ, USA, 2012; pp. 145–176.
- Goossens, J.; Sein, H.; Lu, S.; Radwanska, M.; Muyldermans, S.; Sterckx, Y.G.-J.; Magez, S. Functionalization of gold nanoparticles with nanobodies through physical adsorption. Anal. Methods 2017, 9, 3430–3440. [CrossRef]
- Li, Z.; Torres, J.E.P.; Goossens, J.; Vertommen, D.; Caljon, G.; Sterckx, Y.G.-J.; Magez, S. An Unbiased Immunization Strategy Results in the Identification of Enolase as a Potential Marker for Nanobody-Based Detection of Trypanosoma evansi. Vaccines 2020, 8, 415. [CrossRef]
- Odongo, S.; Sterckx, Y.G.J.; Stijlemans, B.; Pillay, D.; Baltz, T.; Muyldermans, S.; Magez, S. An Anti-proteome Nanobody Library Approach Yields a Specific Immunoassay for Trypanosoma congolense Diagnosis Targeting Glycosomal Aldolase. PLOS Neglected Trop. Dis. 2016, 10, e0004420. [CrossRef]
- Pinto, J.; Odongo, S.; Lee, F.; Gaspariunaite, V.; Muyldermans, S.; Magez, S.; Sterckx, Y.G.-J. Structural basis for the high specificity of a Trypanosoma congolense immunoassay targeting glycosomal aldolase. PLOS Neglected Trop. Dis. 2017, 11, e0005932. [CrossRef]
- Gelkop, S.; Sobarzo, A.; Brangel, P.; Vincke, C.; Romão, E.; Fedida-Metula, S.; Strom, N.; Ataliba, I.; Mwiine, F.N.; Ochwo, S.; et al. The Development and Validation of a Novel Nanobody-Based Competitive ELISA for the Detection of Foot and Mouth Disease 3ABC Antibodies in Cattle. Front. Veter- Sci. 2018, 5, 250. [CrossRef]
- Katz, H.E. Antigen sensing via nanobody-coated transistors. Nat. Biomed. Eng. 2021, 5, 639–640. [CrossRef]
- Campuzano, S.; Salema, V.; Moreno-Guzmán, M.; Gamella, M.; Yáñez-Sedeño, P.; Fernández, L.; Pingarrón, J. Disposable amperometric magnetoimmunosensors using nanobodies as biorecognition element. Determination of fibrinogen in plasma. Biosens. Bioelectron. 2014, 52, 255–260. [CrossRef]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid single-molecule detection of COVID-19 and MERS antigens via nanobody-functionalized organic electrochemical transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [CrossRef]
- Noltes, M.E.; van Dam, G.M.; Nagengast, W.B.; van der Zaag, P.J.; Slart, R.H.J.A.; Szymanski, W.; Kruijff, S.; Dierckx, R.A.J.O. Let’s embrace optical imaging: a growing branch on the clinical molecular imaging tree. Eur. J. Nucl. Med. 2021, 48, 4120–4128. [CrossRef]
- Oliveira, S.; Van Dongen, G.a.M.S.; Stigter-Van Walsum, M.; Roovers, R.C.; Stam, J.C.; Mali, W.; Van Diest, P.J.; Van Bergen En Henegouwen, P.M.P. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-Infrared Fluorescent Anti–Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [CrossRef]
- Kijanka, M.M.; van Brussel, A.S.; van der Wall, E.; Mali, W.P.; van Diest, P.J.; van Bergen en Henegouwen, P.M.; Oliveira, S. Optical imaging of pre-invasive breast cancer with a combination of VHHs targeting CAIX and HER2 increases contrast and facilitates tumour characterization. Ejnmmi Research 2016, 6, 1-13.
- Broisat, A.; Hernot, S.; Toczek, J.; De Vos, J.; Riou, L.M.; Martin, S.; Ahmadi, M.; Thielens, N.; Wernery, U.; Caveliers, V.; et al. Nanobodies Targeting Mouse/Human VCAM1 for the Nuclear Imaging of Atherosclerotic Lesions. Circ. Res. 2012, 110, 927–937. [CrossRef]
- Broisat, A.; Toczek, J.; Dumas, L.S.; Ahmadi, M.; Bacot, S.; Perret, P.; Slimani, L.; Barone-Rochette, G.; Soubies, A.; Devoogdt, N. 99mTc-cAbVCAM1-5 imaging is a sensitive and reproducible tool for the detection of inflamed atherosclerotic lesions in mice. Journal of Nuclear Medicine 2014, 55, 1678-1684.
