1. Introduction
As safety concerns have been alleviated by improved technology and cost concerns by increased efficiency, microwave applications have expanded beyond food cooking to include communications, the development of novel medical treatments and biosensor diagnostics.
Microwaves (MW) are a part of the electromagnetic (EM) spectrum characterised by frequency, wavelength and energy. There are many different MW frequency bands that have been allocated for industrial, scientific and medical (ISM) use, for which equipment is readily available, as shown in
Table 1.
Interactions of the EM field with the human body have been utilized in medicine,
e.g., cardiology, oncology, physiotherapy, and urology since late 1970s. Currently, EM fields are frequently used in a few well-established medical procedures. Good examples in the area of medical diagnostics are computed tomography (CT) and magnetic resonance imaging (MRI) while in the area of therapy, electrosurgery and radiofrequency (RF) heating in physiotherapy and microwaves (MW) are being used [
2].
According to the purpose of how MW are used, the medical applications of MW can be divided into three main groups:
- -
Diagnostics based on permittivity measurements such as MW differential tomography, UWB radar technology, and MW radiometers;
- -
Part of the medical equipment, for example microwave technology as the basis of the linear accelerator;
- -
Therapy, mostly so-called thermal effects are being used.
Medical treatment applications of MW and RF are represented mainly by those based on thermal effects, which can be divided into several basic modalities with respect to the goal temperature level or interval:
- -
Diathermia: this means mild heating up to a maximum of 41°C, e.g. in physiotherapy. The therapeutic effect is based on the principle of heating biological tissue, usually only up to 41 °C. It is used to treat pain in certain rheumatic and degenerative diseases and to treat chronic inflammation resistant to antibiotics, often in rehabilitation and physiotherapy;
- -
Hyperthermia: clinically mostly used in oncology, which means increasing the temperature in the tumour area up to 41–45°C. The biological principle exploits the fact that certain tumour cells are very sensitive to temperatures above 41°C, while normal cells generally survive elevated temperatures up to 45°C. Heating the tumour region to temperatures of 41-45°C can therefore selectively destroy tumour cells;
- -
Thermoablation: this means raising the temperature above 45°C. It is used, for example, in urology for the treatment of BPH and in cardiology for the treatment of fibrillation and/or arrhythmia;
- -
Thermocoagulation - heating to temperatures much higher than 45 °C, usually around 70 °C. An example is MW treatment of benign prostate hyperplasia (BPH), which can replace complicated surgery.
High frequency EM fields can penetrate and propagate through the human body. As EM waves propagate through biological tissues, their energy is gradually absorbed and converted into heat, increasing the temperature of the irradiated area. To such a wave, biological tissue behaves like a lossy dielectric. As MWs pass through the biological tissue, their energy is absorbed and converted to heat, resulting in an increase in the temperature of the biological tissue within the irradiated area. The spatial distribution of the temperature in relation to the depth of MW penetration and the depth of effective treatment depends on several factors, the most important of which are:
- -
The frequency of the wave, which determines the EM wavelength and the penetration depth;
- -
The spatial distribution of the biological tissue in the irradiated volume;
- -
The dielectric and thermal characteristics of each tissue type in a given area;
- -
Blood flow in the treated area.
Microwave (MWA) and radiofrequency (RFA) thermoablation are minimally invasive technologies that use a local increase in temperature to induce the denaturation of cellular membrane proteins and kill cells. These procedures can be used for cancer treatment [
3], pain management [
4], cardiac arrhythmias [
5], benign tumours [
6] and other applications [
7,
8].
There are several differences between the two technologies, which do not mean that one is always more effective than the other [
9]. RFA is a well-established treatment with a long track record of success since the 1990s [
10], while MWA has only been used since the 2000s [
11] and on a large scale in the last decade. Despite this, MWA is a faster technique that can be used more efficiently to treat larger tumours [
12].
Due to the lack of a standard procedure [
13] and the continuous improvements in technology and applications [
14], the skill and experience of the physician play a fundamental role in the choice of procedure type, although this means a greater burden of responsibility and less repeatability of results.
The aim of the work was to determine the influence of different parameters related to the RFA and MWA that facilitate the work of the operator in choosing the most appropriate set of parameters for each clinical case. By observing the size of the ablated areas in ex-vivo tissues, i.e. chicken breast and bovine liver, and correlating it with the MW power used and the intervention time, an ablation reference matrix was obtained that can guide the clinician's choice, after scaling the dimensions based on the tissue type and the expected blood perfusion.
2. Experimental set-up and method
2.1. Microwave and Radiofrequency Generators, Control Circuit and Probes
The wide range of applications makes the market for RFA and MWA equipment extremely buoyant, with strong growth prospects over the next few years. One of the focal points in the development of these technologies and their diffusion is the introduction of solid-state generators. Unlike magnetron-based generators, which use vacuum tubes, in solid-state MW generators the electrons move inside semiconductor materials, i.e. transistors such as LDMOS (laterally diffused metal oxide semiconductor) and GaN (gallium nitride). A solid-state MW generator can generally be broken down into five main blocks, namely the signal source, low power signal conditioning, amplifiers, the combining stage and the power detector. The integration of functions such as frequency sweeping, phase shifting or power control depends on the inclusion of specific devices within the generator.
Similar to industrial magnetron-based MW generators, a solid-state generator can deliver power in continuous wave (CW) or pulsed form, and is capable of producing an excellent frequency spectrum over the full power range from the very first watts. The solid-state MW generators are significantly smaller than magnetron-based generators, are more reliable, have a longer operating life, are easier to handle and offer a higher level of control. Other advantages include frequency and phase variability and control, and better compatibility with other electronic circuitry.
