Introduction
One of the best-studied functions of p53 is the induction of apoptosis. P53 performs this task by directly activating the expression of many pro-apoptotic genes that encode proteins located at various points of programmed-cell-death signaling cascades starting either from death receptors (extrinsic pathway) or from the detectors of internal cellular stress like DNA damage (intrinsic or mitochondrial pathway) (reviewed by Aubrey et al.).
The apoptosis triggered by death receptors plays important role in functioning of the immune system, e.g. the cytotoxic lymphocytes or natural killer cells destroy their targets (e.g. incipient cancer cells) by stimulating death receptors on their surface. The death receptors are characterized by extracellular domain, which binds to their cognate ligands and by intracellular “death domain”, recruiting various proteins that mediate cell death. One of these proteins is called FADD. It binds to the death domain of receptors and to the death effector domain of inactive caspase-8. This aggregate of death receptor, FADD and caspase-8 is called DISC (death-inducing signaling complex). Through a complicated process, the inactive caspase-8 in DISC is cleaved into smaller fragments and forms active caspase-8 consisting of two smaller and two larger subunits. In some cell types (e.g. immune cells), the activation of death receptors leads to the formation of so many active caspase-8 molecules, that they are able to directly activate caspase-3, which is the major executioner of apoptosis. In other cell types (e.g. hepatocytes), the active caspase-8 in order to trigger apoptosis must first engage the intrinsic, mitochondrial pathway with all its consequences: increased permeability of the outer mitochondrial membrane, release of cytochrome c, formation of apoptosome, activation of caspase-9 and finally the activation of caspase-3 (reviewed by Fouque et al., Green and Llambi).
One of the death receptor genes activated by p53 is called FAS. The activation of FAS expression is a part of the pro-apoptotic, p53-dependent transcriptional program. A previous study demonstrated that anticancer drugs can sensitize cancer cells to FAS-mediated cytotoxicity. Anticancer drugs can sensitize cancer cells to the FAS agonist by activating p53, which in turn promotes the expression of the FAS receptor and probably other pro-apoptotic proteins.
In our previous work, we demonstrated that two p53 activators, actinomycin D and nutlin-3a synergize in activation of p53 and consequently in inducing expression of some p53-regulated genes. The mechanism of this synergy is not known. We hypothesize that nutlin-3a (Nut3a), which antagonizes the negative regulator of p53 – the MDM2 protein, makes p53 a better target for activating kinases triggered by actinomycin D (ActD). Whatever the mechanism, in A549 lung cancer cells, the co-treatment with ActD+Nut3a strongly stimulates the expression of many p53-regulated pro-apoptotic genes including FAS receptor gene and caspase-10 gene. However, despite very strong upregulation of pro-apoptotic genes, the apoptotic cells in the cell population exposed to ActD+Nut3a are infrequent. Apparently, a crucial trigger for apoptosis is missing. We started the current work by testing the hypothesis that co-treatment with ActD+Nut3a sensitizes the cancer cells, in a p53-dependent manner, to the apoptosis triggered by FAS ligand (FASLG).
Materials and Methods
Cell Culture and Treatment
We used early passage cell lines obtained from ATCC. A549 cells (RRID: CVCL_0023, lung adenocarcinoma, American Type Culture Collection [ATCC]), and U-2 OS cells (RRID: CVCL_0042, osteosarcoma, ATCC) cells were cultured in low glucose DMEM supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). NCI-H460 (RRID: CVCL_0459, lung cancer, ATCC) were cultured in RPMI-1640 supplemented with 2 mM L-glutamine, 4.5 g/L glucose and 1 mM sodium pyruvate and 10% heat inactivated FBS. AGS cells (RRID:CVCL_0139, gastric adenocarcinoma, ATCC) were cultured in McCoy′s 5A supplemented with 10% fetal bovine serum. Normal human fibroblasts (GM07492, Coriel Cell Repositories, Camden, NJ) of early passage were cultured in low glucose DMEM supplemented with 15% FBS. All cells were cultured at 37°C/5% CO2 and all media were supplemented with a 1% penicillin/streptomycin solution.
The stock solutions of chemicals were prepared in DMSO: actinomycin D (10 μM; Sigma-Aldrich, St. Louis, MI, USA), nutlin-3a (10 mM; Selleck Chemicals LLC, Houston, TX, USA), idasanutlin and RG7112 (10 mM; MedChemExpress LLC, Monmouth Junction, NJ, USA). The stock solution of etoposide (20 mg/ml, 34 mM, concentrate for solution for infusion) was from Accord Healthcare Poland, Warsaw. Stock solutions were diluted in culture medium at the following concentrations: 5 nM ActD, 5 μM Nut3a, 5 μM RG7112, 5 μM Idasanutlin, 5 μM etoposide as indicated in the Results section. Control cells were mock-treated with medium containing DMSO. The human FAS ligand (His Tag, active trimer, cat. no. FAL-H5241) was purchased from Acro Biosystems (Newark, DE, USA). To prepare the stock solution, the lyophilized product was reconstituted in deionized water to a stock solution of 1000 μg/ml.
Live-Cell Imaging
The cells were seeded on chambered coverglases. The next day, they were exposed to ActD+Nut3a or DMSO (Control - mock-treated). After 48h incubation the media (with ActD+Nut3a or control) were removed and the cells were exposed to FASLG (100 ng/ml) or control medium. Subsequently, the dish with cells was transferred to a heating insert with CO2 cover on the stage of inverted microscope (ZEISS Elyra 7 with Lattice SIM²). Images were taken from the same field using a 20x objective. The images were acquired using standard software (ZEN black edition, Carl Zeiss), then images were processing using ZEN 3.1 (ZEN Lite, Carl Zeiss).
Cell Staining on Culture Plates
An equal number of cells were seeded on 12-well culture plates at a calculated 10% confluence. After overnight incubation, cells were treated with indicated drugs or mock-treated for approximately 45 hours. Subsequently, fresh culture medium (with or without FASLG) was added and the cells were incubated for 5h. After this, the medium was changed again and the cells were allowed to recover for 24-70 hours as indicated in the Results. The medium was removed and the cells were fixed by incubation with -20oC methanol for 10 min. Dried cells were stained with 0.01% crystal violet for 2 min. Finally, the cells were washed by distilled water, dried, and the plates were scanned.
Colorimetric Cell Viability Assay
A549 cells were seeded (5 000/well) on 96-well plates. After drug treatment (ActD, ActD+Nut3a, Nut3a) for 45h, the cells were either mock-treated or incubated with FASLG (at indicated concentrations for 5h). After this time, the medium was replaced for 24 h a recovery time. Next, cell viability was determined using CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) (Promega) kit according to the manufacturer’s protocol. The absorbance was read at 490 nm using a microplate reader. Data were normalized to control (no drug or FASLG treatment). Four independent biological replicates were performed.
Western Blotting
Whole-cell lysates were prepared using an IP buffer, supplemented with protease and phosphatase inhibitors as described previously. Aliquots of lysates (15–40 µg) were separated by SDS-PAGE on 8% or 13% gels and electro-transferred onto PVDF membranes. Before incubation with primary antibody, the membranes were incubated for 1h at room temperature in blocking solution (5% skim milk in PBS with 0.1% Tween-20). Anti-cleaved caspase-3 (Asp175) (5A1E) rabbit mAb, anti-caspase-8 (1C12) mouse mAb, anti-caspase-9 polyclonal rabbit Ab anti-FAS rabbit mAb (C18C12) and anti-Apaf-1 (D5C3) rabbit mAb were from Cell Signaling Technology (Danvers, MA, USA). The anti-caspase-10 rabbit polyclonal antibody and anti-caspase-6 mouse monoclonal antibody were from Proteintech (Rosemont, IL, USA). Anti-p53 (DO-1), and loading control anti-HSC70 (B-6) antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA). All incubations with primary antibodies were performed overnight at 4oC in blocking solution. HRP-conjugated secondary antibodies (anti-mouse, anti-rabbit) were detected by chemiluminescence (SuperSignalWest Pico or SuperSignal West Femto Chemiluminescent substrate, Thermo Fisher Scientific, Waltham, MA, USA).
Flow Cytometry
The apoptotic cells were analyzed using PE Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to manufacturer's protocol using BD FACSCanto™ cytometer (Becton Dickinson, San Jose, CA, USA). The data were analyzed using BD FACSDiva™ software. The expression of FASLG (CD95L) on cell surface was determined using BD Pharmingen™ APC Mouse Anti-Human CD95 (Becton, Dickinson and Company, NJ, USA) and APC Mouse IgG1 kappa Isotype Control (P3.6.2.8.1) (eBioscience™, San Diego, USA).
Statistical Analysis
The graphs and statistical significance of the differences analyzed in cell viability or death (apoptosis/necrosis) was calculated using GraphPad Prism version 10.0.0 for Windows, GraphPad Software, Boston, Massachusetts USA,
www.graphpad.com. The employed tests and the level of significance are presented in the Results.
