2. Serial Modifications Added to SRC
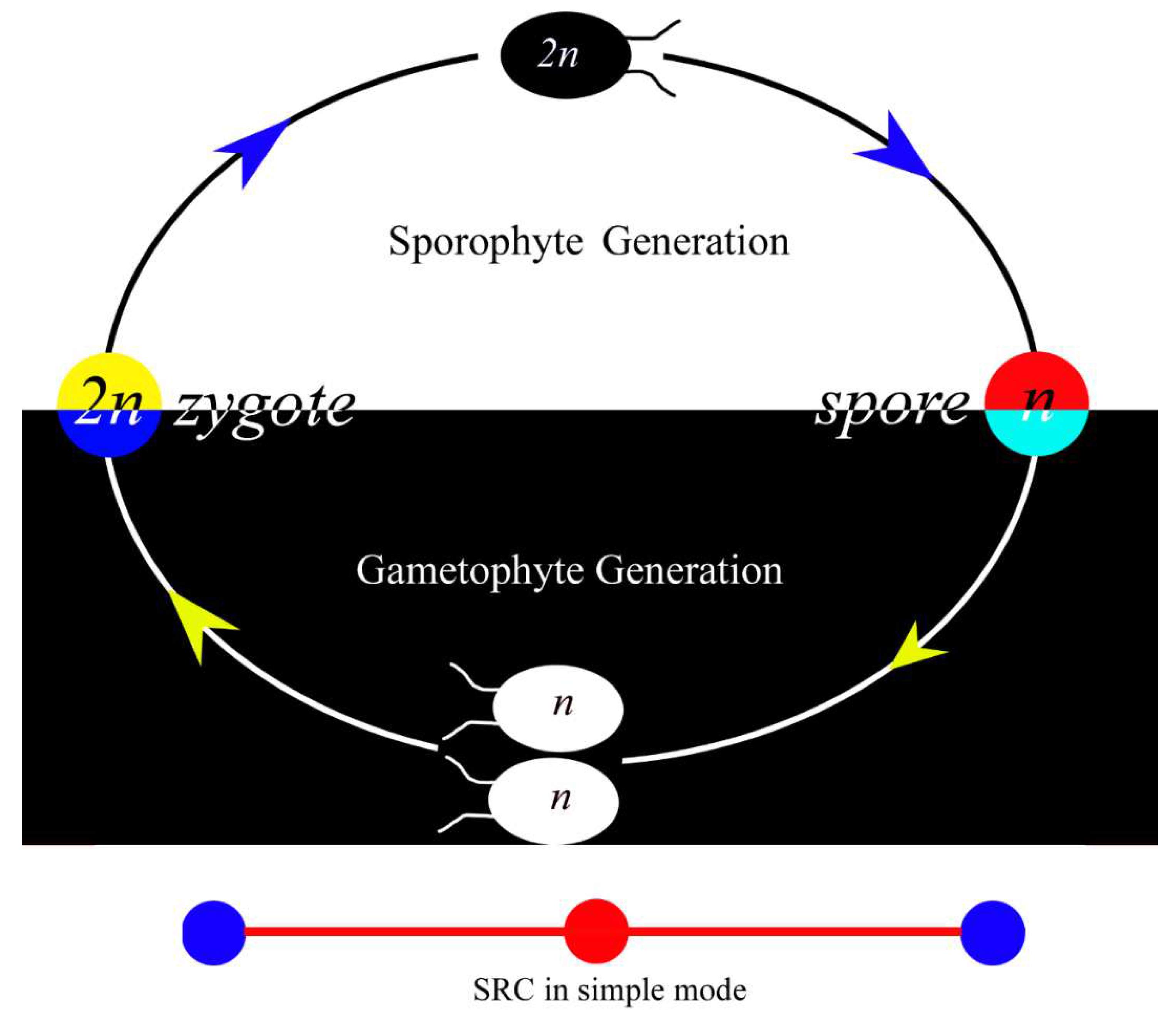
The above described SRC mode is the simplest one. Although simple, it places the foundation for later evolution of plants (and animals). During later evolution, plants add various modifications on this foundation and thus give rise to various plant groups bearing different characteristic organs.
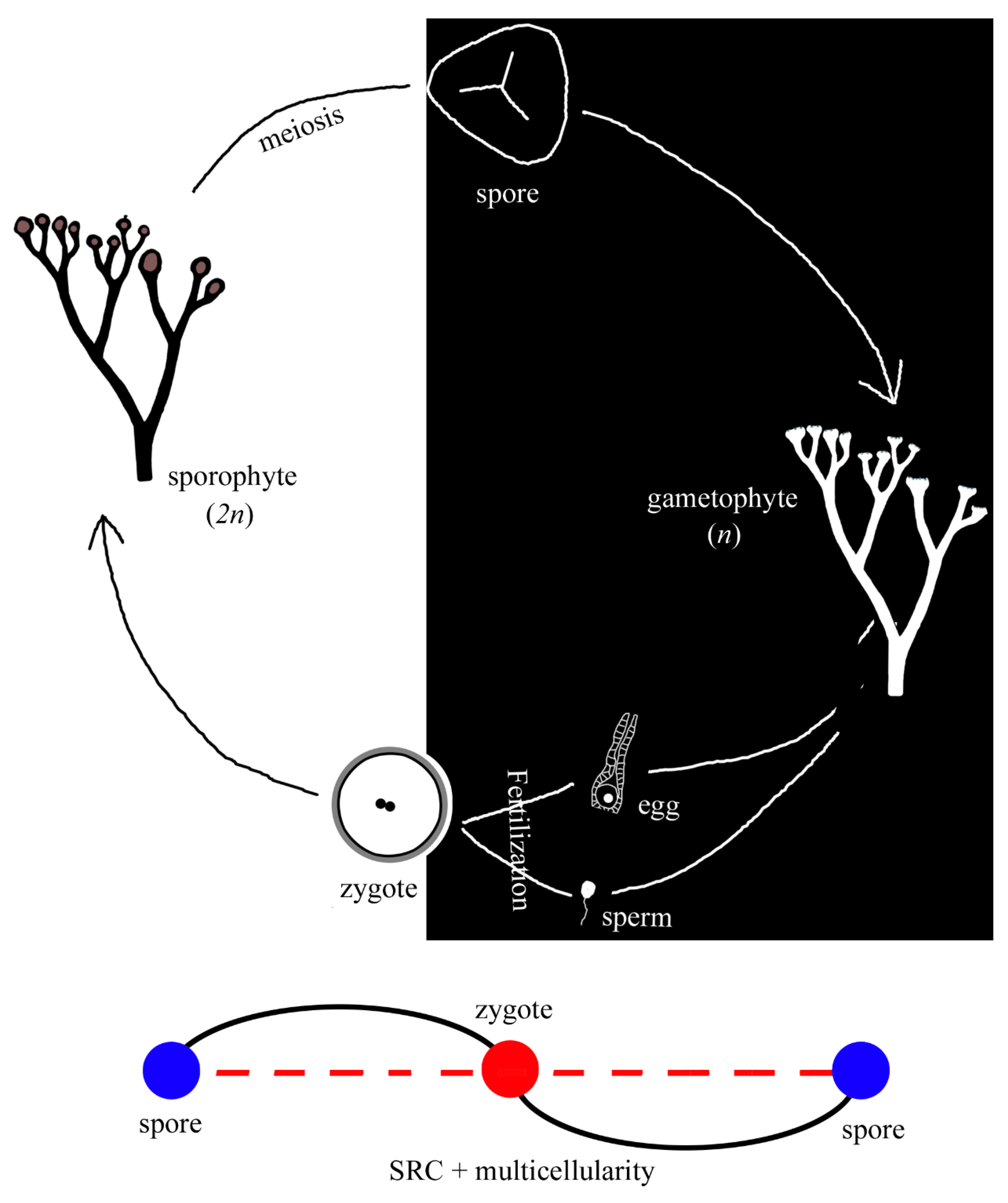
In a favorable condition, an infinite number of the above simple SRC can be connected head-and-tail and thus forms the so-called phylogeny. The negative effects inflicting such a simple SRC mode include that unicellularity restricts the organisms from complications and diversifications. The actual biological history is that organisms did add various modifications to this simple SRC foundation and thus enabled the diversification of organisms.