2.1.1. Microwave Generator
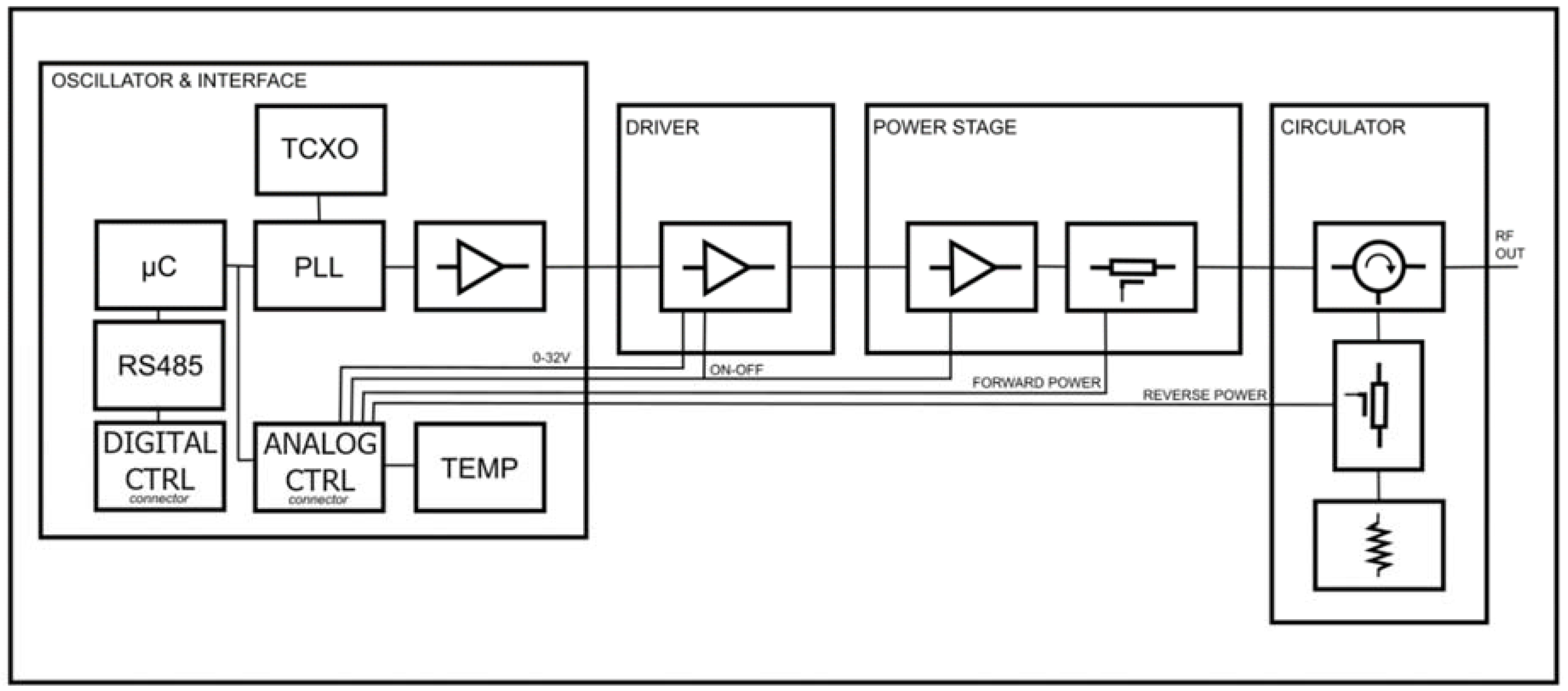
The MW generator used in this study is shown in
Figure 1; the generator operates at frequencies between 2400 MHz and 2500 MHz and provides up to 250 W of MW power. All the stages required for generation and amplification are contained in an aluminium box mounted on an air-cooled heat sink. The selected frequency is generated by a phase-locked loop (PLL) oscillator, which can provide a stable signal over temperature variations. The signal is then pre-amplified prior to the driver stage, which uses an LDMOS amplification system to raise the signal level to a level suitable for the final power stage. All stages are temperature compensated by a bias circuit. At the end of the amplification chain is the output circulator, which isolates the power stage from any reflected power due to mismatch conditions, protecting it and diverting the power to an internal load.
Using a suitable directional coupler, it is possible to measure the forward and reflected power, opening up several possible algorithms to manage the control level in order to obtain a stable power output in time and temperature, also taking into account the integrity of the system, which can protect itself and the connected system (like a catheter in a patient) from dangerous outputs. These control systems can be digital or analogue, in order to be able to react quickly to certain possible hazardous scenarios. Solid-state technology also allows a signal to be delivered at a single frequency that is optimally matched to the connected load, allowing maximum energy transfer.
