Submitted:
24 October 2023
Posted:
26 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mitochondrial Dysfunction and MASLD
2.1. ROS and MASLD
2.2. Impaired Mitochondrial Quality Control (MQC) and MASLD
3. Hypothyroidism and MASLD
4. Thyroid Hormone and MASLD: From Underlying Mechanisms to Therapeutic Implications
4.1. Mechanisms of Action of TH
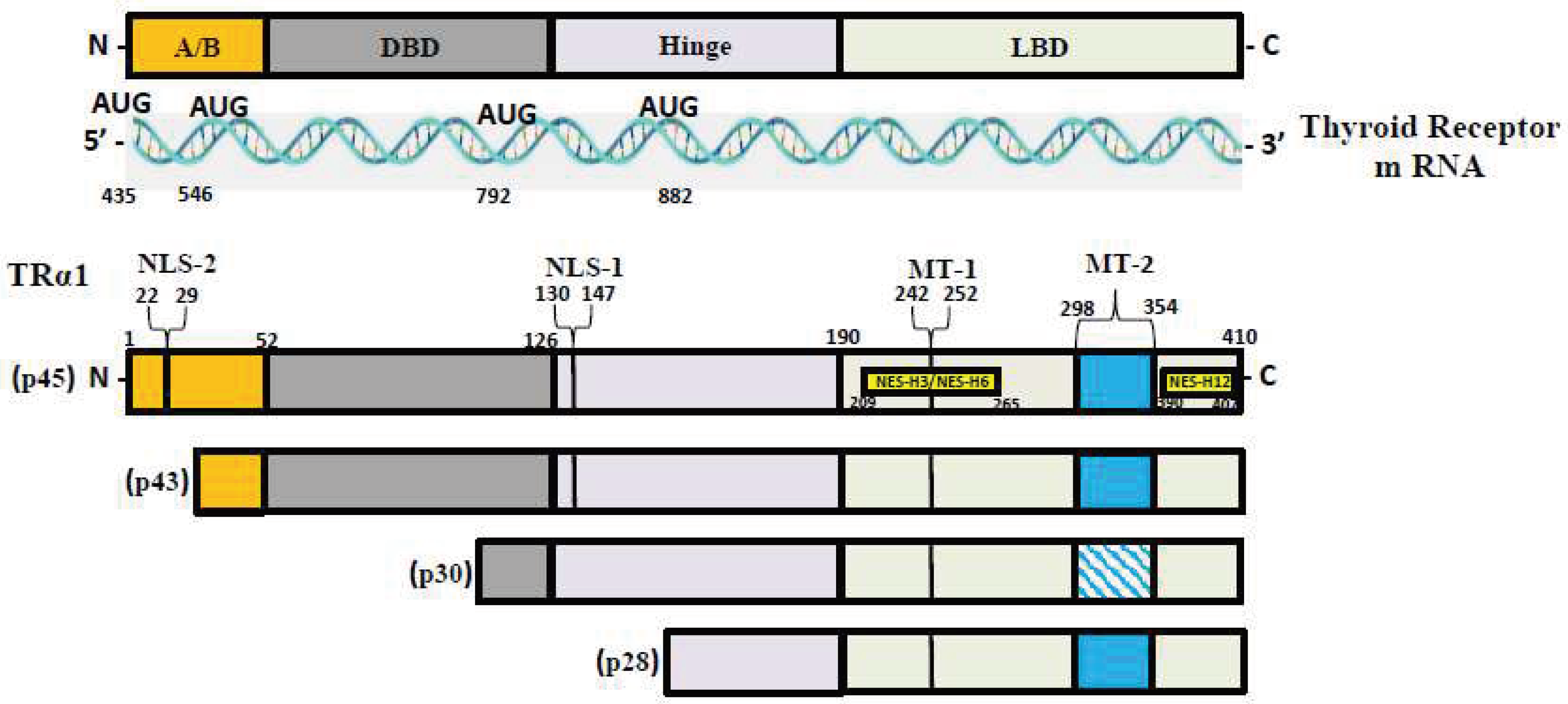
4.2. TH and Its Isoform
4.3. TH and MASLD
4.3.1. TH and FAO
4.3.2. TH and Mitochondrial Biogenesis
4.3.3. TH and Mitophagy
4.4. Potential Therapeutic Use of TH and Its Analogs in MASLD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The Natural History of Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global Epidemiology of NAFLD-Related HCC: Trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat Med 2018, 24, 908–922. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El-Serag, H.B. Association between Nonalcoholic Fatty Liver Disease and Risk for Hepatocellular Cancer, Based on Systematic Review. Clin Gastroenterol Hepatol 2012, 10, 1342–1359.e2. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and Outcomes of Liver Transplantation for Nonalcoholic Steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol 2019, 17, 748–755.e3. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of Inflammation in Nonalcoholic Fatty Liver Disease: The Multiple Parallel Hits Hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Role of Mitochondria in Nonalcoholic Fatty Liver Disease. Int J Mol Sci 2014, 15, 8713–8742. [Google Scholar] [CrossRef]
- Peng, K.Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial Dysfunction-Related Lipid Changes Occur in Nonalcoholic Fatty Liver Disease Progression. J Lipid Res 2018, 59, 1977–1986. [Google Scholar] [CrossRef]
- Li, R.; Toan, S.; Zhou, H. Role of Mitochondrial Quality Control in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Aging (Albany NY) 2020, 12, 6467–6485. [Google Scholar] [CrossRef]
- Ibdah, J.A.; Perlegas, P.; Zhao, Y.; Angdisen, J.; Borgerink, H.; Shadoan, M.K.; Wagner, J.D.; Matern, D.; Rinaldo, P.; Cline, J.M. Mice Heterozygous for a Defect in Mitochondrial Trifunctional Protein Develop Hepatic Steatosis and Insulin Resistance. Gastroenterology 2005, 128, 1381–1390. [Google Scholar] [CrossRef]
- Rector, R.S.; Morris, E.M.; Ridenhour, S.; Meers, G.M.; Hsu, F.F.; Turk, J.; Ibdah, J.A. Selective Hepatic Insulin Resistance in a Murine Model Heterozygous for a Mitochondrial Trifunctional Protein Defect. Hepatology 2013, 57, 2213–2223. [Google Scholar] [CrossRef]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised Hepatic Mitochondrial Fatty Acid Oxidation and Reduced Markers of Mitochondrial Turnover in Human NAFLD. Hepatology 2022. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Zhang, H.X.; Guo, J.R.; Lam, C.W.K.; Wang, C.Y.; Zhang, W. Mitochondria-Mediated Pathogenesis and Therapeutics for Non-Alcoholic Fatty Liver Disease. Mol Nutr Food Res 2019, 63, e1900043. [Google Scholar] [CrossRef]
- Vazquez-Calvo, C.; Suhm, T.; Büttner, S.; Ott, M. The Basic Machineries for Mitochondrial Protein Quality Control. Mitochondrion 2020, 50, 121–131. [Google Scholar] [CrossRef]
- Sheldon, R.D.; Meers, G.M.; Morris, E.M.; Linden, M.A.; Cunningham, R.P.; Ibdah, J.A.; Thyfault, J.P.; Laughlin, M.H.; Rector, R.S. eNOS Deletion Impairs Mitochondrial Quality Control and Exacerbates Western Diet-Induced NASH. Am J Physiol Endocrinol Metab 2019, 317, E605–E616. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Columbano, A.; Perra, A. Thyroid Hormones, Thyromimetics and Their Metabolites in the Treatment of Liver Disease. Front Endocrinol (Lausanne) 2018, 9, 382. [Google Scholar] [CrossRef]
