Introduction
Kidney stone disease is a global health burden as it affects around 10% of the population, with an increase of its prevalence and of its incidence over the past few decades [
1,
2,
3]. Its annual cost exceeds 2 billion USD in the USA [
4]. In the absence of a personalized preventive medical treatment, recurrence of renal calculi at 10 years is high, around 30 to 50% [
2,
5]. Kidney stones can range from an asymptomatic incidental finding on imaging evaluation for other indications to a painful ureteral obstruction that can be complicated by a urinary tract infection. In addition, nephrolithiasis may be associated with the development of chronic kidney disease [
6,
7,
8].
Diet and Lithogenesis Mechanisms
Kidney stones are heterogeneous. Calcium containing stones are the most common type of kidney stones accounting for more than 80% of cases, with most being composed of calcium oxalate or more rarely of calcium phosphate [
5,
9,
10]. Uric acid comprises 5% to 10% of all stones, struvite 1% to 5%, and rare stone (eg: cystine, ammonium urate, drug, dihydroxyadenine) 3% or less [
9,
10].
The different types of stones find their origin according to the underlying pathologies and several superadded lithogenesis mechanisms [
11]. Stone formation is a multifactorial process that can be associated to :
- –
-anatomical abnormalities, eg: medullary sponge kidney, ureteropelvic junction obstruction, horseshoe kidney,
- –
-rare inherited monogenic metabolic disorders, eg: cystinuria, primary hyperoxaluria, 2,8-dihydroxyadeninuria due to adenine phosphoribosyltransferase deficiency, autosomal dominant or recessive distal renal tubular acidosis type I, Dent disease, hereditary hypophosphataemic rickets with hypercalciuria) [
12],
- –
-general diseases : primary hyperparathyroidism, granulomatous diseases such as sarcoidosis, gastrointestinal diseases inducing enteric hyperoxaluria like jejuno-ileal bypass [
13], metabolic syndrome [
14], and diabetes mellitus [
15]
Of note some drugs can also directly or indirectly induce nephrolithiasis [
16]. Poorly soluble medications with high urine excretion are susceptible to crystallize in urine (eg: amoxicillin, indinavir) and some treatments may also indirectly impair urine composition (eg: acetazolamide which dramatically increases urinary pH) [
17]. Finally, chronic or recurrent urinary tract infections with urease-producing microorganisms inducing ammoniogenesis cause struvite stones [
18].
Besides these causes, diet is one of the most important factors of lithogenesis. For example, very common calcium oxalate monohydrate (whewellite) subtype Ia stones are primarily related to high consumption of oxalate-rich foods and precursors of oxalate such as hydroxyproline-rich foods, to low calcium intake (thereby increasing oxalate absorption in the gut), and to insufficient water intake [
11].
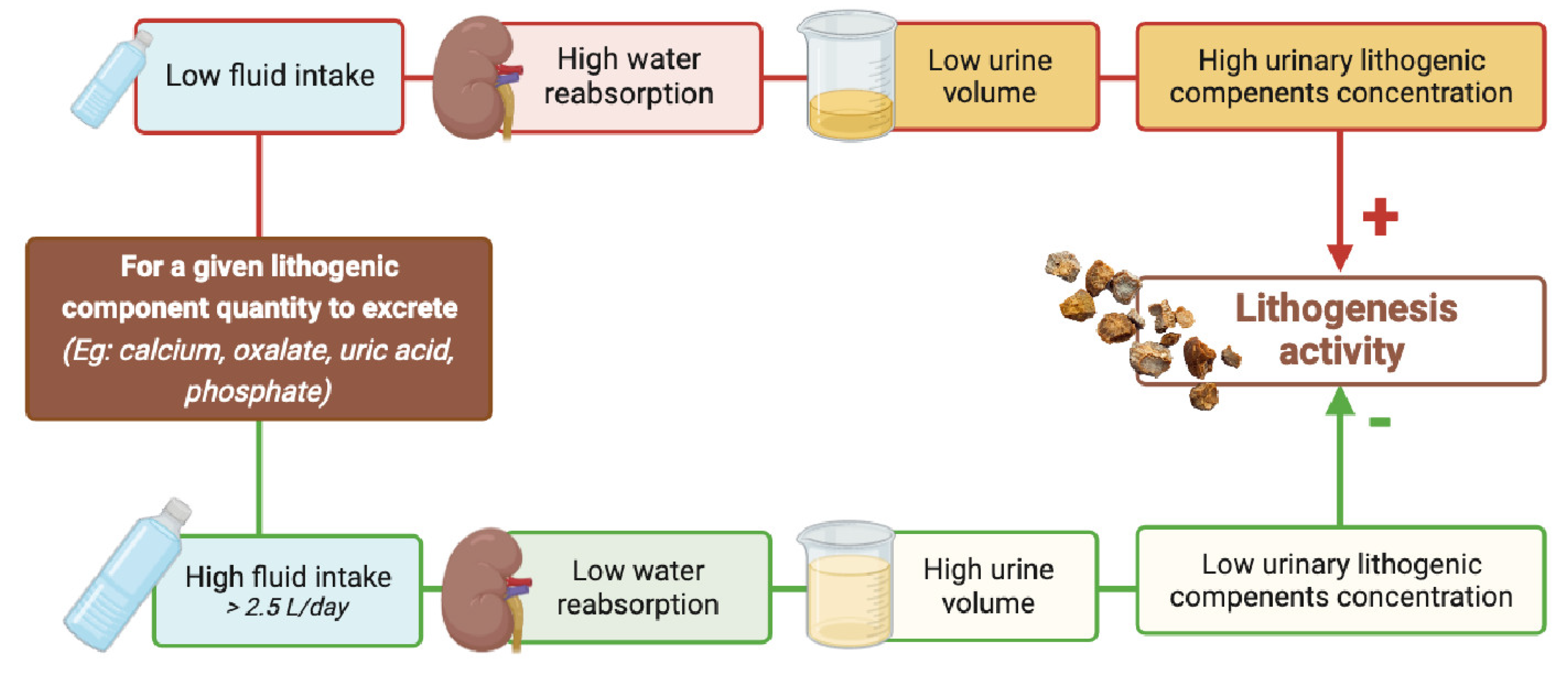
Regardless of the stone type, stone formation involves urine supersaturation, crystal nucleation, growth and aggregation of stone forming salts such as calcium oxalate, calcium phosphate or uric acid. Urine supersaturation, which is a necessary condition for the development of stones, is always due to reduced urine volume and/or to an excess of solutes in urine. Importantly, whereas it is not always possible to decrease urinary daily excretion of stones precursors, it is nearly always possible to increase daily urine volume explaining why increasing fluid intake is the cornerstone of the preventive medical treatment for all types of stones.
