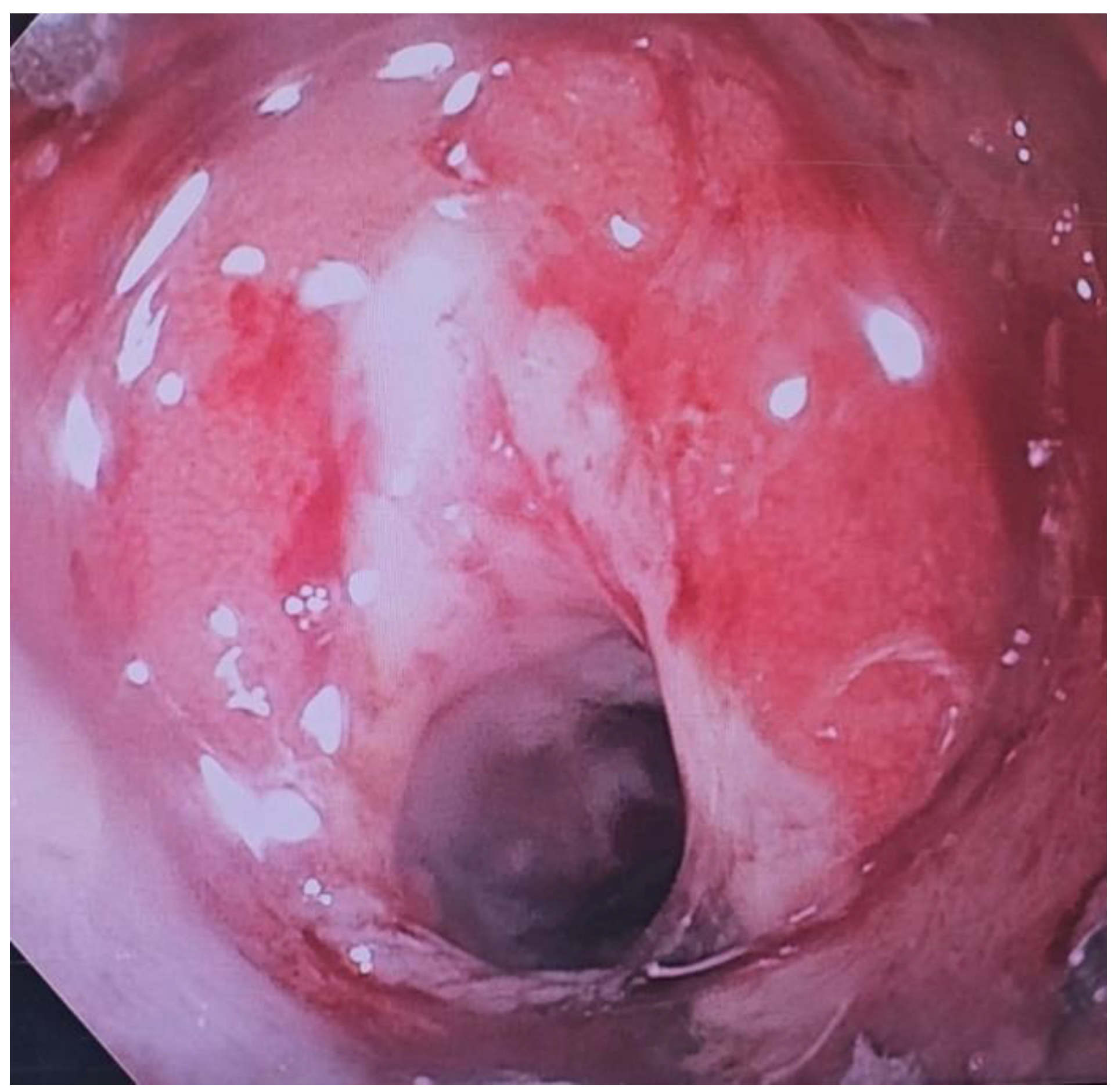
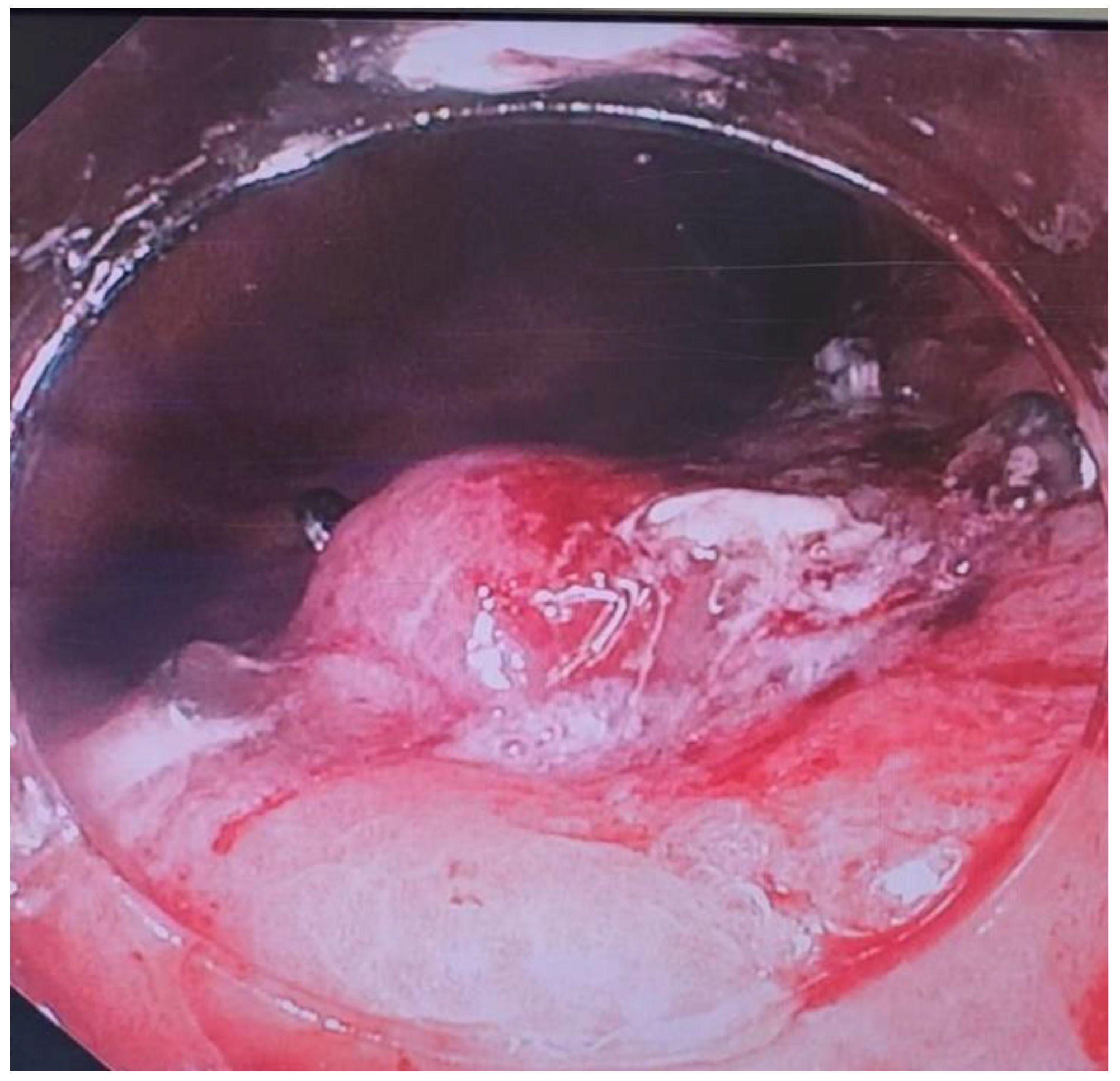
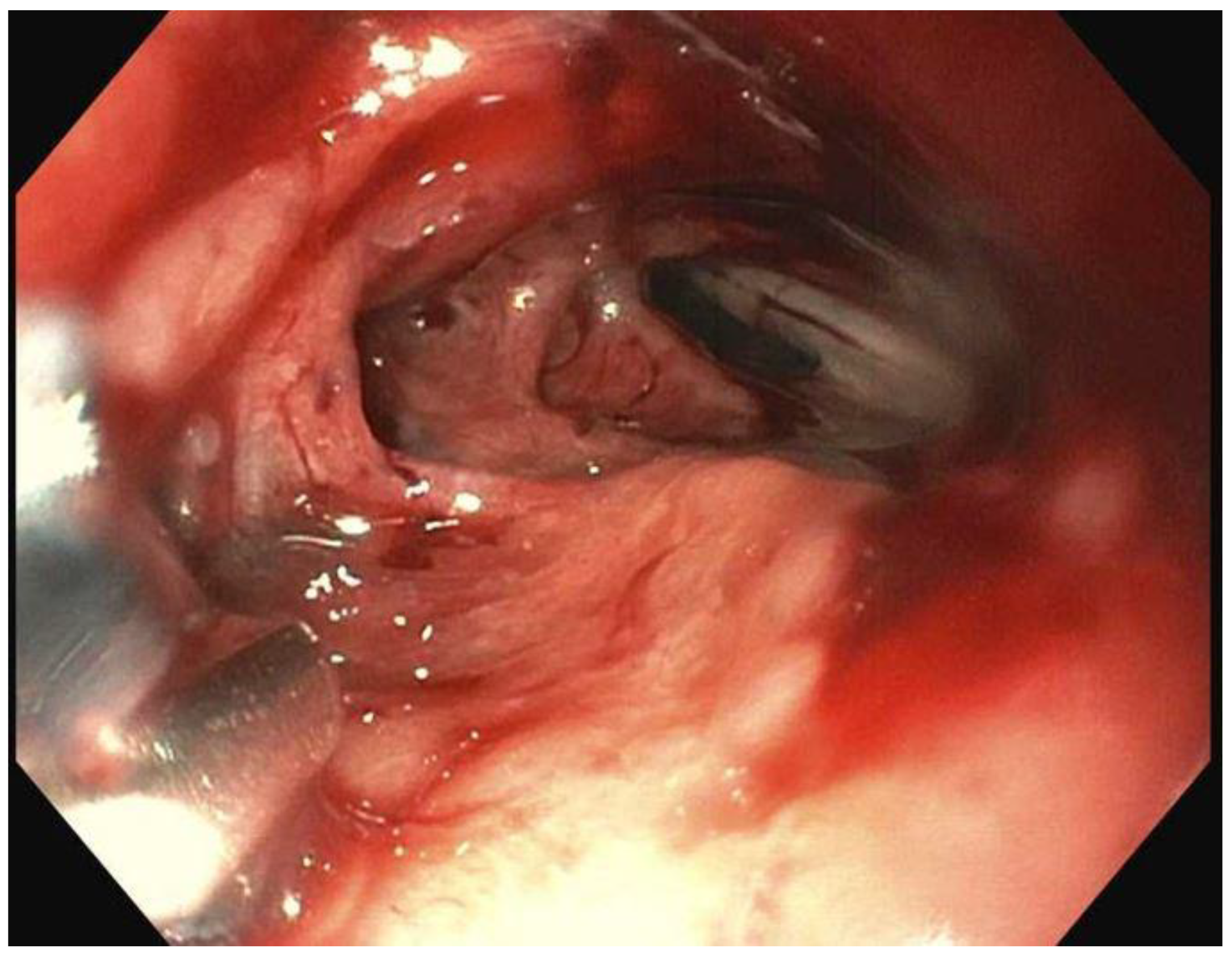
Over the past two decades techniques and innovation in endoscopy have made it easier to treat patients non-surgically. However, the same techniques have also increased risk of causing iatrogenic perforations. A perforation may result in need for an emergency surgery but in most cases, endoscopy is successful in closing perforations. Perforations can happen either during the procedure or can be delayed, often times days later (1). Clips (through-the scope (TTSC), and over-the scope (OTSC)), metal stents, suturing and more recently endovacuum therapy (EVT), glue therapy have all been used to attempt closure of perforations. Although a few retrospective studies have been published previously, prospective outcome studies on endoscopic therapy of perforations are limited. In this review we shall discuss ways to manage gastric perforations, leaks and how the etiology of perforation may determine the approach and the outcome of an endoscopic attempt at closure.
