Introduction
Hepatocellular carcinoma (HCC) is the leading primary cancer of the liver. It is one of the most common malignancies globally and the cause of substantial health-related problems, making it the third most frequent cause of cancer-related deaths in the world 1. The Barcelona Clinic Liver Cancer (BCLC) staging system is the most widely used treatment algorithm worldwide 2. Patients with early-stage HCC based on the BCLC staging system 2 are treated with curative therapies such as surgical resection, transplantation, or locoregional therapies, which include radiofrequency ablation (RFA) and microwave thermosphere ablation (MTA) 3.
Recently, percutaneous RFA has been widely performed because of its ease of use, safety profile, and effectiveness for treating HCC in patients with chronic liver disease 4-7. The fact that it can be performed repeatedly makes it particularly valuable for controlling intrahepatic recurrences 8.
Microwave ablation (MWA) has been developed as another percutaneous thermal ablation therapy for HCC. Compared to RFA, MWA has the advantage of faster heating and less susceptibility to heat sink effects due to the higher temperature generated 9. However, conventional MWA had several major disadvantages. First, the ablation zone in conventional MWA has a teardrop shape. Therefore, after the development of systems with greater power, conventional MWA had a high risk of thermal damage to subcutaneous tissues and skin when ablation is performed for subcapsular tumors in the liver 9. Second, the size of the ablation area in conventional MWA is not predictable with changes in surrounding tissues 9. In order to overcome these disadvantages, Emprint™ (Covidien, Boulder, CO, USA) was developed as a next-generation MWA system with MTA technology. MTA is able to make predictable spherical ablation zones by incorporating field control, thermal control, and wavelength control technologies into the system. This new system was approved for use in the United States in April 2014 and in Japan in July 2017. In this study, we investigated the clinical outcomes of patients with HCC who underwent next-generation MTA at our hospital.
Materials and Methods
Patients
All procedures in this retrospective analysis of database records complied with the Declaration of Helsinki. The study protocol was approved by the institutional ethics committee of the Japanese Red Cross Society Himeji Hospital (IRB No. H30-34) based on the Guidelines for Clinical Research issued by the Ministry of Health, Labour and Welfare of Japan. Informed consent to analyze the data was obtained from all patients.
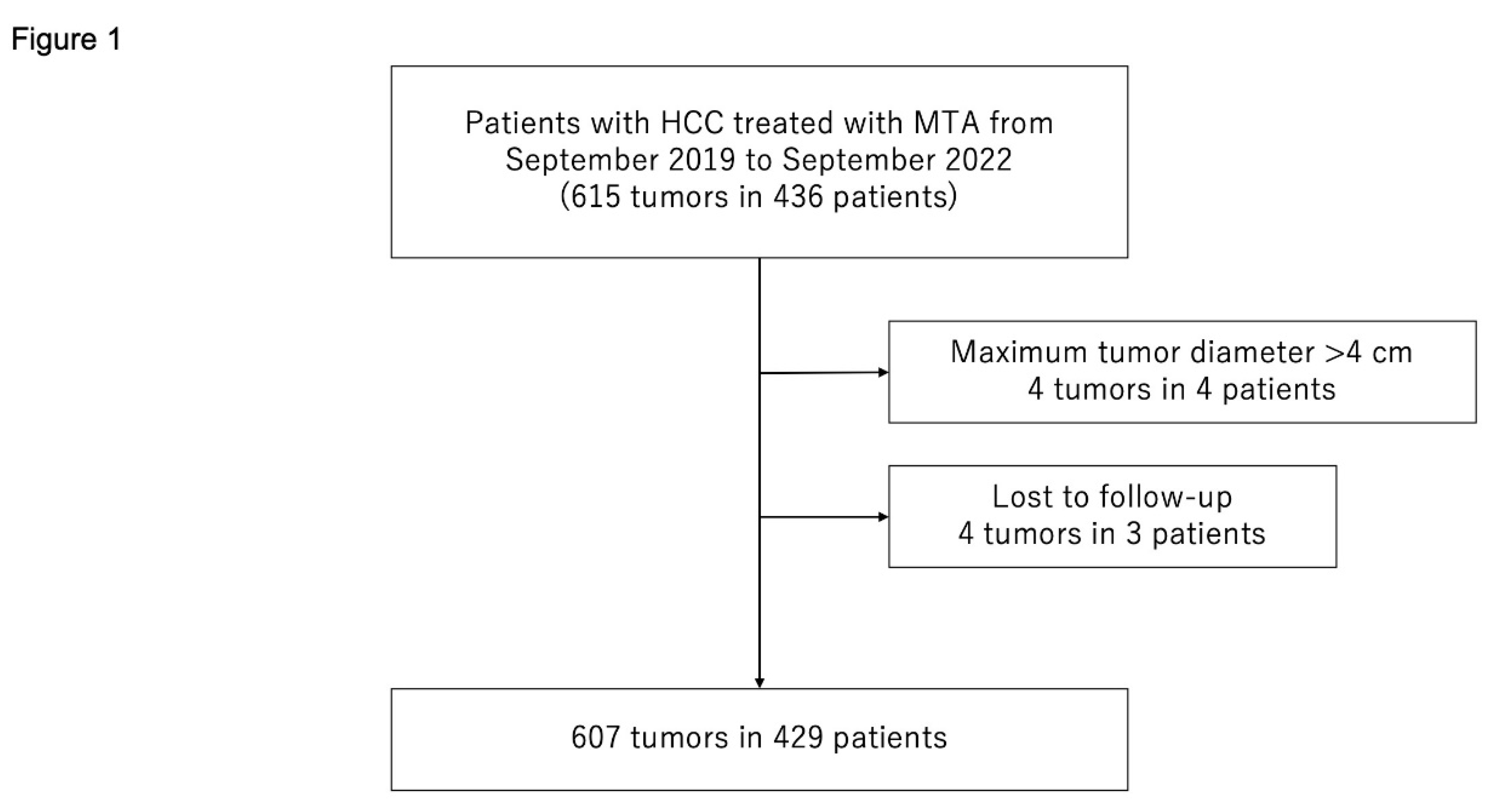
Between September 2019 and September 2022, 436 consecutive patients with 615 HCCs were treated with MTA at the Japanese Red Cross Society Himeji Hospital. We excluded patients who met the following criteria: (1) maximum tumor diameter >40 mm (4 patients with 4 HCCs) or (2) lost to follow-up (3 patients with 4 HCCs). Consequently, 429 patients with 607 HCCs were enrolled in the study (
Figure 1).