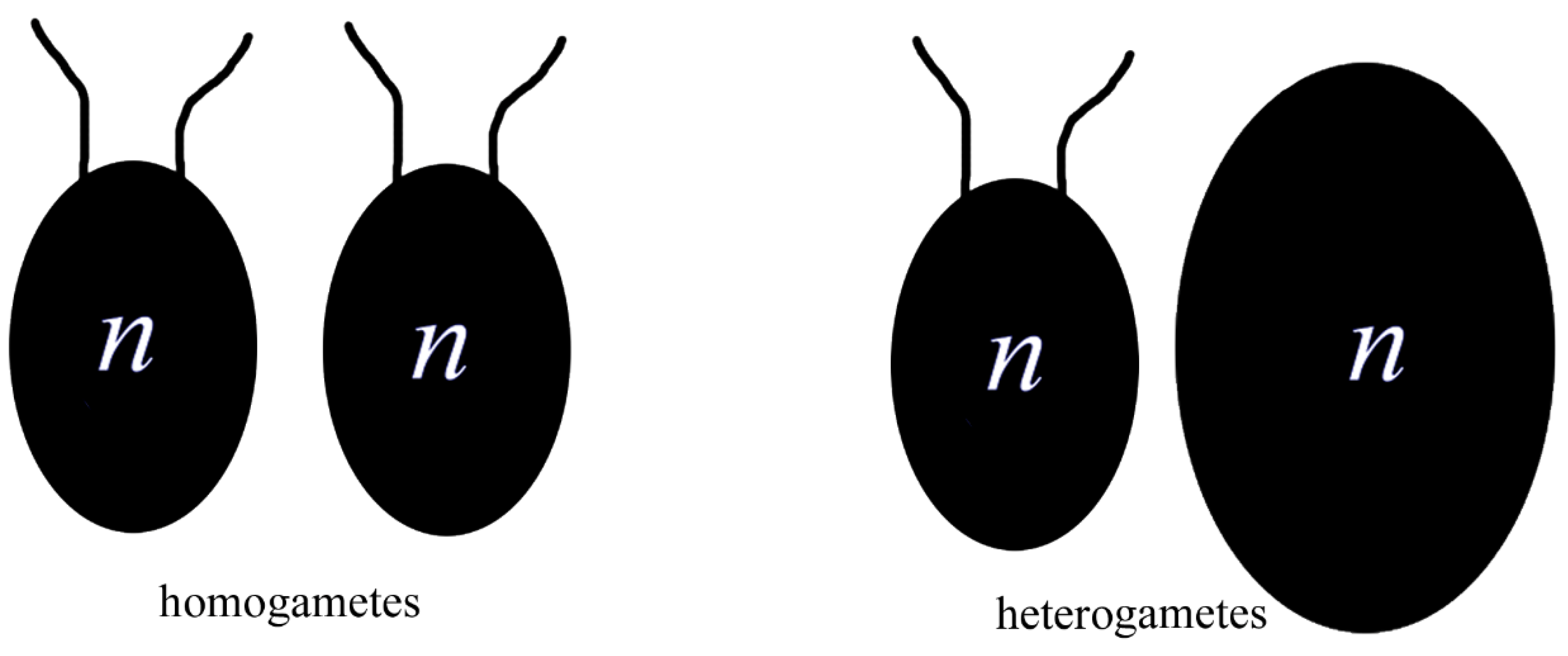
The first important modification added to SRC is
differentiation of gametes, namely, the former isomorphic gametes in
Figure 1 differentiate into two categories, as shown in
Figure 2. The differences between these two categories of gametes may be in various aspects,
e.g. size, form, chemical features, or gene composition. Such differences make two types of gametes distinguishable, which correspond closely to male and female gametes, namely, smaller and more mobile gametes are male, while bigger and more immobile gametes are female. Needless to say, fertilization is restricted between two different gametes. Relative to isomorphic gametes, this differentiation allocates more resources to female gametes, which play a more involving role in the development of offspring. This modification is significant in the history of organisms as it initiates the history of sex and gender in common sense.