- Xavier, C.; Vaneycken, I.; D’huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. Journal of Nuclear Medicine 2013, 54, 776-784.
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68Ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30, iii25–iii26. [CrossRef]
- D'Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted Radionuclide Therapy with A 177Lu-labeled Anti-HER2 Nanobody. Theranostics 2014, 4, 708–720. [CrossRef]
- Bannas, P.; Hambach, J.; Koch-Nolte, F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front. Immunol. 2017, 8, 1603. [CrossRef]
- Verhaar, E.R.; Woodham, A.W.; Ploegh, H.L. Nanobodies in cancer. In Proceedings of Seminars in immunology; p. 101425.
- nbsp;Roovers, R.C.; Vosjan, M.J.W.D.; Laeremans, T.; El Khoulati, R.; De Bruin, R.C.G.; Ferguson, K.M.; Verkleij, A.J.; Van Dongen, G.A.M.S.; Van Bergen en Henegouwen, P.M.P.A biparatopic anti-EGFR nanobody efficiently inhibits solid tumour growth. Int. J. Cancer 2011, 129, 2013–2024. [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [CrossRef]
- Broos, K.; Lecocq, Q.; Xavier, C.; Bridoux, J.; Nguyen, T.T.; Corthals, J.; Schoonooghe, S.; Lion, E.; Raes, G.; Keyaerts, M.; et al. Evaluating a Single Domain Antibody Targeting Human PD-L1 as a Nuclear Imaging and Therapeutic Agent. Cancers 2019, 11, 872. [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [CrossRef]
- Sadeghi, A.; Behdani, M.; Muyldermans, S.; Habibi-Anbouhi, M.; Kazemi-Lomedasht, F. Development of a mono-specific anti-VEGF bivalent nanobody with extended plasma half-life for treatment of pathologic neovascularization. Drug testing and analysis 2020, 12, 92-100.
- Sanaei, M.; Setayesh, N.; Sepehrizadeh, Z.; Mahdavi, M.; Yazdi, M.H. Nanobodies in Human Infections: Prevention, Detection, and Treatment. Immunol. Investig. 2019, 49, 875–896. [CrossRef]
- Mei, Y.; Chen, Y.; Sivaccumar, J.P.; An, Z.; Xia, N.; Luo, W. Research progress and applications of nanobody in human infectious diseases. Front. Pharmacol. 2022, 13, 963978. [CrossRef]
- Xiang, Y.; Nambulli, S.; Xiao, Z.; Liu, H.; Sang, Z.; Duprex, W.P.; Schneidman-Duhovny, D.; Zhang, C.; Shi, Y. Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2. Science 2020, 370, 1479-1484.
- Zupancic, J.M.; Schardt, J.S.; Desai, A.A.; Makowski, E.K.; Smith, M.D.; Pornnoppadol, G.; Garcia de Mattos Barbosa, M.; Cascalho, M.; Lanigan, T.M.; Tessier, P.M. Engineered multivalent nanobodies potently and broadly neutralize SARS-CoV-2 variants. Advanced therapeutics 2021, 4, 2100099.
- Saied, A.A.; Metwally, A.A.; Alobo, M.; Shah, J.; Sharun, K.; Dhama, K. Bovine-derived antibodies and camelid-derived nanobodies as biotherapeutic weapons against SARS-CoV-2 and its variants: a review article. International Journal of Surgery 2022, 98, 106233.
- Detalle, L.; Stohr, T.; Palomo, C.; Piedra, P.A.; Gilbert, B.E.; Mas, V.; Millar, A.; Power, U.F.; Stortelers, C.; Allosery, K.; et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 6–13. [CrossRef]
- Ibañez, L.I.; De Filette, M.; Hultberg, A.; Verrips, T.; Temperton, N.; Weiss, R.A.; Vandevelde, W.; Schepens, B.; Vanlandschoot, P.; Saelens, X. Nanobodies With In Vitro Neutralizing Activity Protect Mice Against H5N1 Influenza Virus Infection. J. Infect. Dis. 2011, 203, 1063–1072. [CrossRef]
- Wichgers Schreur, P.J.; van de Water, S.; Harmsen, M.; Bermudez-Mendez, E.; Drabek, D.; Grosveld, F.; Wernike, K.; Beer, M.; Aebischer, A.; Daramola, O. Multimeric single-domain antibody complexes protect against bunyavirus infections. Elife 2020, 9, e52716.