Results
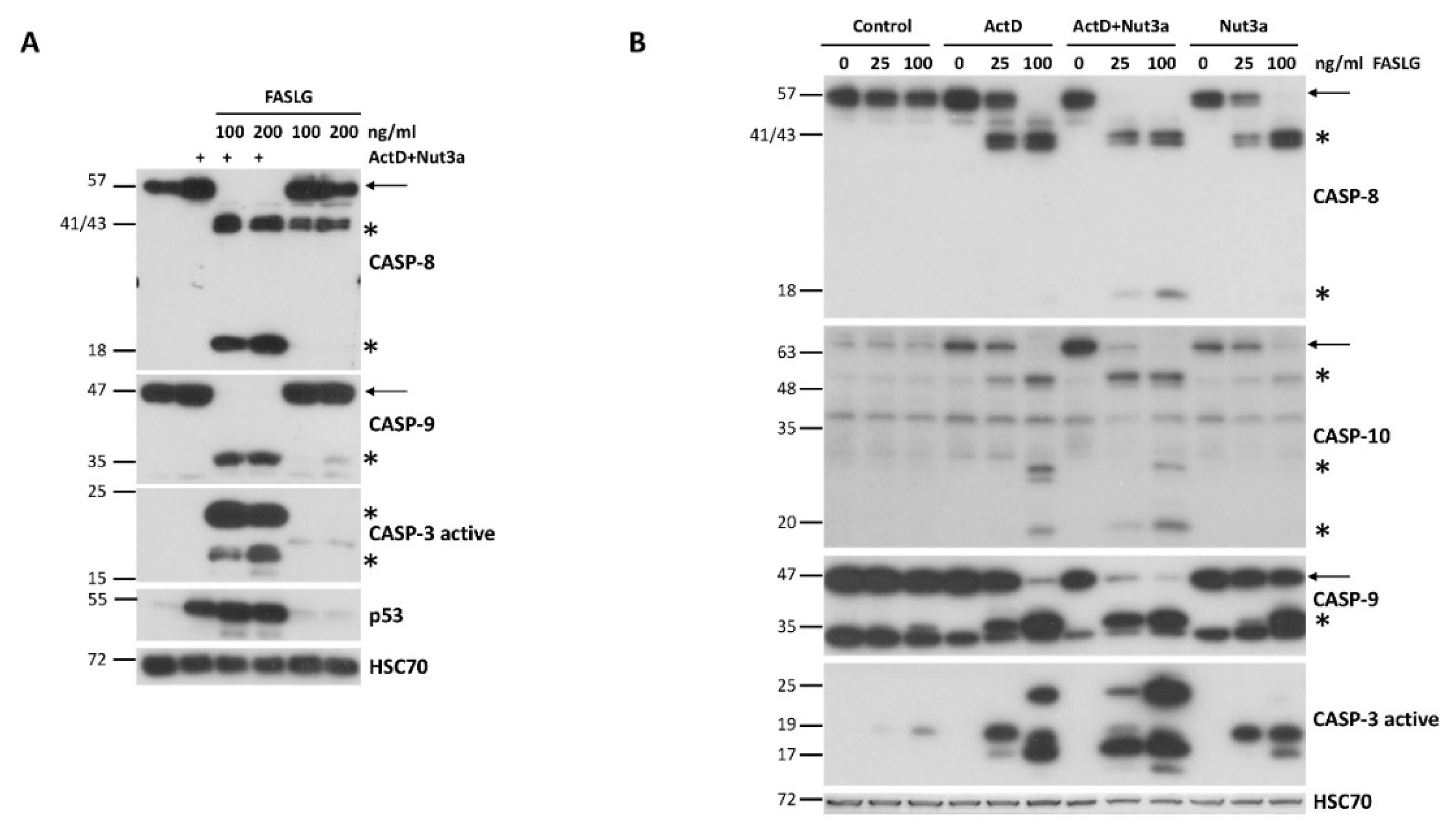
Most cancer cells are resistant to FAS-mediated apoptosis. To find out if we could break this resistance, we pre-exposed A549 lung cancer cells to ActD and Nut3a for 48h and next we treated them for 2.5h with FASLG at 100 or 200 ng/ml concentrations. Expectedly, strong accumulation of p53 appeared, which is concomitant with its activation in cells exposed to ActD+Nut3a (
Figure 1A). In cells pre-exposed to ActD+Nut3a, FASLG induced splitting of caspase-8 (57 kDa) into cleaved intermediates (41 and 43 kDa) and into an active fragment (18 kDa) (
Figure 1A). It was associated with the disappearance of full-length caspase-8 suggesting its activation in most cells. This implies that in the majority of cells the apoptosis was initiated. To find out if the intrinsic pathway of apoptosis was also engaged, we examined the activation status of caspase 9. Only the cleaved product of caspase-9 (p35) was detected in cells pre-treated with ActD+Nut3a and exposed to FASLG and no full length protein (p47) was observed (
Figure 1A). This again suggests that apoptosis was initiated in the majority of cells and that the intrinsic apoptotic pathway was activated. We also observed the activation of caspase 3 (17 and 19 kDa fragments). The active caspase 3 appeared only in cells pre-exposed to ActD+Nut3a and treated with FASLG. Exposure of cells only to ActD+Nut3a did not result in detectable activation of either of the examined caspases. Exposure to FASLG alone induced only the appearance of cleaved intermediate of caspase-8 (p41 and p43), indicating that the ligand-mediated apoptosis was initiated, but in most cells it did not reach the stage of activation of caspase-9 or caspase-3, which is consistent with the observations that cancer cells are generally resistant to FASLG-initiated apoptosis.
To find out whether the sensitization to FASLG-induced apoptosis results from the synergistic activity of ActD and Nut3a, we exposed A549 cells either separately to each compound or simultaneously to both of them. After pre-exposure, cells were treated for 2h with FASLG at two concentrations (25 and 100 ng/ml). Subsequently, we examined the activation status of caspase-8, caspase-9 and caspase-3 (
Figure 1B). We observed strong synergy between ActD and Nut3a in sensitizing the cells to FASLG. ActD or Nut3a acting separately sensitized cells to apoptosis induced by 25 ng/ml concentration of FASLG, however, the level of caspase activation was small as compared with ActD+Nut3a pre-treatment (compare the level of full-length caspases 8 and 9). The expression of activated caspase-3 was also higher in cells pre-exposed to ActD+Nut3a (
Figure 1B). Both, actinomycin D and nutlin-3a acting separately sensitized the cells to high concentration (100 ng/ml) of FASLG, but again the effect was much stronger when both compounds acted together. A similar synergy between actinomycin D and nutlin-3a in sensitizing cells to the pro-apoptotic effect of FASLG was observed in three other cell lines with wild-type p53: AGS, NCI-H460 and U-2 OS (
Supplementary Figures, S1 and S2).
Next, we examined the expression level of caspas-10 (
Figure 1B), which is a paralog of caspase-8, is also involved in the death receptor pathway of apoptosis and was found in our transcriptomic data to be activated by ActD+Nut3a in various cancer cell lines. Since caspase-10 gene (
CASP10) is activated by p53, we hypothesized that treatment with ActD+Nut3a promotes the expression of this protein. Our assumption was correct. Actinomycin D and nutlin-3a acting separately stimulated the expression of full-length caspase-10, but when combined, both substances acted in this regard at least additively (
Figure 1B). The addition of FASLG reduced the expression of full-length caspase-10 and resulted in appearance of cleaved forms with the size of approximately 50, 30 and 20 kDa. Regarding cleavage (activation) of caspase-10, actinomycin D and nutlin-3a acted synergistically. FASLG at 25 ng/ml concentration did not reduce the level of full-length protein in cells pre-exposed to actinomycin D or nutlin-3a but in cells pre-exposed to ActD+Nut3a the amount of full-length caspase-10 dropped significantly (
Figure 1B). Therefore, ActD+Nut3a promoted the expression of caspase-10, which was activated after exposure to FASLG.
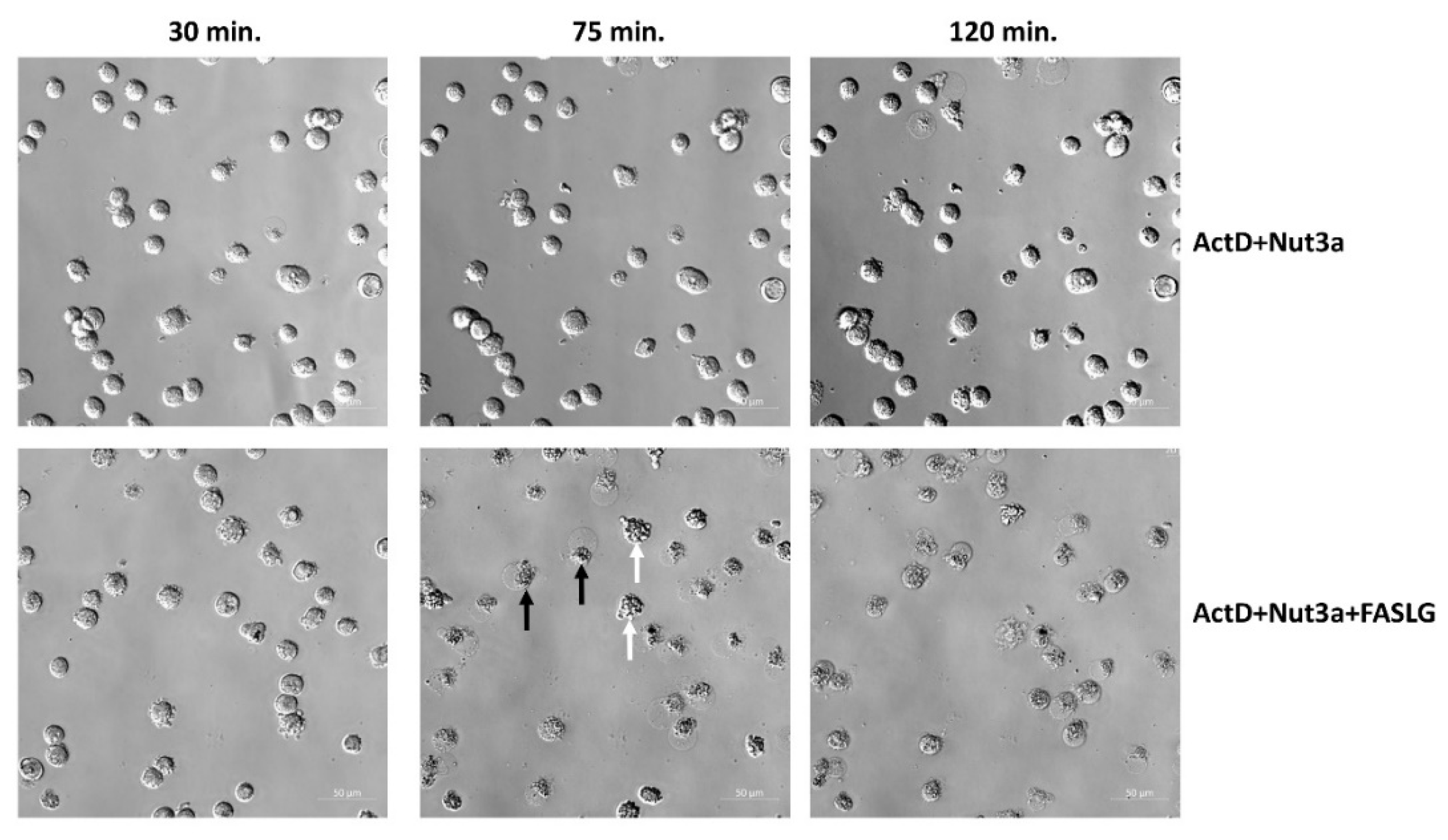
To observe the morphology of cell death, we performed live-cell imaging. As shown in
Figure 2, after 75 min. of incubation with FASLG most cells pre-exposed to ActD+Nut3a show either sign of early apoptosis or late apoptosis/necrosis. In cell population pre-exposed to ActD+Nut3a and subsequently growing in control conditions, cell death was infrequent even after 120 min. This simple observation indicates that the cells pre-exposed to ActD+Nut3a and treated with FASLG die quickly and the death is observed in majority of cells. Similar conclusion can be drawn from live-cell imaging of U-2 OS cells (
supplementary figure, Figure S3).