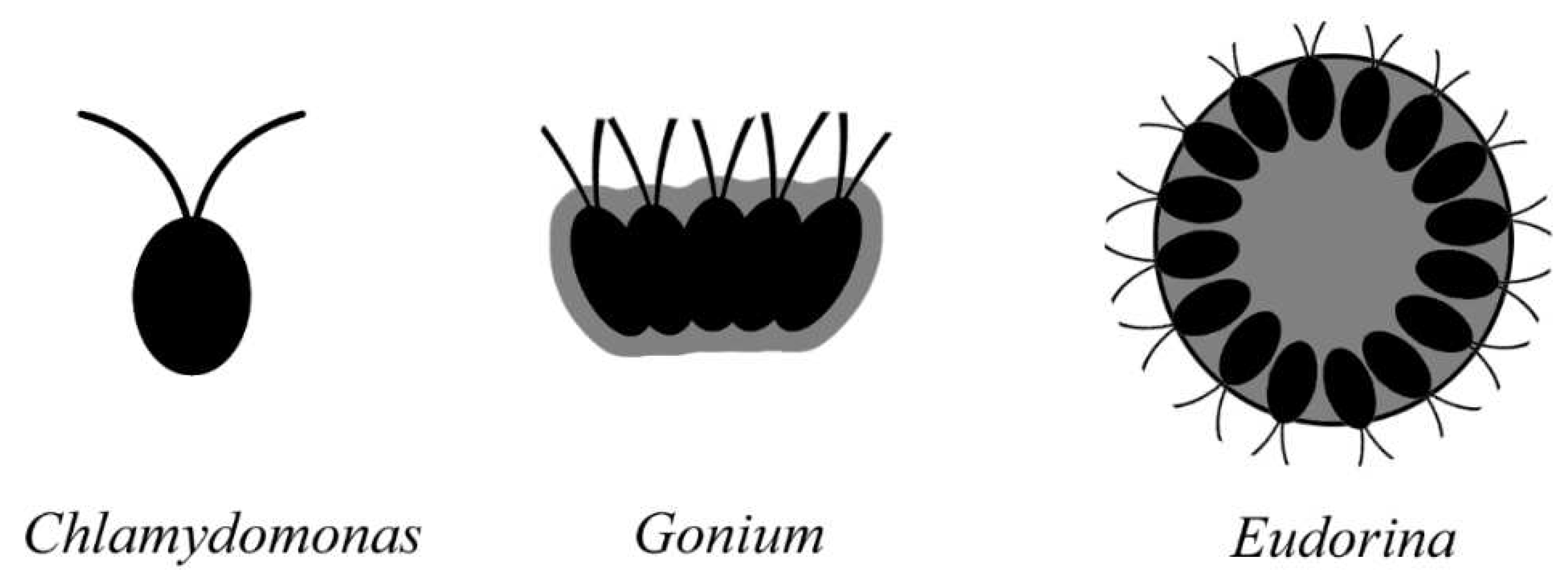
The second important modification added to SRC is
multicellularization, which may have occurred multiple times in the history. Compared to the frequently seen complicated morphs of life, the unique feature of the organisms in
Figure 1 is their unicellularity and simplicity. To derive more complicated morphs of life, mutlicellularity is a must. To carry out this process, a serial unicellular-colonial-multicellular transformations in cell-to-cell adhesion, communication, coordination, and specialization[
2], and probably a multinucleate unicellular progenitor are required[
3]. Multicellularization requires formerly free, independent, simple individual cells to be aggregated into a single organism that has all of its cells integrated, behave and function collectively and coordinately (
Figure 3) [
2]. Apparently, the overhead of such aggregating is that the behaviors of the cells must be coordinated for optimal function. Extracellular matrix (ECM, = cell wall) is a medium that units these cells.
ECM may be taken as a gel mixture of water, polysaccharides, and fibrous proteins, which coordinates cell growth to maintain the morphology of organisms and buffers the effects of local stressors. The macromolecules secreted by adjacent cells are scaffolded into polymers that lend the matrix a viscous consistency. The matrix prevents cellular structures from collapsing and keeps the cells in a tissue separate and physically distinct, although these cells actually remain more or less connected with its adjacent peers through plasmodesmata.
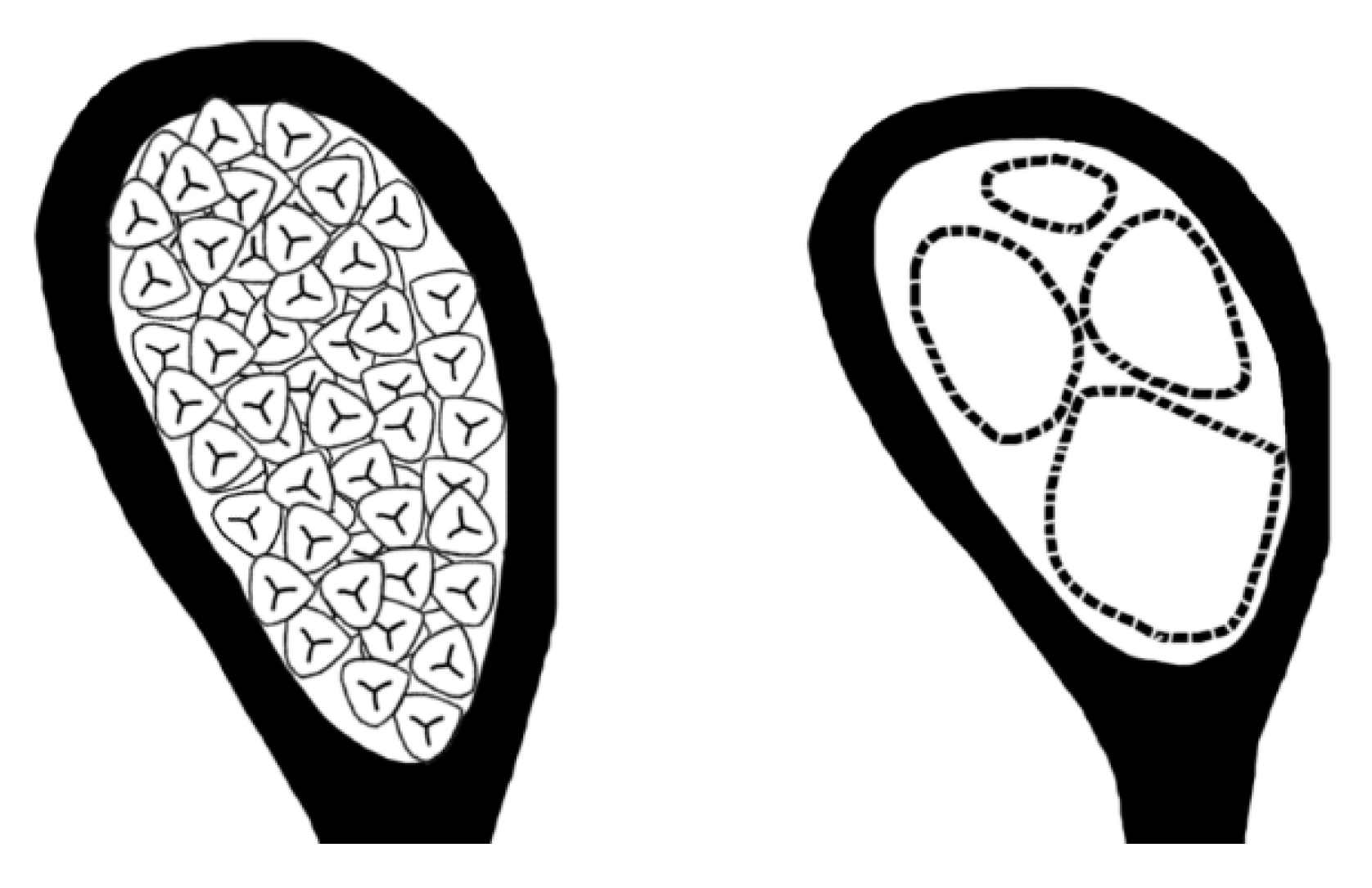
It is inconceivable that organisms always live in favorable environments. In case of unfavorable environment or stress, organisms suspend their physiological activity and generate cysts, in which spores are saved, to preserve their germs for later life. These cysts are cases enclosing spores, homologous to sporangia in spore plants. The occurrence of cysts signifies the third important modification added to SRC,
differentiation between somatic and reproductive parts in organisms, namely, parts of organisms are assigned different functions, becoming somatic parts and reproductive parts, respectively (
Figure 4). The differentiation between these parts can be traced at least back to the Ediacara (600 Ma ago)[
4,
5].