In September 2019, MTA therapy began as percutaneous local ablation therapy for HCC at our hospital. Until September 2019, RFA was performed as percutaneous local ablation therapy for HCC at our hospital. However, after the start of MTA therapy, the number of patients undergoing RFA decreased. Since January 2020, MTA therapy has been performed in all patients with HCC for whom percutaneous local ablation therapy was indicated.
HCC etiology was defined as hepatitis B virus in patients positive for hepatitis B virus surface antigen. It was defined as hepatitis C virus in those positive for hepatitis C virus antibodies.
The start of follow-up was defined as the time when initial MTA therapy was performed. The end of follow-up was defined as the date of the final visit for patients who remained alive or the date of death for patients who died during the follow-up period.
Diagnosis and treatment of HCC
HCC was diagnosed based on increases in levels of tumor markers such as α-fetoprotein, des-γ-carboxy prothrombin, and lens culinaris agglutinin-reactive α-fetoprotein as well as the results of multi-phasic contrast-enhanced computed tomography (CECT), gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)–enhanced magnetic resonance imaging (MRI), contrast-enhanced ultrasonography (CEUS), pathological examination, or a combination of these modalities 10, 11.
The Japanese practice guidelines for HCC 12, 13 state that MTA is indicated for patients with HCC and 3 or fewer lesions (none >3 cm). For patients classified as having more severe HCC, MTA was selected on the basis of discussions among hepatologists, surgeons, and radiologists with consideration of the patient's background such as Eastern Cooperative Oncology Group Performance Status (ECOG-PS), hepatic function, and tolerance for surgery. Informed consent was obtained from each patient.
MTA technique
The Emprint™ ablation system with a 13-gauge standard antenna (20-cm long) was used for MTA. Prior to percutaneous MTA therapy, 15 mg of pentazocine hydrochloride and 25 mg of hydroxyzine hydrochloride were administered intravenously. Local anesthesia was induced by 5 mL of 1% lidocaine injected through the skin into the peritoneum along a predetermined puncture line. MTA-antenna puncture was performed under ultrasound guidance. When the tumor was difficult to visualize using B-mode ultrasound, CEUS or fusion imaging with CECT or Gd-EOB-DTPA–enhanced MRI was used as complementary methods for MTA. B-mode ultrasound and CEUS images were obtained using the Aplio™️ i800 system (Canon Medical Systems, Otawara, Japan) with an 8-MHz convex transducer (PVI-482BX). Artificial pleural effusion or ascites was prepared using 5% glucose solution if needed. When tumor diameter was less than 1.5 cm, output power was set initially at 60 W and changed to 75 W after 1 minute. In tumors with a diameter greater than 1.5 cm, starting output power was set at 60 W, changed to 75 W after 1 minute, and then changed to 100 W after another minute.
MTA therapy was considered completed when the target tumor was entirely covered by a transient hyperechoic zone. If coverage of the transient hyperechoic zone was insufficient for the target tumor, additional punctures and ablation were performed in the same treatment session. Needle track ablation at 75 W was performed to prevent hemorrhage. After taking the antenna out of the liver, the needle track was observed using color Doppler ultrasonography. If hemorrhage from the needle track increased rapidly, we stopped bleeding with re-ablation of the liver surface around the needle track using the MTA-antenna via a new percutaneous puncture.
In this study, we defined the following areas of the liver as those where MTA therapy is difficult to perform: caudate lobe and areas near the primary and secondary branches of the intrahepatic portal vein, inferior vena cava, gallbladder, heart, duodenum, abdominal esophagus, collateral veins around the liver, and spleen. We defined tumors in contact with the hepatic capsule and raised on the liver surface as protruding from the liver surface.
Evaluation of treatment efficacy
Treatment efficacy was evaluated on CECT or MRI at 1–2 days after MTA therapy. Complete ablation was defined as no tumor enhancement with a safety margin ≥5 mm on CECT or a post-ablation zone that included the entire target tumor with a safety margin ≥5 mm on MRI.