Fluid Intake Is the Main Determinant of Urine Volume (Figure 1)
Water Is the Main Constituent of the Human Body
Around 60% and 50% of the body weight in healthy men and women adults respectively is composed of water, owing to differences in body fat [
19]. About two thirds of body water is intracellular whereas one third of body water is in the extracellular space (divided between the plasma and the interstitial compartments (in a ratio of 1:3) [
19]. Thus, a man weighting 70 kg is composed of about 42 L of water, distributed in intracellular compartment for 28 L, in plasma for 3.5 L and in interstitial compartments for 10.5 L.
At steady state, the intracellular volume is in equilibrium with the extracellular space: intra and extracellular osmolalities are equal (about 290 mOsmol/kg H
2O). Almost all cell membranes express water channels (aquaporin) allowing passive water diffusion according to an osmotic gradient while cell membranes are relatively impermeable to solutes that must be actively transported. A change in the amount of water in extracellular compartment without any change in the solute content creates an osmotic gradient between intra and extracellular compartments, resulting in a water flow from one compartment to the other, thereby restoring osmotic equilibrium [
19].
Water Homeostasis Depends on Inflows and Outflows
Water intake mainly comes from beverages and, to a lesser extent, from hydrated foods (vegetables) and from the oxidative metabolism of carbohydrates or fats [
20]. Beverage consumption is influenced by thirst in response to a slight increase in plasma osmolality induced by the absorption of salt and other solutes from food but is also conditioned by social behaviors [
19].
Regarding fluid outputs, water output from the kidneys (urine) constitutes the majority of water outflows from the body and is the only one that is regulated [
20]. Breathing, perspiration, sweating and feces constitute other insensitive water outflows from the body, depending on ambient temperature, physical activity and digestive pathologies.
In physiological conditions and intermediate climatic situation, the insensible losses (from breathing, perspiration, sweating and feces) are roughly equivalent to the insensible intakes (from hydrated foods and metabolism) so that the 24-hour urine volume can be approximately considered to reflect the daily fluid intake.
Kidneys Regulate Water Output and Urine Volume
Kidney’s urine concentrating and diluting mechanisms are mandatory to maintain a nearly constant blood plasma osmolality without continuous access to water [
21]. When water intake is large enough to dilute plasma, more diluted urine is produced to normalize plasma osmolality. In contrast, when water intake is so low that plasma is concentrated, concentrated urine is produced to restore normal plasma osmolality [
21].
In healthy adult kidneys, the glomeruli filter approximately 180 L per day of ultrafiltrate from plasma. Most of it is reabsorbed in the tubule, with a massive and non-regulated water reabsorption in the proximal tubule and Henle loop and a more moderate but tightly regulated water reabsorption in the collecting duct. Indeed, the final setting of water reabsorption depends on the water permeability of the collecting duct, enabled by the expression of aquaporin channels controlled by the level of an antidiuretic hormone, plasma arginine vasopressin (AVP) [
19,
22,
23].
Under physiological conditions, the most important determinant of AVP secretion by magnocellular neurons in the hypothalamic neuropituitary tract is the effective osmolality [
19]. If plasma osmolality increases (for example if water intake is low or if extra renal water losses increase (eg: diarrhea or sweating), the plasma AVP concentration increases. In turn, AVP activates V2-receptor in the renal collecting duct, leading to an increased production of aquaporin and to their expression at the cell membrane [
22,
24]. Consequently, the epithelial cells of the collecting duct become permeable to water, allowing water reabsorption from tubular fluid to blood thanks to the osmotic gradient formed in the renal medulla (the vascular organization favors the accumulation and the concentration of solutes in the renal medulla, generating the osmotic driving force) [
19,
25]. As a result, urine volume becomes lower and urine concentration higher. At the opposite, in case of high fluid intake associated with low AVP secretion, these renal epithelial cells, as well as their tight junctions, are impermeable to water. Therefore, a larger volume of filtrate is excreted as dilute urine to maintain the plasma osmolality within a narrow range [
23].
Furthermore, chronic polyuria interferes with the maintenance of the medullary concentration gradient, decreasing the maximum concentration ability of urine. The extent of the decrease varies proportionally to the urine volume [
26].
Reducing Urine Volume May Increase the Risk of Developing First Kidney Stones
Patients exposed to hot climate, working or performing physical activities at high ambient temperature have a high incidence of kidney stones [
27,
28,
29,
30,
31,
32,
33,
34,
35,
36]. This is likely due, at least in part, to “dehydration”, attributed to heat-induced sweating, thus reducing urine volume and increasing urine supersaturation of stone-forming salts. An educational program to increase fluid intake in a desert town was associated with a slight increase in urine volume and to a subsequent decrease in the prevalence of urolithiasis [
37].
On the other hand, population that purposefully limit fluid intake to reduce urinary frequency, yielding more concentrated urine may be at higher risk to develop kidney stones, such as taxi cab drivers or health care professional working in an operating room without proper access to bathroom facilities [
36,
38,
39].
Borghi
et al. evaluated urine volume in patients suffering from their first stone episode. They reported that urine volume was significantly lower in 199 stone-forming men and women with a first episode of calcium stone than in 101 healthy control subjects (1,057 ± 238
vs 1,401 ± 562 ml/day in males, p<0.0001; 990 ± 230 vs 1,239 ± 440 ml/day in females, p<0.0001) [
40].
