Northern bottlenose whales Hyperoodon ampullatus (Forster 1770) are large-sized beaked whales (up to 9.7m) that inhabit deep, subarctic to temperate waters of the North Atlantic Ocean (south to ca. 37 °N) where they primarily feed on squid species of the genus Gonatus (Whitehead et al. 2003, 2021, Moors-Murphy 2018). Bottlenose whales were heavily hunted throughout their range in the period 1880 to 1920 and, then again, in 1937 to 1973, causing an estimated 40% reduction of their population (Taylor et al. 2008, Whitehead & Hooker 2012). Though precise estimations are not available, the entire North Atlantic population is estimated to number at least 20,000 individuals. Currently, anthropogenic sound from military mid-frequency sonar and seismic surveys as well as bycatch in fisheries are the main threats for this species (Whitehead & Hooker 2012). As a result of the historical depletion, the uncertainty of abundance estimates, and the exposition to harmful anthropogenic factors, northern bottlenose whales have been listed as ‘near threatened’ on the IUCN Red List of Threatened Species (Whitehead et al. 2021).
There are only few reports on the pathologies of H. ampullatus, even in stranded individuals. Dagleish et al. (2007) described severe mycotic encephalitis caused by the fungus Aspergillus fumigatus in a juvenile male H. ampullatus stranded in North Kessock, Scotland, in October 2006. Tattoo skin disease, a cetacean poxvirus infection, was also observed in this individual (Dagleish et al. 2008) as well as in a juvenile female that died in the Thames River in 2006 (Van Bressem et al. 2009). Tomilin (1967) reported that the ‘upper parts of the epidermis of H. ampullatus are sometimes affected by a pathological process of mycotic nature, resulting in the appearance of large white patches on the skin’. Feyrer et al. (2021) described anthropogenic marks, including probable entanglement and propeller-vessel strike scars, in 6.6% of the Endangered Scotian Shelf population (Nova Scotia) photographed from 1988 to 2019. The skeleton of a young northern bottlenose whale from the Shetland Islands, curated at the Royal Belgian Institute of Natural Sciences at Brussels (RBINS), shows exostosis of the spiny processes of the caudal vertebrae. Another skeleton, at Naturalis Biodiversity Center, Leiden, is reported to have a ‘bony growth on the rib’ (Slijper 1938, Tomilin 1967). Slijper (1938), possibly referring to the RBINS specimen, also mentioned spondylitis deformans in this species, while Kompanje (1999) reported discarthrosis and zygarthrosis without further details. Knowledge of osteopathology in southern bottlenose whales Hyperoodon planifrons Flower, 1882 is even more restricted (Barcellos 1977). In this context, it is important to promote studies of pathologies in beaked whales as to better understand the natural factors that may negatively impact their conservation.
During a revision of cetacean specimens kept at the Academy of Natural Sciences of the Drexel University of Philadelphia, one of us (JA) observed a large, bulbous exostosis in the right pterygoid of an adult female H. ampullatus (specimen ANSP-3004) that had washed ashore on the US Atlantic coast in the 19th century. Here, we describe the case. which represents the first record of this type of pathology in ziphiids.
The 823 cm female H. ampullatus stranded with her calf on the shore of Narrangansett Bay near Tiverton, Newport County, Rhode Island, USA, in February 1867, after being hunted and probably harpooned, based on images associated with the description (Cope 1869, Mitchell & Kosicki 1975). Cope (1869) provided the following information on the adult specimen ‘Its most striking feature is the relatively longer and more slender beak, and less elevated and prominent front. Thus, in the Newport specimen, it is one-twelfth of the [body] length, or 2 1/4 feet: equal three-fourths the distance between the eye and the spout hole. The prominent swollen front is in the Newport whale. It is considerably compressed and the eye is placed in a strong longitudinal prominence on each side of the head’. Cope (1869) further mentioned that the ‘muzzle’ [rostrum] of the female was longer than represented for European specimens, but this has not been confirmed. The skull was collected and donated to the museum where it was entered as ANSP-3004, two years after collection, i.e., in 1869.