2.1.2. Radiofrequency Generator
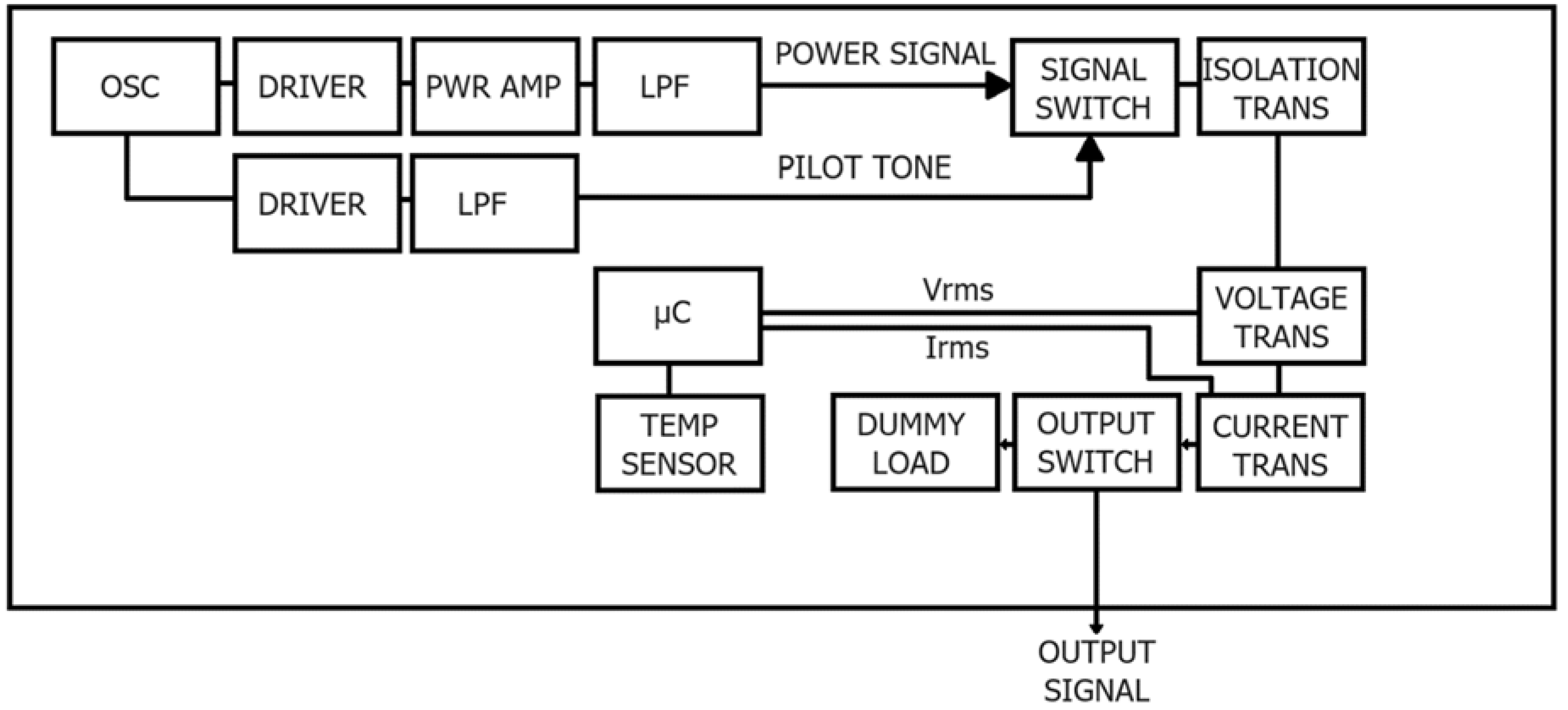
The RF generator in
Figure 2 operates at a frequency of 480 kHz and can deliver up to 100 W of RF power. The frequency is generated by a quartz oscillator and amplified by two different gain chains to provide the power signal for therapy and the pilot tone for impedance measurement. The potential-free output is provided by the isolation transformer to reduce the risk of creating unwanted alternative current paths, which can lead to unwanted burns in many parts of the patient.
Power and impedance measurements are obtained from the RMS voltage and current measured directly by dedicated transformers and detectors. The output can be routed to a dummy load to perform internal tests for accuracy of readings and ability to deliver rated power.
2.1.3. Hybrid Microwaves and Radiofrequency Generator
The dual frequency generator shown in
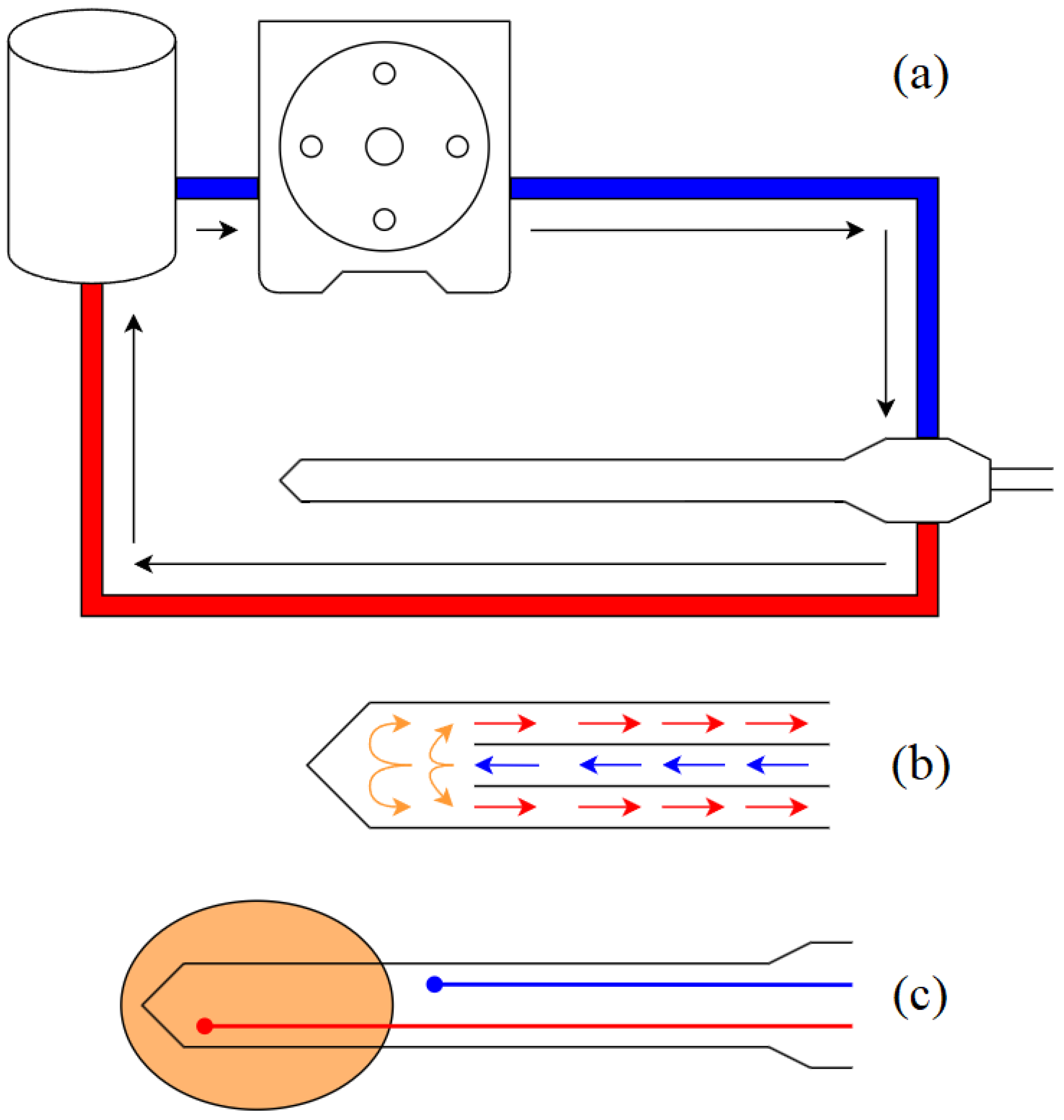
Figure 3 is capable of delivering microwave power up to 250 W at 50 Ω at a frequency of 2450 MHz and up to 50 W at 100 Ω RF power at a frequency of 480 kHz. The main feature of the unit in RF mode is the management of the output power as a function of the measured tissue impedance and temperature profile. In MW mode, the generator uses a low RF power level for measuring tissue impedance only, without increasing tissue temperature. The unit includes a peristaltic pump,
Figure 4a, for cooling the probe/catheter with saline solution. Energy is delivered to the tissue in continuous wave (CW) mode and the temperature of the ablation volume and the cooled zone is measured and used to control the power output of the hybrid generator.