Large observational studies including non-stone-forming patients have reported the preventive effect of high fluid intake on kidney stone risk (primary prevention), the risk of kidney stones being inversely correlated with fluid intake. In a prospective observational cohort study of 45,619 men who had no previous history of kidney stones (The National Health Professionals cohort), an inverse association between fluid intake and the risk of kidney stones was found during four years of follow-up [
41]. In the quintile consuming over 2,500 ml of fluid per day, compared to the quintile consuming less than 1,275 ml per day, the relative risk dropped from 1 to 0.71 (95 percent confidence interval 0.52 to 0.97; (p=0.003)) [
41]. Taylor
et al. reported similar results regarding the beneficial effect of fluid intake in this cohort after a follow-up of 14 years: the relative risk for the men in the highest as compared with the lowest quintile group for fluid intake was 0.71 (95%CI, 0.59 to 0.85; p<0.001 for trend) [
42]. This result was also confirmed in large prospective cohorts of women (Nurses' Health Studies (NHS)) with a follow-up of 8 and 12 years [
43,
44].
Curhan
et al. examined 24-hour urine chemistries in 907 men and women with a history of kidney stone disease and 239 without history of kidney stone disease who where participants in three large cohort studies (the NHS I (N= 297 cases; N=99 controls) and II (N=169 cases, N=30 controls) and the Health Professionals Follow-up Study (HPFS; N=341 cases, N=110 controls)) [
45]. The mean age and weight were similar for cases and controls within the respective cohorts. Mean 24-hour urine volume was significantly lower in cases than controls in NHS I (Mean (SD) 1.76 L (0.70)
vs 2.15 L (0.91), p≤0.01) and HPFS (Mean (SD) 1.66 L (0.64)
vs 1.87 L (0.76), p≤0.01) [
45] but the difference was not significant in NHS II (Mean (SD) 1.51 L (0.67)
vs 1.75 L (0.79)) possibly due to a lack of power [
45].
In a UK population-based prospective cohort of 439,072 participants over a mean of 6.1 years of follow-up, total fluid intake was inversely associated with the risk of developing a first incident kidney stone [
46]. When compared to fluid intake of zero to six glasses per day (approximately 1.2 L), individuals who drank 13 glasses or more (equivalent to ≥ 2.3 L) had a 50% reduced risk of developing kidney stones [
46]. For every additional drink (200 ml) consumed per day of total fluid, the risk of kidney stones declined by 13% (hazard ratio=0.87, 95% confidence interval 0.85-0.89, p value for trend ≤0.001) [
46].
Increasing Urine Volume in Stone Formers May Decrease the Risk of Stone Recurrence
The effect of dilution on crystallization of stone-forming salts in patients with kidney stones and in normal subjects was evaluated by Pak
et al. Dilution of urine was achieved
in vitro by adding distilled water to urine or
in vivo by the intake of distilled water. Urine dilution significantly reduced the urinary activity product ratio of calcium phosphate (brushite), calcium oxalate, and monosodium urate and increased the minimum supersaturation needed to elicit spontaneous nucleation of calcium oxalate, thereby reducing the risk of crystal nucleation [
47]
.
To assess the effect of urine dilution, Borghi
et al. examined natural and diluted overnight 8-hour urine collections. The overnight 8-hour urine was collected from 11.30 pm to 07.30 am under normal hydration conditions and after a 500-mL load of oligomineral water administered at 11.30 pm in 15 male calcium oxalate stone formers without metabolic urinary anomalies (hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia and hypomagnesuria) and 12 male controls. The water load reduced the calcium oxalate saturation [
48]. It also increased the urine tolerance to oxalate loads (a test consisting of adding increasing amounts of oxalate in order to determine the minimum quantity of oxalate required to start crystallization). Importantly, it did not modify the power of the macromolecules to inhibit calcium oxalate crystallization, despite the concurrent dilution of the calcium crystallization inhibitors induced by the water load [
48].
As regards the prevention of recurrences, Hosking
et al. reviewed the clinical courses of 108 patients with idiopathic calcium urolithiasis (86.1% of whom had recurrent stones) advised to increase fluid intake to achieve a daily urine output of at least 2,500 mL. Patients who had no evidence of stone growth or new stone formation during follow-up had significantly higher urine volume than those with recurrent stone formation (approximately 2,100 mL/day vs. 1,700 mL/day (p<0.005)) [
49]. In this study, patients were not controlled for other predisposing factors to urinary stone disease such as diet [
49].
In a prospective cohort study of idiopathic calcium oxalate stone formers (including 22% (n=40) first stone formers and 78% (n=141) multiple stone formers before being referred), Daudon
et al. reported a higher mean urine volume in patients without stone recurrence (mean ± SEM: 2,260 ± 50 mL/day) compared to patients with stone recurrence (1,740 ± 60 mL/day, p<0.0001), each patient having a follow-up of at least 3 years (within a mean follow-up of 6.8 years). The risk of recurrence decreased by 68% for each 1,000 mL/day increase in daily urine output [
50].
Only two randomized trials have been published regarding this topic. In the first one, Sarica
et al. randomized 70 stone formers prior to shock wave lithotripsy for calcium oxalate stones into three groups: 25 patients receiving calcium channel blocker, 25 patients undergoing an enforced fluid intake program to achieve a daily urinary output of more than 2,500 mL and 20 patients not receiving any specific medication and/or measure apart from close follow-up after shock wave lithotripsy [
51]. The mean follow-up period was 30.4 months (24 – 36 months). Among patients who became stone free (12/25, 48%) in the group advised to increase fluid intake, only one patient (1/12, 8.3%) experienced a new stone formation during follow-up, while the figure was 55% (5/9) in patients receiving no specific measure [
51]. It should be noted that the characteristics of the subset of participants who become stone-free were not compared to assess that the groups were comparable at baseline and urine volumes were not reported in this study.