Images of the ANSP-3004 skull were examined by the authors and compared to ‘normal’ female
H. ampullatus specimens kept at the Naturalis Biodiversity Center, Leiden, Netherlands (courtesy P. Kamminga), at the Museum of Comparative Zoology Mammalogy, Harvard University (MCZ; courtesy M. Omura) and the Royal Belgian Institute of Natural Sciences (RBINS; courtesy O. Lambert). Fourteen cranial measurements were taken (
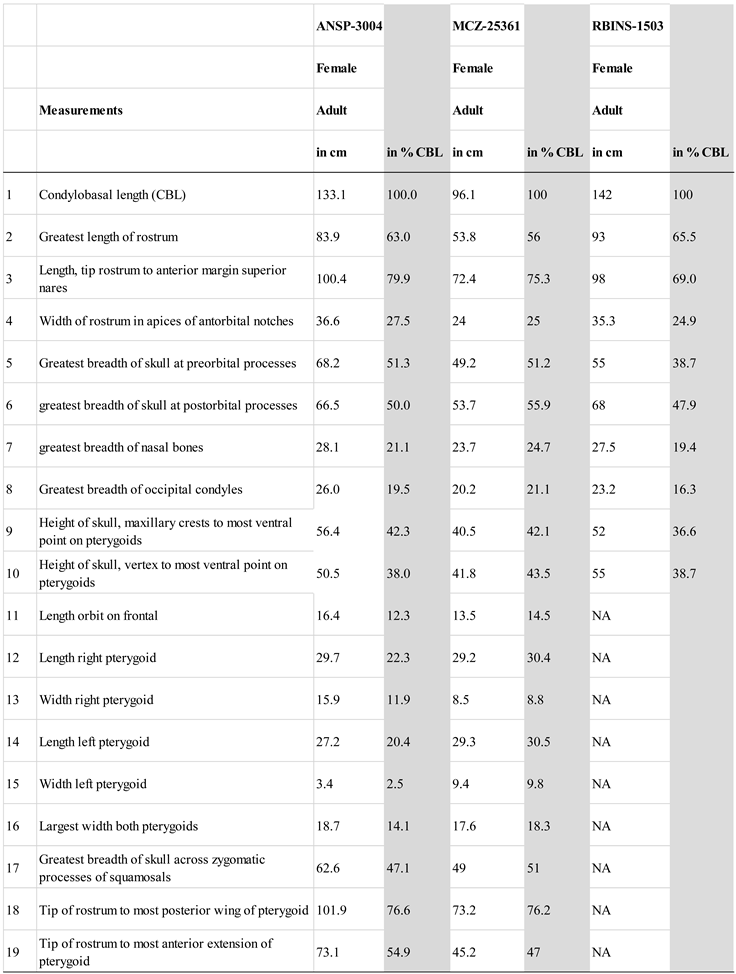
Table 1) and compared with unpublished measurements for two other adult, female specimens: MCZ- 25362, stranded in Beverly Farms, Massachusetts, in October 1923, and RBINS-1503, stranded in Zeeland, The Netherlands, in September 1840. Craniometrics were taken by different persons, so inter-observer variability may be significant. Surface scans of ANSP-3004 were made with a Faro Design Arm 1.0 scanner at 75µm resolution. Due to the size of the specimen, several scans were realized and stitched together using the 3D Systems Geomagic 2017 software to make a single surface model.
The condylobasal length (CBL) of ANSP-3004 was 133.1 cm. Other measurements are provided in
Table 1. Overall, the cranial dimensions of ANSP-3004, measured as % of CBL, were comparable to normal
H. ampullatus skulls without osteopathy (see
Table 1), with the exception of the pterygoid bones. Values for, respectively, ANSP-3004 and MCZ-25362, both adult females, were (in % CBL): length of left pterygoid (27.2 vs. 29.3), length of right pterygoid (29.7 vs. 29.2), and width of left pterygoid (3.4 vs 9.4). Only the width of the right pterygoid (15.9 vs. 8.5) was greater in ANSP-3004 due to the deformation.
The form and structure of the right pterygoid were abnormal (
Figure 1 and
Figure 2). Its distal (palatinal) extremity was strongly curved in a labial direction while its proximal (ventral-most) extremity was deformed by a large (143 x 125 mm), bulbous exostosis that extended laterally and medially, occluding the space between the pterygoids and partially obstructing the right nasal passage (Figs. 1, 3). There was also evidence of osteolysis exposing deep resorption lacunae in the posterior end of the osseous mass (
Figure 3). The left pterygoid was thin (34 mm), and slightly displaced laterally (
Figure 1).