Next, we used the MTS assay to measure the cells viability (
Figure 3A). The A549 cells were pre-exposed for 45h and subsequently they were treated for 5h with increasing concentrations of FASLG (5, 10 and 25 ng/ml). FASLG acting on untreated cells only marginally reduced their viability (88% of the control for 25 ng/ml). When cells were pre-exposed to ActD+Nut3a and subsequently treated with 25 ng/ml FASLG, their viability dropped to less than 1% of control. Thus, consistent with the Western blotting and microscopy observations, pretreatment with ActD+Nut3a and subsequent exposure to FASLG with at least 25 ng/ml concentration killed most cells. To better illustrate the influence of FASLG, we transformed this graph by setting the viability of cells not exposed to FASLG (control, ActD, ActD+Nut3a or Nut3a) to 100% (
Figure 3B). The effect of synergy between ActD and Nut3a on the sensitization to FASLG was best visible at 25 ng/ml concentration. When cells were pre-exposed either to actinomycin D or nutlin-3a, the addition of FASLG reduced viability to approximately 65%, whereas when two compounds were combined, FASLG reduced cell viability to 0.8%. To visualize this effect macroscopically, the experiment was performed on cells seeded on to 12-well plate. The results are presented on
Figure 3C. In the columns, cells were either mock-treated or exposed (45h) as shown. Subsequently, in the rows the cells were exposed to FASLG (25 and 100 concentration) for 5h or were left untreated (the top row). After treatment, the cells were left for 70h in fresh medium to allow the surviving cells to regrow. The staining revealed that on the plate pre-exposed either to actinomycin D or nutlin-3a and treated with FASLG (25 ng/ml) the cells reached confluence after recovery, while the cells in the well exposed to ActD+Nut3a and FASLG did not regrow (no visible staining). Thus, overwhelming majority of cells pretreated with ActD+Nut3a and exposed to FASLG at 25 ng/ml concentration did not survive the treatment. A similar effect was observed in other cell lines with wild-type p53 - U-2 OS and NCI-H460 (
Supplementary Figure S4). Another assay helped to estimate that FASLG reduced the clonogenic potential of A549 cells exposed to ActD+Nut3a by a factor of roughly 1000 (
Figure S5).
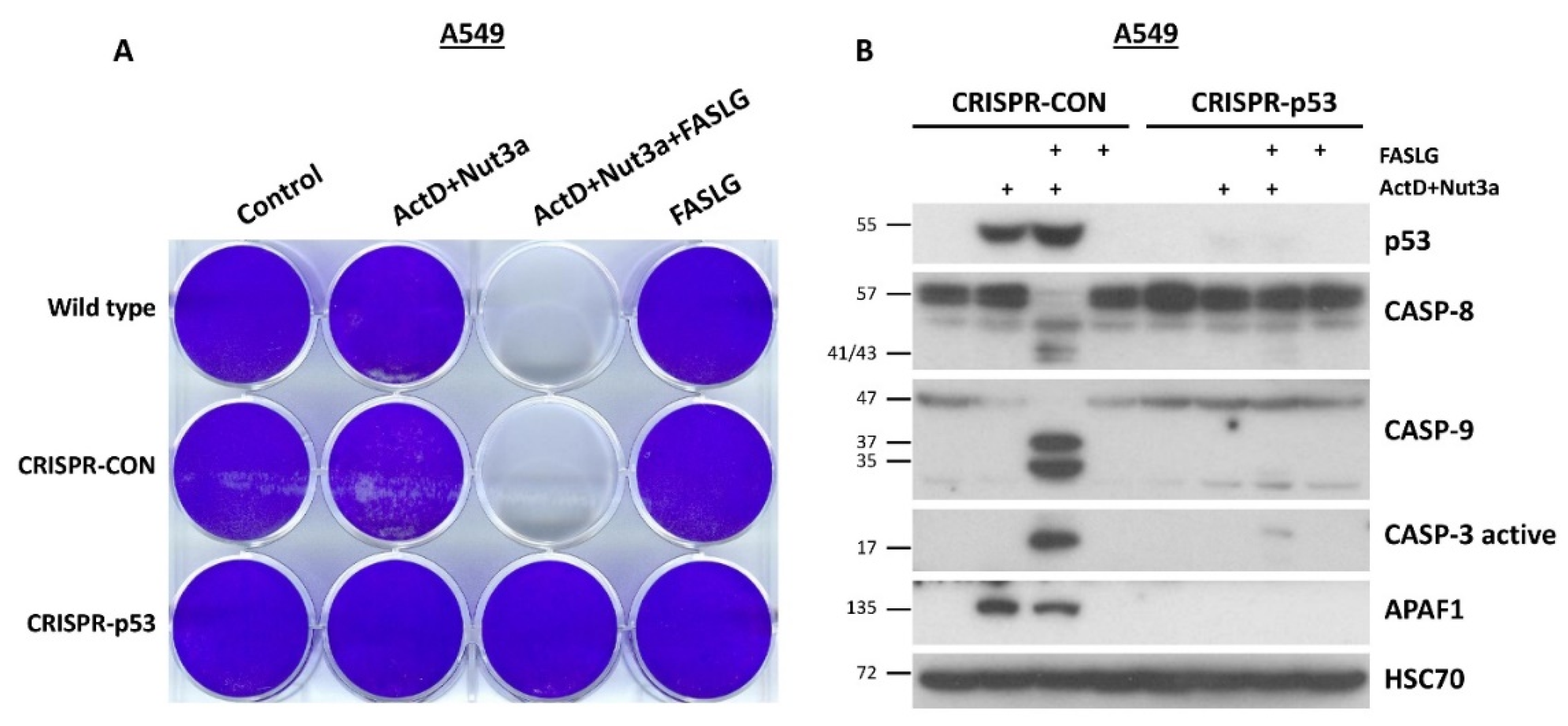
Next, we tested if p53 participates in the sensitization. We started by using the method of cell staining on a 12-well plate. We used parental A549 cells, A549 cells with the expression of p53 knocked-down by CRISPR/Cas9 technology and the controls for knock-down created previously. These cells were seeded in the rows of the plate (
Figure 4A). The cells in columns were mock-treated or exposed to ActD+Nut3a, ActD+Nut3a+FASLG or to FASLG. After the recovery, no cell staining was visible in wells of parental cells or controls exposed to ActD+Nut3a+FASLG, whereas the p53 knockdown cells reached the confluence. Therefore, the sensitizing effect of ActD+Nut3a was strictly dependent on the presence of wild-type p53. Next, we performed a similar experiment; however, the treatment time with FASLG was shorter (2.5h) and cells were collected directly after treatment for cell lysate preparation. The Western blot presented on
Figure 4B shows that the activation of caspases -8, -9 and -3 was detectable only in control cells for knockdown. The APAF1 is a protein coded by p53 regulated gene, which is involved in apoptosome formation and activation of caspase-9 [
3,
12]. The lack of accumulation of APAF1 in p53 knockdown cells exposed to ActD+Nut3a confirms that they have no functional p53. The experiment carried out in NCI-H460 cells with p53 expression knocked down by CRISPR/Cas9 technology using the same method described for A549 cells yielded similar results (
Supplementary Figure S6).