In the second trial, Borghi
et al. randomized 220 patients after their first episode of idiopathic calcium stone episode in two different follow-up programs lasting 5 years to either increase water intake with a goal urine volume equal to or greater than 2 L per day without any changes in diet or to no treatment. One hundred and ninety-nine patients completed the study, including 99 patients in the intervention group. After the 5-year follow-up period, patients treated with high fluid intake had significantly fewer recurrences (assessed as episodes of renal colic or stone expulsion episodes or at annual imaging) (12.1 % of patients) compared with control group (27% of patients) (p=0.008). In addition, the mean time to relapse was also significantly longer in the treated group than in the control group (38.7 ± 13.2 months
versus 25.1 ± 16.4 months, p=0.016). The high-water intake caused a large increase in urine volume associated to a strong reduction of supersaturation of lithogenous salts throughout the follow-up period. After 5 years, the mean urine volume in the high fluid intake group was 2.6 L/day compared to 1 L/day in the controls, highlighting the protective role of a high fluid intake against recurrence after the first idiopathic calcium stone episode [
40]. Of note, patients included in this study were stone free after their first lithiasis episode and had no metabolic pathology, excluding patients most at risk of recurrence.
Systematic review and meta-analyses confirmed the significantly reduced risk of kidney stones with high fluid consumption (pooled risk ratios 0.20 (95% confidence interval 0.09 - 0.44) calculated according to 7 observational studies) [
52].
To our best knowledge, no prospective randomized trial evaluated the effect of urine dilution in high-risk stone formers, such as in case of kidney anatomical abnormalities or general or genetic diseases associated with stone formation [
16].
Increasing Urine Volume in Cystinuric Patients May Decrease Stone Recurrence
The rationale for the amount of urine dilution can be discussed in cystinuric patients. Cystinuria (OMIM 220100), an autosomal recessive disorder, is the most frequent monogenic cause of kidney stones and is responsible for 1% of nephrolithiasis in adults [
53]. It affects renal cystine reabsorption. Because of its poor solubility at a typical urine pH lower than 7, excess cystine results in urinary cystine stone recurrent formation. Cystine solubility is <1.05 mmol/L at pH <6 and reaches 2.1 mmol/L at pH >7.5 [
53]. The amount of cystine excreted by cystinuric patients is typically >1.6 mmol/day and usually reaches 2.5–6 mmol/day [
53]. Alkaline hyperdiuresis is the cornerstone of the medical preventive therapy [
53]. Fluid intake should guarantee a diuresis large enough to maintain a urine cystine concentration below 1 mmol/L [
53]. Barbey
et al. reported in 27 cystinuric patients that the daily urine volume was significantly higher in patients with arrested stone formation (3,151 ± 587 versus 2,446 ± 654 mL/24 hours, p = 0.006), suggesting that maintaining a daily urine volume of greater than 3 L is essential for therapeutic success [
54]. Moreover, the probability of cystine crystalluria that is associated with stone formation among patients with cystinuria [
55] increased as urine specific gravity increased from 1.005 to 1.010 g/cm
3 [
56]. It is interesting to underline that if fluid intake is well distributed over the 24-h period, there is an almost linear relationship between morning urine specific gravity and 24-h urine volume. Moreover, a morning specific gravity of 1.005 g/cm3 corresponds to a 3 L/24 h diuresis [
57], reinforcing the concordance between the two above mentioned targets.
Nonetheless, further studies are needed to investigate the effect of increased fluid intake on preventing stone recurrence in different types of stone formers including non-calcium stone formers.
How Could Physicians Help Their Patients to Increase Their Fluid Intake and Pay Attention to Its Composition?
Practical Ways to Increase Fluid Intake and Adherence to This Measure
Adequate fluid intake is a powerful and inexpensive strategy in urolithiasis prevention. Increased water intake for the prevention of urolithiasis recurrence has a significant cost saving potential [
64]. A sustained increase in urine volume can be achieved over two decades in stone formers followed in a clinic dedicated to stone prevention, but the authors cannot document exactly how this was accomplished [
65]. Patients should benefit from dietitian counseling and education on fluid intake. However, patients may have difficulties adhering to suggested interventions. In order to increase patient compliance, stone formers should first be informed that they are at high risk of recurrence. Behavior change can be obtained if patients perceived benefits of high fluid intake behind the constraint. There are many reasons why stone formers do not drink adequate amounts of fluids: water availability, aversion to the taste of water, feel abdominal discomfort after ingesting high volume of fluids, lack of thirst, forgetting to drink water and voiding frequency. Overactive bladder, bladder outlet obstruction and voiding frequency incompatible with the job activity such as for example professional drivers or schoolteachers or in case of limited access to bathroom or are common barriers preventing from increasing fluid intake [
60,
66,
67].
Practical ways to increase fluid intake and daily routines should be advised such as drinking at set times during the day, drinking regularly despite lack of thirst, maintaining a water bottle in all places where significant time is spent or carrying a water bottle and eating foods with a high content in water such as fruits and vegetables [
60]. Self-monitoring using simple urine color chart, urine dipstick for urine specific gravity or 24h urine volume can help to succeed in increasing water intake [
68,
69,
70]. Of note, 24h urine volume should be performed preferably before each medical consultation to assess all risk factors for stone recurrence, including of course direct measurement of urine volume.
Digital tools and smart water bottles may also promote fluid consumption [
71,
72,
73] however the effect on urine volume may be similar to counseling [
74]. Smart water bottles that record fluid intake and associated digital applications, sync to the user’s smartphone, can provide fluid intake reminders and improve patients’ compliance [
75]. Moreover, thanks to these connected tools, fluid volume consumed can be accessible for physicians to evaluate their patients’ compliance.
Safety data on high fluid intake are limited [
52]. Patients who eat little and drink a large volume may become overhydrated and subsequently hyponatremic while excreting maximally dilute urine; water ingestion exceeding the kidney’s capacity for water excretion, as in the case of a “tea-and-toast” diet [
76]. Urinary dilution can also be altered in case of advanced renal failure [
77] or with thiazide diuretics use [
78], or in case of reduced effective circulating volume associated to non-osmotic mediated AVP release (eg: congestive heart failure, cirrhosis) [
76], leading to a risk of hyponatremia. In these patients, blood sodium concentration should be closely monitored and advice regarding urine volume should be individualized and adjusted as necessary.
Advice Regarding Types of Fluid Intake
Fructose intake is associated with an increased risk of kidney stones [
79] and urinary oxalate excretion increased after cola beverage consumption [
80]. Consumption of sugar-sweetened soda [
81,
82], grapefruit juice [
83], apple juice [
83] and punch [
81] is associated with a higher risk of stone formation, whereas consumption of coffee [
81,
83,
84,
85], tea [
81,
83], beer [
81,
83], wine [
81,
83] is associated with a lower risk.