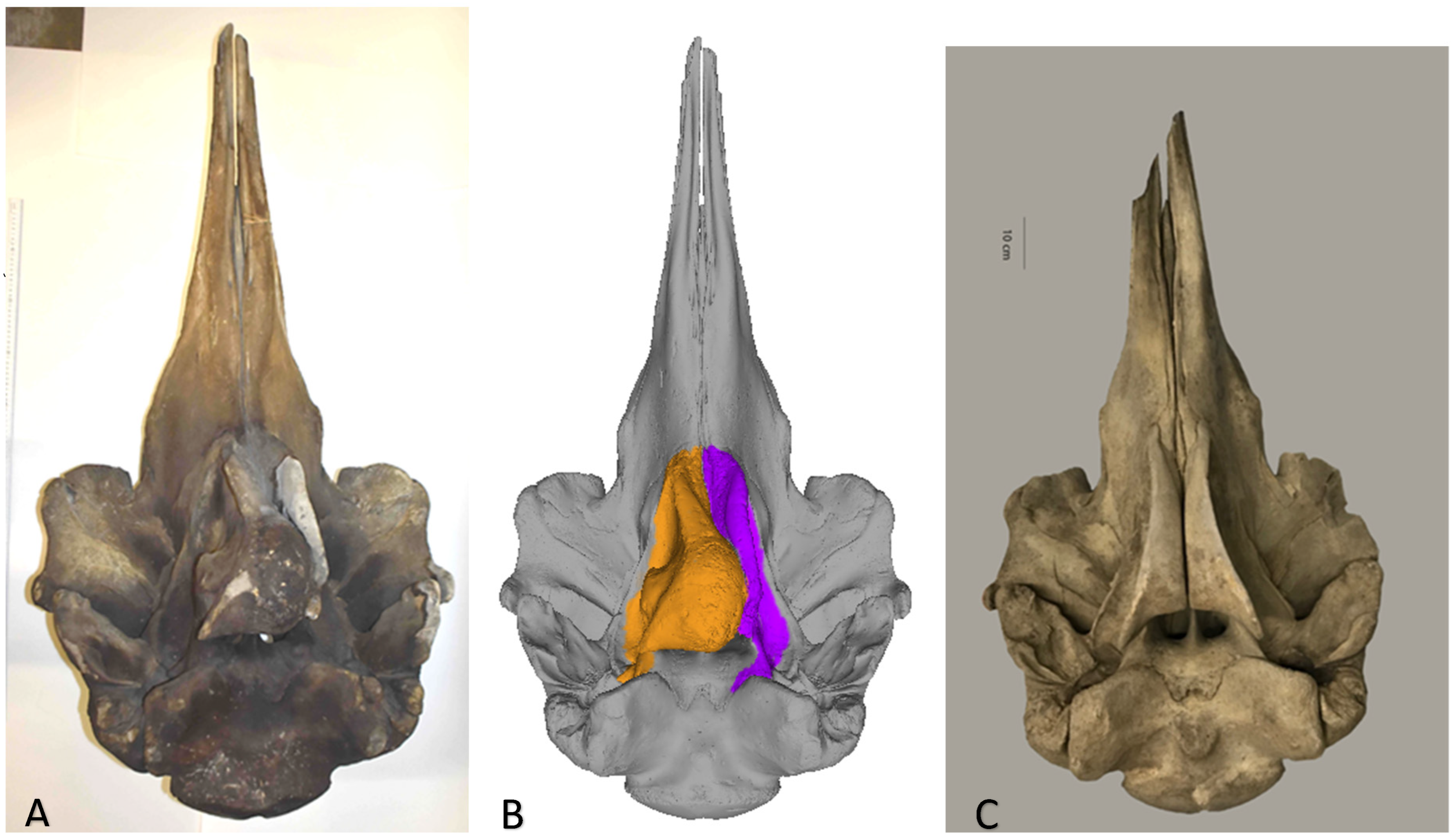
Figure 1.
(A) Ventral view of study specimen Hyperoodon ampullatus ANSP-3004; (B) surface model of this specimen and (C) in comparison with a ventral view of the normal skull of adult female H. ampullatus (MCZ-25362) from the Museum of Comparative Zoology, Mammalogy Department, Harvard University.
Figure 1.