- Chung, G.E.; Kim, D.; Kim, W.; Yim, J.Y.; Park, M.J.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Non-Alcoholic Fatty Liver Disease across the Spectrum of Hypothyroidism. J Hepatol 2012, 57, 150–156. [Google Scholar] [CrossRef]
- Weitzel, J.M.; Iwen, K.A. Coordination of Mitochondrial Biogenesis by Thyroid Hormone. Mol Cell Endocrinol 2011, 342, 1–7. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. NAFLD in Some Common Endocrine Diseases: Prevalence, Pathophysiology, and Principles of Diagnosis and Management. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, M.; Han, B.; Qi, X. Association of Non-Alcoholic Fatty Liver Disease with Thyroid Function: A Systematic Review and Meta-Analysis. Dig Liver Dis 2018, 50, 1153–1162. [Google Scholar] [CrossRef]
- Mandato, C.; D’Acunzo, I.; Vajro, P. Thyroid Dysfunction and Its Role as a Risk Factor for Non-Alcoholic Fatty Liver Disease: What’s New. Dig Liver Dis 2018, 50, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Shum, M.; Ngo, J.; Shirihai, O.S.; Liesa, M. Mitochondrial Oxidative Function in NAFLD: Friend or Foe? Mol Metab 2021, 50, 101134. [Google Scholar] [CrossRef]
- Begriche, K.; Massart, J.; Robin, M.A.; Bonnet, F.; Fromenty, B. Mitochondrial Adaptations and Dysfunctions in Nonalcoholic Fatty Liver Disease. Hepatology 2013, 58, 1497–1507. [Google Scholar] [CrossRef]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease and Alcohol Related Liver Disease. Transl Gastroenterol Hepatol 2021, 6, 4. [Google Scholar] [CrossRef]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol Metab 2017, 28, 250–260. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Méndez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA Cycle Function in the Pathology of Diet-Induced Hepatic Insulin Resistance and Fatty Liver. J Lipid Res 2012, 53, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Mansouri, A.; Fromenty, B. Nonalcoholic Steatosis and Steatohepatitis. V. Mitochondrial Dysfunction in Steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2002, 282, G193-9. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Lukens, J.R.; Kanneganti, T.D. Mitochondria: Diversity in the Regulation of the NLRP3 Inflammasome. Trends Mol Med 2015, 21, 193–201. [Google Scholar] [CrossRef]
- Pessayre, D. Role of Mitochondria in Non-Alcoholic Fatty Liver Disease. J Gastroenterol Hepatol 2007, 22 Suppl 1, S20-7. [Google Scholar] [CrossRef]
- Haouzi, D.; Lekéhal, M.; Moreau, A.; Moulis, C.; Feldmann, G.; Robin, M.A.; Lettéron, P.; Fau, D.; Pessayre, D. Cytochrome P450-Generated Reactive Metabolites Cause Mitochondrial Permeability Transition, Caspase Activation, and Apoptosis in Rat Hepatocytes. Hepatology 2000, 32, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ricchelli, F.; Sileikytė, J.; Bernardi, P. Shedding Light on the Mitochondrial Permeability Transition. Biochim Biophys Acta 2011, 1807, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Fromenty, B.; Robin, M.A.; Igoudjil, A.; Mansouri, A.; Pessayre, D. The Ins and Outs of Mitochondrial Dysfunction in NASH. Diabetes Metab 2004, 30, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, X.; Nie, J.; Zhang, J.; Kimball, S.R.; Zhang, H.; Zhang, W.J.; Jefferson, L.S.; Cheng, Z.; Ji, Q.; et al. ALCAT1 Controls Mitochondrial Etiology of Fatty Liver Diseases, Linking Defective Mitophagy to Steatosis. Hepatology 2015, 61, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Tait, S.W.G. Mitochondrial Quality Control: From Molecule to Organelle. Cell Mol Life Sci 2021, 78, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, Y.; Gooz, M.; Li, L.; Lemasters, J.J.; Zhong, Z. Role of Mitochondrial Depolarization and Disrupted Mitochondrial Homeostasis in Non-Alcoholic Steatohepatitis and Fibrosis in Mice. Int J Physiol Pathophysiol Pharmacol 2019, 11, 190–204. [Google Scholar]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Edmunds, L.R.; Xie, B.; Mills, A.M.; Huckestein, B.R.; Undamatla, R.; Murali, A.; Pangburn, M.M.; Martin, J.; Sipula, I.; Kaufman, B.A.; et al. Liver-Specific Prkn Knockout Mice Are More Susceptible to Diet-Induced Hepatic Steatosis and Insulin Resistance. Mol Metab 2020, 41, 101051. [Google Scholar] [CrossRef]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef]