Surveillance for HCC recurrence after MTA therapy
Follow-up consisted of regular blood tests and monitoring of tumor markers such as α-fetoprotein, des-γ-carboxy prothrombin, and lens culinaris agglutinin-reactive α-fetoprotein every 3 months. Multi-phasic CECT, Gd-EOB-DTPA–enhanced MRI, or both were performed every 3–6 months after HCC treatment. When HCC recurrence or disease progression was detected based on radiologic findings, the most appropriate therapy was initiated in each patient based on the Japanese practice guidelines for HCC 12, 13.
Evaluation of outcomes after MTA therapy
The primary endpoint of this study was local tumor recurrence, which represents treatment failure. Local tumor recurrence was defined as tumor growth that touched the inside or outside of the post-ablation zone.
Safety evaluation
To evaluate the safety of MTA therapy, the profile and incidence of complications were investigated. Major complications were defined as events leading to substantial morbidity or disability, higher level of care, hospital admission, or substantially extended hospital stay 14.
Statistical analysis
Continuous variables are expressed as medians (interquartile range). The chi-square test with Fisher’s exact test was used for categorical variables. Actuarial analysis of cumulative local tumor progression was performed using the Kaplan–Meier method; differences were evaluated using the log-rank test. Univariable and multivariable Cox proportional hazards models were used to analyze local tumor progression. In addition, univariable and multivariable regression were used to evaluate the relationship between clinical factors and complications. In this study, the number of events was small and only factors that were significant in the univariable analysis were included as covariates in the multivariable analysis.
Statistical significance was defined as p<0.05. Statistical analysis was performed using JMP® (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
The characteristics of the 429 patients at admission are shown in