(A) Ventral view of study specimen Hyperoodon ampullatus ANSP-3004; (B) surface model of this specimen and (C) in comparison with a ventral view of the normal skull of adult female H. ampullatus (MCZ-25362) from the Museum of Comparative Zoology, Mammalogy Department, Harvard University.
On the 3D model, much of the space of the right pterygoid sinus appeared filled with osseous tissue while the left pterygoid sinus was unobstructed (
Figure 1B). To what degree adjacent cranial bones, i.e., vomer, mesethmoid, ectecmoids, sphenoid, and (right) palatine, were affected could not be determined without (destructive) osteohistology and hands-on examination by (all) authors.
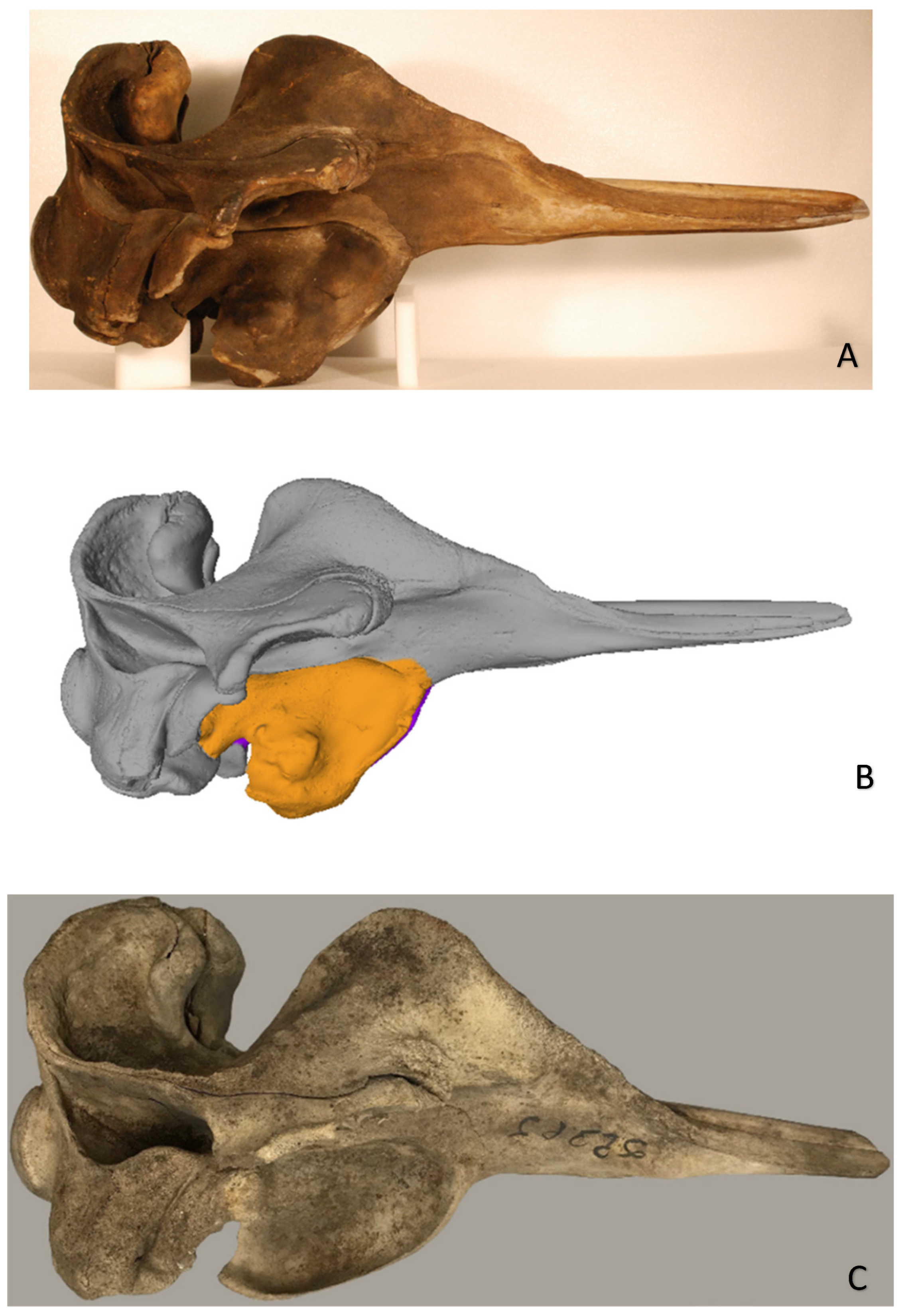
Figure 2.