- Kennedy, P.J.; Oliveira, C.; Granja, P.L.; Sarmento, B. Monoclonal antibodies: technologies for early discovery and engineering. Crit. Rev. Biotechnol. 2017, 38, 394–408. [CrossRef]
- Li, H.-C.; Lo, S.-Y. Hepatitis C virus: Virology, diagnosis and treatment. World journal of hepatology 2015, 7, 1377.
- Liver, E.A.f.T.S.o.T. EASL recommendations on treatment of hepatitis C 2016. Journal of hepatology 2017, 66, 153-194.
- Wilken, L.; McPherson, A. Application of camelid heavy-chain variable domains (VHHs) in prevention and treatment of bacterial and viral infections. Int. Rev. Immunol. 2017, 37, 69–76. [CrossRef]
- Barta, M.L.; Shearer, J.P.; Arizmendi, O.; Tremblay, J.M.; Mehzabeen, N.; Zheng, Q.; Battaile, K.P.; Lovell, S.; Tzipori, S.; Picking, W.D.; et al. Single-domain antibodies pinpoint potential targets within Shigella invasion plasmid antigen D of the needle tip complex for inhibition of type III secretion. J. Biol. Chem. 2017, 292, 16677–16687. [CrossRef]
- Smolarek, D.; Hattab, C.; Hassanzadeh-Ghassabeh, G.; Cochet, S.; Gutiérrez, C.; de Brevern, A.G.; Udomsangpetch, R.; Picot, J.; Grodecka, M.; Wasniowska, K.; et al. A recombinant dromedary antibody fragment (VHH or nanobody) directed against human Duffy antigen receptor for chemokines. Cell. Mol. Life Sci. 2010, 67, 3371–3387. [CrossRef]
- Stijlemans, B.; Caljon, G.; Natesan, S.K.A.; Saerens, D.; Conrath, K.; Pérez-Morga, D.; Skepper, J.N.; Nikolaou, A.; Brys, L.; Pays, E.; et al. High Affinity Nanobodies against the Trypanosome brucei VSG Are Potent Trypanolytic Agents that Block Endocytosis. PLOS Pathog. 2011, 7, e1002072. [CrossRef]
- Obishakin, E.; Stijlemans, B.; Santi-Rocca, J.; Vandenberghe, I.; Devreese, B.; Muldermans, S.; Bastin, P.; Magez, S. Generation of a Nanobody Targeting the Paraflagellar Rod Protein of Trypanosomes. PLOS ONE 2014, 9, e115893. [CrossRef]
- Bocancia-Mateescu, L.-A.; Stan, D.; Mirica, A.-C.; Ghita, M.G.; Stan, D.; Ruta, L.L. Nanobodies as Diagnostic and Therapeutic Tools for Cardiovascular Diseases (CVDs). Pharmaceuticals 2023, 16, 863. [CrossRef]
- Broggini, L.; Giono, M.; Speranzini, V.; Barzago, M.; Palladini, G.; Diomede, L.; Pappone, C.; Ricagno, S. Nanobodies as novel potential drugs to target cardiac light chain amyloidosis. Cardiovasc. Res. 2022, 118. [CrossRef]
- Wingler, L.M.; Feld, A.P. Nanobodies as Probes and Modulators of Cardiovascular G Protein–Coupled Receptors. J. Cardiovasc. Pharmacol. 2021, 80, 342–353. [CrossRef]
- McMahon, C.; Staus, D.P.; Wingler, L.M.; Wang, J.; Skiba, M.A.; Elgeti, M.; Hubbell, W.L.; Rockman, H.A.; Kruse, A.C.; Lefkowitz, R.J. Synthetic nanobodies as angiotensin receptor blockers. Proc. Natl. Acad. Sci. 2020, 117, 20284–20291. [CrossRef]