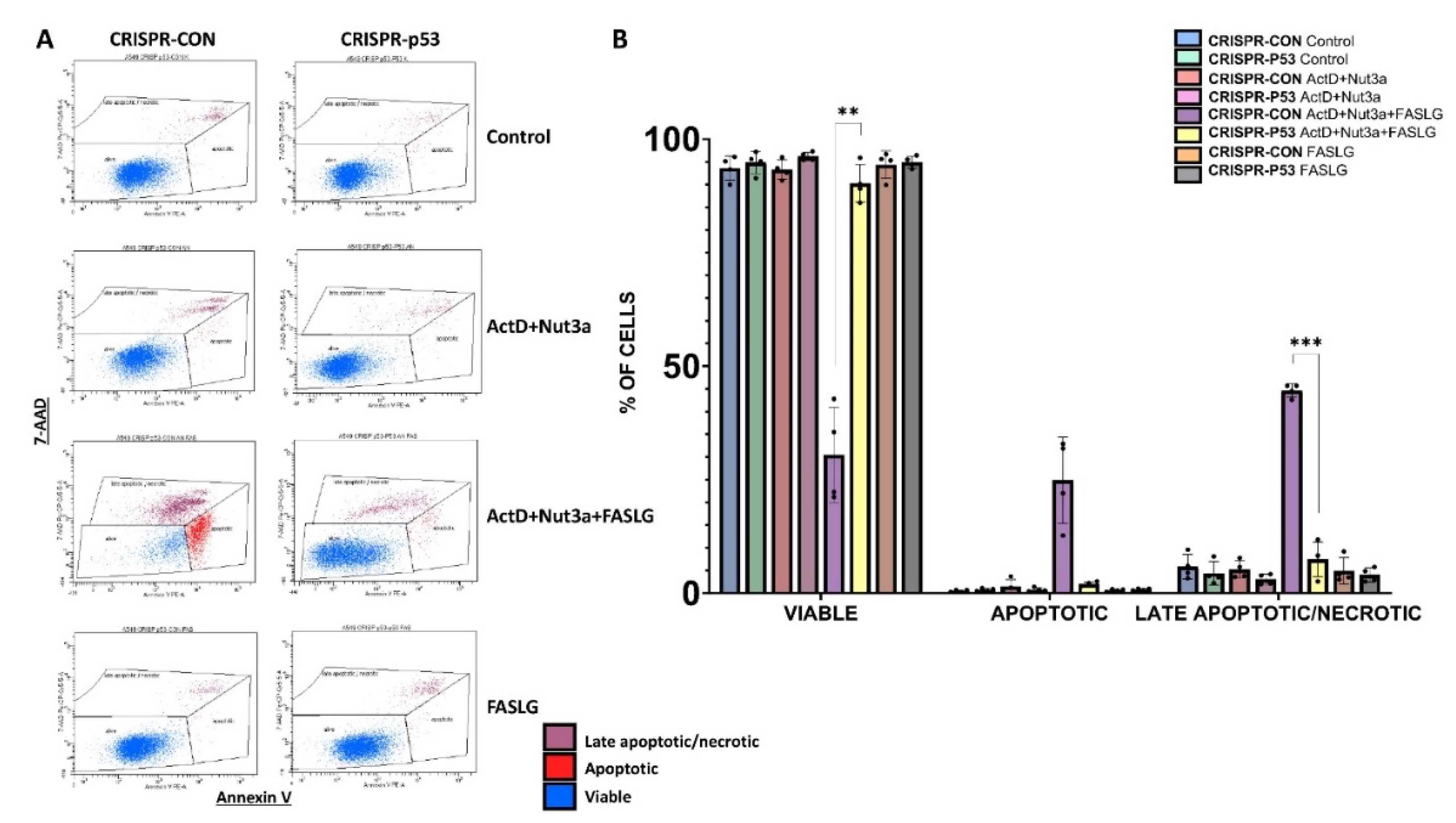
Subsequently, we measured the frequency of apoptotic cells by flow cytometry. The raw data of one biological replicate are presented on
Figure 5A and the graph shows the data from four biological replicates (
Figure 5B). Apoptotic cells were very rare after exposure to ActD+Nut3a. The frequency of both early apoptotic cells (high staining for Annexin V and low staining for 7-AAD) and late apoptotic/necrotic cells (high staining with 7-AAD) increased strongly in cells pre-exposed to ActD+Nut3aand treated with FASLG, but only in the case of p53-proficient cells. Consequently, the frequency of viable p53-proficient cells exposed to ActD+Nut3a+FASLG was significantly lower than in case of p53-deficient cells. This experiment further confirms that p53 is required to induce apoptosis in cells exposed to ActD+Nut3a+FASLG. In this experiment we have more viable cells than in the MTS test, because for cytometric analysis, the cells were exposed to FASLG only for 2.5 hours.
What is the mechanism used by p53 to overcome resistance of cells to FASLG? The data presented on Western blot (
Figure 4B) indicate that p53-deficient cells are not able to activate caspase-8, thus the defect is already present at the beginning of the signaling pathway, which starts with the FAS receptor. Hence, the logical conclusion is that the combination of ActD+Nut3a stimulates the expression of the
FAS gene. Our transcriptomic data show that this is the case (
Figure 6A). However, the synergy of actinomycin D and nutlin-3a in sensitizing cells to FASLG-dependent apoptosis cannot arise from the activation of
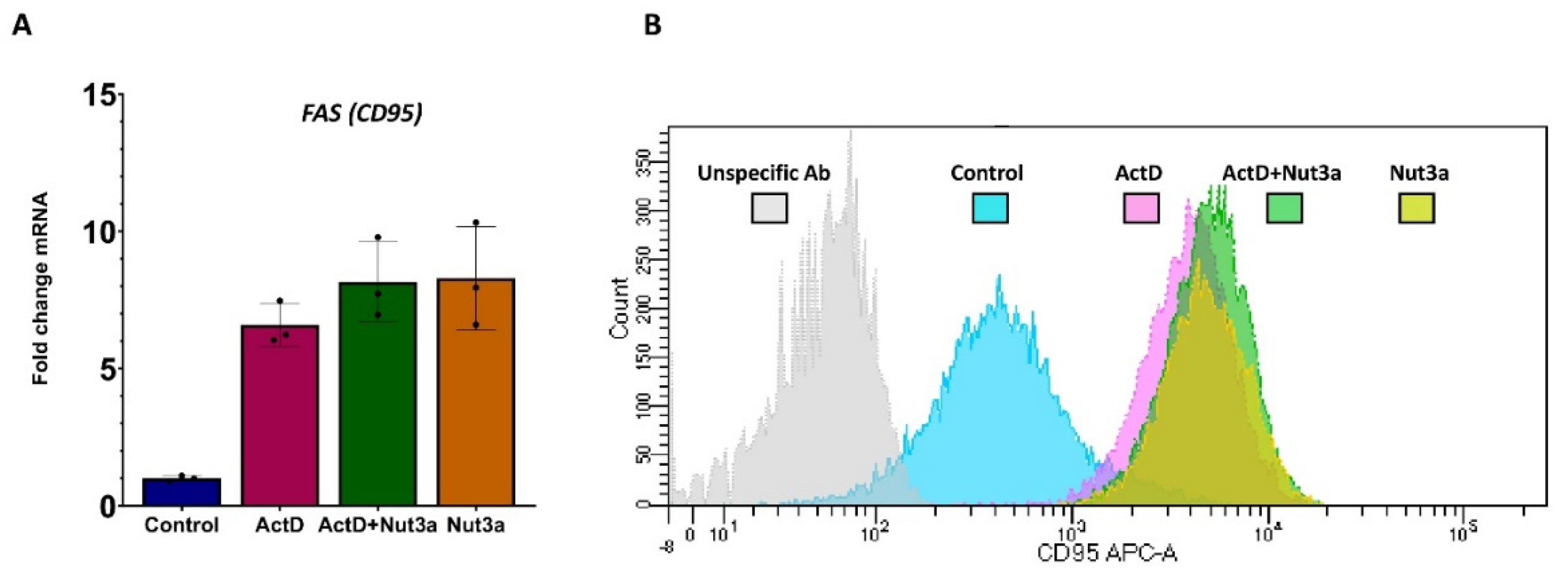
FAS gene because these substances do not synergize in its expression (Fig 6A). The other possibility is that actinomycin D and nutlin-3a synergize in augmenting expression of FAS receptor on the plasma membrane. However, the cytometric measurement of FAS on the cell surface showed its similar expression in all treatment conditions (
Figure 6B).
One of the proteins located on the top of signaling pathway starting from death receptors is the aforementioned caspase-10.