(A) Right lateral view of study specimen northern bottlenose whale Hyperoodon ampullatus ANSP-3004; (B) single surface scan model of this specimen showing deformation of the right pterygoid bone (orange); (C) lateral view of an adult female H. ampullatus (MCZ-25362) with normal pterygoids at the Museum of Comparative Zoology, Mammalogy, Harvard University.
Figure 2.
(A) Right lateral view of study specimen northern bottlenose whale Hyperoodon ampullatus ANSP-3004; (B) single surface scan model of this specimen showing deformation of the right pterygoid bone (orange); (C) lateral view of an adult female H. ampullatus (MCZ-25362) with normal pterygoids at the Museum of Comparative Zoology, Mammalogy, Harvard University.
Figure 3.
Osteopathy of the right pterygoid bone in Hyperoodon ampullatus ANSP-3004: (Left) Occipital view of the upturned skull with (anteriad) the bulbous exostosis seen protruding from the right pterygoid; (Right) close-up view of the osseous mass exposing resorption lacunae, apparently from osteolysis.
Figure 3.
Osteopathy of the right pterygoid bone in Hyperoodon ampullatus ANSP-3004: (Left) Occipital view of the upturned skull with (anteriad) the bulbous exostosis seen protruding from the right pterygoid; (Right) close-up view of the osseous mass exposing resorption lacunae, apparently from osteolysis.
Based on available observations, ANSP-3004 presented a marked ventral cranial malformation linked to a severe bulbous exostosis enveloping the right pterygoid, of unknown aetiology.
Table 1.
Cranial measurements of female Hyperoodon ampullatus ANSP-3004 compared to two mature females (MCZ-25361 & RBINS-1503). NA= not available.
Table 1.
Cranial measurements of female Hyperoodon ampullatus ANSP-3004 compared to two mature females (MCZ-25361 & RBINS-1503). NA= not available.
Lesions of the skull are poorly documented in the Ziphiidae family, with only one brief mention of osteolysis of the rostrum tip (premaxillary-maxillary bones) in an adult male Peruvian or lesser beaked whale Mesoplodon peruvianus Reyes, Mead and Van Waerebeek, 1991 stranded in Peru and curated at the Museo de Delfines, Lima, as MJE-001 (Montes et al. 2004, Reyes & Van Waerebeek 2018). The case described here represents the first record of an osteopathy of the pterygoids in ziphiids. Nematodes of the genus Crassicauda commonly infest the cranial sinuses of several Delphinidae species and cause trabecular or basket-like lytic lesions in the pterygoids, referred to as crassicaudiasis (Raga et al. 1982, Van Bressem et al. 2020). However, the pathology observed in ANSP-3004 bears no resemblance to those. Besides, these helminth parasites have never been reported in the cranial sinuses of Ziphiidae, although they have been found in their vascular and genito-urinary systems (Tajima et al. 2015, Díaz-Delgado et al. 2016). A possible condition that may explain the osteopathy documented in the right pterygoid of ANSP-3004 includes osteoma, a benign, slow growing tumor that is ‘histologically similar to ossifying fibroma, fibrous dysplasia, heterotopic ossification and exostosis’ (Blood & Studdert 1999). In veterinary practice, osteomas are seen most commonly on jaws and in the nasal sinuses of horses and cattle (Blood and Studdert 1999). The latter (Bovinae) are closely related to Cetacea phylogenetically and share the same Order (Artiodactyla). Chronic osteitis following parasitic, fungal or bacterial sinusitis (Dagleish et al. 2007, Belda et al. 2018), or a combination thereof, may have contributed to exostosis formation, but is not thought to be the primary aetiology. A definitive diagnosis could not be reached as destructive sampling and histologic analysis of the exostosis was not permitted on this intangible, historical specimen.
The thick and robust pterygoid bones of beaked whales enclose large sinuses, each approximately a litre in volume in H. ampullatus (Scholander 1940), lined with a rich venous plexus that play an important role in deep diving (Rommel et al. 2006, Costidis & Rommel 2012). The malformation, including the severe bulbous exostosis in the right pterygoid, a partial obstruction of the right internal nare as well as a reduction of the left pterygoid width may have affected respiration and diving to some degree. However, the impact of this osteopathy on deep diving, and thus foraging (Rommel et al. 2006), appeared still inconsequential at the time of capture, considering that the female was able to nurse a calf. No other data were available that would allow an assessment of the general health status of both mother and calf.