Differentiation of vegetative parts A noticeable feature of earliest vascular plants is that almost all bifurcated branches are fertile, namely, almost every branch bears a sporangium on its terminal. Ensuing evolution introduces differentiation among these initially equal branches, and some of these branches became sterilized and lost their reproductive functions. These sterilized branches underwent various metamorphosis and gave rise to megaleaves. Although this process appears in a direction opposite to what observed in the individual development, it is a historical fact. The functions of these vegetative parts are not restricted to photosynthesis, instead they are recruited to play various auxiliary roles in forming reproductive organs (as seen below).
The differentiation of reproductive parts makes the occurrence of sporangia in the earliest land plants rather expected. In the Rhynie Chert Flora,
Cooksonia plants comprise only branches and sporangia.
Cooksonia and its allies are representatives of land plant pioneers[
6,
7]. Sporangia in these plants are the precursors of sporangia, sori, pollen sacs, anthers, megasporangia, metamegasporangia (= ovules), and the core part in primary, secondary, and various tertiary metamegasporangium complexes. The following is a brief characterization of these novelties in plants.
Sporangia are almost universally isomorphic in sporophytes of early land plants. Just as cysts mentioned above, sporangia protect and ensure the spores within to survive harsh times and revive later, through nutrition supplied through tapetum. The isomorphy is not restricted to general morphology, spores in the sporangia are universally trilete and hard to distinguish each other. When mature, spores escape through a sporangium slit. Those spores that perch in favorable niches germinate and give rise to gametophytes. A gametophyte of early spore plants usually is simple, bearing antheridia/archegonia on its terminals (
Figure 5). When a gametophyte matures, sperms from the antheridia swim through a film of water, fuse with and fertilize the eggs in archegonia. The fusion of a sperm and an egg is termed as fertilization, a process that produces a zygote. A zygote is the starting point of a new sporophyte, which bears terminal sporangia when mature. This completes the SRC of early spore plants (
Figure 5).
Figure 5.