Table 1. There were 293 (68.3%) males and 136 (31.7%) females, with a median age of 76.0 (71.0–82.0) years. The primary etiologies of HCC were hepatitis-related: 259 (60.4%) cases of HCV, 31 (7.3%) cases of HBV, and two instances of both. Median maximum tumor diameter was 15.0 (10.0–21.0) mm. There were 483 (79.6%) HCCs with maximum tumor diameter of ≤20 mm, 107 (17.6%) with maximum diameter of 20–30 mm, and 17 (2.8%) with maximum diameter of 30–40 mm. There were 304 (70.9%) patients with 1 tumor, 81 (18.9%) patients with 2 tumors, 24 (5.6%) patients with 3 tumors, and 20 (4.7%) patients with ≥4 tumors.
Table 1 includes patients who received multiple treatments during the study period; 304 unique patients were treated with MTA. The characteristics of these 304 patients are shown in
Table 2. There were 201 (66.1%) males and 103 (33.9%) females, with a median age of 73.0 (71.0–81.0) years. The median follow-up period was 23.0 (15.3–30.7) months.
Table 3 shows the number of tumors in areas where MTA is difficult (n=86, including duplications). The most common difficult-to-treat nodules were located near the primary and secondary branches of the intrahepatic portal vein (26 nodules). There were 598 (98.5%) nodules that needed 1 ablation session, 8 (1.3%) that needed 2 sessions, and 1 (0.2%) nodule that needed 3 sessions.
Complete ablation was achieved in 98.7% (599/607) of nodules.
Local tumor recurrence
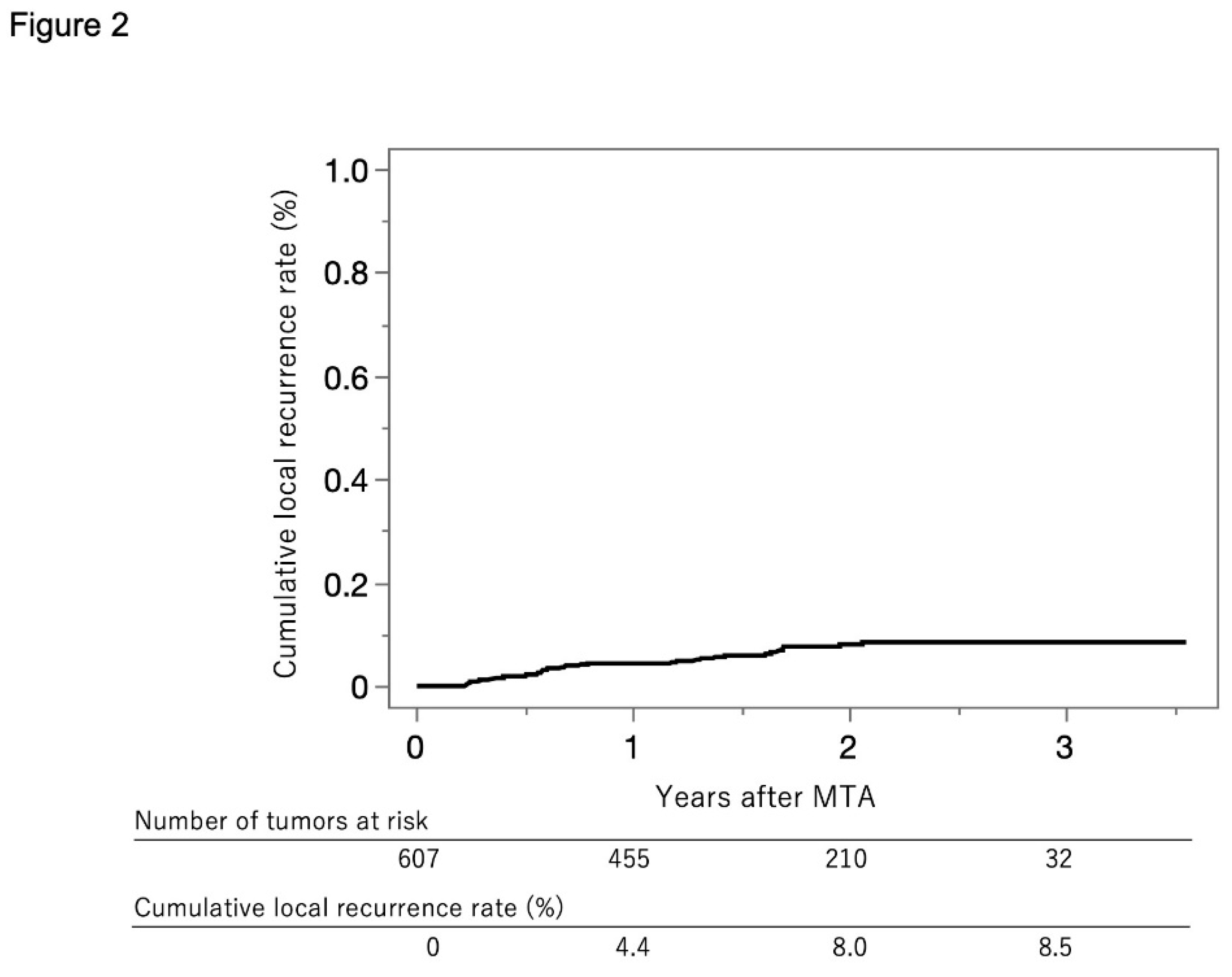
Figure 2 shows the curve for local tumor recurrence in this cohort. The cumulative local tumor recurrence rate was 4.4% at 1 year, 8.0% at 2 years, and 8.5% at 3 years.
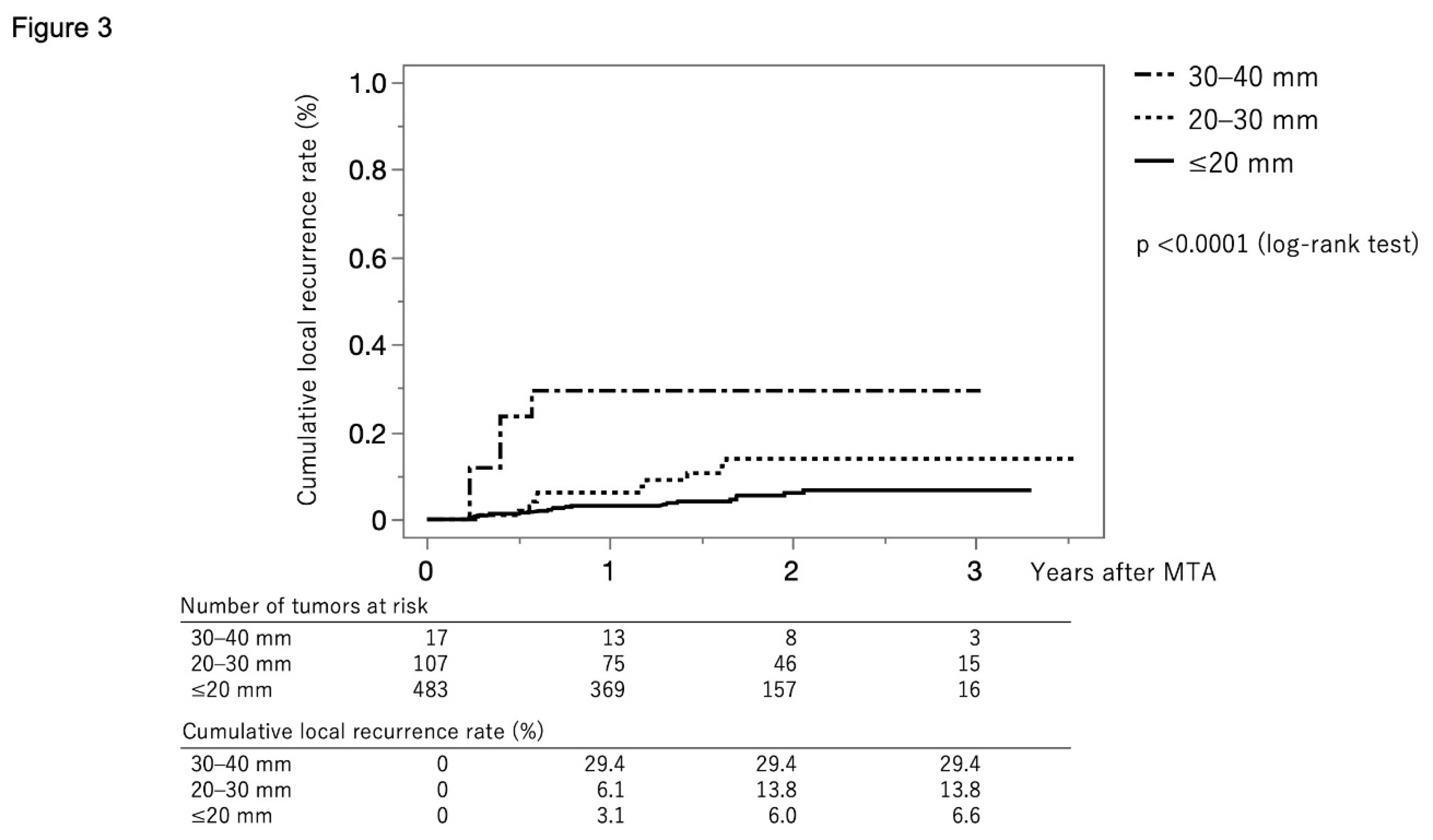
Figure 3 shows the curves for local tumor recurrence stratified by tumor size group. The cumulative local tumor recurrence rate at 1, 2, and 3 months were 3.1%, 6.0%, and 6.6%, respectively, in the tumor size ≤20 mm group (solid line). The rates were 6.1%, 13.8%, and 13.8%, respectively, in the tumor size 20–30 mm group (dotted line) and 29.4%, 29.4%, and 29.4%, respectively, in the tumor size ≥30 mm group (dash-dot-dash line) (p<0.001, log-rank test).