Although non-iatrogenic causes of gastric perforation remain uncommon such as gastric ulcers, procedural causes of gastric perforation have been steadily increasing, particularly as Endoscopic Mucosal resection (EMR) and endoscopic submucosal dissection (ESD) gain in popularity. Diagnostic endoscopy rarely results in a perforation, however therapeutic endoscopy procedures mentioned earlier are associated with higher rates (2). Yamamoto and co-workers estimated gastric perforation rate from ESD estimating to about 3%. 98% of the patients in those instances recovered without intervention (3). In addition to the growth of EMR and ESD, bariatric procedures have also been rising due to the ongoing obesity crisis (4). This has resulted in a higher number of postoperative leaks and other complications (5,6). Recognizing perforations early is key in minimizing morbidity and mortality. To that end, in 2017, the Sydney Classification of deep mural injury was proposed (7). This was primarily proposed to evaluate depth of injury and diagnose early endoscopic perforations in resection of colon polyps, however this can be extended to gastric defects, as the type of injury is classified as per the mucosal injury (
Table 1). Most perforations that occur as a result of endoscopic procedures can be treated endoscopically without need for surgery. By the end of this review, the reader will come to appreciate that Type 1-4 injuries, and type 5 in many instances, can be managed endoscopically. Despite endoscopic closure many of these patients still need to be monitored as inpatients
Gastric surgery can also result in post-operative anastomotic leaks or complete breakdown at the surgical site. This remains a major post-operative clinical problem, and delayed diagnosis can result in mortality rates as high as 60% (8-10). Fever, elevated C-reactive protein level, septic shock, and leukocytosis are major clinical signs of leakage of gastrointestinal contents into the peritoneum (8,9).