2.1.4. Radiofrequency and Microwave Probes
The RF probe consists of two cables that pass through the applicator to connect to two electrodes whose size and spacing determine the shape of the ablation volume. The MW probe consists of a coaxial cable and a coaxial connector that acts as an antenna. Due to the unbalanced nature of coaxial cables, current could flow along the surface of the outer conductor, causing unwanted heating along the cable [
15]. Therefore, the antenna must be properly matched to the load for maximum energy transfer and must have a system to suppress current flow along the outer conductor. Both probes incorporate a micro-hydraulic circuit, shown in
Figure 4b, for shaft cooling to prevent unwanted heating of tissue outside the target volume caused by the Joule effect associated with conductor losses. The probes also have two thermocouples -
Figure 4c - one near the tip to monitor the ablation temperature and one immediately after the ablation zone, where the tissue temperature must not increase for any reason, to protect tissue outside the target volume.
2.2. Experimental Method
In order to obtain an ablation matrix that can guide the physician in selecting the correct set of parameters based on the clinical case, it is necessary to use equipment capable of providing reliable and repeatable results.
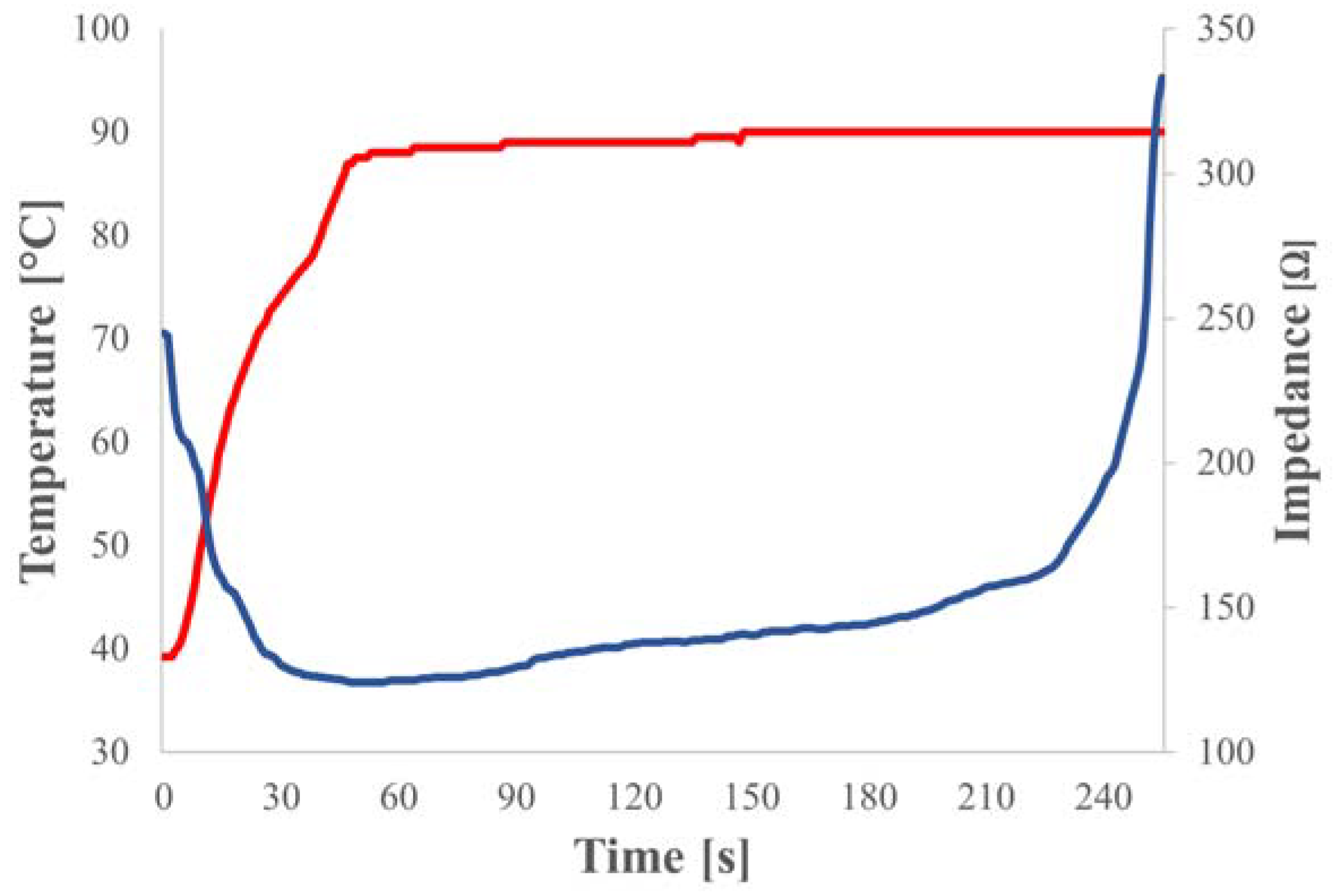
During the tests, as shown in
Figure 3, the ablation temperature was controlled by closed-loop algorithms that matched the delivered EM power to the desired temperature profile as measured by a thermocouple sensor embedded in the probe. Highly accurate and repeatable results were achieved thanks to the extreme flexibility and reliability of the solid-state technology used for both RFA and MWA testing.