- Perra, A.; Simbula, G.; Simbula, M.; Pibiri, M.; Kowalik, M.A.; Sulas, P.; Cocco, M.T.; Ledda-Columbano, G.M.; Columbano, A. Thyroid Hormone (T3) and TRbeta Agonist GC-1 Inhibit/Reverse Nonalcoholic Fatty Liver in Rats. FASEB J 2008, 22, 2981–2989. [Google Scholar] [CrossRef]
- Cable, E.E.; Finn, P.D.; Stebbins, J.W.; Hou, J.; Ito, B.R.; van Poelje, P.D.; Linemeyer, D.L.; Erion, M.D. Reduction of Hepatic Steatosis in Rats and Mice after Treatment with a Liver-Targeted Thyroid Hormone Receptor Agonist. Hepatology 2009, 49, 407–417. [Google Scholar] [CrossRef]
- Sinha, R.A.; Yen, P.M. Thyroid Hormone-Mediated Autophagy and Mitochondrial Turnover in NAFLD. Cell Biosci 2016, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Bianco, A.C.; Jonklaas, J.; Peeters, R.P. Hypothyroidism. Lancet 2017, 390, 1550–1562. [Google Scholar] [CrossRef]
- Notariza, K.R.; Wisnu, W. The Risk of Developing Non-Alcoholic Fatty Liver Disease in Adult Patients with Subclinical Hypothyroidism Compared to Euthyroid: An Evidence-Based Case Report. Acta Med Indones 2019, 51, 179–188. [Google Scholar] [PubMed]
- He, W.; An, X.; Li, L.; Shao, X.; Li, Q.; Yao, Q.; Zhang, J.A. Relationship between Hypothyroidism and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 2017, 8, 335. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.; Lu, M.; Chen, W.; Song, Y.; Jing, F.; Guan, Y.; Wang, L.; Lin, Y.; Bo, T.; et al. Thyrotropin Increases Hepatic Triglyceride Content through Upregulation of SREBP-1c Activity. J Hepatol 2014, 61, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Jornayvaz, F.R. Pathophysiology of NASH in Endocrine Diseases. Endocr Connect 2021, 10, R52–R65. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Mantovani, A.; Nascimbeni, F.; Lugari, S.; Targher, G. Pathogenesis of Hypothyroidism-Induced NAFLD: Evidence for a Distinct Disease Entity? Dig Liver Dis 2019, 51, 462–470. [Google Scholar] [CrossRef]
- Liebe, R.; Esposito, I.; Bock, H.H.; Vom Dahl, S.; Stindt, J.; Baumann, U.; Luedde, T.; Keitel, V. Diagnosis and Management of Secondary Causes of Steatohepatitis. J Hepatol 2021, 74, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N. Update in Lipid Alterations in Subclinical Hypothyroidism. J Clin Endocrinol Metab 2012, 97, 326–333. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Gusdon, A.M.; Qu, S. Cross-Talk between the Thyroid and Liver: A New Target for Nonalcoholic Fatty Liver Disease Treatment. World J Gastroenterol 2013, 19, 8238–8246. [Google Scholar] [CrossRef] [PubMed]
- Nanda, N.; Bobby, Z.; Hamide, A. Inflammation and Oxidative Stress in Hypothyroids: Additive Effects on Cardiovascular Risk. Indian J Physiol Pharmacol 2011, 55, 351–356. [Google Scholar]
- Baskol, G.; Atmaca, H.; Tanriverdi, F.; Baskol, M.; Kocer, D.; Bayram, F. Oxidative Stress and Enzymatic Antioxidant Status in Patients with Hypothyroidism before and after Treatment. Exp Clin Endocrinol Diabetes 2007, 115, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Kizivat, T.; Maric, I.; Mudri, D.; Curcic, I.B.; Primorac, D.; Smolic, M. Hypothyroidism and Nonalcoholic Fatty Liver Disease: Pathophysiological Associations and Therapeutic Implications. J Clin Transl Hepatol 2020, 8, 347–353. [Google Scholar] [CrossRef]