Previously, a perforation often times resulted in immediate surgical intervention, and endoscopy to close a potential defect was contraindicated due to worry of making a defect larger. With advent of newer devices such as TTSC, OTSC and endoscopic suturing, endoscopic procedures to close defects have become the first line therapy in closure of leaks and perforations, and in many cases second and third line as well.
Endoclips
Two major types of endoclips are used for closure of gastric perforations and leaks. Through-the-scope clips are the most commonly used. First used in 1975, these have become very popular due to their ease of use. In the last decade, TTSC have been developed that can be rotated and newer clips have been developed with “teeth” or where only one arm of the clip can be opened (11) (Microtech;Boston Sci). All of these makes usage of these clips to close defects as large at 2-3 cm easier. As a general rule, majority of the TTSC can be used to close gastric defects less than 2cm in size. Clipping is also very safe with minimal risk. Magdeburg and co-workers published a retrospective review of 117 patients, in which they showed closure with clips was successful in 98.3% of patients (115/117) (12). A recent Expert opinion suggested TTSC to be ideal for defects smaller than 1cm (1), although in our opinion the location of the defect within the stomach may make it difficult to place these TTSC. Although there are several other uses and indications for usage of these clips, these applications will not be discussed here as that is beyond the scope of this review.
The second type of endoclip is the OTSC. There are 2 popular OTSC systems in use. The Padlock clip (
Figure 1) (Aponos Medical Corp., Kingston, New Hampshire) and the OTSC-Bear Claw (
Figure 2) by Ovesco Endoscopy (Ovesco Endoscopy AG, Tubingen, Germany). Both of these clips have similar deployment mechanisms, although the loading of the clips is slightly different between the two systems. Different sizes and types are available. For instance, the ‘gc’ type is ideal for gastrocutaneous fistula closure, whereas the ‘t’ type is considered ideal for traumatic leaks and defects such as perforations (
Figure 3 and
Figure 4). The OTSC system by Ovesco has two dedicated accessories, anchor and twin grasper forceps (
Figure 5) that can help facilitate closure of perforations and leaks. Because the OTSC are larger in diameter, these are ideal for defects that are 1-3cm in size. A major limitation of the OTSC is that the endoscopist has only 1 attempt to close the defect. A twin grasper forceps can be employed in closure of defects with an OTSC. The twin grasper forceps has 2 jaws, and each one can be opened independent of the other. This allows each end of a perforation to be grabbed independently of the other. Once both ends are grabbed, the site can be pulled into OTSC cap, prior to clip deployment. Care has to be taken to ensure the clip is not deployed over the twin grasper forceps. Similar to the twin grasper forceps made by Ovesco that can aid in defect closure, a recent TTSC has also been developed by Boston Scientific (
Figure 6) in closure of defects up to 3cm in size (Mantis, Boston Scientific, Marlborough, MA). Another limitation of OTSC that has been reported is the inadvertent inclusion of organs/vessels adjacent to the site of the perforation/defect if not carefully deployed (13). In some cases, a combined approach can be utilized whereby a TTSC and OTSC are both used to close defects (14). Once a defect is larger than 3cm, options are limited. One option is suturing, discussed below, another is using an endoloop in conjunction with a TTSC (1). In this option, 2 TTSC are placed at either end of the defect, followed by using an endoloop/detachable snare to place around the defect and then tightening the snare, that results is closure of the defect. Endoscopic suturing, however is generally preferred over other options for defects larger than 3cm.
Endoscopic Suturing
Endoscopic suturing has become increasingly popular for endobariatric procedures, however can be successfully utilized to close large or small defects. Endoscopic suturing has been used to close both acute perforations and chronic fistulas. Two separate studies have shown the feasibility and high rates of closure of gastro-gastric fistulas after bariatric surgery (15, 16). It is well known, that surgical repair of such fistulas carry a high morbidity and mortality, as well as long hospital stays (15). The Apollo Overstitch device (
Figure 7) allows one to have a full-thickness purchase of tissue, particularly the gastric wall, and allows the endoscopist to successfully appose to opposite ends in an intermittent or a running pattern. In initial studies, it appeared that the limit of closure would be a defect size of 10mm or less, however, later studies and changes to the early endoscopic suturing system has resulted in successful closures of larger defects, with no obvious limits as it pertains to defect sizes. (15-20). In some cases, a defect can also be closed by pulling omental fat into the gastric lumen and closing opposite sides of a defect with the omental fat (17,18). A few studies exist where endoscopic suturing has been shown to be successful in preventing both delayed bleeding or perforation after ESD (19, 20). Perforation remains a feared complication of ESD, with rates as high as ~5%. Majority of these perforations occur during the ESD, with small subset being in a delayed manner. Hence, closure of ESD defects is crucial in minimizing risk of perforation. Hammad and co-workers, showed in a small prospective single-center study, that closure of defects in an interrupted suture pattern was feasible, safe and effective in preventing delayed bleeding and perforation. In fact, employing their technique of closure post-ESD resulted in 0 delayed bleeding or perforations—an impressive statistic (19).