The CASP10 gene is activated by p53. Therefore, by up-regulating caspase-10, p53 can also promote the extrinsic apoptotic pathway. We demonstrated that actinomycin D and nutlin-3a cooperate in upregulation of caspase-10 in A549 cells (
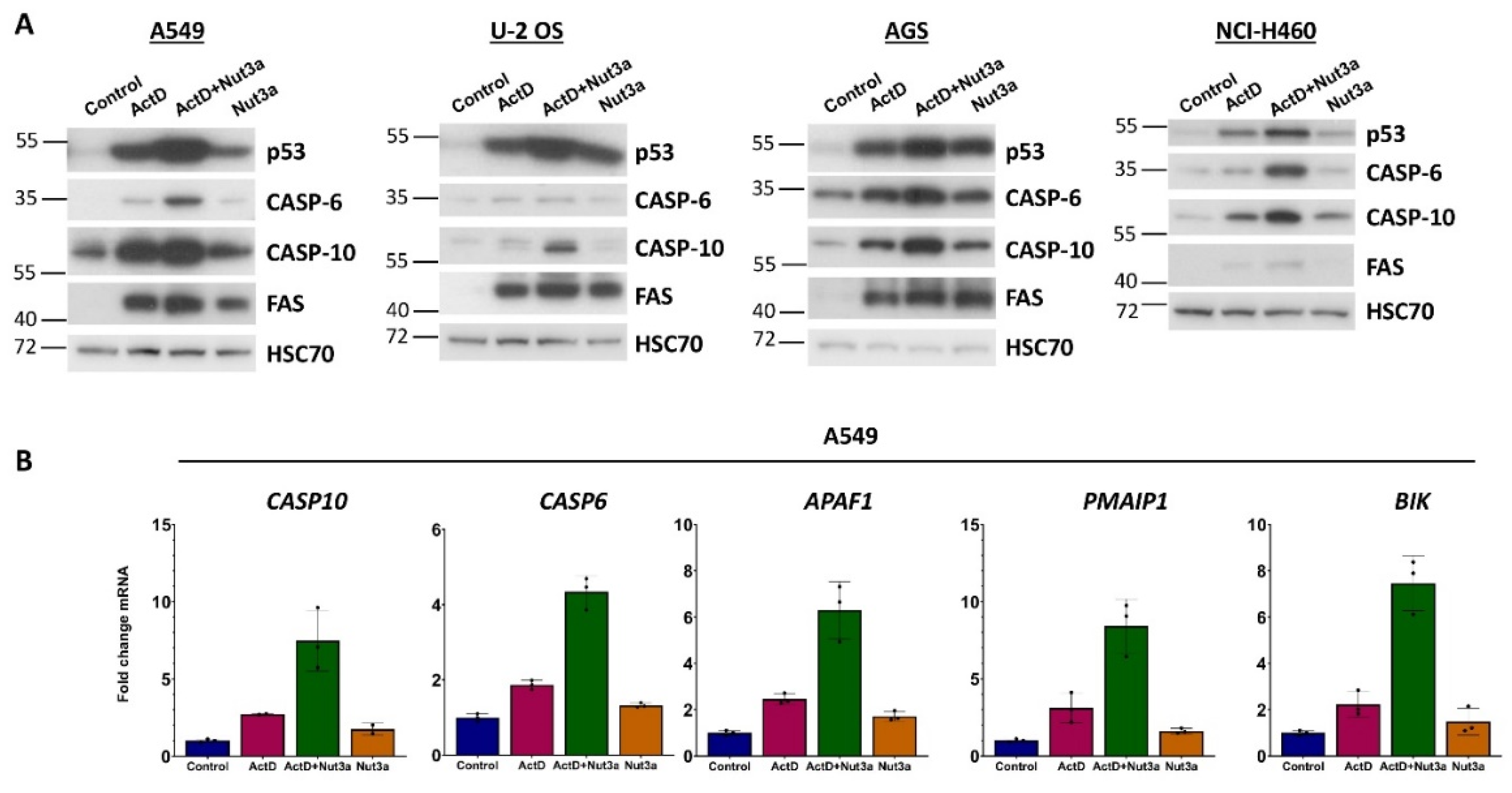
Figure 1B). To find out if similar effect occurs in other cell types, we performed the treatment of various cell lines with wild-type p53. The data are shown on
Figure 6A. In all four cell lines, treatment with ActD+Nut3a leads to accumulation of caspase-10. Moreover, in three cell lines actinomycin D and nutlin-3a do not synergize in upregulation of FAS receptor (
Figure 6A). We also examined the expression of caspase-6, which is coded by the gene (
CASP6) activated by ActD+Nut3a as shown by our transcriptomic data [
7,
8]. Caspase-6 is an executioner caspase but it can also directly activate caspase-8 following activation of mitochondrial pathway of apoptosis, which can create a positive feedback loop. Furthermore,
CASP6 is directly activated by p53. In three cell lines, the amount of caspase-6 increased following exposure to ActD+Nut3a (
Figure 6A). Thus, the upregulation of
CASP6 by treatment with ActD+Nut3a must be considered as a plausible element of increased sensitivity of cells to FASLG.
Our transcriptomic data demonstrated that in A549 cells actinomycin D and nutlin-3a cooperate in activation of
CASP10 and
CASP6 (
Figure 7B), which is consistent with our Western blot results (
Figure 7A). Other proteins may also contribute to the sensitizing effect of actinomycin D and nutlin-3a because these substances synergize (
Figure 7B) in the activation of other p53-regulated, pro-apoptotic genes like
APAF1,
PMAIP1 and
BIK.
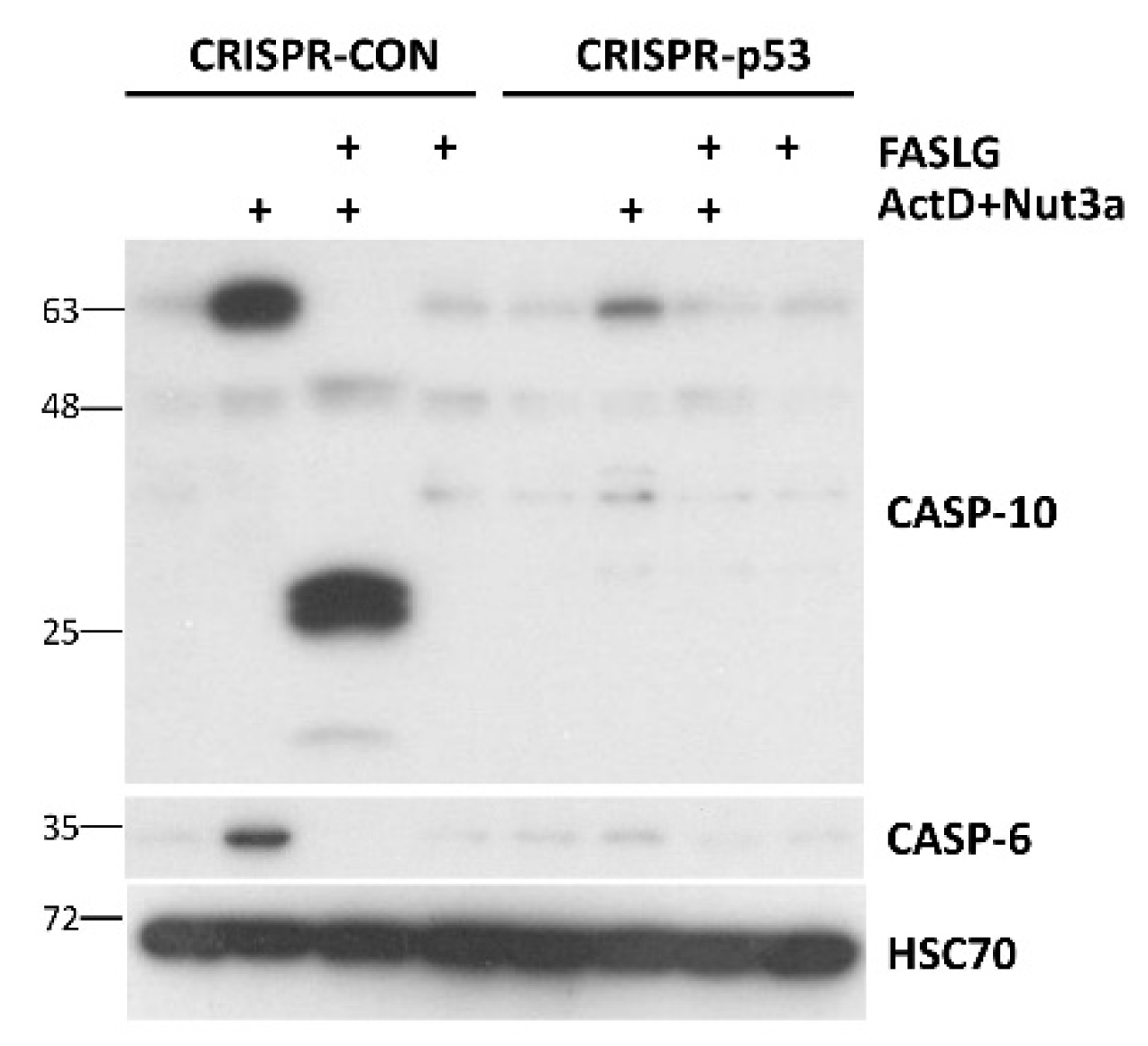
Regulation of
CASP6 and
CASP10 by p53, as mentioned above, was detected by others [
11,
15], however, it was not extensively studied. Our Western blot performed on lysates from p53-proficient and p53-deficient A549 cells (
Figure 8) confirm, that these two genes are activated in a p53-dependent manner. It should be noted that that similar to caspase-10, the full-length caspase-6 disappears in cells exposed to FASLG, probably due to activation by cleavage.