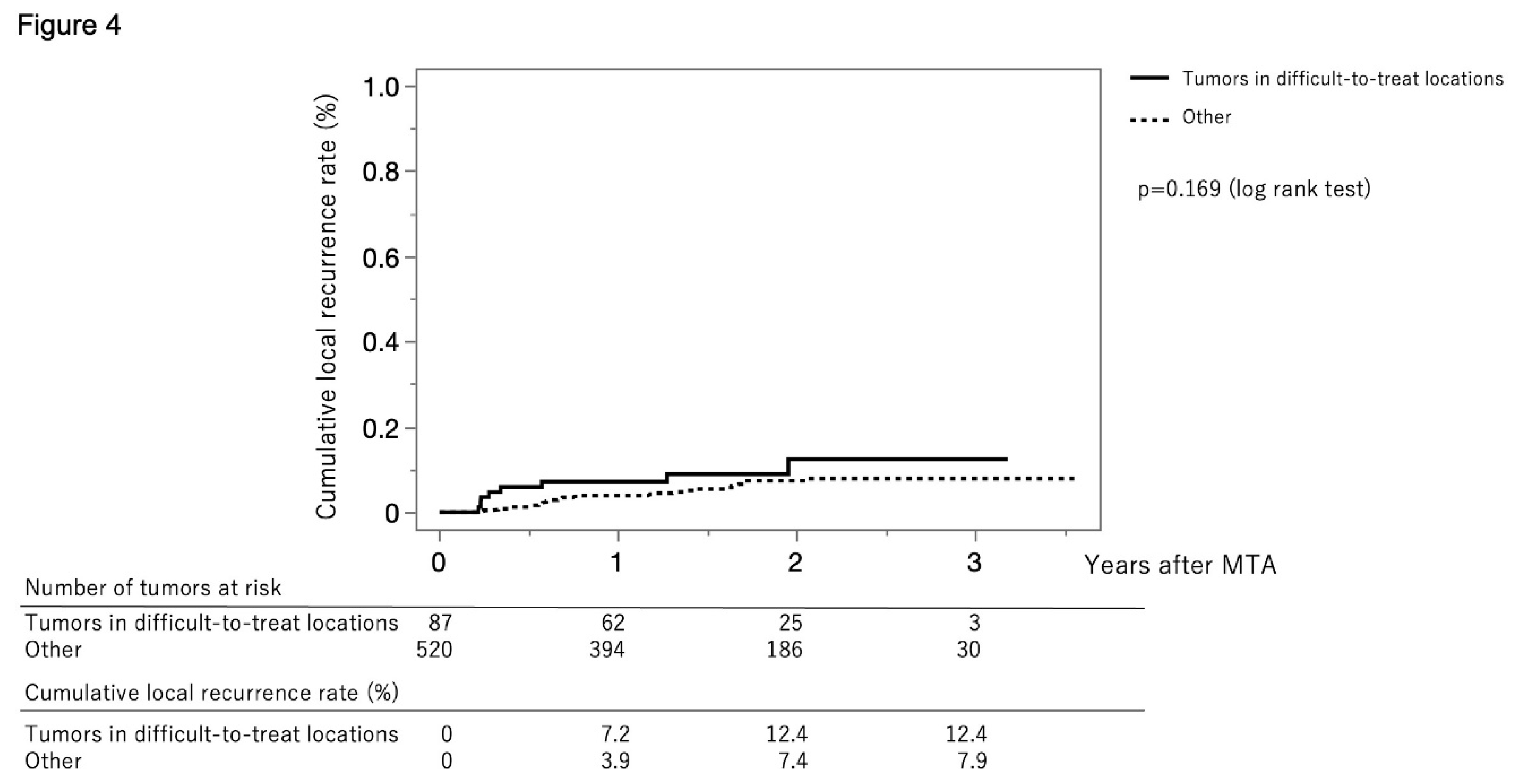
Figure 4 shows the curves for local tumor recurrence stratified by difficult-to-treat status. The cumulative local tumor recurrence rates at 1, 2, and 3 months were 7.2%, 12.4%, and 12.4%, respectively, in the difficult-to-treat group (solid line) and 3.9%, 7.4%, and 7.9%, respectively, in the non–difficult-to-treat group (dotted line) (p=0.169, log-rank test).