The selected ex-vivo tissues on which the procedures have been performed are chicken breast and bovine liver. These tissues have been preheated to start each procedure at a temperature of approximately 37 °C to simulate as closely as possible the environment inside a living body.
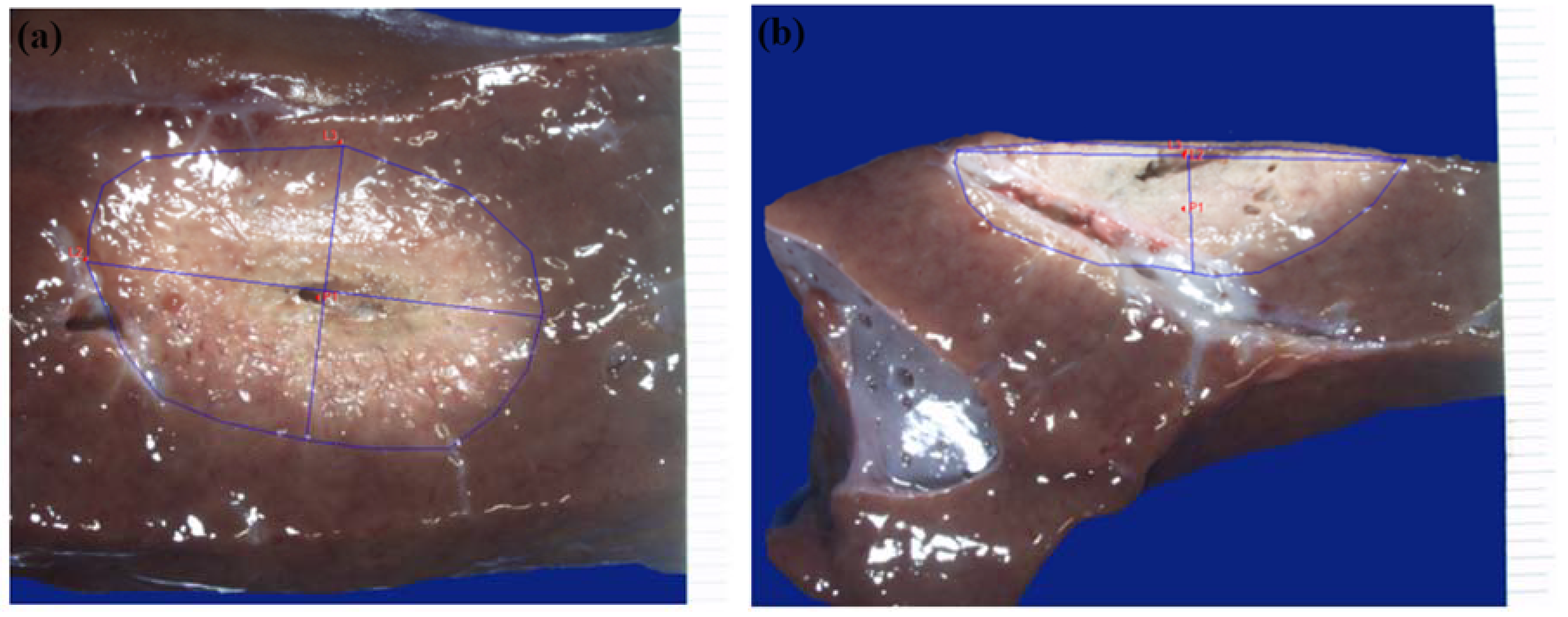
The liver specimens were sectioned in relation to the ablation area and the section of liver where the ablation area was more obvious was photographed using a dimensional marker with a Leica Z6 APO stereoscopic microscope. Macroscopic examinations were performed to assess the absence of carbonisation and tissue integrity. Morphometric analyses were performed on the image using the Image-Pro Insight program to calculate the ablation area and the A and B dimensions -
Figure 5a. After the initial morphometric analysis, a second cut was made halfway through the ablation using a scalpel to determine the centrality of the ablation. The section of liver where the ablation area is more obvious was photographed with a dimensional marker using a Leica Z6 APO stereoscopic microscope. Morphometric analyses were performed on the images using the Image-Pro Insight program to calculate the roundness index and the A and B dimensions,
Figure 5b.
After macroscopic examination, a sample containing the lesion and surrounding non-ablative tissue was taken from all sections of the ablated liver. A further 'control' sample was taken from an area of liver away from all ablation procedures. Each section was embedded in paraffin and serially sectioned at 6-7μm using a Leica RM2155 microtome. Sections were stained with haematoxylin and eosin (morphological stain to highlight tissue components). Slides were observed using a Nikon Eclipse E600 biological microscope and photographed using a Leica DFC450 camera.
3. Results and Discussion
Looking at data from different national registries [
16,
17], the number of patients treated by RFA or MWA ranges from a few thousand to several tens of thousands per country. The average cost of disposable equipment for each procedure ranges from a few thousand dollars to several thousand dollars [
18]. In addition, an increasing number of patients are candidates for non-pharmacological therapies, especially for cardiovascular diseases such as atrial fibrillation; the cost of each ablation system can range from tens to hundreds of thousands of dollars. As a result, the medical ablation market appears to be booming and has certainly not yet peaked.
Thermal ablation aims to heat tissue to a temperature of >60 °C to denature its proteins and induce coagulative necrosis of the cells. The technological challenge is to cover the entire volume occupied by the lesion in the shortest possible time, while trying to preserve the surrounding healthy tissue. For this reason, the most typical application of this therapy uses needle or catheter probes introduced into the target tissue by minimally invasive surgery [
19].