A simple spore plant and its SRC. Note that both sporophyte and gametophyte are similar in morphology. Compared to the SRC primitive mode in
Figure 1, a spore is connected to a gamete by a curve (instead of a straight line as in
Figure 1), as multicellularity is inserted in between.
Figure 5.
A simple spore plant and its SRC. Note that both sporophyte and gametophyte are similar in morphology. Compared to the SRC primitive mode in
Figure 1, a spore is connected to a gamete by a curve (instead of a straight line as in
Figure 1), as multicellularity is inserted in between.
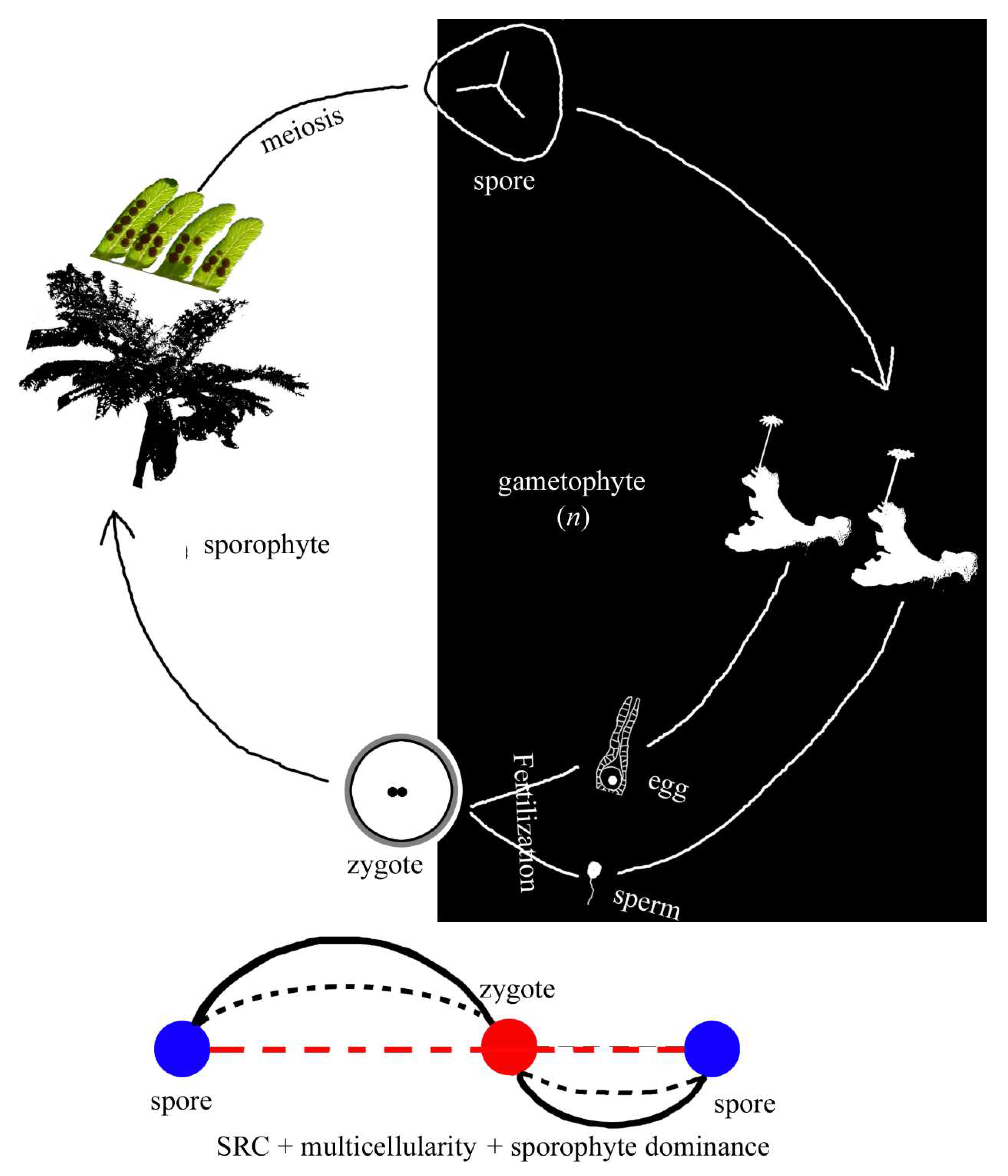
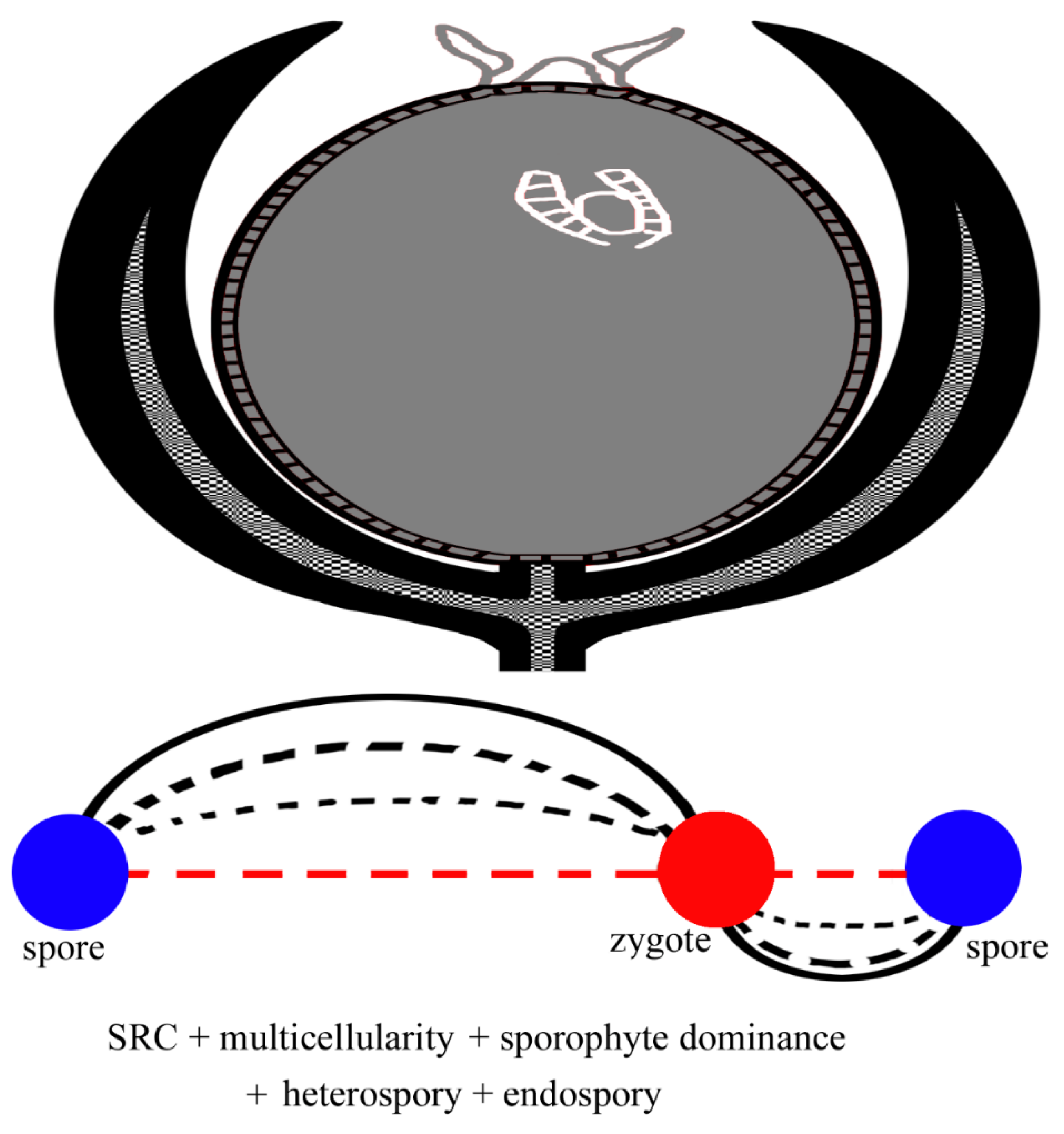
Figure 6.
A fern and its SRC. Note that the sporophyte (generation) is dominant, and the gametophyte (generation) is reduced. Compared to the SRC mode in
Figure 2, the dominance of sporophyte (generation) is added to the SRC.
Figure 6.
A fern and its SRC. Note that the sporophyte (generation) is dominant, and the gametophyte (generation) is reduced. Compared to the SRC mode in
Figure 2, the dominance of sporophyte (generation) is added to the SRC.
The spores in these pioneering land plants are termed
homospores, against those later derived spores of two distinct morphologies, which are termed
heterospores. The occurrence of heterospores in plants signifies the occurrence of
heterospory. Thenceafter, the differentiation of former identical spores into spores of two distinct morphologies (especially sizes) is completed, correspondingly, former sporangia differentiate into
microsporangia and
megasporangia (
Figure 7). The megaspores in megasporangia are fewer in number and each allocated more resources as they are destined to play a more involving role and take more responsibility in the development of offspring. In contrast, microspores in microsporangia are greater in number and each allocated less nutrition as the responsibility for them in reproduction is restricted to passing genes in 1
n chromosomes to ovule to form a diploid zygote.
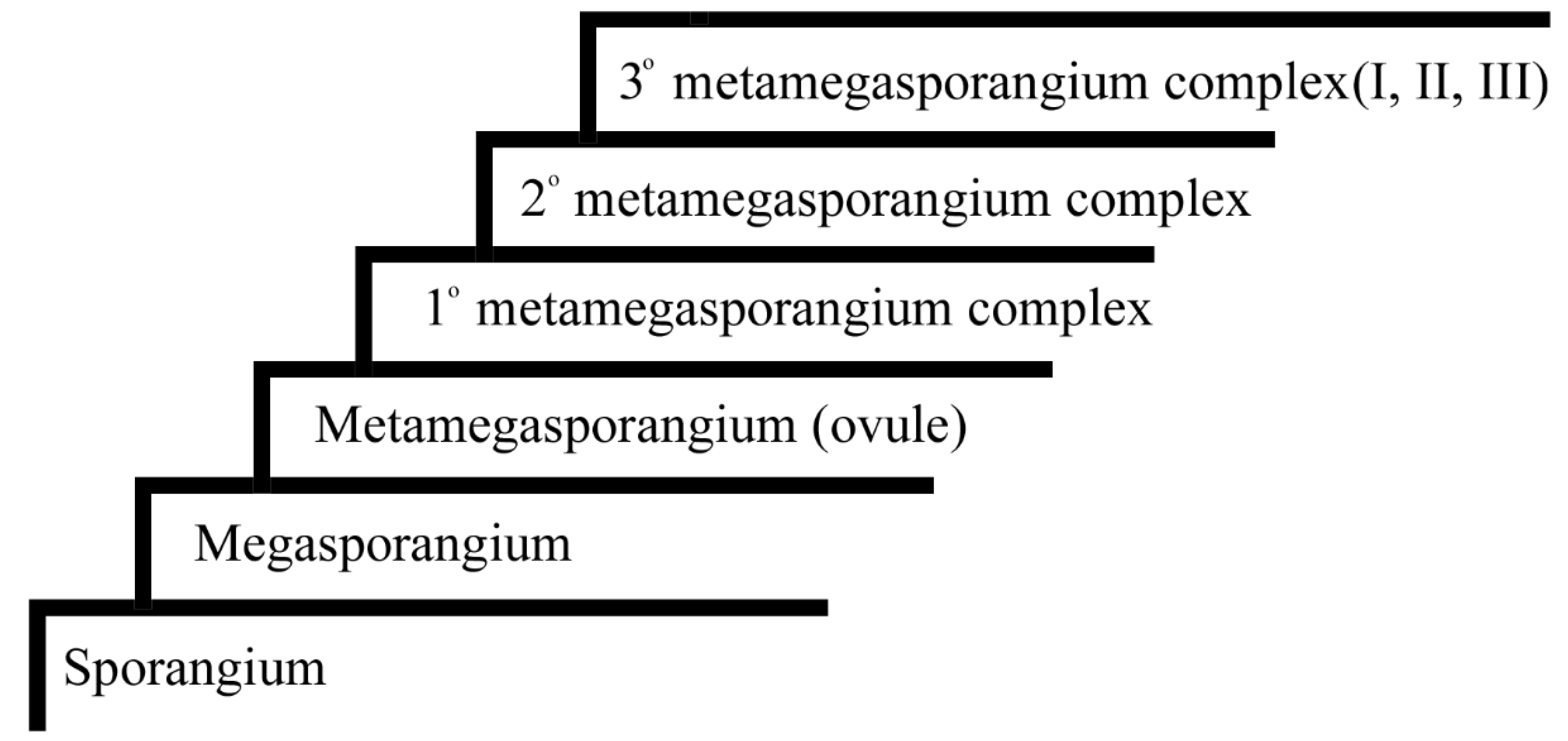
Although each megaspore in megasporangia is allocated more resources, the amount of resources allocated to each megaspore is limited by the dimensions of megaspore itself, as megaspores leave their parent plants, live independently, and are cut from further nutrition supply afterward. Such a limit apparently sets up an upper limit for the vitality of the megaspore and its offspring, endangering the continuity of a phylogeny. Plants lift such a resource limit by evolving a character termed