Findings on the impact of orange juice intake on urinary risk factors for stone formation are inconsistent [
86]. Curhan
et al. reported no association between orange juice intake and the risk of stone formation [
83] whereas Ferraro
et al. reported a lower risk of stone formation with the consumption of orange juice [
81]. Low-calorie orange juice can increase urinary citrate [
87].
A meta-analysis has investigated the association between different types of beverages and risk of kidney stones [
88]. Authors confirmed that increased water intake was associated with a reduced risk of developing kidney stones [
88]. They also concluded that increased intake of coffee, tea and alcohol showed potential benefits on stones prevention [
88], although water remains the preferred fluid [
16]. Patients should be advised to reduce the intake of sodas, in particular those acidified by phosphoric acid (typically colas) [
82] and calorie-containing fluids and alcohol that may be detrimental to health [
16]. Of note, black tea and green tea contain varying amounts of oxalate depending on the origin, quality, time of harvest and preparation [
86]. Consequently, tea should not be very steeped to avoid excessive oxalate intake.
Concretely, physicians should advice their patients to preferentially drink water as it is free from calories, fructose and alcohol. However, the composition of different mineral waters has to be taken into account since the mineral composition of bottled drinkable water and of tap water varies significantly [
89,
90,
91,
92,
93] and may influence urinary composition regarding stone promotors and inhibitors.
Bicarbonate is a natural constituent of mineral water. Alkaline beverage will increase urinary pH and citrate excretion [
94,
95,
96] so that drinking alkalizing beverages such as bicarbonate-rich mineral water can support alkalization therapy for uric acid and cystine stones while neutral pH beverages are recommended in general preventive measures for other type of stones [
16]. Cystinuric patients are advised to maintain urine pH between 7.5 and 8 in each freshly voided urine portion [
16,
53]. In case of stones containing uric acid, the targeted urine pH is 6.2-6.8 for prevention and 6.5-7.2 for chemolitholysis [
16].
Calcium-rich mineral water can be used if daily calcium intake achieved by consuming dairy products such as milk, cheese or yoghurt is insufficient. Patients should be aware of the calcium content of the water and be advised to have a normal calcium diet that is associated with a reduced risk of oxalate stone formation [
58]. Importantly, calcium intake, whatever its source (food or beverage) must be spread over the three daily meals since intestinal calcium binds oxalate, brought with each meal, thereby decreasing its intestinal absorption and urinary excretion [
97].
Finally, magnesium rich mineral water can be used in case of hypomagnesemia or of low urinary excretion of magnesium (<3 mmol/24h [
16]) related to poor dietary intake or to reduced intestinal absorption. Physician should keep in mind that magnesium rich mineral waters are most of the time also rich in calcium.
Conclusions
In conclusion, increasing water intake to increase urine dilution is a recognized therapeutic approach to reduce the risk of kidney stones in all stone formers and represents the cornerstone of the preventive medical treatment for all types of nephrolithiasis. Patients should be informed and supported in this difficult process of increasing urine dilution through increasing their fluid intake. This measure should be considered within a holistic approach to health. Further studies are needed in patients at higher risk of developing kidney stones to determine the target of urine volume.
Author Contributions
Conceptualization: CPB and MC; Methodology: CPB; Writing—original draft preparation: CPB, ST, EB, AM, LP, SB and MC; Writing—review and editing: CPB, ST, EB, AM, LP, SB and MC; Supervision: CPB and MC; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shoag, J.; Tasian, G.E.; Goldfarb, D.S.; Eisner, B.H. The new epidemiology of nephrolithiasis. Adv Chronic Kidney Dis 2015, 22, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the true burden of kidney stone disease. Nat Rev Nephrol 2020, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Calhoun, E.A.; Curhan, G.C.; Urologic Diseases of America, P. Urologic diseases in America project: urolithiasis. J Urol 2005, 173, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Enders, F.T.; Vaughan, L.E.; Bergstralh, E.J.; Knoedler, J.J.; Krambeck, A.E.; Lieske, J.C.; Rule, A.D. Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community. Mayo Clin Proc 2015, 90, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Rule, A.D.; Bergstralh, E.J.; Melton, L.J., 3rd; Li, X.; Weaver, A.L.; Lieske, J.C. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 2009, 4, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Gillen, D.L.; Worcester, E.M.; Coe, F.L. Decreased renal function among adults with a history of nephrolithiasis: a study of NHANES III. Kidney Int 2005, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- El-Zoghby, Z.M.; Lieske, J.C.; Foley, R.N.; Bergstralh, E.J.; Li, X.; Melton, L.J., 3rd; Krambeck, A.E.; Rule, A.D. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol 2012, 7, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Rule, A.D.; Krambeck, A.E.; Williams, J.C.; Bergstralh, E.J.; Mehta, R.A.; Moyer, T.P. Stone composition as a function of age and sex. Clin J Am Soc Nephrol 2014, 9, 2141–2146. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Lechevallier, E.; Saussine, C. [Epidemiology of urolithiasis]. Prog Urol 2008, 18, 802–814. [Google Scholar] [CrossRef]
- Corrales, M.; Doizi, S.; Barghouthy, Y.; Traxer, O.; Daudon, M. Classification of Stones According to Michel Daudon: A Narrative Review. Eur Urol Focus 2021, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Harris, P.C.; Sas, D.J.; Lieske, J.C. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol 2022, 18, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, L.; Puri, S.; Goldfarb, D.S. Enteric hyperoxaluria: an important cause of end-stage kidney disease. Nephrol Dial Transplant 2016, 31, 375–382. [Google Scholar] [CrossRef]
- West, B.; Luke, A.; Durazo-Arvizu, R.A.; Cao, G.; Shoham, D.; Kramer, H. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am J Kidney Dis 2008, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005, 68, 1230–1235. [Google Scholar] [CrossRef]
- Skolarikos, A.; Jung, H.; Neisius, A.; Petřík, A.; Somani, B.; Tailly, T.; Gambaro, G. EAU Guidelines on Urolithiasis. European Association of Urology. 2023. Available online: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Urolithiasis-2023.pdf.