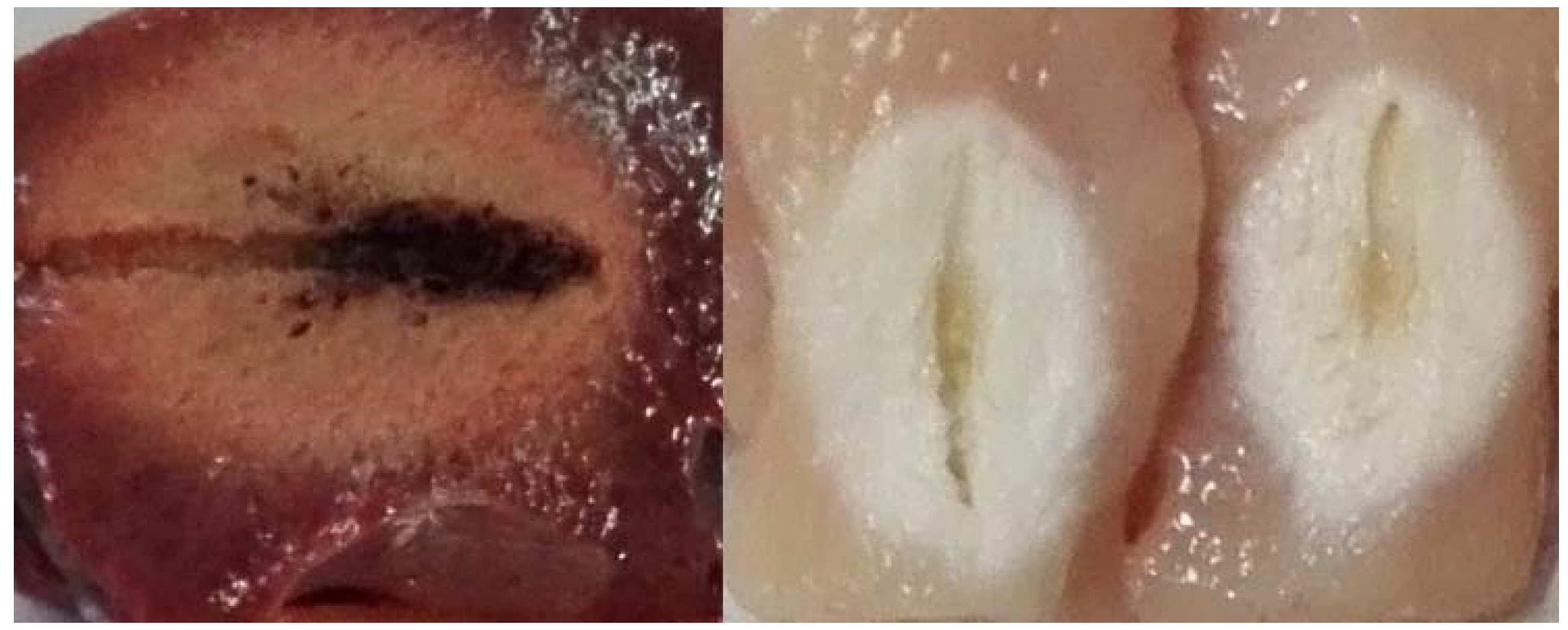
Although the principle behind the effectiveness of the therapy is the same, there are significant differences between the two technologies that must be taken into account in order to make a rational choice based on the specific clinical case. The heating in RFA is due to the joule associated with the current flow, which is based on the presence of dissolved ions in the fluids perfusing the tissue. This means that as the tissue dries, it conducts less and less current until it becomes charred as shown in
Figure 6. For this reason, RFA should be supported by impedance control algorithms to avoid dehydrating tissue too quickly before the target reaches the desired temperature and necrosis.
MWA transfers EM energy to the polar molecules of the tissue (mainly water), raising their temperature. This makes the technique less susceptible to drying phenomena and allows a larger volume to be heated uniformly in less time [
20].
While it may seem that MWA is always more effective than RFA, there are some details that should not be overlooked. Firstly, the costs associated with RFA are lower due to the greater simplicity and wider adoption of the technology. In addition, RF conductive coupling is very useful when it is desired to selectively ablate heterogeneous tissue, as the current will always follow the path with the lowest impedance,
e.g. very effective for ablation of bone or spinal metastases [
21]. Another advantage of RFA is the ability to create applicators of very different shapes to better tailor the ablation pattern to the application, as well as the ability to greatly reduce the size of the probe by eliminating the need to use coaxial cables.
Overall, RFA is less expensive and very effective for performing controlled ablations with minimally invasive probes, such as cardiac pathologies, small lesions, tissues with particular composition, while MWA is optimal for performing large ablations in highly vascularised tissues, such as liver cancer, where it is necessary to achieve higher temperatures.
Based on previous experience with different applications on different organs and tissues, the aim has been to perform ablations as large as possible and with the highest roundness index. This is because the uniform size of a sphere lends itself very well to operations that aim to hit only the target tissue, sparing nearby healthy tissue as much as possible. This is not easily achieved due to the tail effect associated with unwanted heating of the part of the catheter closest to the emission zone, or even due to a change in the electrical properties of the ablated tissue, which no longer allows the shortest path between the poles to coincide with the preferred signal path.
The roundness index is a value between 0 and 1; a high value of this index represents a tendency for the shape to be spherical and can be calculated as the ratio between two ablation diameters. Assuming that the tissue is homogeneous, and given the difficulty of measuring both the transverse and longitudinal diameters due to the incision that must be made on the ablated tissue, the two are reasonably assumed to be equal, as shown in
Figure 7. This leads to the planar roundness index (Eq. 1) being considered as the most representative of the sphericity of the ablation.
Procedures were performed using a closed-loop temperature control algorithm with the maximum output power set and the procedure duration fixed at 10 minutes. The algorithm runs a constant temperature ramp until the target is reached. Since protein denaturation begins at around 60 °C and the boiling temperature of water is around 100 °C, a good target to set is 95°C to heat the tissue as much as possible without creating bubbles that may mismatch the load in MWA mode or reduce the contact area with the RFA electrodes.
As predicted by theory, the results in
Table 2 show higher roundness index and size in MWA. In general, all sizes of ablated tissue are smaller with RFA due to the occlusive effect of the dried tissue with its high impedance at radiofrequency. The dimensional difference can also be observed between the results obtained on chicken breast and bovine liver -
Figure 8, due to the different electrical properties and the less homogeneous composition of bovine liver, which has a denser vascularisation that limits heat propagation.