- Lugari, S.; Mantovani, A.; Nascimbeni, F.; Lonardo, A. Hypothyroidism and Nonalcoholic Fatty Liver Disease - a Chance Association? Horm Mol Biol Clin Investig 2018, 41. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Physiol Rev 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct Effects of Thyroid Hormones on Hepatic Lipid Metabolism. Nat Rev Endocrinol 2018, 14, 259–269. [Google Scholar] [CrossRef]
- Cordeiro, A.; Souza, L.L.; Einicker-Lamas, M.; Pazos-Moura, C.C. Non-Classic Thyroid Hormone Signalling Involved in Hepatic Lipid Metabolism. J Endocrinol 2013, 216, R47–57. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, Y.; Gagné, D.J.; Papasavas, P.; Hayetian, F.; Maurer, J.; Bononi, P.; Caushaj, P.F. Improvement of Hypothyroidism after Laparoscopic Roux-En-Y Gastric Bypass for Morbid Obesity. Obes Surg 2004, 14, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Loomis, A.K.; Kabadi, S.; Preiss, D.; Hyde, C.; Bonato, V.; St Louis, M.; Desai, J.; Gill, J.M.; Welsh, P.; Waterworth, D.; et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab 2016, 101, 945–952. [Google Scholar] [CrossRef]
- Mahdavi, M.; Amouzegar, A.; Mehran, L.; Madreseh, E.; Tohidi, M.; Azizi, F. Investigating the Prevalence of Primary Thyroid Dysfunction in Obese and Overweight Individuals: Tehran Thyroid Study. BMC Endocr Disord 2021, 21, 89. [Google Scholar] [CrossRef]
- Ritter, M.J.; Amano, I.; Hollenberg, A.N. Thyroid Hormone Signaling and the Liver. Hepatology 2020, 72, 742–752. [Google Scholar] [CrossRef]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173–1191. [Google Scholar] [CrossRef]
- Gereben, B.; McAninch, E.A.; Ribeiro, M.O.; Bianco, A.C. Scope and Limitations of Iodothyronine Deiodinases in Hypothyroidism. Nat Rev Endocrinol 2015, 11, 642–652. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the Local Control of Thyroid Hormone Action. J Clin Invest 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological Actions of “nonclassical” Thyroid Hormones. J Endocrinol 2014, 221, R1–12. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Sidhaye, A.R.; Wondisford, F.E. Thyroid Hormone Receptors and Resistance to Thyroid Hormone Disorders. Nat Rev Endocrinol 2014, 10, 582–591. [Google Scholar] [CrossRef]
- Weinberger, C.; Thompson, C.C.; Ong, E.S.; Lebo, R.; Gruol, D.J.; Evans, R.M. The C-Erb-A Gene Encodes a Thyroid Hormone Receptor. Nature 1986, 324, 641–646. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Tennyson, G.E.; Nikodem, V.M. Alternative Splicing Generates Messages Encoding Rat C-erbA Proteins That Do Not Bind Thyroid Hormone. Proc Natl Acad Sci U S A 1988, 85, 5804–5808. [Google Scholar] [CrossRef]
- Mitsuhashi, T.; Nikodem, V.M. Regulation of Expression of the Alternative mRNAs of the Rat Alpha-Thyroid Hormone Receptor Gene. J Biol Chem 1989, 264, 8900–8904. [Google Scholar] [CrossRef]
- Bigler, J.; Eisenman, R.N. C-erbA Encodes Multiple Proteins in Chicken Erythroid Cells. Mol Cell Biol 1988, 8, 4155–4161. [Google Scholar] [CrossRef]
- Bigler, J.; Hokanson, W.; Eisenman, R.N. Thyroid Hormone Receptor Transcriptional Activity Is Potentially Autoregulated by Truncated Forms of the Receptor. Mol Cell Biol 1992, 12, 2406–2417. [Google Scholar] [CrossRef]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Mitochondrial T3 Receptor and Targets. Mol Cell Endocrinol 2017, 458, 112–120. [Google Scholar] [CrossRef]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic Actions of Thyroid Hormone. Nat Rev Endocrinol 2016, 12, 111–121. [Google Scholar] [CrossRef]
- Bassett, J.H.; Harvey, C.B.; Williams, G.R. Mechanisms of Thyroid Hormone Receptor-Specific Nuclear and Extra Nuclear Actions. Mol Cell Endocrinol 2003, 213, 1–11. [Google Scholar] [CrossRef]