endospory, which means that mature megaspores are retained in sporangia on their mother plants and mature megaspores remain nutritionally bonded with their mother plant. Endospory extends the time length of the nutritional bond between mother plant and megaspores, providing extra physical protection for megaspores and making the developmental environment of female gametophytes more congenial and controllable until seed maturation. This novel nutritional relationship turns a former megaspore into a
metamegaspore (= an
ovule), which is the developmental precursor of a seed (
Figure 8), and thus gives rise to a new feature of plants,
seed, and initiates a new phylogeny of plants,
seed plants. The occurrence of ovule/seed may be the single most significant event in the evolution of plants, as it led to the great diversity of seed plants (including more than 300,000 species in angiosperms) in the current earth ecosystem. Besides the above mentioned extended nutritional bond, another significant concomitant change is that sporophyte generation becomes more dominant in SRC and of longer time in a SRC, and correspondingly gametophyte generation is more reduced, parasitic on the sporophytes, and of shorter time in a SRC.
An ovule is not strictly homologous or equivalent to a megaspore. Instead, an ovule is homologous or equivalent to a megasporangium and its adjacent accessories (integument or integuments), which are sterilized sporangia and play a protective role for the
nucellus (a megasporangium now centrally positioned) [
8,
9]. Previously independently living gametophytes (in spore plants) are now highly reduced and develop within the nucellus, which continuously derives its nutrition through the nutritional bond from the parent plant (sporophyte) and thus supports the parasitic gametophyte. In this way, a gametophyte, including egg-bearing archegonia, develops and matures within the nucellus. Various pollination mechanisms transfer pollen (= microspores) to the termini of nucellus, where pollen germinate and complete male gametophyte development and finally produce sperms that swim to egg cell in the archegonia within nucellus to fulfill fertilization process. The fusion between a sperm and an egg results in a zygote. This process (fertilization) gives a start to a new sporophyte (zygote) of seed plants. This completes a SRC of a primitive seed plant, such as