The results obtained are consistent with those documented in the literature [
22] and with the data provided by the main players in the metastasis ablation market using MWA and RFA. Certainly, the performance of the procedures can be significantly increased if the generator, with its thermal profile and power control algorithms, is combined with probes designed with advanced technologies and simulation systems. A good combination of this technology with state-of-the-art probes could significantly reduce procedure times, expose the patient to less risk and maximise healthy tissue preservation.
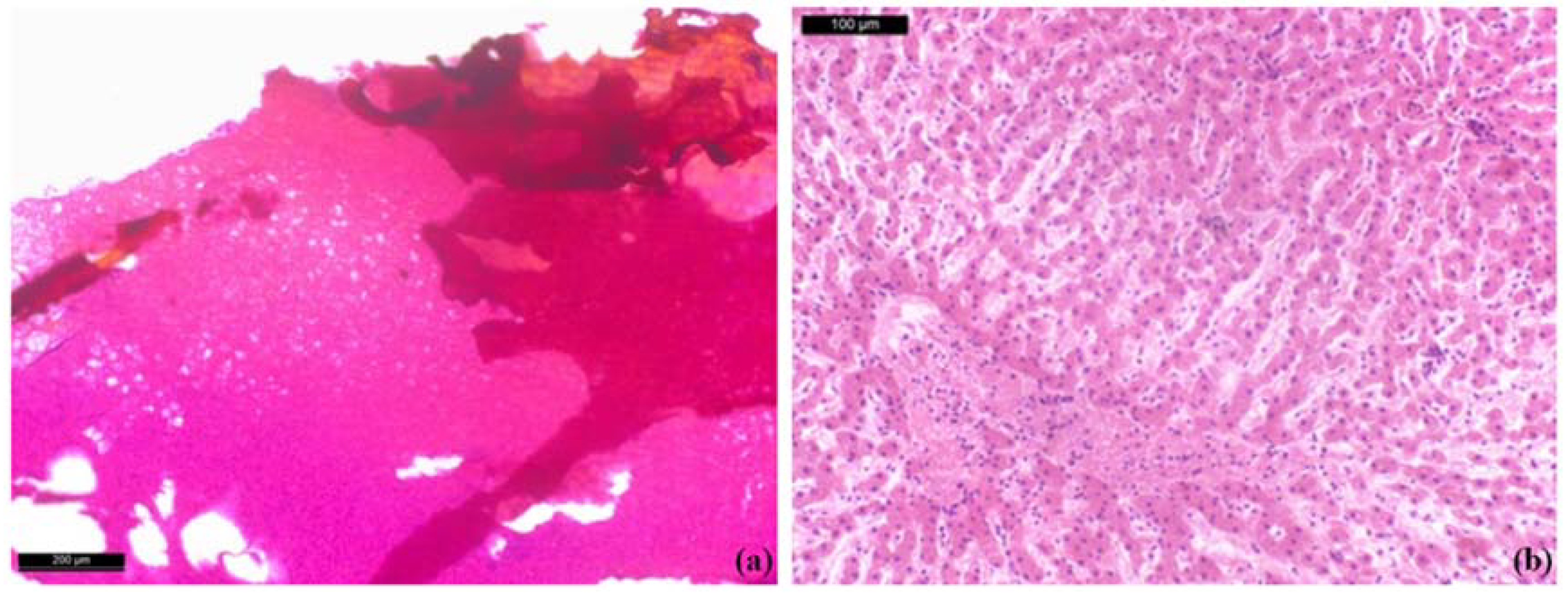
Morpho-histological analysis shows that the MWA and RFA experiments confirmed the safety of the ablation procedures in bovine liver tissue. Macroscopic examination shows whitish ellipsoidal areas due to ablation and no carbonisation was observed in the ablated soft tissue samples. Histologically, near the probe there are some small areas of parenchymal destruction and some areas of coagulative necrosis due to the high temperatures achieved during the ablation process -
Figure 9a. Around the disrupted area there is hyalinisation and vacuolar degeneration of the cytoplasm and the nuclei appear slightly reduced and pyknotic -
Figure 9b.
4. Conclusion
Microwave heating is based on the ability of certain liquids and solids to convert electromagnetic energy into heat. In the context outlined above, this mode of in situ energy conversion directly within tumours sensitive to these waves is of great interest for cancer ablation. In medical applications of microwaves, the parameters to be considered are related to i) the tumour, e.g. volume/mass to be treated, and ii) the equipment, e.g., frequency, type of generator, energy transfer system and its control. One of the key points in the development of adapted ablation equipment and its use is the introduction of solid-state generators and the precision of their frequency and power delivery capabilities, together with higher process control capabilities.
The hybrid dual frequency microwave and radiofrequency generator used in this research has demonstrated increased accuracy due to its proprietary hardware, which incorporates the latest technologies and algorithms to control the emitted electromagnetic power vs. temperature profile of the treated sample. In particular, the hybrid generator incorporates control systems based on impedance or reflected power measurements that allow controlled ablation without causing unwanted carbonisation and without including areas where tissue damage is not desired.
Now that the efficacy of radiofrequency and microwave ablation has been proven and the number of procedures has increased over the years, it is important for the medical industry to focus on improving the performance of these procedures. For radiofrequency ablation, it is necessary to invest in delivery algorithms according to electrical parameters related to tissue hydration. In addition, multi-probe applications can be a good way to increase the volume to be treated with the same procedure time. For microwave ablation, it is necessary to invest in antennas that allow greater customisation of ablation shapes and control of the high temperatures that can be achieved with this technology. The issue of higher cost may be offset over time by a reduction in hospital stay and the likelihood of metastatic recurrence. These improvements can be combined to get the best of each technology, thanks to the use of hybrid generators that allow specific therapy for each case. Following ablation by both radiofrequency and microwave techniques, it would be important for the medical industry to focus on improving the performance of the probes to detect more histologically evident necrosis.