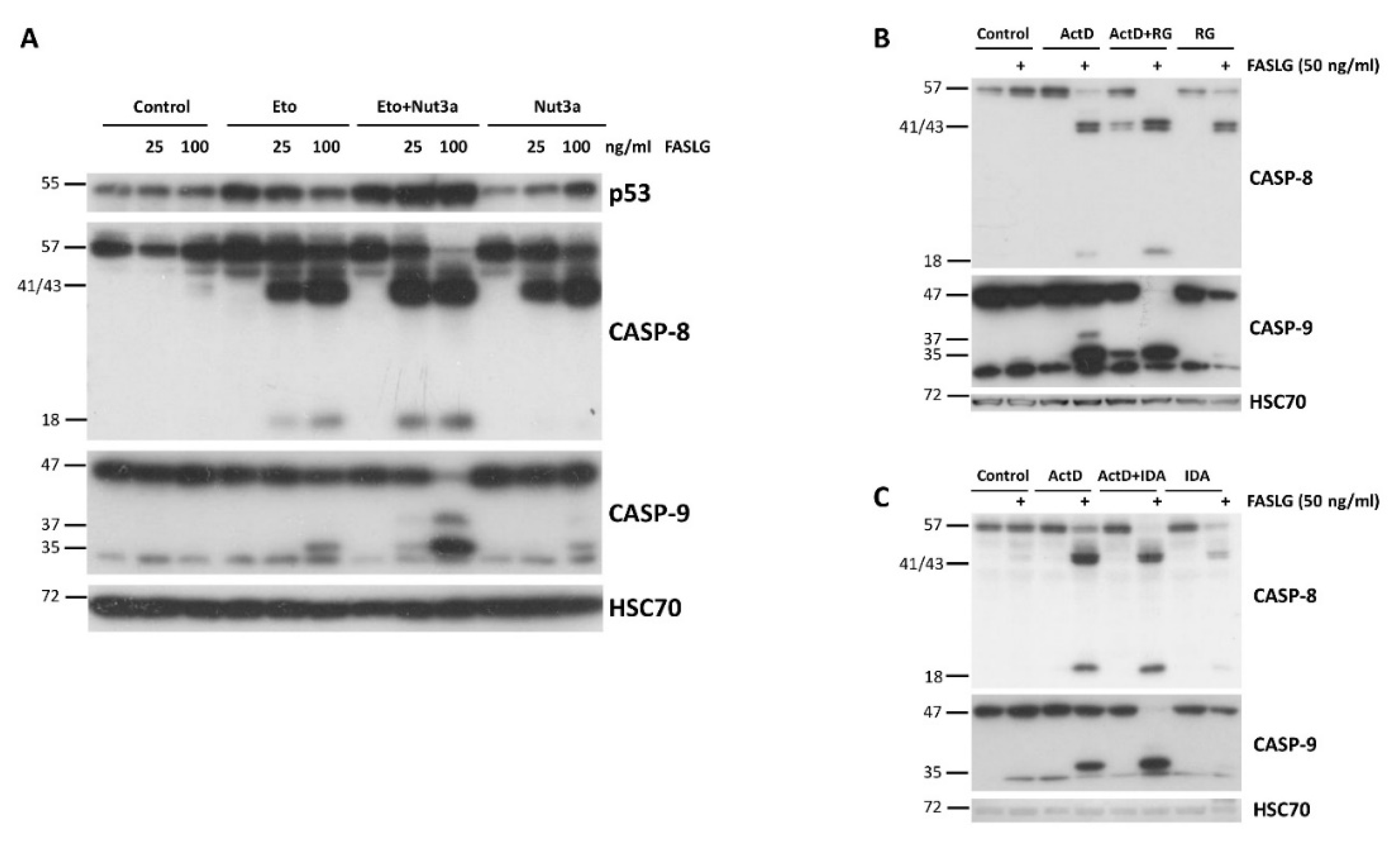
The next experiments were designed to find out if actinomycin D and nutlin-3a can be substituted by other compounds to reach similar, proapoptotic effect. First, we substituted actinomycin D with etoposide (Eto), which can also activate p53. The Western blot shows clear synergy between Eto and nutlin-3a (
Figure 9A). Moreover, we substituted nutlin-3a with other antagonists with p53-MDM2 interaction, idasanutlin or RG7112 [
19,
20]. Their co-incubation with actinomycin D also synergistically sensitized A549 cells to the pro-apoptotic activity of FASLG (
Figure 9B).
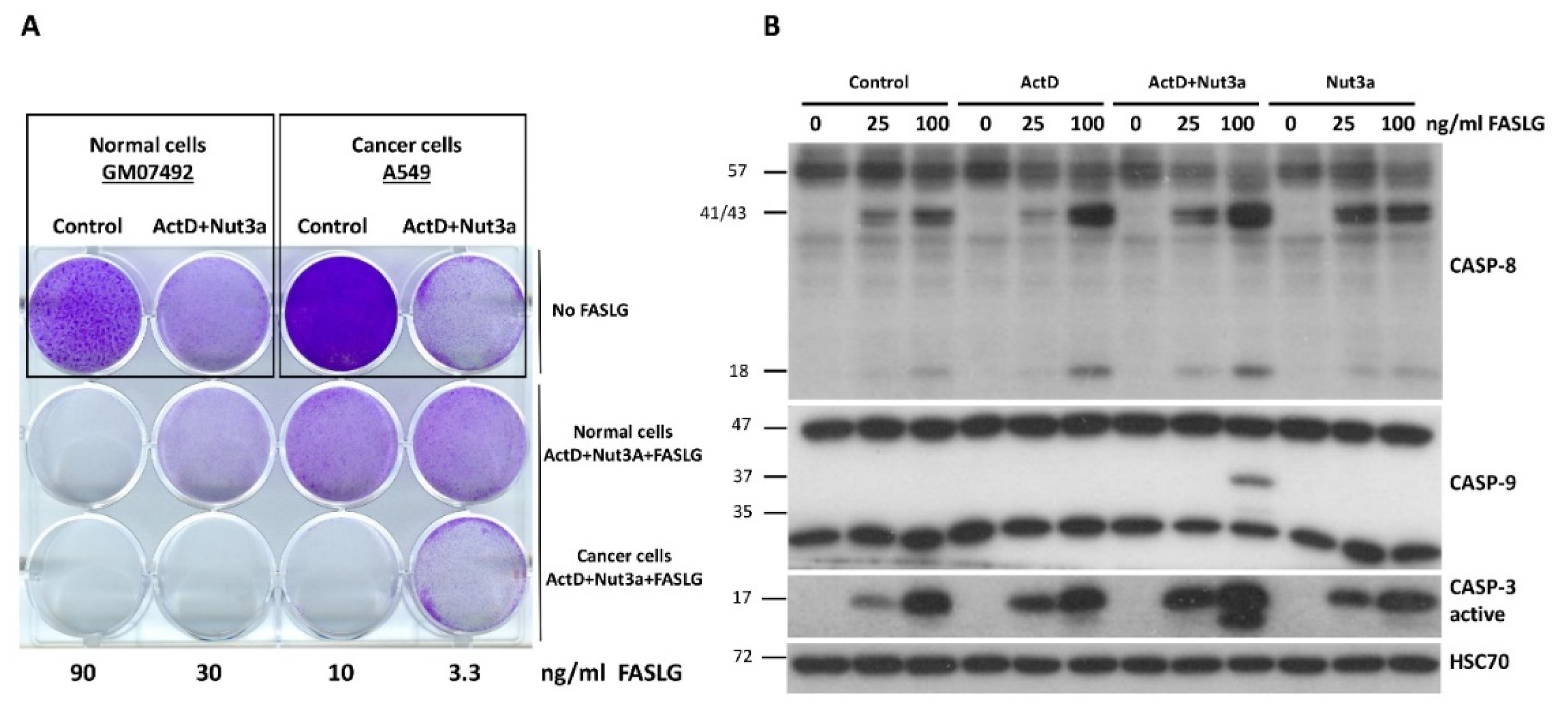
To find out how noncancerous cells react to the treatment with ActD+Nut3a+FASLG, we compared how normal human fibroblasts (GM07492) and lung cancer cells (A549) grow after pre-treatment with ActD+Nut3a with subsequent exposure to FASLG (
Figure 10A). Using cell staining, we noticed that cancer cells were more susceptible to death at the intermediate concentration of FASLG (10 and 30 ng/ml). We could not perform the viability assay because we noticed that these fibroblasts poorly metabolize the MTS used for the test. Western blot performed in cells treated as shown on
Figure 10B, analogously to the treatment of cancer cells presented on
Figure 1B, revealed that there is no strong sensitizing effect of ActD+Nut3a combination on FASLG-induced apoptosis. ActD+Nut3a pretreatment enables FASLG to activate caspase-9 but only when the ligand was applied at a high concentration (100 ng/ml). Moreover, even in these conditions the activation of caspase-9 was not very strong. The activation of caspase-3 was very similar after the addition of FASLG regardless of earlier pre-treatment. Because in these fibroblasts, caspase-3 can be activated by FASLG without caspase-9 activation, these cells apparently belong to the type, which can directly activate caspase-3 by active caspase-8. If other normal cells are also less sensitive to ActD+Nut3a+FASLG combination, there may be a therapeutic window for this treatment modality.
Discussion
In this report we demonstrated that actinomycin D and nutlin-3a synergize to sensitize cancer cells to apoptosis induced by FASLG. This finding may have therapeutic implications. Shortly after the discovery of the pro-apoptotic activity of FASLG, it was considered a candidate anticancer drug. However, an unexpected major obstacle appeared, namely, the FAS agonist antibodies induced death of hepatocytes leading to fulminant hepatitis and death of treated animals. In principle, our method of sensitization of cancer cells to FAS agonists creates an opportunity to use them at lower dose. Experiments with modified FASLG performed by others suggest that preventing hepatotoxicity is feasible [
22,
23]. Moreover, our observations may improve therapeutic application of the antagonists of MDM2-p53 interaction like nutlin-3a, idasanutlin, RG7112 and many other. Their therapeutic use is limited by their inefficiency of killing cancer cells – they mainly induce the cell cycle arrest. However, combined with actinomycin D (or Eto), the antagonists of MDM2 can sensitize cancer cells to the destruction by patient’s own cytotoxic lymphocytes or natural killer cells, which employ FASLG on their surface to induce the death of target cells.
The mechanism of sensitization of cancer cells to FASLG by actinomycin D and nutlin-3a, or similar drug combinations, is not known. It strictly depends on wild-type p53. Apparently, there is something extraordinary about the set of genes regulated by p53 in cells exposed to ActD+Nut3a. Some of these genes make cancer cells extremely sensitive to FASLG. The most obvious hypothesis was that actinomycin D and nutlin-3a synergistically increase the expression of the FAS receptor. However, analysis of its mRNA level, the total amount of FAS protein or its expression on the cell surface did not support this hypothesis (Figs. 6 and 7). We noticed that actinomycin D and nutlin-3a cooperate at least additively in the activation of
CASP10 gene, which encodes caspase-10, the protein that, like caspase-8, also conveys the pro-apoptotic signal from the FAS receptor to executioner caspases. The upregulation of caspase-10 probably contributes to the sensitizing effect but may not be the single factor. In addition to
CASP10, actinomycin D and nutlin-3a cooperate in activation of other p53-regulated, pro-apoptotic genes such as
CASP6,
APAF1,
PMAIP1, BIK and many others [
7,
8]. Thus, it is very likely that there is no single p53 target gene, which is a crucial element of this sensitizing mechanism. Probably, every p53 target gene related to apoptosis contributes partially to the sensitization, however this hypothesis requires further testing. Our recently published transcriptomic data on various cell lines exposed to ActD+Nut3a may help to generate such hypotheses.