- Kalyanaraman, H.; Schwappacher, R.; Joshua, J.; Zhuang, S.; Scott, B.T.; Klos, M.; Casteel, D.E.; Frangos, J.A.; Dillmann, W.; Boss, G.R.; et al. Nongenomic Thyroid Hormone Signaling Occurs through a Plasma Membrane-Localized Receptor. Sci Signal 2014, 7, ra48. [Google Scholar] [CrossRef]
- Carazo, A.; Levin, J.; Casas, F.; Seyer, P.; Grandemange, S.; Busson, M.; Pessemesse, L.; Wrutniak-Cabello, C.; Cabello, G. Protein Sequences Involved in the Mitochondrial Import of the 3,5,3’-L-Triiodothyronine Receptor P43. J Cell Physiol 2012, 227, 3768–3777. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, G.; Kaspari, R.R.; Spadaro, O.; Reyna-Neyra, A.; Perry, R.J.; Cardone, R.; Kibbey, R.G.; Shulman, G.I.; Dixit, V.D.; Carrasco, N. Pathogenesis of Hypothyroidism-Induced NAFLD Is Driven by Intra- and Extrahepatic Mechanisms. Proc Natl Acad Sci U S A 2017, 114, E9172–E9180. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, J.; Boes, T.; Kim, E.Y.; Dearie, F.; Kim, B.W.; Schroeder, J.; Mun, E.; Nasser, I.; Park, P.J.; Bianco, A.C.; et al. Thyroid Hormone-Related Regulation of Gene Expression in Human Fatty Liver. J Clin Endocrinol Metab 2009, 94, 3521–3529. [Google Scholar] [CrossRef]
- Bohinc, B.N.; Michelotti, G.; Xie, G.; Pang, H.; Suzuki, A.; Guy, C.D.; Piercy, D.; Kruger, L.; Swiderska-Syn, M.; Machado, M.; et al. Repair-Related Activation of Hedgehog Signaling in Stromal Cells Promotes Intrahepatic Hypothyroidism. Endocrinology 2014, 155, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Bruinstroop, E.; Dalan, R.; Cao, Y.; Bee, Y.M.; Chandran, K.; Cho, L.W.; Soh, S.B.; Teo, E.K.; Toh, S.A.; Leow, M.K.S.; et al. Low-Dose Levothyroxine Reduces Intrahepatic Lipid Content in Patients With Type 2 Diabetes Mellitus and NAFLD. J Clin Endocrinol Metab 2018, 103, 2698–2706. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol Rev 2014, 94, 355–382. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Gnoni, A.; Siculella, L. Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Nascimbeni, F.; Lonardo, A.; Zoppini, G.; Bonora, E.; Mantzoros, C.S.; Targher, G. Association Between Primary Hypothyroidism and Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Thyroid 2018, 28, 1270–1284. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Reciprocal Crosstalk Between Autophagic and Endocrine Signaling in Metabolic Homeostasis. Endocr Rev 2017, 38, 69–102. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; You, S.H.; Zhou, J.; Siddique, M.M.; Bay, B.H.; Zhu, X.; Privalsky, M.L.; Cheng, S.Y.; Stevens, R.D.; Summers, S.A.; et al. Thyroid Hormone Stimulates Hepatic Lipid Catabolism via Activation of Autophagy. J Clin Invest 2012, 122, 2428–2438. [Google Scholar] [CrossRef]
- Lombardi, A.; De Matteis, R.; Moreno, M.; Napolitano, L.; Busiello, R.A.; Senese, R.; de Lange, P.; Lanni, A.; Goglia, F. Responses of Skeletal Muscle Lipid Metabolism in Rat Gastrocnemius to Hypothyroidism and Iodothyronine Administration: A Putative Role for FAT/CD36. Am J Physiol Endocrinol Metab 2012, 303, E1222–33. [Google Scholar] [CrossRef]
- Lombardi, A.; de Lange, P.; Silvestri, E.; Busiello, R.A.; Lanni, A.; Goglia, F.; Moreno, M. 3,5-Diiodo-L-Thyronine Rapidly Enhances Mitochondrial Fatty Acid Oxidation Rate and Thermogenesis in Rat Skeletal Muscle: AMP-Activated Protein Kinase Involvement. Am J Physiol Endocrinol Metab 2009, 296, E497–502. [Google Scholar] [CrossRef]
- Chocron, E.S.; Sayre, N.L.; Holstein, D.; Saelim, N.; Ibdah, J.A.; Dong, L.Q.; Zhu, X.; Cheng, S.Y.; Lechleiter, J.D. The Trifunctional Protein Mediates Thyroid Hormone Receptor-Dependent Stimulation of Mitochondria Metabolism. Mol Endocrinol 2012, 26, 1117–1128. [Google Scholar] [CrossRef]
- Raghu Ramanathan, S.A.J.; Jamal, A. Ibdah THYROID HORMONE INCREASES HEPATIC MITOCHONDRIAL FATTY ACID OXIDATION AND RESCUES NAFLD IN MICE.; WILEY, 2021; Vol. 74, pp. 1095A-1095A.