- Li, T.; Shen, Y.; Lin, F.; Fu, W.; Liu, S.; Wang, C.; Liang, J.; Fan, X.; Ye, X.; Tang, Y.; et al. Targeting RyR2 with a phosphorylation site–specific nanobody reverses dysfunction of failing cardiomyocytes in rats. FASEB J. 2019, 33, 7467–7478. [CrossRef]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7. [CrossRef]
- De Genst, E.; Foo, K.S.; Xiao, Y.; Rohner, E.; de Vries, E.; Sohlmér, J.; Witman, N.; Hidalgo, A.; Kolstad, T.R.S.; Louch, W.E.; et al. Blocking phospholamban with VHH intrabodies enhances contractility and relaxation in heart failure. Nat. Commun. 2022, 13, 1–18. [CrossRef]
- Callewaert, F.; Roodt, J.; Ulrichts, H.; Stohr, T.; van Rensburg, W.J.; Lamprecht, S.; Rossenu, S.; Priem, S.; Willems, W.; Holz, J.-B. Evaluation of efficacy and safety of the anti-VWF Nanobody ALX-0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood The Journal of the American Society of Hematology 2012, 120, 3603–3610. [CrossRef]
- Marturano, A.; Hendrickx, M.L.; Falcinelli, E.; Sebastiano, M.; Guglielmini, G.; Hassanzadeh-Ghassabeh, G.; Muyldermans, S.; Declerck, P.J.; Gresele, P. Development of anti-matrix metalloproteinase-2 (MMP-2) nanobodies as potential therapeutic and diagnostic tools. Nanomedicine: Nanotechnology, Biol. Med. 2019, 24, 102103. [CrossRef]
- Jooss, N.J.; Smith, C.W.; Slater, A.; Montague, S.J.; Di, Y.; O'shea, C.; Thomas, M.R.; Henskens, Y.M.; Heemskerk, J.W.; Watson, S.P. Anti-GPVI nanobody blocks collagen-and atherosclerotic plaque–induced GPVI clustering, signaling, and thrombus formation. Journal of Thrombosis and Haemostasis 2022, 20, 2617-2631.
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nature reviews Disease primers 2021, 7, 33.
- Habiba, U.; Descallar, J.; Kreilaus, F.; Adhikari, U.K.; Kumar, S.; Morley, J.W.; Bui, B.V.; Koronyo-Hamaoui, M.; Tayebi, M. Detection of retinal and blood Aβ oligomers with nanobodies. Alzheimer's Dementia: Diagn. Assess. Dis. Monit. 2021, 13, e12193. [CrossRef]
- Danis, C.; Dupré, E.; Zejneli, O.; Caillierez, R.; Arrial, A.; Bégard, S.; Mortelecque, J.; Eddarkaoui, S.; Loyens, A.; Cantrelle, F.-X.; et al. Inhibition of Tau seeding by targeting Tau nucleation core within neurons with a single domain antibody fragment. Mol. Ther. 2022, 30, 1484–1499. [CrossRef]
- Marino, M.; Zhou, L.; Rincon, M.Y.; Callaerts-Vegh, Z.; Verhaert, J.; Wahis, J.; Creemers, E.; Yshii, L.; Wierda, K.; Saito, T. AAV-mediated delivery of an anti-BACE1 VHH alleviates pathology in an Alzheimer's disease model. EMBO molecular medicine 2022, 14, e09824.
- Marino, M.; Holt, M.G. AAV Vector-Mediated Antibody Delivery (A-MAD) in the Central Nervous System. Front. Neurol. 2022, 13, 870799. [CrossRef]
- Zhao, L.; Meng, F.; Li, Y.; Liu, S.; Xu, M.; Chu, F.; Li, C.; Yang, X.; Luo, L. Multivalent Nanobody Conjugate with Rigid, Reactive Oxygen Species Scavenging Scaffold for Multi-Target Therapy of Alzheimer's Disease. Advanced Materials 2023, 2210879.
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson's disease. The Lancet 2021, 397, 2284-2303.