Leanfa is a company based in Italy, member of Muegge group, designing and manufacturing solid-state microwave and radio-frequency generators for Industrial, Scientific, and Medical (ISM) applications. Leanfa has a team of highly qualified engineers, from hardware to software design, with several years of experience in high frequency medical and laboratory devices design. In addition to cutting-edge technologies such as MW and RF generators, Leanfa is specialized in laser, neuromuscular stimulation and ultrasound devices. Leanfa is ISO 9001 and AEOF certified company.
Life and Device is a service company specialized in the study of biocompatibility for medical devices and biological interfaces. Life and Device is specialized in anatomical-morphological, histochemical and morphometric analyses, suitable and diversified based on the study to be conducted and the type of device. The particular specificity of the analyses conducted, the great experience in the sector, the collaboration closely with university structures, able to conduct in vivo studies, and the availability of technologically advanced equipment and tools, make the team one of the most competent in the Community sector of implantable devices. Life and Device is ISO 9001 certified and as a laboratory capable of operating in accordance with GLP principles.
References
- Decareau, R.; Teterson, R. Microwave Processing and Engineering; Ellis Horwood: Chicester, England, 1986. [Google Scholar]
- David Vrba, Jan Vrba, Ondrej Fiser, Jesus Cumana, Milan Babak, Jan Vrba Senior. Applications of Microwaves in Medicine and Biology. [CrossRef]
- G. Carrafiello, D. Laganà, M. Mangini, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. International journal of surgery, London, England, vol. 6, Suppl 1, p. 65-69, Dec. 2008. [CrossRef]
- D.K. Filippiadis, S. Yevich, F. Deschamps, J.W. Jennings, S. Tutton, A. Kelekis “The Role of Ablation in Cancer Pain Relief.” Current oncology reports. 2019, 21, 105. [CrossRef]
- W. Wisser, C. Khanzen, E. Deviatko, et al. “Microwave and radiofrequency ablation yield similar success rates for treatment of chronic atrial fibrillation.”, European Journal of Cardio-Thoracic Surgery: official journal of the European Association for Cardio-thoracic Surgery. 2004, 25, 1011–1017. [CrossRef]
- J. Reis, Y. Chang, A.K. Sharma “Radiofrequency ablation vs microwave ablation for osteoid osteomas: long-term results.”, Skeletal radiology. 2020, 49, 1995–2000. [CrossRef]
- L. Bennardo, I. Fusco, C. Cuciti, et al. “Microwave Therapy for Cellulite: An Effective Non-Invasive Treatment.”, Journal of clinical medicine. 2022, 11, 515. [CrossRef]
- S.J. Goodyear, I.K. Nyamekye “Radiofrequency ablation of varicose veins: Best practice techniques and evidence.”, Phlebology. 2015, 30, 9–17. [CrossRef]
- F. Izzo, V. Granata, R. Grassi, et al. “Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update.”, The oncologist. 2019, 24, 990–1005. [CrossRef]
- D.I. Rosenthal, A. Alexander, A.E. Rosenberg, A.D. Springfield “Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure.”, Radiology. 1992, 183, 29–33. [CrossRef]
- D.E. Dupuy, R.J. Zagoria, W. Akerley, W.W. Mayo-Smith, P.V. Kavanagh, H. Safran “Percutaneous radiofrequency ablation of malignancies in the lung.”, AJR. American journal of roentgenology. 2000, 174, 57–59. [CrossRef]
- K. Suwa, T. Seki, K. Aoi, et al. “Efficacy of microwave ablation versus radiofrequency ablation for hepatocellular carcinoma: a propensity score analysis.”, Abdominal radiology, New York. 2021, 46, 3790–3797. [CrossRef]
- H. Rathke, B. Hamm, F. Güttler, et al. “Comparison of four radiofrequency ablation systems at two target volumes in an ex vivo bovine liver model.”, Diagnostic and interventional radiology, Ankara, Turkey. 2014, 20, 251–258. [CrossRef]
- A. Pfannenstiel, J. Iannuccilli, F.H. Cornelis, D.E. Dupuy, W.L. Beard, P. Prakash “Shaping the future of microwave tumor ablation: a new direction in precision and control of device performance.”, International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2022, 39, 664–674. [CrossRef]
- Y. Mohtashami, H. Luyen, J. F. Sawicki, et al., "Tools for Attacking Tumors: Performance Comparison of Triaxial, Choke Dipole, and Balun-Free Base-Fed Monopole Antennas for Microwave Ablation," IEEE Antennas and Propagation Magazine. 2018, 60, 52–57. [CrossRef]
- N. Molitor, E. Yalcinkaya,A. Auricchio, et al. “Swiss National Registry on Catheter Ablation Procedures: Changing Trends over the Last 20 Years.”, Journal of clinical medicine, Basel, Switzerland. 2021, 10, 3021. [CrossRef]
- J. Criado, A. Quesada, R. Cózar, “18th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2018).”, Revista Española de Cardiología, Alicante, Spain. 2019, 72, 1031–1042.
- T. Hunter, S. Palli, J. Rizzo, “Cost comparison of radiofrequency catheter ablation versus cryoablation for atrial fibrillation in hospitals using both technologies.”, Journal of medical economics. 2016, 19, 959–964. [CrossRef]
- H. Takahashi, B. Kahramangil, E. Berber, “Local recurrence after microwave thermosphere ablation of malignant liver tumors: results of a surgical series.”, Surgery. 2017, 163, 709–713. [CrossRef]
- P. Donlon, M. Dennedy, “Thermal ablation in adrenal disorders: a discussion of the technology, the clinical evidence and the future.”, Current Opinion in endocrinology, diabetes and obesity. 2021, 28, 291–302. [CrossRef]
- M. Eckmann, M. Martinez, S. Lindauer, et al., “Radiofrequency ablation near the bone-muscle interface alters soft tissue lesion dimensions.”, Regional Anesthesia & Pain Medicine. 2015, 40, 270–275. [CrossRef]
- P. Afaghi, M. Lapolla, K. Ghandi, “Percutaneous microwave ablation applications for liver tumors: recommendations for COVID-19 patients.” Heliyon, 2021, 7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).