- Harper, M.E.; Seifert, E.L. Thyroid Hormone Effects on Mitochondrial Energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef]
- Thakran, S.; Sharma, P.; Attia, R.R.; Hori, R.T.; Deng, X.; Elam, M.B.; Park, E.A. Role of Sirtuin 1 in the Regulation of Hepatic Gene Expression by Thyroid Hormone. J Biol Chem 2013, 288, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Lammel Lindemann, J.A.; Angajala, A.; Engler, D.A.; Webb, P.; Ayers, S.D. Thyroid Hormone Induction of Human Cholesterol 7 Alpha-Hydroxylase (Cyp7a1) in Vitro. Mol Cell Endocrinol 2014, 388, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bonde, Y.; Plösch, T.; Kuipers, F.; Angelin, B.; Rudling, M. Stimulation of Murine Biliary Cholesterol Secretion by Thyroid Hormone Is Dependent on a Functional ABCG5/G8 Complex. Hepatology 2012, 56, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheng, S.Y. New Insights into Regulation of Lipid Metabolism by Thyroid Hormone. Curr Opin Endocrinol Diabetes Obes 2010, 17, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Moreno, M.; Lombardi, A.; de Lange, P.; Silvestri, E.; Ragni, M.; Farina, P.; Baccari, G.C.; Fallahi, P.; Antonelli, A.; et al. 3,5-Diiodo-L-Thyronine Powerfully Reduces Adiposity in Rats by Increasing the Burning of Fats. FASEB J 2005, 19, 1552–1554. [Google Scholar] [CrossRef]
- Cavallo, A.; Taurino, F.; Damiano, F.; Siculella, L.; Sardanelli, A.M.; Gnoni, A. Acute Administration of 3,5-Diiodo-L-Thyronine to Hypothyroid Rats Stimulates Bioenergetic Parameters in Liver Mitochondria. J Bioenerg Biomembr 2016, 48, 521–529. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid Hormone Induction of Mitochondrial Activity Is Coupled to Mitophagy via ROS-AMPK-ULK1 Signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef]
- Singh, B.K.; Sinha, R.A.; Tripathi, M.; Mendoza, A.; Ohba, K.; Sy, J.A.C.; Xie, S.Y.; Zhou, J.; Ho, J.P.; Chang, C.Y.; et al. Thyroid Hormone Receptor and ERRα Coordinately Regulate Mitochondrial Fission, Mitophagy, Biogenesis, and Function. Sci Signal 2018, 11. [Google Scholar] [CrossRef]
- Krotkiewski, M. Thyroid Hormones and Treatment of Obesity. Int J Obes Relat Metab Disord 2000, 24 Suppl 2, S116-9. [Google Scholar] [CrossRef]
- Oh, S.S.; Kaplan, M.L. Early Treatment of Obese (Ob/Ob) Mice with Triiodothyronine Increases Oxygen Consumption and Temperature and Decreases Body Fat Content. Proc Soc Exp Biol Med 1994, 207, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Grover, G.J.; Egan, D.M.; Sleph, P.G.; Beehler, B.C.; Chiellini, G.; Nguyen, N.H.; Baxter, J.D.; Scanlan, T.S. Effects of the Thyroid Hormone Receptor Agonist GC-1 on Metabolic Rate and Cholesterol in Rats and Primates: Selective Actions Relative to 3,5,3’-Triiodo-L-Thyronine. Endocrinology 2004, 145, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.U.; Swanson, E.; Gloss, B.; Wang-Iverson, D.B.; Zhang, H.; Volodarsky, T.; Grover, G.J.; Baxter, J.D.; Chiellini, G.; Scanlan, T.S.; et al. The Thyroid Hormone Receptor-Beta-Selective Agonist GC-1 Differentially Affects Plasma Lipids and Cardiac Activity. Endocrinology 2000, 141, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Wohlfart, P.; Madsen, A.N.; Veidal, S.S.; Feigh, M.; Schmoll, D. Activation of Thyroid Hormone Receptor-β Improved Disease Activity and Metabolism Independent of Body Weight in a Mouse Model of Non-Alcoholic Steatohepatitis and Fibrosis. Br J Pharmacol 2021, 178, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Deng, Z.; Wang, W.; Liao, G.; Zhao, Y.; Zhong, H.; Zhang, Q.; Liu, J.; Mao, X.; Chen, B.; et al. CS27109, A Selective Thyroid Hormone Receptor-β Agonist Alleviates Metabolic-Associated Fatty Liver Disease in Murine Models. Int J Endocrinol 2023, 2023, 4950597. [Google Scholar] [CrossRef]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Zhou, J.; Waskowicz, L.R.; Lim, A.; Liao, X.H.; Lian, B.; Masamune, H.; Refetoff, S.; Tran, B.; Koeberl, D.D.; Yen, P.M. A Liver-Specific Thyromimetic, VK2809, Decreases Hepatosteatosis in Glycogen Storage Disease Type Ia. Thyroid 2019, 29, 1158–1167. [Google Scholar] [CrossRef]
- Berkenstam, A.; Kristensen, J.; Mellström, K.; Carlsson, B.; Malm, J.; Rehnmark, S.; Garg, N.; Andersson, C.M.; Rudling, M.; Sjöberg, F.; et al. The Thyroid Hormone Mimetic Compound KB2115 Lowers Plasma LDL Cholesterol and Stimulates Bile Acid Synthesis without Cardiac Effects in Humans. Proc Natl Acad Sci U S A 2008, 105, 663–667. [Google Scholar] [CrossRef]
- Ramanathan, R.; Johnson, S.; Ibdah, J.A. Ramanathan, R.; Johnson, S.; Ibdah, J.A. Low Dose Thyroid Hormone Improves Hepatic Mitochondrial Fatty Acid Oxidation and Rescues Non-Alcoholic Fatty Liver Disease in Mice.; J Hep 2022, Vol. 77, pp. S692–S693 (Elsevier: London).