Clinical factors associated with local tumor recurrence
Table 4 shows the univariable and multivariable Cox proportional hazards models associated with local tumor recurrence. Tumor size (per 1 mm) (hazard ratio [HR], 1.07; 95% confidence interval [CI], 1.03–1.11; p<0.001) and ablative margin (per 1 mm) (HR, 0.81; 95% CI, 0.70–0.92; p=0.002) were significantly associated with local tumor recurrence.
Safety
Table 5 shows the complications in patients overall and stratified by tumor size group. The overall complication rate differed significantly across tumor size groups (p<0.001).
Table 6 shows the univariable and multivariable regression results of factors associated with complications. Tumor size (per 1 mm) (odds ratio, 1.08; 95% CI, 1.02–1.15; p=0.015) was significantly associated with complications.
Discussion
In this study, we clarified the clinical outcomes of next-generation MTA in patients with HCC. Although this study only included patients with HCC who received MTA therapy at a single center in Japan, it included over 400 patients with 600 HCCs. This study showed that the cumulative local tumor recurrence rates at 1 and 3 years were 4.4% and 8.5%, respectively. In this study, there were 86 tumors in areas that were difficult to treat with MTA therapy; approximately 30% of those were located near the primary and secondary branches of the intrahepatic portal vein. However, this study did not identify any significant differences in local tumor recurrence by difficult-to-treat status. In the multivariable analysis, tumor size was significantly associated with local tumor recurrence. In fact, the cumulative recurrence rate was significantly higher in the group with large tumor size. In addition, ablative margin was inversely associated with local tumor recurrence. Regarding complications, only tumor size was significantly associated with complications, whereas difficult-to-treat status was not. These results suggest that MTA therapy for HCC located in areas where MTA is difficult is not associated with local tumor progression or post-procedure complications.
Tamai et al. 15 reported a significant difference in 3-year local tumor progression rate between the RFA group (22%) and the MTA group (8%) (p<0.001) in an analysis of 513 patients with 630 HCCs (≤3 cm) who underwent percutaneous RFA (174 patients, 214 HCCs) or MTA (339 patients, 416 HCCs). They found that ablation procedure (MTA; HR, 0.565; 95% CI, 0.437–0.731; p<0.001) and tumor diameter (per mm; HR, 1.070; 95% CI, 1.030–1.113; p=0.001) were independent factors associated with local tumor progression in multivariable analysis 15. In addition, they found that the total complication rate was significantly lower in the MTA group (8.0%, 26/339) than in the RFA group (14.0%, 25/174) (p<0.05), particularly for bile duct injury (3.0% (11/339) versus 9.0% (15/174); p=0.010). In this study, we found that the cumulative local tumor recurrence rate at 3 years was 8.5% in our cohort, which included patients with tumors ≥3 cm. In this cohort, the total complication rate was 5.4% (33/607). These results suggest that MTA therapy is able to achieve good outcomes in patients with HCC for whom percutaneous ablation therapy is indicated. The advantage of this study is that our study included more patients and tumors that underwent MTA than the study by Tamai et al.
The Emprint™ Ablation System with Thermosphere™ Technology (Covidien) is an improved version of the Evident™ Microwave Ablation System developed by the same manufacturer. The new device uses a frequency of 2,450 MHz and consists of a 100 W generator with a high-efficiency reusable cable and an ablation pump that cools the antenna during ablation. The device was approved for use by the United States Food and Drug Administration on April 28, 2014 and in Japan on November 1, 2016. This new generation MWA system attempts to address the limitations of conventional systems. It was designed to create a large predictable spherical ablation zone that is unaffected by changes in the tissue environment 16. Thus, it can provide predictable ablation results and outcomes regardless of target site or tissue type 17. A large, accurate, and predictable spherical ablation zone is maintained throughout the procedure with 3 different types of controls: field control, thermal control, and wavelength control 18. Thermosphere™ technology maintains wavelength control by creating a constant, stable environment around the probe shaft and circulating sterile saline solution along the shaft. This minimizes changes in the dielectric constant directly under the probe and maintains shorter wavelengths. It also ensures that the migration pattern of electrons in the probe is maintained, providing reliable field control. However, there have been few studies regarding MTA therapy that have evaluated treatment of HCC in real-world clinical practice. In this study, we clarified the outcomes of this therapy for HCC at a high-volume center in Japan.
The main limitations of this study include its small number of patients and retrospective nature at a single center in Japan. Further prospective studies with more patients from multiple centers are warranted. Another limitation of this study was that the outcomes have not been compared with those of RFA, which had been the mainstay of local ablation therapy for HCC. Further prospective studies that compare clinical outcomes of MTA and RFA in the real-world clinical setting should be performed.
In conclusion, MTA is a safe and effective local ablation therapy for HCC, even when the tumor is located in an area of the liver where local ablation therapy is difficult. Further studies are warranted to confirm these findings in other populations.
Financial support
There was no grant support or other financial support for this study.
Disclosure of Ethical Statements
Approval of the research protocol: The study protocol was approved by the institutional ethics committee of the Japanese Red Cross Society Himeji Hospital (IRB No. H30-34).
Registry and the Registration No. of the study/trial
N/A
Research involving recombinant DNA
N/A
List of abbreviations
HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; RFA, radiofrequency ablation; MTA, microwave thermosphere ablation; MWA, microwave ablation; CECT, contrast-enhanced computed tomography; Gd-EOB-DTPA, gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid; MRI, magnetic resonance imaging; CEUS, contrast-enhanced ultrasonography; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; CI, confidence interval.