- Haddad, F.; Sawalha, M.; Khawaja, Y.; Najjar, A.; Karaman, R. Dopamine and Levodopa Prodrugs for the Treatment of Parkinson’s Disease. Molecules 2017, 23, 40. [CrossRef]
- Vuchelen, A.; O’day, E.; De Genst, E.; Pardon, E.; Wyns, L.; Dumoulin, M.; Dobson, C.M.; Christodoulou, J.; Hsu, S.-T.D. 1H, 13C and 15N assignments of a camelid nanobody directed against human α-synuclein. Biomol. NMR Assignments 2009, 3, 231–233. [CrossRef]
- De Genst, E.J.; Guilliams, T.; Wellens, J.; O'Day, E.M.; Waudby, C.A.; Meehan, S.; Dumoulin, M.; Hsu, S.-T.D.; Cremades, N.; Verschueren, K.H.; et al. Structure and Properties of a Complex of α-Synuclein and a Single-Domain Camelid Antibody. J. Mol. Biol. 2010, 402, 326–343. [CrossRef]
- Guilliams, T.; El-Turk, F.; Buell, A.K.; O'Day, E.M.; Aprile, F.A.; Esbjörner, E.K.; Vendruscolo, M.; Cremades, N.; Pardon, E.; Wyns, L.; et al. Nanobodies Raised against Monomeric α-Synuclein Distinguish between Fibrils at Different Maturation Stages. J. Mol. Biol. 2013, 425, 2397–2411. [CrossRef]
- El-Turk, F.; Newby, F.N.; De Genst, E.; Guilliams, T.; Sprules, T.; Mittermaier, A.; Dobson, C.M.; Vendruscolo, M. Structural Effects of Two Camelid Nanobodies Directed to Distinct C-Terminal Epitopes on α-Synuclein. Biochemistry 2016, 55, 3116–3122. [CrossRef]
- Hmila, I.; Vaikath, N.N.; Majbour, N.K.; Erskine, D.; Sudhakaran, I.P.; Gupta, V.; Ghanem, S.S.; Islam, Z.; Emara, M.M.; Abdesselem, H.B. Novel engineered nanobodies specific for N-terminal region of alpha-synuclein recognize Lewy-body pathology and inhibit in-vitro seeded aggregation and toxicity. The FEBS Journal 2022, 289, 4657-4673.
- Singh, R.K.; Soliman, A.; Guaitoli, G.; Störmer, E.; von Zweydorf, F.; Dal Maso, T.; Oun, A.; Van Rillaer, L.; Schmidt, S.H.; Chatterjee, D. Nanobodies as allosteric modulators of Parkinson’s disease–associated LRRK2. Proceedings of the National Academy of Sciences 2022, 119, e2112712119.
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front. Immunol. 2022, 13, 978513. [CrossRef]
- Steeland, S.; Puimège, L.; Vandenbroucke, R.E.; Van Hauwermeiren, F.; Haustraete, J.; Devoogdt, N.; Hulpiau, P.; Leroux-Roels, G.; Laukens, D.; Meuleman, P.; et al. Generation and Characterization of Small Single Domain Antibodies Inhibiting Human Tumor Necrosis Factor Receptor 1. J. Biol. Chem. 2015, 290, 4022–4037. [CrossRef]
- Steeland, S.; Van Ryckeghem, S.; Van Imschoot, G.; De Rycke, R.; Toussaint, W.; Vanhoutte, L.; Vanhove, C.; De Vos, F.; Vandenbroucke, R.E.; Libert, C. TNFR1 inhibition with a Nanobody protects against EAE development in mice. Sci. Rep. 2017, 7, 13646. [CrossRef]
- Sadeghian-Rizi, T.; Behdani, M.; Khanahmad, H.; Sadeghi, H.M.; Jahanian-Najafabadi, A. Generation and characterization of a functional nanobody against inflammatory chemokine CXCL10, as a novel strategy for the treatment of multiple sclerosis. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 2019, 18, 141-148.
- van de Water, J.A.J.M.; Bagci-Onder, T.; Agarwal, A.S.; Wakimoto, H.; Roovers, R.C.; Zhu, Y.; Kasmieh, R.; Bhere, D.; Henegouwen, P.M.P.V.B.E.; Shah, K. Therapeutic stem cells expressing variants of EGFR-specific nanobodies have antitumor effects. Proc. Natl. Acad. Sci. 2012, 109, 16642–16647. [CrossRef]
- Figarella-Branger, D.; Appay, R.; Metais, A.; Tauziède-Espariat, A.; Colin, C.; Rousseau, A.; Varlet, P. La classification de l’OMS 2021 des tumeurs du système nerveux central. In Proceedings of Annales de Pathologie; pp. 367-382.