- Daudon, M.; Jungers, P. Drug-induced renal calculi: epidemiology, prevention and management. Drugs 2004, 64, 245–275. [Google Scholar] [CrossRef]
- Daudon, M.; dessombz, A.; Frochot, V.; Letavernier, E.; Haymann, J.P.; Jungers, P.; Bazin, D. Comprehensive morphoconstitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Comptes Rendus Chimie 2016, 19, 1470–1491. [Google Scholar] [CrossRef]
- Robertson, G.L. Thirst and Vasopressin. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Fifth edition 2013; Volume 1, p. 1441.
- Jequier, E.; Constant, F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr 2010, 64, 115–123. [Google Scholar] [CrossRef]
- Rivard, C.J.; Wang, W.; Chan, L. Hypernatremic States. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Fifth edition 2013; Volume 1, p. 1541.
- Nielsen, S.; Kwon, T.H.; Dimke, H.; Skott, M.; Frøkiær, J. Aquaporin Water Channels in Mammalian Kidney. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Fifth edition 2013; Volume 1, p. 1405.
- Bichet, D.G. GENETICS IN ENDOCRINOLOGY Pathophysiology, diagnosis and treatment of familial nephrogenic diabetes insipidus. Eur J Endocrinol 2020, 183, R29–R40. [Google Scholar] [CrossRef]
- Nielsen, S.; Frokiaer, J.; Marples, D.; Kwon, T.H.; Agre, P.; Knepper, M.A. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 2002, 82, 205–244. [Google Scholar] [CrossRef]
- Sands, J.F.; Layton, H.E. The Urine Concentrating Mechanism and Urea Transporters. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Fifth edition 2013; Volume 1, p. 1463.
- Robertson, G.L. Differential diagnosis of polyuria. Annu Rev Med 1988, 39, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Better, O.S.; Shabtai, M.; Kedar, S.; Melamud, A.; Berenheim, J.; Chaimovitz, C. Increased incidence of nephrolithiasis (N) in lifeguards (LG) in Israel. Adv Exp Med Biol 1980, 128, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Turner, L.R.; Hurst, C.; Mai, H.; Zhang, Y.; Tong, S. Exposure to ambient heat and urolithiasis among outdoor workers in Guangzhou, China. Sci Total Environ 2014, 472, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Antonelli, J.; Jimenez, I.B.; Gharbi, H.; Herring, R.; Beaver, A.; Dennis, A.; Von Merveldt, D.; Carter, S.; Cohen, A.; et al. The kidney stone and increased water intake trial in steel workers: results from a pilot study. Urolithiasis 2017, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur J Clin Nutr 2003, 57 Suppl 2, S47–51. [Google Scholar] [CrossRef]
- Atan, L.; Andreoni, C.; Ortiz, V.; Silva, E.K.; Pitta, R.; Atan, F.; Srougi, M. High kidney stone risk in men working in steel industry at hot temperatures. Urology 2005, 65, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Meschi, T.; Amato, F.; Novarini, A.; Romanelli, A.; Cigala, F. Hot occupation and nephrolithiasis. J Urol 1993, 150, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Pin, N.T.; Ling, N.Y.; Siang, L.H. Dehydration from outdoor work and urinary stones in a tropical environment. Occup Med (Lond) 1992, 42, 30–32. [Google Scholar] [CrossRef]
- Masterson, J.H.; Jourdain, V.J.; Collard, D.A.; Choe, C.H.; Christman, M.S.; L'Esperance, J.O.; Auge, B.K. Changes in urine parameters after desert exposure: assessment of stone risk in United States Marines transiently exposed to a desert environment. J Urol 2013, 189, 165–170. [Google Scholar] [CrossRef]
- Fakheri, R.J.; Goldfarb, D.S. Ambient temperature as a contributor to kidney stone formation: implications of global warming. Kidney Int 2011, 79, 1178–1185. [Google Scholar] [CrossRef]
- Malieckal, D.A.; Goldfarb, D.S. Occupational kidney stones. Curr Opin Nephrol Hypertens 2020, 29, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; De Vries, A. Prevention of urolithiasis. Education to adequate fluid intake in a new town situated in the Judean Desert Mountains. Arch Environ Health 1966, 13, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.J.; Rangel, L.J.; Krambeck, A.E. The effect of work location on urolithiasis in health care professionals. Urolithiasis 2013, 41, 327–331. [Google Scholar] [CrossRef]
- Mass, A.Y.; Goldfarb, D.S.; Shah, O. Taxi cab syndrome: a review of the extensive genitourinary pathology experienced by taxi cab drivers and what we can do to help. Rev Urol 2014, 16, 99–104. [Google Scholar] [PubMed]
- Borghi, L.; Meschi, T.; Amato, F.; Briganti, A.; Novarini, A.; Giannini, A. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996, 155, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Stampfer, M.J. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993, 328, 833–838. [Google Scholar] [CrossRef]
- Taylor, E.N.; Stampfer, M.J.; Curhan, G.C. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol 2004, 15, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Arch Intern Med 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Spiegelman, D.; Stampfer, M.J. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997, 126, 497–504. [Google Scholar] [CrossRef]
- Curhan, G.C.; Willett, W.C.; Speizer, F.E.; Stampfer, M.J. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 2001, 59, 2290–2298. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Neal, N.L.; Bradbury, K.E.; Heers, H.; Allen, N.E.; Turney, B.W. Fluid Intake and Dietary Factors and the Risk of Incident Kidney Stones in UK Biobank: A Population-based Prospective Cohort Study. Eur Urol Focus 2020, 6, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.Y.; Sakhaee, K.; Crowther, C.; Brinkley, L. Evidence justifying a high fluid intake in treatment of nephrolithiasis. Ann Intern Med 1980, 93, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Meschi, T.; Schianchi, T.; Briganti, A.; Guerra, A.; Allegri, F.; Novarini, A. Urine volume: stone risk factor and preventive measure. Nephron 1999, 81 Suppl 1, 31–37. [Google Scholar] [CrossRef]
- Hosking, D.H.; Erickson, S.B.; Van den Berg, C.J.; Wilson, D.M.; Smith, L.H. The stone clinic effect in patients with idiopathic calcium urolithiasis. J Urol 1983, 130, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Hennequin, C.; Boujelben, G.; Lacour, B.; Jungers, P. Serial crystalluria determination and the risk of recurrence in calcium stone formers. Kidney Int 2005, 67, 1934–1943. [Google Scholar] [CrossRef] [PubMed]