A limitation of suturing has been its technical expertise, that whilst growing is still not as widespread as endoclips. A more recent addition to the armamentarium is the X-tack device, which is through the scope suturing using 3-0 polypropelene sutures. This is a simple technique which can aid in closure of gastric defects. Unlike the endoscopic suturing device, this does not provide full thickness closure, and may not always be successful. Nonetheless, this when combined with other techniques such as endoclips, or OTSC can provide an option for closure of mucosal defects. One of the biggest advantages of the X-tack device is that it can be used with both a standard adult gastroscope and colonoscope, making deployment of these devices much easier in management of otherwise difficult to reach spaces. This can also be used in conjunction with OTSC or TTSC.
Unfortunately, in the case of chronic fistulas, although initial technical success rates are high, long-term results are less than impressive (21).
Endoscopic Vacuum Therapy (EVT)
Endoscopic Vacuum therapy is a recent technique developed to treat anastomotic leaks (5). The technique has gained prominence in last few years particularly in treatment of leaks after bariatric procedures. It has shown promise in treatment of gastroesophageal junction perforations and also in Boerhaave’s syndrome (22). It is essentially a technique that can best be described as surgical wound management initiated endoscopically. There have been long term efficacy studies of EVT for anastomotic leaks after colorectal surgery as well. Success rates in these retrospective studies are well over 75% (23). Brangewitz and co-workers retrospectively studied stent placement vs EVT for closure of intrathoracic leaks, and found significant benefit for the EVT group compared to the stent group (24). Studies done since have shown a 10% difference in favor of EVT, in post-procedural mortality and a 16% difference in closure of fistula rate, also in favor of EVT. (25)
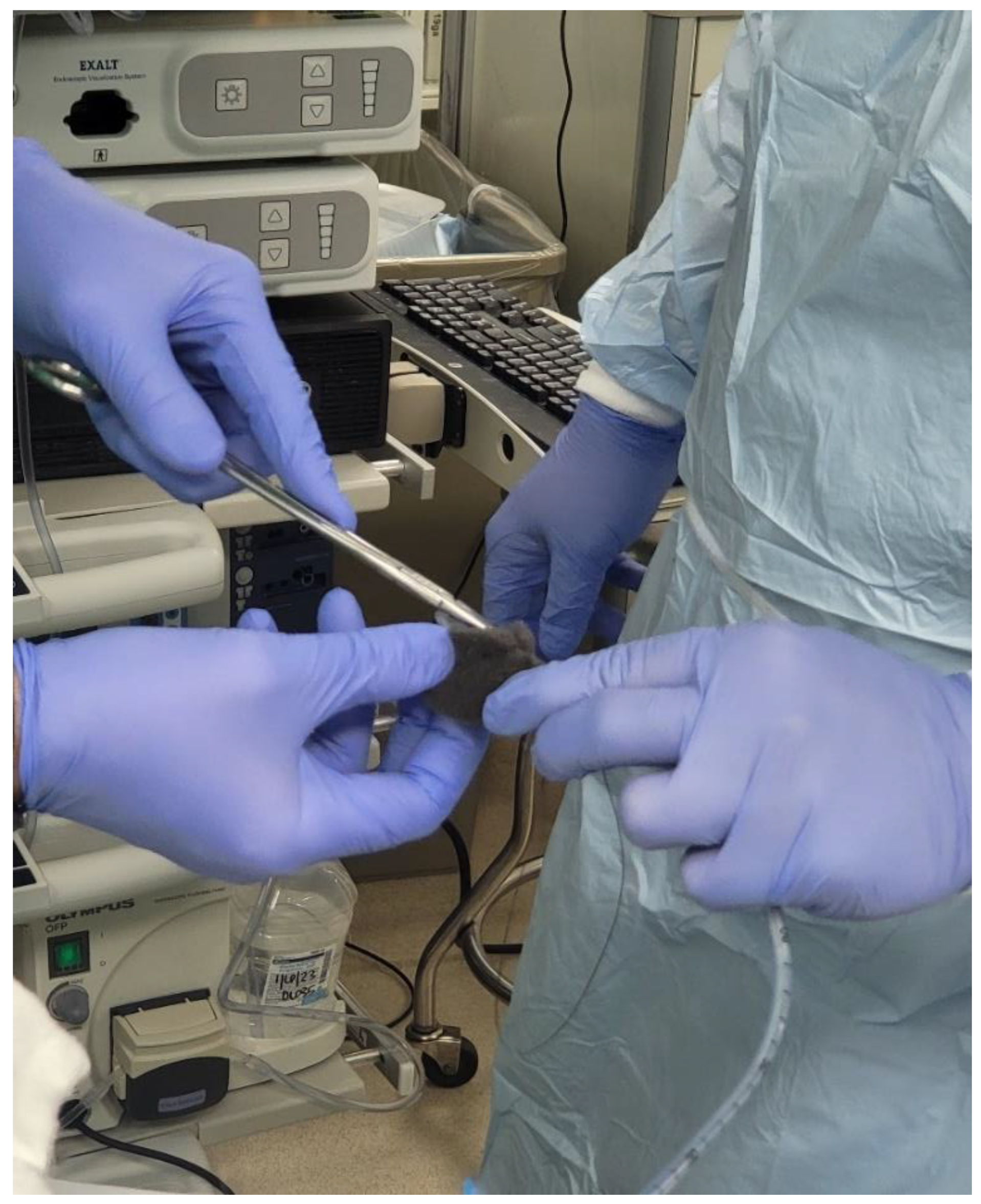
There are two big limitations of EVT seems to be placement of a sponge into the defect in question and the frequency with which the sponge has to be changed. At the time of writing, there is no device available in the US for placement/deployment of a sponge which can be used for EVT. A device is available in Europe, however (Eso-SPONGE, Braun Medical, UK). Because of the lack of resources, treatment typically involves using a sponge that is cut out to the size of the wound defect and cavity. This is then sutured to a nasogastric tube (NG), and then introduced into the cyst cavity with endoscopic guidance (
Figure 8,
Figure 9 and
Figure 10). The longer the path to the defect, the harder it becomes to place the sponge into the cavity, due to friction generated by the sponge as well as the increased probability of inadvertently pulling the sponge out
after it has been placed. The second limitation of EVT is the need to change the sponge every 3-4 days until the wound has healed. In between treatments the NG is attached to suction, and the patient usually remains as an inpatient, which can result in high costs.
Stents