While case studies as this one are very useful because yielding initial insights, what is needed are systematic future studies of diseases and traumas in representative samples of beaked whale skeletal material, available in many marine mammal collections. Such research should provide insights on the aetiology, prevalence and severity of ziphiid osteopathies, and help elucidate individual and potential population impact.
Acknowledgements
We thank Ned Gilmore from the Academy of Natural Sciences of Drexel University for providing access to the main study specimen as well as supplying images and measurements. P. Kamminga (Naturalis Biodiversity Center, Leiden) is thanked for supplying photos of healthy H. ampullatus skulls as comparative material. We thank Kyle Luckenbill (Academy of Natural Sciences of Drexel University) for 3D scanning the study specimen and Drs Barbara Grandstaff (University of Pennsylvania) and Allison Tumarkin-Deratzian (Temple University) for taking additional photos and measurements. We kindly thank Dr. O. Lambert from the Royal Belgian Institute of Natural Sciences for sharing images of northern bottlenose whales, for helping with measuring specimen RBINS-1503 and providing information. Lastly, we are most grateful to Mark Omura (Harvard Museum of Comparative Zoology) for supplying photos and measurements from specimen MCZ-25361. CMED-CEPEC are volunteer study groups.
Conflict of interest
The authors declare no conflicting interests related to any aspect of this case report.
References
- Barcellos LP (1977) Nota sobre osteopatologia em um exemplar de Hyperoodon planifrons (Ziphiidae–Cetacea), Rio Grande do Sul, Brasil. Atlantica 2(1):118–123.
- Belda B, Petrovitch N, Mathews KG (2018) Sinonasal aspergillosis: Outcome after topical treatment in dogs with cribriform plate lysis. J Vet Intern Med 32: 1353–1358. [CrossRef]
- Blood DC, Studdert VP (1999) Saunders Comprehensive Veterinary Dictionary. 2nd Edition. WB Saunders, London. 1380pp.
- Cope ED (1869) Of the species of the cetaceans of the west coast of North America. Proc Acad Nat Sci Phila 21: 14-32.
- Costidis A, Rommel SA (2012) Vascularization of air sinuses and fat bodies in the head of the bottlenose dolphin (Tursiops truncatus): morphological implications on physiology. Front. Physiol. 3: 1-23. [CrossRef]
- Dagleish MP, Barley J, Howie FE, Reid RJ, Herman J, Foster G (2007) Isolation of Brucella species from a diseased atlanto-occipital joint of an Atlantic white-sided dolphin (Lagenorhynchus acutus). Vet Rec 160: 876–878.
- Dagleish M, Foster G, Howie F, Reid RJ, Barley JP (2008) Fatal mycotic encephalitis caused by Aspergillus fumigatus in a northern bottlenose whale (Hyperoodon ampullatus). Vet Rec 163: 602-604.
- Díaz-Delgado J, Fernández A, Xuriach A, Sierra E and others (2016) Verminous Arteritis Due to Crassicauda sp. in Cuvier’s Beaked Whales (Ziphius cavirostris) Vet Pathol 53:1233-1240. [CrossRef]
- Feyrer LJ, Stewart M, Yeung J, Soulier C and Whitehead H (2021) Origin and Persistence of Markings in a Long-Term Photo-Identification Dataset Reveal the Threat of Entanglement for Endangered Northern Bottlenose Whales (Hyperoodon ampullatus). Front Mar Sci 8: 620804. [CrossRef]
- Flower, W.H. (1882) On the whales of the genus Hyperoodon. Proc Zool Soc London 1882: 722-726. [CrossRef]
- Forster, 1770 (on Balaena ampullata Forster, 1770) in Kalm, P. (1770). Travels into North America: containing its natural history, and a circumstantial account of its plantations and agriculture in general, with the civil, ecclesiastical and commercial state of the country, the manners of the inhabitants, and several curious and important remarks on various subjects. 1:1-400, available online at https://www.biodiversitylibrary.org/page/23971020 page(s): 18; note: In the footnote [details].