- Sarica, K.; Inal, Y.; Erturhan, S.; Yagci, F. The effect of calcium channel blockers on stone regrowth and recurrence after shock wave lithotripsy. Urol Res 2006, 34, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Rossetti, S.; Friend, K.; Erickson, S.B.; Lieske, J.C. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. J Nephrol 2016, 29, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Thomas, K.; Dello Strologo, L.; Sayer, J.A.; Bekri, S.; Bertholet-Thomas, A.; Bultitude, M.; Capolongo, G.; Cerkauskiene, R.; Daudon, M.; et al. Cystinuria: clinical practice recommendation. Kidney Int 2021, 99, 48–58. [Google Scholar] [CrossRef]
- Barbey, F.; Joly, D.; Rieu, P.; Mejean, A.; Daudon, M.; Jungers, P. Medical treatment of cystinuria: critical reappraisal of long-term results. J Urol 2000, 163, 1419–1423. [Google Scholar] [CrossRef]
- Daudon, M.; Cohen-Solal, F.; Barbey, F.; Gagnadoux, M.F.; Knebelmann, B.; Jungers, P. Cystine crystal volume determination: a useful tool in the management of cystinuric patients. Urol Res 2003, 31, 207–211. [Google Scholar] [CrossRef]
- Prot-Bertoye, C.; Lebbah, S.; Daudon, M.; Tostivint, I.; Jais, J.P.; Lillo-Le Louët, A.; Pontoizeau, C.; Cochat, P.; Bataille, P.; Bridoux, F.; et al. Adverse events associated with currently used medical treatments for cystinuria and treatment goals: results from a series of 442 patients in France. BJU Int 2019, 124, 849–861. [Google Scholar] [CrossRef]
- Daudon, M.; Traxer, O.; Jungers, P. Lithiase Urinaire (seconde edition). Medecine-Sciences Publications, Paris, Lavoisier.
- Borghi, L.; Schianchi, T.; Meschi, T.; Guerra, A.; Allegri, F.; Maggiore, U.; Novarini, A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002, 346, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Stampfer, M.J.; Mount, D.B.; Curhan, G.C. DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol 2010, 5, 2315–2322. [Google Scholar] [CrossRef]
- Bhojani, N.; Bjazevic, J.; Wallace, B.; Lee, L.; Kaler, K.S.; Dion, M.; Cowan, A.; Sultan, N.; Chew, B.H.; Razvi, H. UPDATE - Canadian Urological Association guideline: Evaluation and medical management of kidney stones. Can Urol Assoc J 2022, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C., Jr.; Gambaro, G.; Rodgers, A.; Asplin, J.; Bonny, O.; Costa-Bauza, A.; Ferraro, P.M.; Fogazzi, G.; Fuster, D.G.; Goldfarb, D.S.; et al. Urine and stone analysis for the investigation of the renal stone former: a consensus conference. Urolithiasis 2021, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M. [Crystalluria]. Nephrol Ther 2015, 11, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Letavernier, E.; Frochot, V.; Haymann, J.P.; Bazin, D.; Jungers, P. Respective influence of calcium and oxalate urine concentration on the formation of calcium oxalate monohydrate or dihydrate crystals. Comptes Rendus Chimie 2016, 19, 1504–1513. [Google Scholar] [CrossRef]
- Lotan, Y.; Buendia Jimenez, I.; Lenoir-Wijnkoop, I.; Daudon, M.; Molinier, L.; Tack, I.; Nuijten, M.J. Increased water intake as a prevention strategy for recurrent urolithiasis: major impact of compliance on cost-effectiveness. J Urol 2013, 189, 935–939. [Google Scholar] [CrossRef]
- Parks, J.H.; Coe, F.L. Evidence for durable kidney stone prevention over several decades. BJU Int 2009, 103, 1238–1246. [Google Scholar] [CrossRef]
- McCauley, L.R.; Dyer, A.J.; Stern, K.; Hicks, T.; Nguyen, M.M. Factors influencing fluid intake behavior among kidney stone formers. J Urol 2012, 187, 1282–1286. [Google Scholar] [CrossRef]
- Tarplin, S.; Monga, M.; Stern, K.L.; McCauley, L.R.; Sarkissian, C.; Nguyen, M.M. Predictors of Reporting Success With Increased Fluid Intake Among Kidney Stone Patients. Urology 2016, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.X.; Prasad, N.S.; Rangan, G.K.; Allman-Farinelli, M.; Rangan, A.M. A systematic review to determine the most effective interventions to increase water intake. Nephrology (Carlton) 2016, 21, 860–869. [Google Scholar] [CrossRef]
- Khorami, M.H.; Hashemi, R.; Bagherian-Sararoudi, R.; Sichani, M.M.; Tadayon, F.; Shahdoost, A.A.; Arezegar, S.H. The assessment of 24 24-h urine volume by measurement of urine specific gravity with dipstick in adults with nephrolithiasis. Adv Biomed Res 2012, 1, 86. [Google Scholar] [CrossRef] [PubMed]
- Travers, S.; Prot-Bertoye, C.; Daudon, M.; Courbebaisse, M.; Baron, S. How to Monitor Hydration Status and Urine Dilution in Patients with Nephrolithiasis. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Conroy, D.E.; West, A.B.; Brunke-Reese, D.; Thomaz, E.; Streeper, N.M. Just-in-time adaptive intervention to promote fluid consumption in patients with kidney stones. Health Psychol 2020, 39, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Borofsky, M.S.; Dauw, C.A.; York, N.; Terry, C.; Lingeman, J.E. Accuracy of daily fluid intake measurements using a "smart" water bottle. Urolithiasis 2018, 46, 343–348. [Google Scholar] [CrossRef]
- Cohen, R.; Fernie, G.; Roshan Fekr, A. Monitoring fluid intake by commercially available smart water bottles. Sci Rep 2022, 12, 4402. [Google Scholar] [CrossRef]
- Wright, H.C.; Alshara, L.; DiGennaro, H.; Kassis, Y.E.; Li, J.; Monga, M.; Calle, J.; Sivalingam, S. The impact of smart technology on adherence rates and fluid management in the prevention of kidney stones. Urolithiasis 2022, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Stout, T.E.; Lingeman, J.E.; Krambeck, A.E.; Humphreys, M.R.; Zisman, A.; Elfering, S.; Large, T.; Dahm, P.; Borofsky, M. A Randomized Trial Evaluating the Use of a Smart Water Bottle to Increase Fluid Intake in Stone Formers. J Ren Nutr 2022, 32, 389–395. [Google Scholar] [CrossRef]
- Seay, N.W.; Lehrich, R.W.; Greenberg, A. Diagnosis and Management of Disorders of Body Tonicity-Hyponatremia and Hypernatremia: Core Curriculum 2020. Am J Kidney Dis 2020, 75, 272–286. [Google Scholar] [CrossRef]
- Sterns, R.H.; Silver, S.M.; Hix, J.K. Hyponatremia. In Seldin and Giebisch’s The Kidney, Physiology and Pathophysiology, Alpern, R.J., Caplan, M.J., Moe, O.W., Eds.; Elsevier: Fifth edition 2013; Volume 1, p. 1511.