Author contributions statement
Concept and study design: Shinichiro Nakamura and Toshifumi Tada. Data acquisition: All authors. Data analysis: Shinichiro Nakamura and Toshifumi Tada. Statistics: Shinichiro Nakamura. Supervision: Hiroyuki Okada. Manuscript preparation: Toshifumi Tada
Data availability statement
The datasets are available from the corresponding author upon reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Llovet JM, Villanueva A, Marrero JA, et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73 (Suppl 1), 158–191. [CrossRef]
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012, 262, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008, 48 (Suppl 1), S20–S37. [Google Scholar] [CrossRef] [PubMed]
- Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005, 129, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Local ablation therapy for hepatocellular carcinoma: current status and future perspectives. J Gastroenterol. 2004, 39, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011, 53, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Lubner MG, Brace CL, Hinshaw JL, Lee FT, Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. Journal of vascular and interventional radiology : JVIR. 2010, 21, S192–S203. [Google Scholar] [CrossRef] [PubMed]
- Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa K, Takemura N, Yamashita T, et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol Res. 2023, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kudo M, Kawamura Y, Hasegawa K, et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021, 10, 181–223. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia. 2016, 32, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Tamai H, Okamura J. New next-generation microwave thermosphere ablation for small hepatocellular carcinoma. Clinical and molecular hepatology. 2021, 27, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Alonzo M, Bos A, Bennett S, Ferral H. The Emprint Ablation System with Thermosphere Technology: One of the Newer Next-Generation Microwave Ablation Technologies. Semin Intervent Radiol. 2015, 32, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi P, Schena E, Massaroni C, et al. Temperature monitoring during microwave ablation in ex vivo porcine livers. Eur J Surg Oncol. 2015, 41, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Hubner F, Schreiner R, Reimann C, et al. Ex vivo validation of microwave thermal ablation simulation using different flow coefficients in the porcine liver. Med Eng Phys. 2019, 66, 56–64. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Flow chart of patient selection. MTA, microwave thermosphere ablation; HCC, hepatocellular carcinoma.
Figure 1.
Flow chart of patient selection. MTA, microwave thermosphere ablation; HCC, hepatocellular carcinoma.
Figure 2.
Curve for local tumor recurrence. The cumulative local tumor recurrence rates at 1, 2, and 3 years were 4.4%, 8.0%, and 8.5%, respectively.
Figure 2.
Curve for local tumor recurrence. The cumulative local tumor recurrence rates at 1, 2, and 3 years were 4.4%, 8.0%, and 8.5%, respectively.
Figure 3.
Curves for local tumor recurrence stratified by tumor size group. The cumulative local tumor recurrence rates at 1, 2, and 3 months are 3.1%, 6.0%, and 6.6%, respectively, in the tumor size ≤20 mm group (solid line). They were 6.1%, 13.8%, and 13.8%, respectively, in the tumor size 20–30 mm group (dotted line) and 29.4%, 29.4%, and 29.4%, respectively, in the tumor size ≥30 mm group (dash-dot-dash line) (p<0.001, log-rank test).
Figure 3.
Curves for local tumor recurrence stratified by tumor size group. The cumulative local tumor recurrence rates at 1, 2, and 3 months are 3.1%, 6.0%, and 6.6%, respectively, in the tumor size ≤20 mm group (solid line). They were 6.1%, 13.8%, and 13.8%, respectively, in the tumor size 20–30 mm group (dotted line) and 29.4%, 29.4%, and 29.4%, respectively, in the tumor size ≥30 mm group (dash-dot-dash line) (p<0.001, log-rank test).
Figure 4.
Curves for local tumor recurrence stratified difficult-to-treat status. The cumulative local tumor recurrence rates at 1, 2, and 3 months were 7.2%, 12.4%, and 12.4%, respectively, in the difficult-to-treat group (solid line) and 3.9%, 7.4%, and 7.9%, respectively, in the non-difficult-to-treat group (dotted line) (p=0.169, log-rank test). MTA, microwave thermosphere ablation.
Figure 4.
Curves for local tumor recurrence stratified difficult-to-treat status. The cumulative local tumor recurrence rates at 1, 2, and 3 months were 7.2%, 12.4%, and 12.4%, respectively, in the difficult-to-treat group (solid line) and 3.9%, 7.4%, and 7.9%, respectively, in the non-difficult-to-treat group (dotted line) (p=0.169, log-rank test). MTA, microwave thermosphere ablation.
Table 1.
Characteristics of patients and HCCs at the time of admission (n=429).
Table 1.
Characteristics of patients and HCCs at the time of admission (n=429).
| Age at admission (years) |
76.0 (71.0–82.0) |
| Gender (male/female) * |
293/136 |
| HCC etiology (hepatitis B virus / hepatitis C virus / B+C / non-B, non-C) |
31/259/2/137 |
| Child-Pugh classification (A/B) |
379/50 |
| ECOG-PS (0/1/2) |
384/41/4 |
| Maximum tumor diameter (mm) * |
15.0 (10.0–21.0) |
| Number of tumors (1/2/3/≥4) |
304/81/24/20 |
| Number of prior treatments for HCC (0/1/≥2) |
120/99 /210 |
| α-fetoprotein (ng/mL) * |
6.1 (3.3–13.4) |
|
Lens culinaris agglutinin-reactive α-fetoprotein (%) * |
0.0 (0.0–9.6) |
| Des-γ-carboxy prothrombin (mAU/mL) * |
28.0 (19.0–64.1) |
Table 2.