Our results clearly indicate that even strong activation of many p53-regulated, pro-apoptotic genes may not be sufficient to induce apoptosis as long as a crucial trigger is missing. In case of cells with p53 activated by ActD+Nut3a this trigger is FASLG. We believe that treatment with ActD+Nut3a resembles a naturally occurring stress factor, which activates p53 in a similar fashion and leads to a strong response to FASLG. Considering that infected cells are physiological targets of FASLG-bearing lymphocytes, we speculate that treatment with ActD+Nut3a resembles stress within the infected cells, which activates p53 and makes them susceptible to FASLG.
Unexpectedly, it was found that FAS ligand can in some conditions promote tumor development. For instance, FASLG-containing microvesicles derived from cancer cells can kill T lymphocytes what promote tumor growth. Thus, tumor cells can use FASLG to suppress the attack by immune system. Furthermore, FAS receptor can engage non-apoptotic, growth promoting pathways. Unexpectedly, some cancer cells depend on the constitutive activity of FAS, stimulated by cancer-produced FASLG, for optimal growth. The microenvironment of some cancers appears to be permeated with a high level of FASLG [
29,
30]. If this is the case, treatment with ActD+Nut3a can change FASLG from the promoter of cancer growth to its inhibitor. Our observations clearly point toward novel approaches to cancer therapy employing the pro-apoptotic pathway starting from the FAS ligand or other agonists of death receptors. Moreover, they support the notion that p53 is an important element of immunity.
Figure 1.
FASLG in A549 cells pre-exposed to actinomycinD and nutlin-3a activates caspases of extrinsic (caspase-8) and intrinsic (caspase-9) apoptotic pathways, as well as of executioner caspase-3. A. A549 cells were pre-exposed to actinomycinD and nutlin-3a (ActD+Nut3a) for 48h and subsequently were treated for 2.5h with FASLG in two concentrations. Control cells were mock-treated, while other cells were exposed only to ActD+Nut3a or FASLG. Full length caspases are marked by arrows. The cleaved forms of caspases are marked by asterisks. Left-side numbers represent protein sizes in kDa. B. A549 cells were pre-exposed with actinomycinD (ActD), nutlin-3a (Nut3a), or both substances for 45 hours and subsequently they were exposed for 2h to FASLG at indicated concentrations. Some cells were only exposed to FASLG. Control cells were mock-treated. The expression of the indicated caspases was examined by Western blotting. HSC70 is the leading control.
Figure 1.
FASLG in A549 cells pre-exposed to actinomycinD and nutlin-3a activates caspases of extrinsic (caspase-8) and intrinsic (caspase-9) apoptotic pathways, as well as of executioner caspase-3. A. A549 cells were pre-exposed to actinomycinD and nutlin-3a (ActD+Nut3a) for 48h and subsequently were treated for 2.5h with FASLG in two concentrations. Control cells were mock-treated, while other cells were exposed only to ActD+Nut3a or FASLG. Full length caspases are marked by arrows. The cleaved forms of caspases are marked by asterisks. Left-side numbers represent protein sizes in kDa. B. A549 cells were pre-exposed with actinomycinD (ActD), nutlin-3a (Nut3a), or both substances for 45 hours and subsequently they were exposed for 2h to FASLG at indicated concentrations. Some cells were only exposed to FASLG. Control cells were mock-treated. The expression of the indicated caspases was examined by Western blotting. HSC70 is the leading control.
Figure 2.
Microscope observations reveal frequent death of cells pre-exposed to actinomycin D and nutlin-3a and subsequently incubated with FASLG. A549 cells were pre-exposed to ActD+Nut3a for 45h and subsequently they were incubated with or without 100 ng/ml FASLG. At the indicated times the cells on the same fragment were photographed. White arrows mark examples of cells with early apoptotic morphology and black arrows mark the cells with late apoptotic/necrotic morphology.
Figure 2.
Microscope observations reveal frequent death of cells pre-exposed to actinomycin D and nutlin-3a and subsequently incubated with FASLG. A549 cells were pre-exposed to ActD+Nut3a for 45h and subsequently they were incubated with or without 100 ng/ml FASLG. At the indicated times the cells on the same fragment were photographed. White arrows mark examples of cells with early apoptotic morphology and black arrows mark the cells with late apoptotic/necrotic morphology.
Figure 3.
Actinomycin D and nutlin-3a synergize in sensitizing A549 cells to cell death induced by FASLG. A. The relative cell viability measured by MTS assay. The viability of cells treated neither with drugs nor with FASLG was set to 100%. B. The data from graph A presented in modified fashion. The viability of mock-treated cells or cells exposed to any drag without subsequent exposure to FASLG was set to 100%. This allows for visualizing of the effect of FASLG alone (at indicated concentrations ng/ml) on cell viability. The statistical significance of the differences was calculated by two way ANOVA, Tukey's multiple comparisons test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001). C. The cells attached to the wells of the culture plate (stained by crystal violet) following the indicated treatment regimen. The cells were pretreated with drugs for 46h and subsequently were left untreated (the top row) or were exposed to indicated concentrations of FASLG for 5h. Surviving cells were allowed to recover for 70h before fixation and staining.
Figure 3.
Actinomycin D and nutlin-3a synergize in sensitizing A549 cells to cell death induced by FASLG. A. The relative cell viability measured by MTS assay. The viability of cells treated neither with drugs nor with FASLG was set to 100%. B. The data from graph A presented in modified fashion. The viability of mock-treated cells or cells exposed to any drag without subsequent exposure to FASLG was set to 100%. This allows for visualizing of the effect of FASLG alone (at indicated concentrations ng/ml) on cell viability. The statistical significance of the differences was calculated by two way ANOVA, Tukey's multiple comparisons test (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001). C. The cells attached to the wells of the culture plate (stained by crystal violet) following the indicated treatment regimen. The cells were pretreated with drugs for 46h and subsequently were left untreated (the top row) or were exposed to indicated concentrations of FASLG for 5h. Surviving cells were allowed to recover for 70h before fixation and staining.
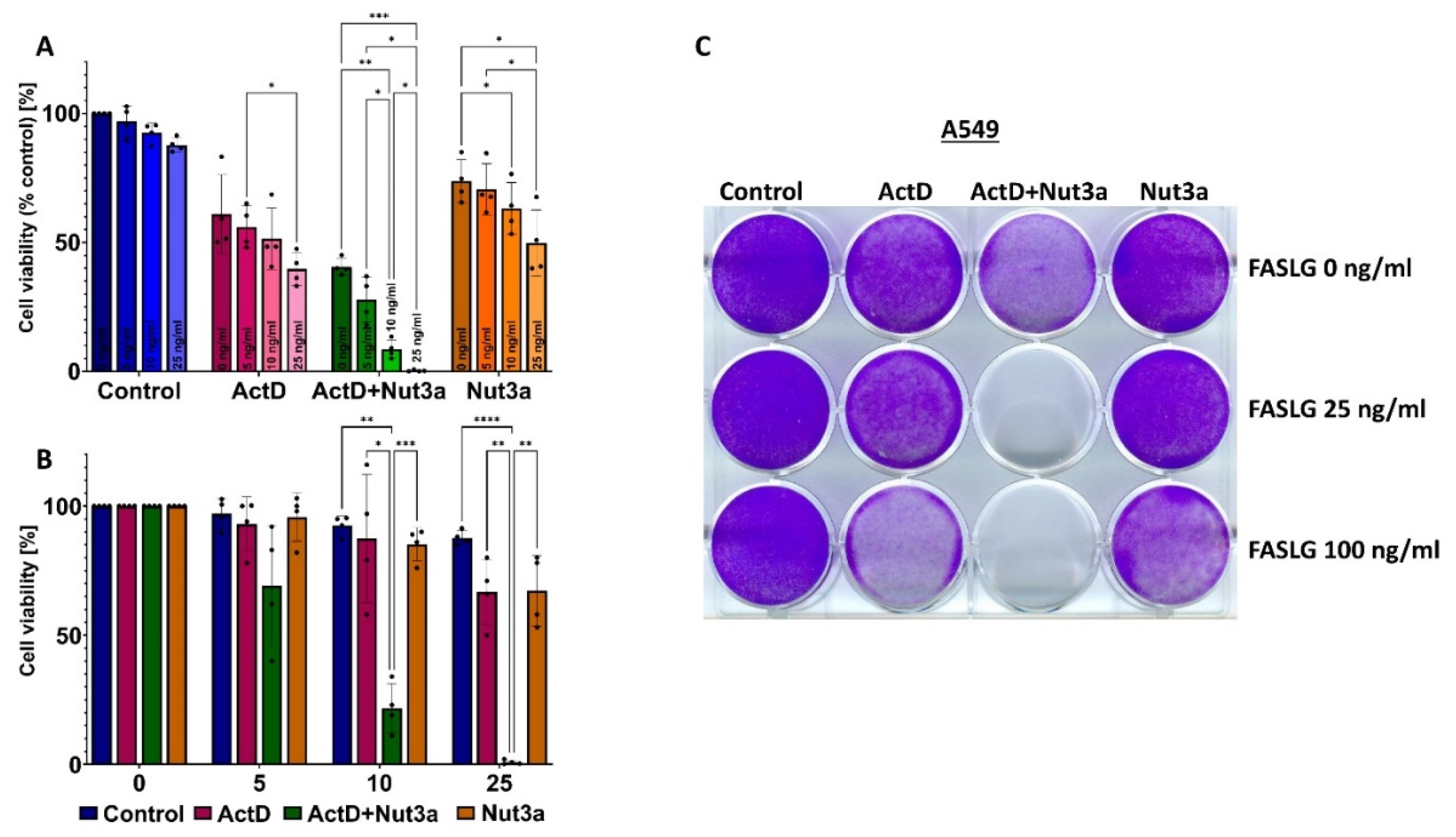
Figure 4.