- Filippone, E.J.; Ruzieh, M.; Foy, A. Thiazide-Associated Hyponatremia: Clinical Manifestations and Pathophysiology. American Journal of Kidney Diseases 2020, 75, 256–264. [Google Scholar] [CrossRef]
- Taylor, E.N.; Curhan, G.C. Fructose consumption and the risk of kidney stones. Kidney Int 2008, 73, 207–212. [Google Scholar] [CrossRef]
- Rodgers, A. Effect of cola consumption on urinary biochemical and physicochemical risk factors associated with calcium oxalate urolithiasis. Urol Res 1999, 27, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013, 8, 1389–1395. [Google Scholar] [CrossRef]
- Shuster, J.; Jenkins, A.; Logan, C.; Barnett, T.; Riehle, R.; Zackson, D.; Wolfe, H.; Dale, R.; Daley, M.; Malik, I.; et al. Soft drink consumption and urinary stone recurrence: a randomized prevention trial. J Clin Epidemiol 1992, 45, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Rimm, E.B.; Spiegelman, D.; Stampfer, M.J. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol 1996, 143, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Caffeine intake and the risk of kidney stones. Am J Clin Nutr 2014, 100, 1596–1603. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Mao, Z.; He, X.; Zhang, Q.; Zhang, D. A meta-analysis of coffee intake and risk of urolithiasis. Urol Int 2014, 93, 220–228. [Google Scholar] [CrossRef]
- Siener, R. Nutrition and Kidney Stone Disease. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Large, T.; Williams, J., Jr.; Asplin, J.R.; Krambeck, A. Using Low-Calorie Orange Juice as a Dietary Alternative to Alkali Therapy. J Endourol 2020, 34, 1082–1087. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, C.; Wang, X.L.; Liu, T.Z.; Zeng, X.T.; Li, S.; Duan, X.W. Self-Fluid Management in Prevention of Kidney Stones: A PRISMA-Compliant Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Medicine (Baltimore) 2015, 94, e1042. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Kamphuis, G.M.; Geraghty, R.; Vogt, L.; Henderickx, M.; Hameed, B.M.Z.; Ibrahim, S.; Pietropaolo, A.; Jamnadass, E.; Aljumaiah, S.M.; et al. Global Variations in the Mineral Content of Bottled Still and Sparkling Water and a Description of the Possible Impact on Nephrological and Urological Diseases. J Clin Med 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; DeConinck, V.; Talso, M.; et al. Variations in the Mineral Content of Bottled "Still" Water Across Europe: Comparison of 182 Brands Across 10 Countries. J Endourol 2021, 35, 206–214. [Google Scholar] [CrossRef]
- Stoots, S.J.M.; Geraghty, R.; Kamphuis, G.M.; Jamnadass, E.; Henderickx, M.; Ventimiglia, E.; Traxer, O.; Keller, E.X.; De Coninck, V.; Talso, M.; et al. Variations in the mineral content of bottled 'carbonated or sparkling' water across Europe: a comparison of 126 brands across 10 countries. Cent European J Urol 2021, 74, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Michael, K.; Somani, B.K. Variation in Tap Water Mineral Content in the United Kingdom: Is It Relevant for Kidney Stone Disease? J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Hubert, J.; Hubert, C.; Jungers, P.; Daudon, M.; Hartemann, P. [Drinking water and urinary stones. Which drinking water and which modalities of diuresis?]. Prog Urol 2002, 12, 692–699. [Google Scholar] [PubMed]
- Siener, R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis 2016, 44, 51–56. [Google Scholar] [CrossRef]
- Kessler, T.; Hesse, A. Cross-over study of the influence of bicarbonate-rich mineral water on urinary composition in comparison with sodium potassium citrate in healthy male subjects. Br J Nutr 2000, 84, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Siener, R.; Jahnen, A.; Hesse, A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. Eur J Clin Nutr 2004, 58, 270–276. [Google Scholar] [CrossRef]
- Dai, J.C.; Pearle, M.S. Diet and Stone Disease in 2022. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Qaseem, A.; Dallas, P.; Forciea, M.A.; Starkey, M.; Denberg, T.D.; Clinical Guidelines Committee of the American College of, P. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014, 161, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M.; et al. Medical management of kidney stones: AUA guideline. J Urol 2014, 192, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Groothoff, J.W.; Metry, E.; Deesker, L.; Garrelfs, S.; Acquaviva, C.; Almardini, R.; Beck, B.B.; Boyer, O.; Cerkauskiene, R.; Ferraro, P.M.; et al. Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope. Nat Rev Nephrol 2023, 19, 194–211. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).