Self-expanding metal stents have a role in alleviating obstructions throughout the GI tract, including esophageal/gastric/duodenal/biliary and colonic. Fully covered Self expanding metal stents (FC-SEMS) have been shown to be effective in treating anastomotic leaks and fistulas. The main purpose of placement of a FC-SEMS is to cover a defect so as to minimize leakage of gastric contents into the peritoneum. They are a minimally invasive option, particularly if other options maybe limited, or they can be used as an adjunct in some cases (26-27). Stents, when used in conjunction with drains, can be highly effective in controlling or sealing the leak and negating the need for a surgery. These stents have a silicone covering preventing the defect from being exposed to the gastric contents and the acidic environment of the stomach. It should be noted that this is rarely ever 100% effective in completely preventing that exposure, due to seepage around the stent; however, there is some utility in even decreasing the exposure, as this seems to help in wound healing.
A major limitation of a FC-SEMS, is that they tend to migrate easily with rates estimated as high as 40% in long term follow up studies (28). It is hypothesized that the smooth surface of the stents may contribute to the higher rate of migration. Furthermore, within the stomach, the curvature and peristalsis may increase the probability of the migration as well. In cases of gastric surgery, the size of the anastomosis may be prohibitive to placement of a stent, depending on how large the anastomosis is. Furthermore, to minimize risk of stent migration endoscopic suturing may have to be performed to anchor the stent in place, and this may be difficult based on the anatomy.
A future and an actively studied field is one of combining EVT with stents particularly in management of gastroesophageal disruptions and some anastomotic fistulas/disruptions.
Other Options
One common tissue sealant in use is glue, also known as fibrin glue or cyanoacrylate (29, 30). Fibrin glue is most effective when applied onto a dry area and hence endoscopic debridement of necrotic tissue remnants and pus is necessary. N-butyl-2-cyanoacrylate is the most common cyanoacrylate used. It can be in cases of gastrocutaneous fistulas be used transcutaneously to apply within the fistula tract. It is not affected by gastric secretions or pancreatic enzymes. It also has antibacterial properties and can be applied to an infected area (29, 31). We recommend denuding the mucosa of the fistula/leak with either argon plasma coagulation or with a cytology brush prior to using an endoscopic sealant. This has been shown to aid in healing of mucosal defects (32). One publication even showed effectiveness of glue at 25 years in closure of anastomotic leaks (29). Availability of fibrin glue however is limited, and we recommend using glue in conjunction with another closure method. For instance, fibrin glue could be used in conjunction with a stent (33). Small studies have also shown benefit of endoscopic therapy over surgical options in closure of gastric defects, however larger studies need to be done (34). Nonetheless, fibrin glue is another tool in closure of gastric leaks and fistulas.