- Kompanje EJO (1999) Considerations on the comparative pathology of the vertebrae in Mysticeti and Odontoceti; evidence for the occurrence of discarthrosis, zygarthrosis, infectious spondylitis and spondyloarthritis. Zool Med 73: 99−130.
- Mitchell E, Kozicki VM (1975) Autumn Stranding of a Northern Bottlenose Whale (Hyperoodon ampullatus) in the Bay of Fundy, Nova Scotia. J Fish Res Board Can 1975, 32(7): 1019-1040. [CrossRef]
- Montes DI, Chavera AC, Van Bressem M-F, Perales RC, Falcón NP, Van Waerebeek K (2004) Descripción y evaluación anatómica de lesiones óseas cráneo-mandibulares en cetáceos odontocetos del mar peruano. Rev Invest Vet Perú 15: 13-24.
- Moors-Murphy HB (2018) Bottlenose Whales Hyperoodon ampullatus and H.planifrons. In: Encyclopedia of Marine Mammals. pp. 130-132. (eds. B. Würsig, J.G.M. Thewissen and Kovacs, K.M. Academic Press, Third Edition.
- Raga JA, Casinos A, Filella S, Raduan MA (1982) Notes on cetaceans of the Iberian Coasts. V. Crassicauda grampicola Johnston and Mawson, (1941 Nematoda), cause of injuries in the pterygoids of some specimens of Grampus griseus. Saugetierkdl Mitt 30: 315−318.
- Reyes JC, Mead JG, Van Waerebeek K (1991) A new species of beaked whale Mesoplodon peruvianus sp.n. (Cetacea: Ziphiidae) from Peru. Mar Mamm Sci 7: 1-24. [CrossRef]
- Reyes JC and Van Waerebeek K (2018) The Lesser beaked whale Mesoplodon peruvianus Reyes, Mead & Van Waerebeek 1991 revisited, with biological observations on new specimens from Peru. Journal of Marine Biology & Oceanography 7(4): 1-10. [CrossRef]
- Rommel SA, Costidis AM, Fernandez A, Jepson PD and others (2006) Elements of beaked whale anatomy and diving physiology and some hypothetical causes of sonar-related stranding. J Cetacean Res Manage 7 (3):189–209.
- Scholander PF (1940) Experimental investigations on the respiratory function in diving mammals and birds. Hvalrad Skr 22: 1-131.
- Slijper EJ (1938) Die Sammlung rezenter Cetacea des Musée Royal d’Histoire Naturelle de Belgique. Bull Mus R Hist Nat Belg 14 (10) :1-33.
- Tajima Y, Maeda K, Yamada TK (2015) Pathological findings and probable causes of the death of Stejneger’s beaked whales (Mesoplodon stejnegeri) stranded in Japan from 1999 and 2011. J Vet Med Sci 77: 45-51. [CrossRef]
- Taylor BL, Baird R, Barlow J, Dawson SM and others (2008) Hyperoodon ampullatus. The IUCN Red List of Threatened Species 2008: e.T10707A3208523. [CrossRef]
- Tomilin AG (1967) Cetacea. Mammals of the USSR and adjacent countries. 717 p. (Transl. from Russian by Israel Program for Sci Transl, Jerusalem No. 1124.
- Van Bressem M-F, Van Waerebeek K, Aznar FJ, Raga JA and others (2009) Epidemiological pattern of tattoo skin disease: A potential general health indicator for cetaceans. Dis Aquat Org85, 225–237. [CrossRef]
- Van Bressem M-F, Duignan P, Raga JA, Van Waerebeek K, Fraija-Fernández N, Plön S (2020) Cranial crassicaudiasis in two coastal dolphin species from South Africa is predominantly a disease of immature individuals. Dis Aquat Org 139:93-102. [CrossRef]
- Whitehead H, Hooker SK (2012) Uncertain status of the northern bottlenose whale Hyperoodon ampullatus: population fragmentation, legacy of whaling and current threats. Endanger Species Res 19(1): 47-61. [CrossRef]
- Whitehead H, Macleod CD, Rodhouse P (2003) Differences in niche breadth among some teuthivorous mesopelagic marine mammals. Mar Mamm Sci 19(2): 400-405.
- Whitehead H, Reeves R, Feyrer L, Brownell Jr. RL (2021) Hyperoodon ampullatus. The IUCN Red List of Threatened Species 2021: e.T10707A50357742. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).