- Ibdah, J.A. Thyroid Hormone for Treatment of Nonalcoholic Steatohepatitis in Veterans. Available online: https://clinicaltrials.gov/study/NCT05526144.
- Grasselli, E.; Voci, A.; Canesi, L.; De Matteis, R.; Goglia, F.; Cioffi, F.; Fugassa, E.; Gallo, G.; Vergani, L. Direct Effects of Iodothyronines on Excess Fat Storage in Rat Hepatocytes. J Hepatol 2011, 54, 1230–1236. [Google Scholar] [CrossRef]
- Jonas, W.; Lietzow, J.; Wohlgemuth, F.; Hoefig, C.S.; Wiedmer, P.; Schweizer, U.; Köhrle, J.; Schürmann, A. 3,5-Diiodo-L-Thyronine (3,5-T2) Exerts Thyromimetic Effects on Hypothalamus-Pituitary-Thyroid Axis, Body Composition, and Energy Metabolism in Male Diet-Induced Obese Mice. Endocrinology 2015, 156, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Lionetti, L.; Moreno, M.; Lombardi, A.; De Lange, P.; Antonelli, A.; Lanni, A.; Cavaliere, G.; Barletta, A.; Goglia, F. 3,5-Diiodo-l-Thyronine, by Modulating Mitochondrial Functions, Reverses Hepatic Fat Accumulation in Rats Fed a High-Fat Diet. J Hepatol 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Iannucci, L.F.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Yen, P.M.; Sinha, R.A. Metabolomic Analysis Shows Differential Hepatic Effects of T2 and T3 in Rats after Short-Term Feeding with High Fat Diet. Sci Rep 2017, 7, 2023. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Zhou, J.; Tripathi, M.; Yau, W.W.; Boelen, A.; Singh, B.K.; Yen, P.M. Early Induction of Hepatic Deiodinase Type 1 Inhibits Hepatosteatosis during NAFLD Progression. Mol Metab 2021, 53, 101266. [Google Scholar] [CrossRef] [PubMed]
- Kannt, A.; Wohlfart, P.; Madsen, A.N.; Veidal, S.S.; Feigh, M.; Schmoll, D. Activation of Thyroid Hormone Receptor-β Improved Disease Activity and Metabolism Independent of Body Weight in a Mouse Model of Non-Alcoholic Steatohepatitis and Fibrosis. Br J Pharmacol 2021, 178, 2412–2423. [Google Scholar] [CrossRef]
- Vatner, D.F.; Weismann, D.; Beddow, S.A.; Kumashiro, N.; Erion, D.M.; Liao, X.H.; Grover, G.J.; Webb, P.; Phillips, K.J.; Weiss, R.E.; et al. Thyroid Hormone Receptor-β Agonists Prevent Hepatic Steatosis in Fat-Fed Rats but Impair Insulin Sensitivity via Discrete Pathways. Am J Physiol Endocrinol Metab 2013, 305, E89–100. [Google Scholar] [CrossRef]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Cioffi, F.; Zambad, S.P.; Chhipa, L.; Senese, R.; Busiello, R.A.; Tuli, D.; Munshi, S.; Moreno, M.; Lombardi, A.; Gupta, R.C.; et al. TRC150094, a Novel Functional Analog of Iodothyronines, Reduces Adiposity by Increasing Energy Expenditure and Fatty Acid Oxidation in Rats Receiving a High-Fat Diet. FASEB J 2010, 24, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the Treatment of Non-Alcoholic Steatohepatitis: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Taub, R.; Chiang, E.; Chabot-Blanchet, M.; Kelly, M.J.; Reeves, R.A.; Guertin, M.C.; Tardif, J.C. Lipid Lowering in Healthy Volunteers Treated with Multiple Doses of MGL-3196, a Liver-Targeted Thyroid Hormone Receptor-β Agonist. Atherosclerosis 2013, 230, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ladenson, P.W.; McCarren, M.; Morkin, E.; Edson, R.G.; Shih, M.-C.; Warren, S.R.; Barnhill, J.G.; Churby, L.; Thai, H.; O’Brien, T.; et al. Effects of the Thyromimetic Agent Diiodothyropropionic Acid on Body Weight, Body Mass Index, and Serum Lipoproteins: A Pilot Prospective, Randomized, Controlled Study. J Clin Endocrinol Metab 2010, 95, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
| Compound | Model and dose | Study findings | MASLD impact | References |
|---|---|---|---|---|
| Animal studies | ||||
| TH | ||||
| T2 | Hepatocyte isolated from Wistar rats; 10-7 to 10-5 M | Reduction of acyl-CoA oxidase and peroxisomal β-oxidation | Reduction of hepatic lipid accumulation | [114] |
| T2 | C57BL/6J mice; 2.5 µg/100g; ip | Increased fatty acid oxidation and decreased lipogenesis | Inhibition of fat accumulation in liver | [115] |