Characteristics of unique patients that underwent MTA (n=304).
Table 2.
Characteristics of unique patients that underwent MTA (n=304).
| Age at first MTA * |
76.0 (71.0–81.0) |
| Gender (male/female) * |
201/103 |
| Etiology (hepatitis B virus/hepatitis C virus /B+C/non-B, non-C) |
25/176/2/101 |
| Follow-up duration (months) * |
23.0 (15.3–30.7) |
Table 3.
Number of tumors in areas of the liver where MTA is difficult (n= 86; including duplications).
Table 3.
Number of tumors in areas of the liver where MTA is difficult (n= 86; including duplications).
| Primary and secondary branches of intrahepatic portal vein |
26 |
| Adjacent to the heart |
25 |
| Adjacent to the inferior vena cava |
19 |
| Adjacent to the gallbladder |
17 |
| Caudate lobe |
9 |
| Adjacent to the duodenum |
5 |
| Adjacent to the esophagus |
1 |
| Adjacent to a collateral vein |
1 |
Table 4.
Factors associated with local tumor recurrence.
Table 4.
Factors associated with local tumor recurrence.
| Factors |
Univariable analysis |
Multivariable analysis |
| HR |
95% CI |
p |
HR |
95% CI |
p |
| Difficult-to-treat location (yes) |
1.72 |
0.79–3.75 |
0.170 |
1.30 |
0.58–2.89 |
0.530 |
| Tumor size (per 1 mm) |
1.08 |
1.04–1.12 |
<0.001 |
1.07 |
1.03–1.11 |
<0.001 |
| Ablative margin (per 1 mm) |
0.78 |
0.68–0.89 |
<0.001 |
0.81 |
0.70–0.92 |
0.002 |
| Protruding from the liver surface (yes) |
1.17 |
0.58–2.36 |
0.660 |
|
|
|
| Multiple tumors (yes) |
0.65 |
0.34–1.25 |
0.190 |
|
|
|
| Prior HCC treatment (yes) |
0.85 |
0.42–1.71 |
0.640 |
|
|
|
| α-fetoprotein (per 1 ng/mL) |
1.00 |
0.99–1.00 |
0.540 |
|
|
|
|
Lens culinaris agglutinin-reactive α-fetoprotein (per 1 %) |
1.01 |
0.99–1.02 |
0.220 |
|
|
|
| Des-γ-carboxy prothrombin (per 1 mAU/mL) |
1.00 |
0.99–1.00 |
0.840 |
|
|
|
Table 5.
Complications due to MTA.
Table 5.
Complications due to MTA.
Complication
(including duplications) |
Total (n=607) |
Tumor size group |
| ≤20 mm |
20–30 mm |
30–40 mm |
| Any |
33 (5.4%) |
17 (3.5%) |
11 (10.3%) |
5 (29.4%) |
| Portal vein thrombus |
9 (1.5%) |
5 (1.0%) |
4 (3.7%) |
0 (0.0%) |
| Biloma |
9 (1.5%) |
5 (1.0%) |
2 (1.9%) |
2 (11.8%) |
| Bile duct dilatation |
6 (1.0%) |
3 (0.6%) |
2 (1.9%) |
1 (5.9%) |
| Pleural effusion |
4 (0.7%) |
3 (0.6%) |
0 (0.0%) |
1 (5.9%) |
| Hemorrhage |
2 (0.3%) |
1 (0.2%) |
1 (0.9%) |
0 (0.0%) |
| Hepatic infarction |
1 (0.2%) |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
| Pneumothorax |
2 (0.3%) |
0 (0.0%) |
2 (1.9%) |
0 (0.0%) |
| Heat injury of the diaphragm |
1 (0.2%) |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
| Heat injury of the right kidney |
1 (0.2%) |
0 (0.0%) |
0 (0.0%) |
1 (5.9%) |
Table 6.
Factors associated with complications.
Table 6.
Factors associated with complications.
| Factors |
Univariable analysis |
Multivariable analysis |
| Odds ratio |
95% CI |
p |
Odds ratio |
95% CI |
p |
| Difficult-to-treat location (yes) |
2.38 |
1.02–5.16 |
0.046 |
2.27 |
0.92–5.17 |
0.074 |
| Total ablation time (per 1 min) |
1.22 |
1.11–1.33 |
<0.001 |
1.03 |
0.84–1.25 |
0.740 |
| Tumor size (per 1 mm) |
1.10 |
1.06–1.15 |
<0.001 |
1.08 |
1.02–1.15 |
0.015 |
| Number of punctures (per puncture) |
2.87 |
1.58–4.96 |
0.001 |
1.47 |
0.53–3.91 |
0.450 |
| Ablative margin (per 1 mm) |
0.93 |
0.81–1.06 |
0.280 |
|
|
|
| Protruding from the liver surface (yes) |
1.66 |
0.77–3.41 |
0.190 |
|
|
|
| Multiple tumors (yes) |
0.72 |
0.35–1.46 |
0.360 |
|
|
|
| Prior HCC treatment (yes) |
0.55 |
0.27–1.18 |
0.120 |
|
|
|
| Child-Pugh classification (B) |
0.87 |
0.53–1.29 |
0.500 |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).