Ginkgo and other early seed plants.
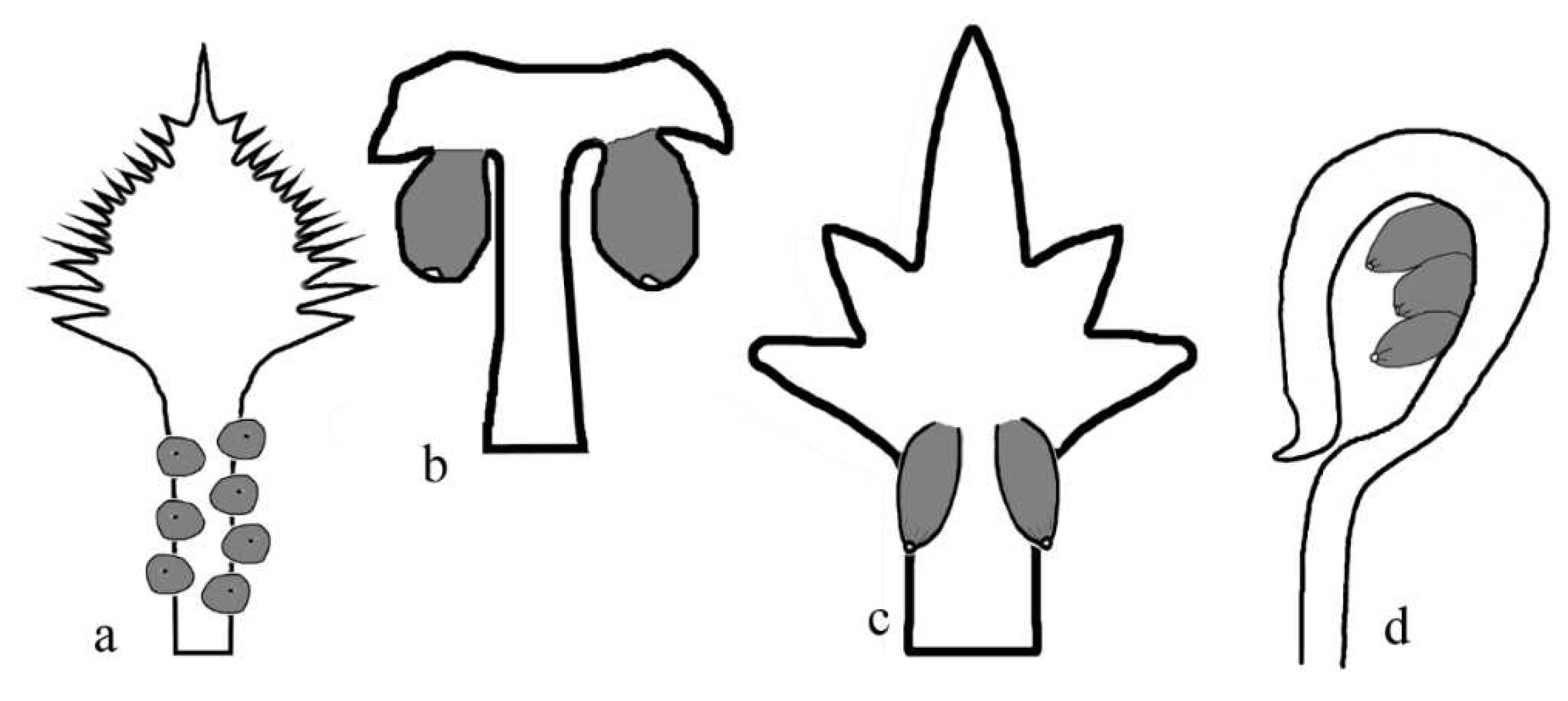
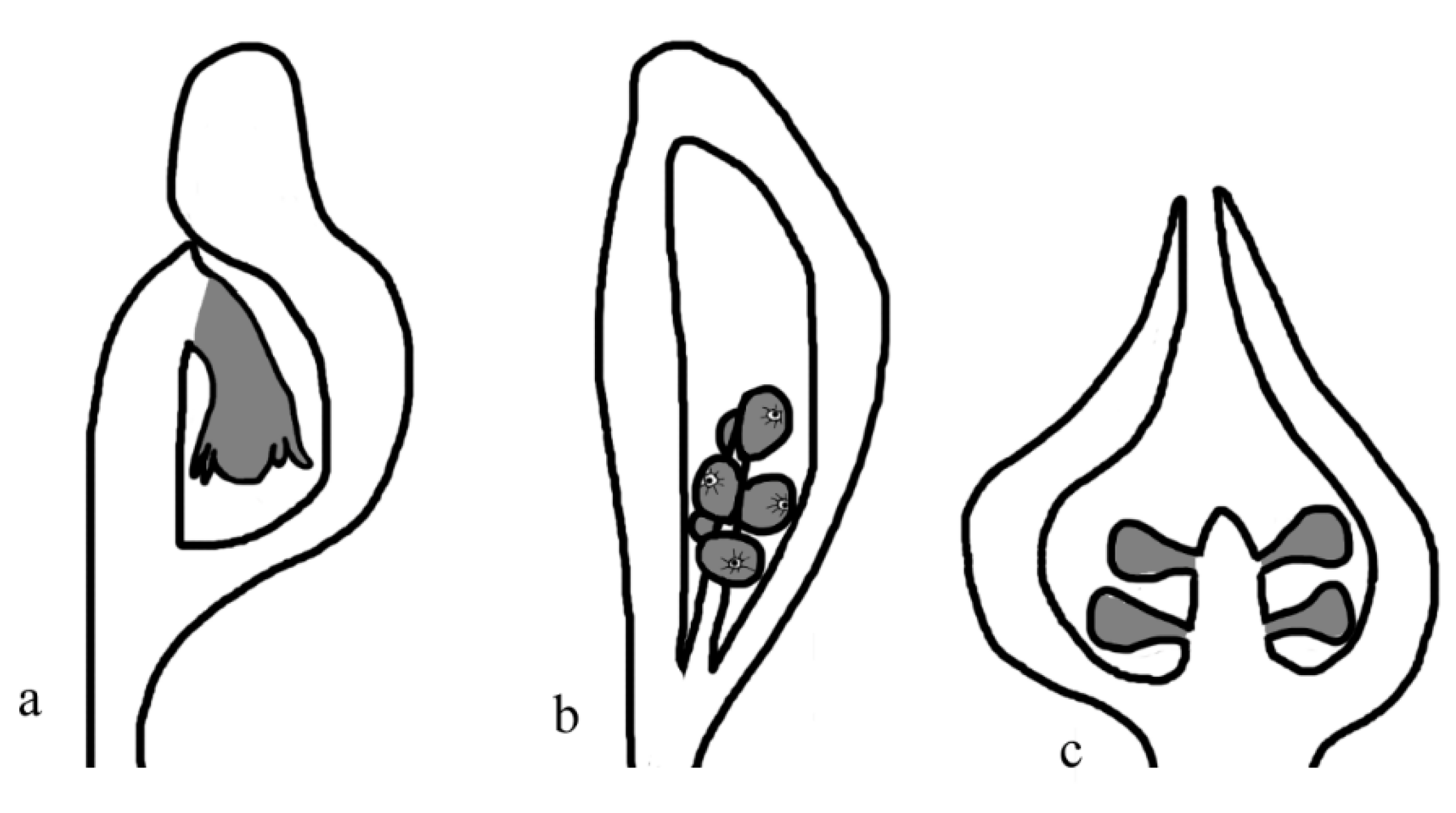
Mature ovules (seeds) are propagules of seed plants. Ovules/seeds in
Ginkgo and early seed plants are all borne on termini of branches and exposed (naked) to the exterior environment, which may be harsh and harmful for the development of ovules/seeds sometimes. Buffering ovules/seeds against potential harms is apparently conducive to their success in survival struggle. It is not surprising that some seed plants, for example, cycads, develop some strategy to shelter their ovules/seeds (especially in the early developmental period). For example, seeds are aggregated on certain branches (frequently termed as sporophylls) of various forms that in turn are aggregated into
strobili, which shelter seeds from harms. In the meantime, seeds in some other seed plants are covered and protected by structures including cupules (
Gnetopsis, Caytoniales, Petriellales, Peltaspermales), interseminal scales (Bennettitales), seed-scales (Coniferales) etc[
10]. Compared to those in
Ginkgo, seeds in these groups are better and more protected (
Figure 9). We term these structures
primary metamegasporangium complexes.
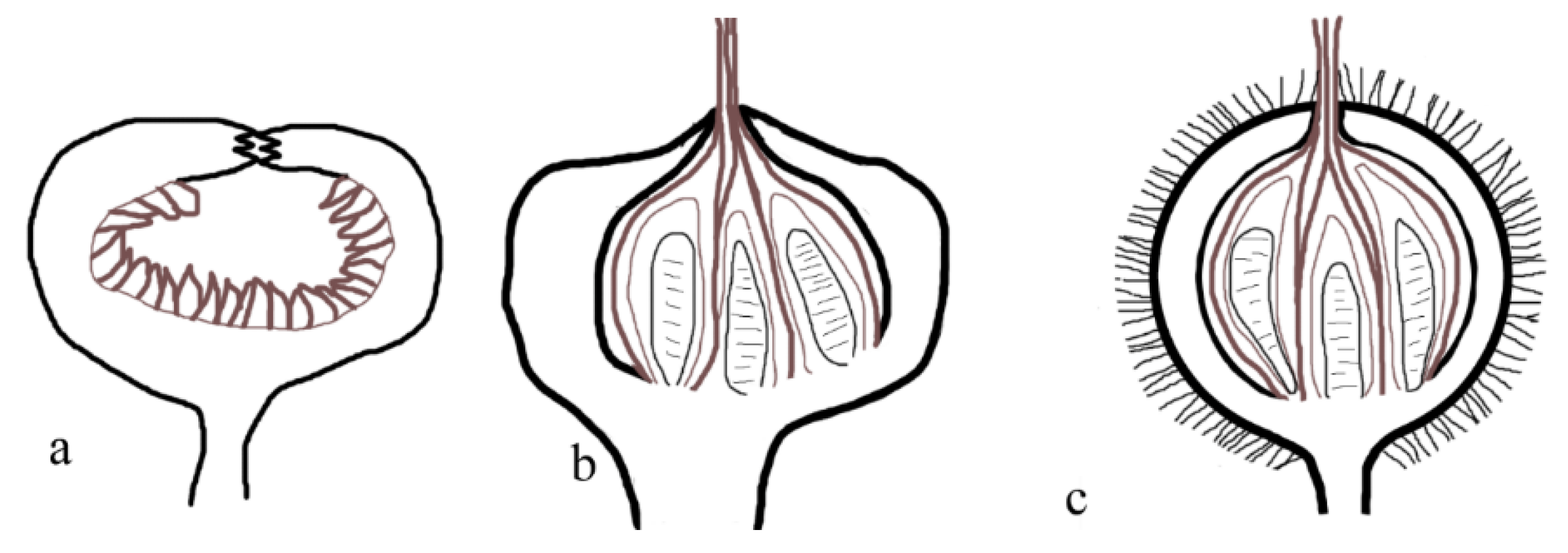
Angiosperms are the single most diversified group of plants in the current world. The key innovation distinguishing angiosperms from their peer gymnosperms is their fuller ovule-protection (
Figure 10). Compared to the above gymnosperms with partial ovule/seed protection/enclosure (
Figure 9), ovule enclosure in angiosperms is fulfilled before pollination (with rare exceptions). This feature, angio-ovuly (metamegasporangia enclosed by accessories) is frequently taken as a feature idiosyncratic to angiosperms. This feature ensures a congenial and favorable developmental environment for the ovules, giving edges to angiosperms in their competition against gymnospermous peers, including pre-zygotic selection of pollen grains, self-incompatibility, outcrossing-enhancing, etc[
11,
12,
13,
14,
15]. The success in reproduction of these novel mechanisms is exemplified by the great diversity of angiosperms (over 300,000 species) in the current world, which enables the origin and survival of the humans.