Conclusion
Regardless of the approach used in management of gastric perforations and leaks, it is vital to have a protocol and a team which is familiar in the various devices listed above, and furthermore it is vital to ensure there is no panic. Over the last 3 decades there has been a tremendous growth in the field of endoscopy, and this has resulted in an increased need for closure of gastric defects, many of which are secondary to advanced procedures. Development of endoscopic suturing and OTSC has resulted in large strides in the field of endoscopic closure. The appropriate management of gastric perforations/fistulas and leaks requires a multidisciplinary approach. Acute perforations during an endoscopic procedure can almost always be closed endoscopically. Large randomized controlled trials comparing the different modalities however are lacking in this field. With advent of endovacuum therapy and other newer modalities of closure, we anticipate further leaps in the field of endoscopic repair of gastric defects.
Author Contributions
Authors equally were involved in writing and formulation of this manuscript.
Funding
No funding sources utilized for this paper.
References
- Lee, J. H.; Kedia, P.; Stavropoulos, S. N.; Carr-Locke, D. AGA Clinical Practice Update on Endoscopic Management of Perforations in Gastrointestinal Tract: Expert Review. Clin Gastroenterol Hepatol 2021, 19 (11), 2252-2261.e2252. [CrossRef]
- Paspatis, G. A.; Dumonceau, J. M.; Barthet, M.; Meisner, S.; Repici, A.; Saunders, B. P.; Vezakis, A.; Gonzalez, J. M.; Turino, S. Y.; Tsiamoulos, Z. P.; et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2014, 46(8), 693–711. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Uedo, N. Management of adverse events related to endoscopic resection of esophageal and gastric neoplasms: Report of consensus meeting. Dig Endosc 2019, 31 Suppl 1, 2–3. [Google Scholar] [CrossRef]
- Alalwan, A. A.; Friedman, J.; Park, H.; Segal, R.; Brumback, B. A.; Hartzema, A. G. US national trends in bariatric surgery: A decade of study. Surgery 2021, 170(1), 13–17. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, P.; Daniluk, J.; Baniukiewicz, A.; Wroblewski, E.; Dabrowski, A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol 2015, 21(37), 10542–10552. [Google Scholar] [CrossRef]
- Rogalski, P.; Hady, H. R.; Baniukiewicz, A.; Dąbrowski, A.; Kaminski, F.; Dadan, J. Gastric band migration following laparoscopic adjustable gastric banding (LAGB): two cases of endoscopic management using a gastric band cutter. Wideochir Inne Tech Maloinwazyjne 2012, 7(2), 114–117. [Google Scholar] [CrossRef] [PubMed]
- Burgess NG, Bassan MS, McLeod D, et al. Deep mural injury and perforation after colonic endoscopic mucosal resection: a new classification and analysis of risk factors. Gut 2017; 66: 1779–1789. [CrossRef]
- Kumar, N.; Thompson, C. C. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol 2013, 11(4), 343–353. [Google Scholar] [CrossRef]
- Kumar, N.; Thompson, C. C. Endoscopic therapy for postoperative leaks and fistulae. Gastrointest Endosc Clin N Am 2013, 23(1), 123–136. [Google Scholar] [CrossRef]
- Urschel, J. D. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995, 169(6), 634–640. [Google Scholar] [CrossRef]
- Hayashi, I.; TM, Y. o.; T, K. ; I. , K. The study on staunch clip for the treatment by endoscopy. Gastrointestinal Endoscopy 1975, 17, 92–101. [Google Scholar] [CrossRef]
- Magdeburg, R.; Collet, P.; Post, S.; Kaehler, G. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc 2008, 22(6), 1500–1504. [Google Scholar] [CrossRef]
- Bartell, N.; Bittner, K.; Kaul, V.; Kothari, T. H.; Kothari, S. Clinical efficacy of the over-the-scope clip device: A systematic review. World J Gastroenterol 2020, 26(24), 3495–3516. [Google Scholar] [CrossRef]
- Maekawa, S.; Nomura, R.; Murase, T.; Ann, Y.; Harada, M. Complete closure of artificial gastric ulcer after endoscopic submucosal dissection by combined use of a single over-the-scope clip and through-the-scope clips (with videos). Surg Endosc 2015, 29(2), 500–504. [Google Scholar] [CrossRef]
- Spaun, G. O.; Martinec, D. V.; Kennedy, T. J.; Swanström, L. L. Endoscopic closure of gastrogastric fistulas by using a tissue apposition system (with videos). Gastrointest Endosc 2010, 71(3), 606–611. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Esparrach, G.; Lautz, D. B.; Thompson, C. C. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis 2010, 6(3), 282–288. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.; Gotoda, T.; Ono, H.; Oda, I.; Hamanaka, H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 2006, 63(4), 596–601. [Google Scholar] [CrossRef]