The p53 is indispensable for the induction of apoptosis of cells pre-exposed to actinomycin D and nutlin-3a. A. The stained cells on culture plate. The cells of parental cell line (A549, wild-type), controls for knocked-down (CRISPR-CON) and the p53-deficient cells (CRISPR-p53) were pre-exposed as indicated for 45h and subsequently treated with FASLG and 100 ng/ml concentration for 5h. The surviving cells were allowed to recover for 72h. The cells were allowed to overgrow to better visualize the scarcity of cells on the wells exposed to ActD+Nut3a+FASLG. B. The expression of indicated proteins as detected by Western blotting. The p53-proficient (CRISPR-CON) and deficient cells (CRISPR-p53) were exposed to ActD+Nut3a for 45h following by exposure to FASLG at 50 ng/ml concentration for 2.5h.
Figure 4.
The p53 is indispensable for the induction of apoptosis of cells pre-exposed to actinomycin D and nutlin-3a. A. The stained cells on culture plate. The cells of parental cell line (A549, wild-type), controls for knocked-down (CRISPR-CON) and the p53-deficient cells (CRISPR-p53) were pre-exposed as indicated for 45h and subsequently treated with FASLG and 100 ng/ml concentration for 5h. The surviving cells were allowed to recover for 72h. The cells were allowed to overgrow to better visualize the scarcity of cells on the wells exposed to ActD+Nut3a+FASLG. B. The expression of indicated proteins as detected by Western blotting. The p53-proficient (CRISPR-CON) and deficient cells (CRISPR-p53) were exposed to ActD+Nut3a for 45h following by exposure to FASLG at 50 ng/ml concentration for 2.5h.
Figure 5.
The flow cytometry shows apoptosis induced by FASLG in p53-proficient cells pre-exposed to actinomycin D and nutlin-3a. A. An example of various cell populations (viable, early apoptotic, late apoptotic/necrotic) detected by cytometric analysis of cells stained for Annexin V and 7-AAD. The cells were treated as indicated. The pre-exposure time to ActD+Nut3a was for 45h, exposure time to FASLG at 50 ng/ml was 2.5h. B. The frequency of indicated cell populations of p53-proficient and p53-deficient A549 cells measured in four biological replicates. The statistical significance of the differences was calculated using Brown-Forsythe ANOVA test and Welch Anova test, Dunnett's T3 multiple comparisons test, **P ≤ 0.01, ***P ≤ 0.001.
Figure 5.
The flow cytometry shows apoptosis induced by FASLG in p53-proficient cells pre-exposed to actinomycin D and nutlin-3a. A. An example of various cell populations (viable, early apoptotic, late apoptotic/necrotic) detected by cytometric analysis of cells stained for Annexin V and 7-AAD. The cells were treated as indicated. The pre-exposure time to ActD+Nut3a was for 45h, exposure time to FASLG at 50 ng/ml was 2.5h. B. The frequency of indicated cell populations of p53-proficient and p53-deficient A549 cells measured in four biological replicates. The statistical significance of the differences was calculated using Brown-Forsythe ANOVA test and Welch Anova test, Dunnett's T3 multiple comparisons test, **P ≤ 0.01, ***P ≤ 0.001.
Figure 6.
Actinomycin D and nutlin-3a do not act synergistically in increasing the expression of FAS mRNA or FAS protein in cell surface. A. The expression of FAS mRNA as measured by RNA-Seq in three biological replicates of treatment of A549 cells with ActD (5nM), Nut3a (5 µM) or ActD+Nut3a for 30 hours. The raw RNA-Seq data were published and deposited in Sequence Read Archive (SRA) under the accession number PRJNA831359. B. The expression of FAS receptor (CD95) as measured by flow cytometry of A549 cells treated as indicated and stained with anti-CD95 (FAS) antibody. The cells were exposed to indicated compounds for 48 h.
Figure 6.
Actinomycin D and nutlin-3a do not act synergistically in increasing the expression of FAS mRNA or FAS protein in cell surface. A. The expression of FAS mRNA as measured by RNA-Seq in three biological replicates of treatment of A549 cells with ActD (5nM), Nut3a (5 µM) or ActD+Nut3a for 30 hours. The raw RNA-Seq data were published and deposited in Sequence Read Archive (SRA) under the accession number PRJNA831359. B. The expression of FAS receptor (CD95) as measured by flow cytometry of A549 cells treated as indicated and stained with anti-CD95 (FAS) antibody. The cells were exposed to indicated compounds for 48 h.
Figure 7.
Actinomycin D and nutlin-3a synergize in activation of selected p53-regulated, pro-apoptotic genes. A. Western blotting showing expression of the p53, caspase-6, caspase-10, and FAS receptor in indicated cell lines exposed as shown for 48h. B. The expression of indicated genes, as measured by aforementioned RNA-Seq, in A549 cells growing in control conditions or exposed to the drugs for 30h.
Figure 7.
Actinomycin D and nutlin-3a synergize in activation of selected p53-regulated, pro-apoptotic genes. A. Western blotting showing expression of the p53, caspase-6, caspase-10, and FAS receptor in indicated cell lines exposed as shown for 48h. B. The expression of indicated genes, as measured by aforementioned RNA-Seq, in A549 cells growing in control conditions or exposed to the drugs for 30h.
Figure 8.
The accumulation of caspase-6 and caspase-10 following treatment with actinomycin D and nutlin-3a does not occur in p53-deficient cells. The Western blot showing expression of caspase-10 (full length and cleaved form) and of caspase-6 (full length form) in p53-proficient and p53-deficient cells treated as described on
Figure 4B.
Figure 8.
The accumulation of caspase-6 and caspase-10 following treatment with actinomycin D and nutlin-3a does not occur in p53-deficient cells. The Western blot showing expression of caspase-10 (full length and cleaved form) and of caspase-6 (full length form) in p53-proficient and p53-deficient cells treated as described on
Figure 4B.
Figure 9.
Drugs other than actinomycin D and nutlin-3a can synergize in sensitizing cells to pro-apoptotic activity of FASLG. A. A549 cells were pre-exposed to etopiside (Eto; 5 µM), nutlin-3a and their combination with subsequent exposure to FASLG for 2.5h. The expression of p53 and the indicated caspases was examined by Western blotting. B. A549 cells were pre-exposed to actinomycin D, RG7112 (RG) or to both substances (A+RG) and subsequently they were treated for 2.5h with 50 ng/ml FASLG. Western blot shows the expression of the indicated proteins. C. A similar experiment as in B was performed with Idasanutlin (IDA) in place of RG7112 as an antagonist of MDM2.
Figure 9.
Drugs other than actinomycin D and nutlin-3a can synergize in sensitizing cells to pro-apoptotic activity of FASLG. A. A549 cells were pre-exposed to etopiside (Eto; 5 µM), nutlin-3a and their combination with subsequent exposure to FASLG for 2.5h. The expression of p53 and the indicated caspases was examined by Western blotting. B. A549 cells were pre-exposed to actinomycin D, RG7112 (RG) or to both substances (A+RG) and subsequently they were treated for 2.5h with 50 ng/ml FASLG. Western blot shows the expression of the indicated proteins. C. A similar experiment as in B was performed with Idasanutlin (IDA) in place of RG7112 as an antagonist of MDM2.
Figure 10.
Normal human fibroblasts are less sensitive than cancer cells (A549) to cytotoxic effect of ActD+Nut3a+FASLG treatment. A. The equal number of early passage normal human fibroblasts were seeded as indicated on the wells of 12-well plate. Subsequently the cells were mock-treated (control) or exposed to ActD+Nut3a for 45 hours. The cells in the middle row were exposed to the increasing concentrations of FASLG as indicated. The cancer cells (A549) were seeded and treated in analogous fashion. Both cell types were grown in the same medium (DMEM+15% FBS) and were exposed (5h) to the same preparation of FASLG in medium. After the treatment cells were allowed to recover for 90h before fixation and staining. B. Normal human fibroblasts were treated as indicated (ActD, Nut3a or their combination for 45h, FASLG for 2.5h) and the expression of caspases was detected by Western blotting.
Figure 10.
Normal human fibroblasts are less sensitive than cancer cells (A549) to cytotoxic effect of ActD+Nut3a+FASLG treatment. A. The equal number of early passage normal human fibroblasts were seeded as indicated on the wells of 12-well plate. Subsequently the cells were mock-treated (control) or exposed to ActD+Nut3a for 45 hours. The cells in the middle row were exposed to the increasing concentrations of FASLG as indicated. The cancer cells (A549) were seeded and treated in analogous fashion. Both cell types were grown in the same medium (DMEM+15% FBS) and were exposed (5h) to the same preparation of FASLG in medium. After the treatment cells were allowed to recover for 90h before fixation and staining. B. Normal human fibroblasts were treated as indicated (ActD, Nut3a or their combination for 45h, FASLG for 2.5h) and the expression of caspases was detected by Western blotting.