- Ellingson, B.M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; Wick, W.; et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro-Oncology 2015, 17, 1188–1198. [CrossRef]
- Zhou, Z.; Vaidyanathan, G.; McDougald, D.; Kang, C.M.; Balyasnikova, I.; Devoogdt, N.; Ta, A.N.; McNaughton, B.R.; Zalutsky, M.R. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol. Imaging Biol. 2017, 19, 867–877. [CrossRef]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011, 25, 2433–2446. [CrossRef]
- Kulkarni, A.; Mochnáčová, E.; Majerova, P.; Čurlík, J.; Bhide, K.; Mertinková, P.; Bhide, M. Single Domain Antibodies Targeting Receptor Binding Pockets of NadA Restrain Adhesion of Neisseria meningitidis to Human Brain Microvascular Endothelial Cells. Front. Mol. Biosci. 2020, 7. [CrossRef]
- Terryn, S.; Francart, A.; Lamoral, S.; Hultberg, A.; Rommelaere, H.; Wittelsberger, A.; Callewaert, F.; Stohr, T.; Meerschaert, K.; Ottevaere, I.; et al. Protective Effect of Different Anti-Rabies Virus VHH Constructs against Rabies Disease in Mice. PLOS ONE 2014, 9, e109367. [CrossRef]
- Terryn, S.; Francart, A.; Rommelaere, H.; Stortelers, C.; Van Gucht, S. Post-exposure Treatment with Anti-rabies VHH and Vaccine Significantly Improves Protection of Mice from Lethal Rabies Infection. PLOS Neglected Trop. Dis. 2016, 10, e0004902. [CrossRef]
- Schut, M.H.; Pepers, B.A.; Klooster, R.; van der Maarel, S.M.; el Khatabi, M.; Verrips, T.; Dunnen, J.T.D.; van Ommen, G.-J.B.; van Roon-Mom, W.M.C. Selection and characterization of llama single domain antibodies against N-terminal huntingtin.. Neurol. Sci. 2014, 36, 429–34. [CrossRef]
- Colby, D.W.; Chu, Y.; Cassady, J.P.; Duennwald, M.; Zazulak, H.; Webster, J.M.; Messer, A.; Lindquist, S.; Ingram, V.M.; Wittrup, K.D. Potent inhibition of huntingtin aggregation and cytotoxicity by a disulfide bond-free single-domain intracellular antibody. Proc. Natl. Acad. Sci. 2004, 101, 17616–17621. [CrossRef]
- Wang, J.; Kang, G.; Yuan, H.; Cao, X.; Huang, H.; de Marco, A. Research Progress and Applications of Multivalent, Multispecific and Modified Nanobodies for Disease Treatment. Front. Immunol. 2022, 12, 838082. [CrossRef]
- Beltran, W.A.; Ripolles-Garcia, A.; Sato, Y.; Gray, A.; Wolfe, J.H.; Luz-Madrigal, A.; Philipps, M.J.; Gamm, D.M.; Aguirre, G.K.; Aguirre, G.D. Structural and functional assessment after subretinal transplantation of human iPSC-derived photoreceptor precursors in a canine model of end stage retinal degeneration. Investigative Ophthalmology & Visual Science 2023, 64, 766-766.
- Liu, X.; Sui, J.; Li, C.; Wang, Q.; Peng, X.; Meng, F.; Xu, Q.; Jiang, N.; Zhao, G.; Lin, J. The preparation and therapeutic effects of β-glucan-specific nanobodies and nanobody-natamycin conjugates in fungal keratitis. Acta Biomater. 2023, 169, 398–409. [CrossRef]
- Wu, A.; Salom, D.; Sander, C.L.; Pardon, E.; Steyaert, J.; Kiser, P.D.; Palczewski, K. NANOBODY rescues adRP phenotype in a P23H adRP cell model, suggesting therapeutic potential. Investigative Ophthalmology & Visual Science 2022, 63, 88–A0061-0088–A0061.
- Navarro-Compán, V.; Ermann, J.; Poddubnyy, D. A glance into the future of diagnosis and treatment of spondyloarthritis. Ther. Adv. Musculoskelet. Dis. 2022, 14. [CrossRef]
- Li, S.; Cao, P.; Chen, T.; Ding, C. Latest insights in disease-modifying osteoarthritis drugs development. Ther. Adv. Musculoskelet. Dis. 2023, 15. [CrossRef]
- Srinivasan, L.; Alzogaray, V.; Selvakumar, D.; Nathan, S.; Yoder, J.B.; Wright, K.M.; Klinke, S.; Nwafor, J.N.; Labanda, M.S.; Goldbaum, F.A.; et al. Development of high-affinity nanobodies specific for NaV1.4 and NaV1.5 voltage-gated sodium channel isoforms. J. Biol. Chem. 2022, 298, 101763. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