- Sachdev, A. H.; Iqbal, S.; Ribeiro, I. B.; de Moura, D. T. H. Use of omental patch and endoscopic closure technique as an alternative to surgery after endoscopic full thickness resection of gastric intestinal stromal tumors: A series of cases. World J Clin Cases 2020, 8(1), 120–125. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wani, S.; Edmundowicz, S. A.; Soetikno, R.; Hammad, H. Feasibility of endoscopic suturing to prevent adverse events and hospitalization after endoscopic submucosal dissection. Endosc Int Open 2020, 8(9), E1212–E1217. [Google Scholar] [CrossRef]
- Kantsevoy, S. V.; Bitner, M.; Mitrakov, A. A.; Thuluvath, P. J. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc 2014, 79(3), 503–507. [Google Scholar] [CrossRef]
- Singh, R. R.; Nussbaum, J. S.; Kumta, N. A. Endoscopic management of perforations, leaks and fistulas. Transl Gastroenterol Hepatol 2018, 3, 85. [Google Scholar] [CrossRef]
- Abdulsada, M.; Sealock, R. J.; Cornwell, L.; Ketwaroo, G. A. Endoluminal vacuum therapy of esophageal perforations. VideoGIE 2020, 5(1), 8–10. [Google Scholar] [CrossRef]
- Arezzo, A.; Verra, M.; Passera, R.; Bullano, A.; Rapetti, L.; Morino, M. Long-term efficacy of endoscopic vacuum therapy for the treatment of colorectal anastomotic leaks. Dig Liver Dis 2015, 47(4), 342–345. [Google Scholar] [CrossRef]
- Brangewitz, M.; Voigtländer, T.; Helfritz, F. A.; Lankisch, T. O.; Winkler, M.; Klempnauer, J.; Manns, M. P.; Schneider, A. S.; Wedemeyer, J. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013, 45(6), 433–438. [Google Scholar] [CrossRef]
- Tavares, G.; Tustumi, F.; Tristão, L.S.; Bernardo, W.M. Endoscopic vacuum therapy for anastomotic leak in esophagectomy and total gastrectomy: A systematic review and meta-analysis. Dis. Esophagus. 2021, 34, doaa132. [Google Scholar] [CrossRef] [PubMed]
- Lamazza, A.; Sterpetti, A. V.; De Cesare, A.; Schillaci, A.; Antoniozzi, A.; Fiori, E. Endoscopic placement of self-expanding stents in patients with symptomatic anastomotic leakage after colorectal resection for cancer: long-term results. Endoscopy 2015, 47(3), 270–272. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J. L.; Louie, B. E.; Farivar, A. S.; Vallières, E.; Aye, R. W. Fully covered self-expanding metal stents are effective for benign esophagogastric disruptions and strictures. J Gastrointest Surg 2013, 17(12), 2045–2050. [Google Scholar] [CrossRef] [PubMed]
- Moyes, L. H.; Mackay, C. K.; Forshaw, M. J. The use of self-expanding plastic stents in the management of oesophageal leaks and spontaneous oesophageal perforations. Diagn Ther Endosc 2011, 2011, 418103. [Google Scholar] [CrossRef] [PubMed]
- Kotzampassi, K.; Eleftheriadis, E. Tissue sealants in endoscopic applications for anastomotic leakage during a 25-year period. Surgery 2015, 157(1), 79–86. [Google Scholar] [CrossRef] [PubMed]
- Böhm, G.; Mossdorf, A.; Klink, C.; Klinge, U.; Jansen, M.; Schumpelick, V.; Truong, S. Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined vicryl plug and fibrin glue. Endoscopy 2010, 42(7), 599–602. [Google Scholar] [CrossRef] [PubMed]
- Pramateftakis, M. G.; Vrakas, G.; Kanellos, I.; Mantzoros, I.; Angelopoulos, S.; Eleftheriades, E.; Lazarides, C. Endoscopic application of n-butyl-2-cyanoacrylate on esophagojejunal anastomotic leak: a case report. J Med Case Rep 2011, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Willingham, F. F.; Buscaglia, J. M. Endoscopic Management of Gastrointestinal Leaks and Fistulae. Clin Gastroenterol Hepatol 2015, 13(10), 1714–1721. [Google Scholar] [CrossRef]
- Victorzon, M.; Victorzon, S.; Peromaa-Haavisto, P. Fibrin glue and stents in the treatment of gastrojejunal leaks after laparoscopic gastric bypass: a case series and review of the literature. Obes Surg 2013, 23(10), 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, J. Y.; Jung, H. Y.; Lee, J. H.; Choi, K. S.; Kim, D. H.; Choi, K. D.; Song, H. J.; Lee, G. H.; Kim, J. H.; et al. Clinical outcomes of endoscopic and surgical management for postoperative upper gastrointestinal leakage. Surg Endosc 2013, 27(11), 4232–4240. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).