| T2 | Male wistar rats; 25 µg/100g; ip |
Reduced hepatic fatty accumulation, enhanced fatty acid oxidation rate and carnitine palmitoyl transferase activity | Activates mitochondrial processes, reverses hepatic steatosis | [116] |
| T2 | Rats injected with 25 µg/100g; ip |
Reduction in Serum TG and cholesterol | Prevents fatty liver by increasing fatty oxidation | [99] |
| T3 | ob/ob mice; 25µg/100g; ip | Lowered body weight and fat, increased oxidative metabolism | Increased oxidative metabolism in brown adipose tissue and liver. | [104] |
| T3 | Male wistar rats; 25µg/100g; ip | Promotes fatty acid peroxisomal and mitochondrial β-oxidation | Prevents hepatic fat accumulation by increasing β-oxidation | [43] |
| T2 and T3 | Wistar rats; 25 and 2.5 µg/100g; ip | Increased CPT-1 levels | Lowering hepatic lipid content, induced autophagy and intra-hepatic acylcarnitine flux. | [117] |
| T4 | Male C57BI/6J mice; | Decreased hepatic triglyceride and cholesterol | Reduce hepatosteatosis and prevent MASH progression. | [118] |
| Thyroid hormome analogues | ||||
| T3 and TRβ agonist GC-1 | Male fischer rats; 4 and 5 mg/kg; ip |
Marked fatty liver with mild hepatitis | Prevents fat accumulation by increasing mitochondrial and peroxisomal oxidation, complete regression of liver steatosis | [43] |
| TRβ agonist GC-1 | Male sprague Dawley rats; 1µg/kg; oral gavage | Reduction in hepatic TG levels | Treatment of obesity and hypercholesterolemia | [105] |
| MB07811 | Male sprague Dawley rats, ob/ob mice; 1 to 50 mg/kg; oral gavage |
Prevents hepatic steatosis, reduced plasma FFA and triglycerides | Increased hepatic fatty acid β-oxidation and mitochondrial respiration rates, as well as lower hepatic triglyceride levels and stimulation of CPT1α expression | [44] |
| Resmetirom (MGL-3196) | C57BI/6J mice; 3mg/kg for 8 weeks by oral gavage |
Lower hepatic triglycerides, lipid peroxidation, steatosis, inflammation and fibrosis |
Improvement in systemic and hepatic metabolism | [119] |
| VK2809 | GSDIa mouse model; 10mg/kg; Subcutaneously |
Restoring autophagy, mitochondrial biogenesis, and β-oxidation of fatty acids | Reduced hepatic lipid accumulation | [110] |
| GC-1 and KB-2115 | Male Sprague-Dawley rats; 164 and 100 µg/kg; ip |
Increased white adipose tissue lipolysis | Reduced hepatic steatosis | [120] |
| TG68 | C57BL mice; 2.8mg/kg in drinking water |
Reduction in liver weight, hepatic steatosis and triglycerides. | Can be used in MASLD | [121] |
| TRC150094 | Male wistar rats for 8 weeks; ip injection (0.750mg/100g b wgt | Reduction of Fat accumulation | Can be used in MASLD | [122] |
| Clinical Trials | ||||
| TH | ||||
| MGL-3196 (Resmetirom) | 36 weeks randomized trial in patients with biopsy proven MASH with fibrosis given 80mg orally daily | Significant reduction of hepatic fat, liver enzymes, lipoprotein, inflammation and fibrosis. | Patients showed reduction of hepatic fat compared to placebo, adverse events were mild and moderate. | [123] |
| 2 weeks randomized trial with 0.25 to 200mg/day | Significant reduction of total cholesterol and triglycerides |
Safe and showed beneficial effect on lipid parameters. | [124] | |
| KB2115 (eprotirome) |
5-day randomized trial in patients given 50 to 2000 µg orally daily |
Reduction in serum TC and LDL in overweight patients |
Reduced body weight | [111] |
| VK2809 | 12-week study of low dose of 5 mg in patients |
Reduction in LDL levels | Improvements in liver fat content in patients with MASLD | [18] |
| Levothyroxine (T4) | Patients with type 2 diabetes and steatosis given 18.75µg/day | Low dose T4 decreased lipid content in euthyroid male patients with type 2 diabetes mellitus. |
Safety and efficacy of TH therapy for MASLD in men | [84] |
| DITPA | 8-week randomized trial in patients with dose from 90 till 360mg/d | Lowered serum cholesterol and decrease in triglycerides |
Reduced body weight | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