In many textbooks, angiosperms seem to culminate the evolution of plants, and angio-ovuly seems to be the ultimate step of plant evolution, as if plants would not evolve thence after. However, this misleading impression apparently does not reflect the truth of plant evolution. A sound theoretical extrapolation is that, beyond angio-ovuly, fruits of angiosperms may be further enclosed and protected by adjacent accessories (
Figure 11). This is not a plain hypothesis: the occurrences of hypanthodium (
Figure 11a-c)[
16] and inflated calyx syndrome (ICS, frequently seen in Solanaceae)[
17] implement extra protection for the fruits in some angiosperms. Hypanthodium uses an expanded axis tip to protect the fruits, namely, it is derived through expansion and invagination of the flower axis tip. These processes not only have been seen in the basalmost angiosperm
Amborella[
18], but also may account for the inferior ovary in a Jurassic flower
Nanjinganthus[
19,
20,
21]. Such a combination of expansion and invagination of the flower axis tip is a phenomenon so far restricted to angiosperms. As a consequence of such a morphological innovation, a mutually beneficial relationship is established between Moraceae and agaonid wasps (
Figure 11a): Moracea invite and trap otherwise free agaonid wasps in the figs for successful pollination, fruit-protecting, and increased seed setting, while agaonid wasps develop various features adapting to their living in host plants[
22]. This may be termed
tertiary metamegasporangium complex I (= angiocarpy, enclosed fruit). Tertiary metamegasporangium complex I has been seen not only in other extant angiosperms (
Siparuna,
Figure 11b), but also in an Early Cretaceous fossil angiosperm,
Chaoyangia liangii (
Figure 11c)[
5].
Other than angio-ovuly and angiocarpy, many angiosperms aggregate multiple flowers into inflorescences of various geometries (
Figure 12). Such an aggregation makes flowers more conspicuous and attractive especially during anthesis. Flowers in a single inflorescence differentiate spatially and temporally in morphology and development (especially anthesis timing) according to their positions in the inflorescence, and thus are conducive to successful pollination and fruitful reproduction. Thus inflorescence may be termed
tertiary metamegasporangium complex II, as it integrates more flowers and accessories into a function unit.
As stated above, one of the major advantages of endomegasporangium is the extension of nutritional bond between mother plants and offspring. This extension seems to culminate in angiosperms that have enclosed ovules/seeds. However, this nutritional bond can be further extended in vivipary.
Vivipary in plants designates that seeds germinate in ovaries/fruits. Apparently, not only ovules and seeds obtain their nutrition from their diploid parent plants, but their derivatives (seedlings) also do so, giving the offspring extra advantages through temporally extended nutritional bond between parent and offspring, namely, mother plants not only supply nutrition to ovules and seeds, but also to newer sporophytes (
Figure 13)[
16]. Such a nutritional bond spanning three generations (including sporophyte, gametophyte, and next generation sporophyte) culminates in Rhizophoraceae[
16], and apparently benefits the survival of their offspring in the unique habitat. This may be termed
tertiary metamegasporangium complex III.
These three types of tertiary metamegasporangium complexes are additional modifications added to SRC of equivalent level, namely, after secondary metamegasporangium complex, therefore they all are termed tertiary metamegasporangium complexes and distinguished by numbers.
3. The Underlying Trends
The above stairs of plant reproduction evolution are morphological reflections of plant evolution, which follows several underlying trends, including ODC, D&A, internalization, E&I, increasing dominance of sporophyte.
ODC (Offspring Development Conditioning) designates that, following the evolution path of a specific plant phylogeny, plants gain increasing control over the developmental environment of their offspring. ODC is implemented either as a strengthened and extended nutritional bond, enhanced physical protection, or their combinations in various lineages of plants[
23].
The organization of plant reproductive organs becomes increasingly complicated and canalized during the evolution. A logical and natural consequence of such evolution is D&A (differentiation & aggregations), during which former equivalent parts are differentiated into the core part and the peripheral protective accessory parts, and both of which become indispensable parts of a more complicated organ. This is well demonstrated in the deriving of ovules and flowers.
Unavoidably in this process, the core reproductive part is shifted from a previously outer and more exposed position into an inner and more sheltered position. Such a morphological change can be termed as
internalization. It is intriguing that internalization not only accounts for the evolution of plant reproductive organs, but also accounts for the evolution of the reproductive organs in vertebrates as well as the humans[
23].
A previously largely ignored morphological change idiosyncratic of angiosperms is
E&I (Expansion & Invagination) of flower axis. This trend has been demonstrated in
Amborella [
18], and it may account for the derivation of inferior ovary in the currently earliest fossil flower,
Nanjinganthus [
19,
20,
21] (
Figure 14) and hypanthodia in some extant angiosperms (
Figure 11a)[
16,
25,
26,
27].
Among vascular plants, a general evolution trend is increasing dominance and longer time of sporophyte generation in a SRC. Gametophyte plays an increasingly reduced and subdued role in a SRC, and gametophyte generation occupies an increasingly shorter time in SRC. There is little information on the evolution of gametophyte-dominating plants (bryophytes) yet.
A phylogeny of plants may be taken as a link composed of serial connected SRC of different individuals. As a link tends to break at its weakest point and the weakest point in a SRC is its initial vulnerable and weak period, naturally, whoever protects and nurtures its offspring better wins in evolution. This is the logic underlying ODC and other trends demonstrated in plant reproduction evolution. Decent applying of these rules contributes to the success of various land plants and accounts for their diversity in the current world.