Submitted:
25 October 2023
Posted:
26 October 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Results
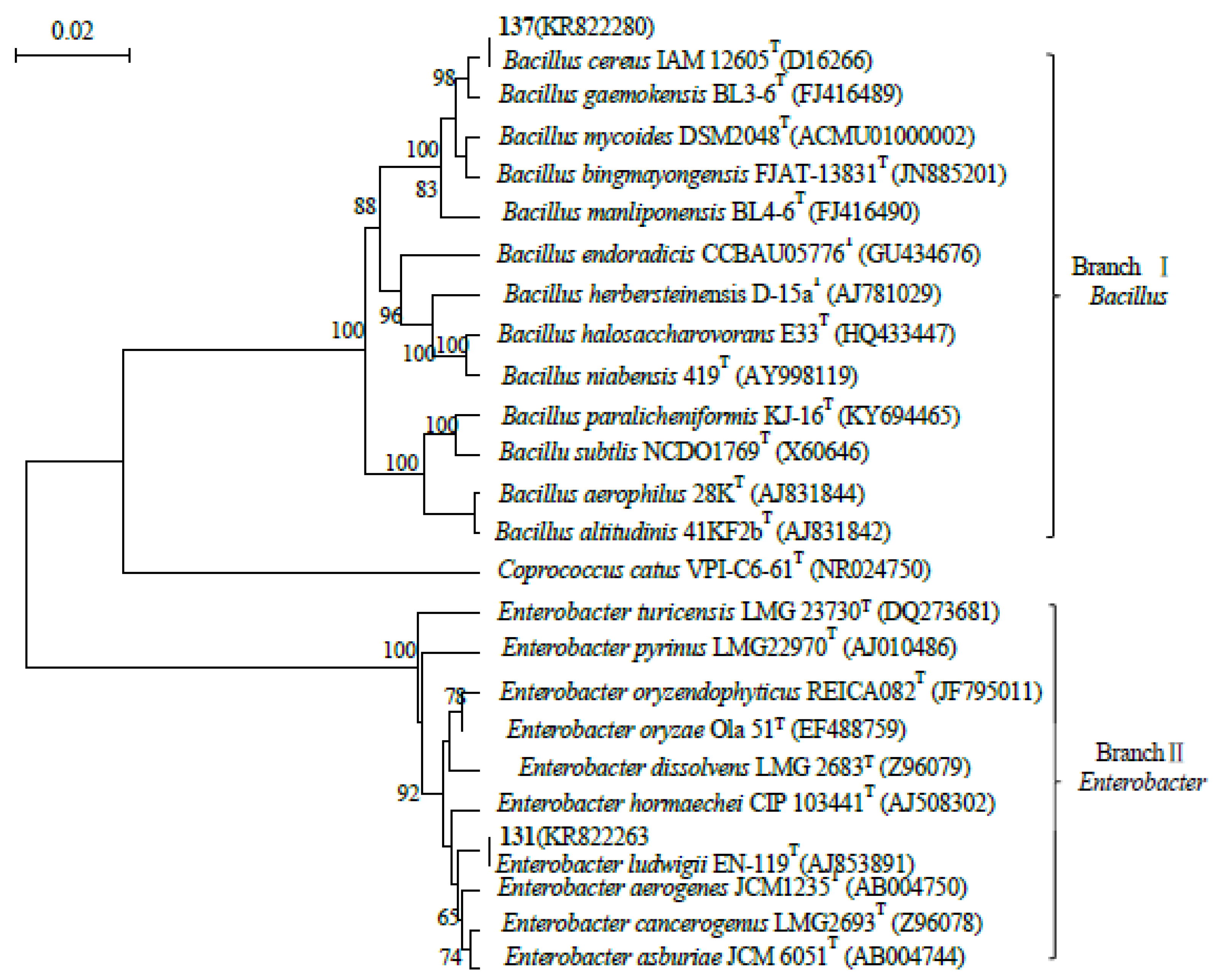
2.1. Sequence Determination and Phylogenetic Status of 16S rRNA Gene in Endophytic Bacteria
2.2. SOD Activity Analysis
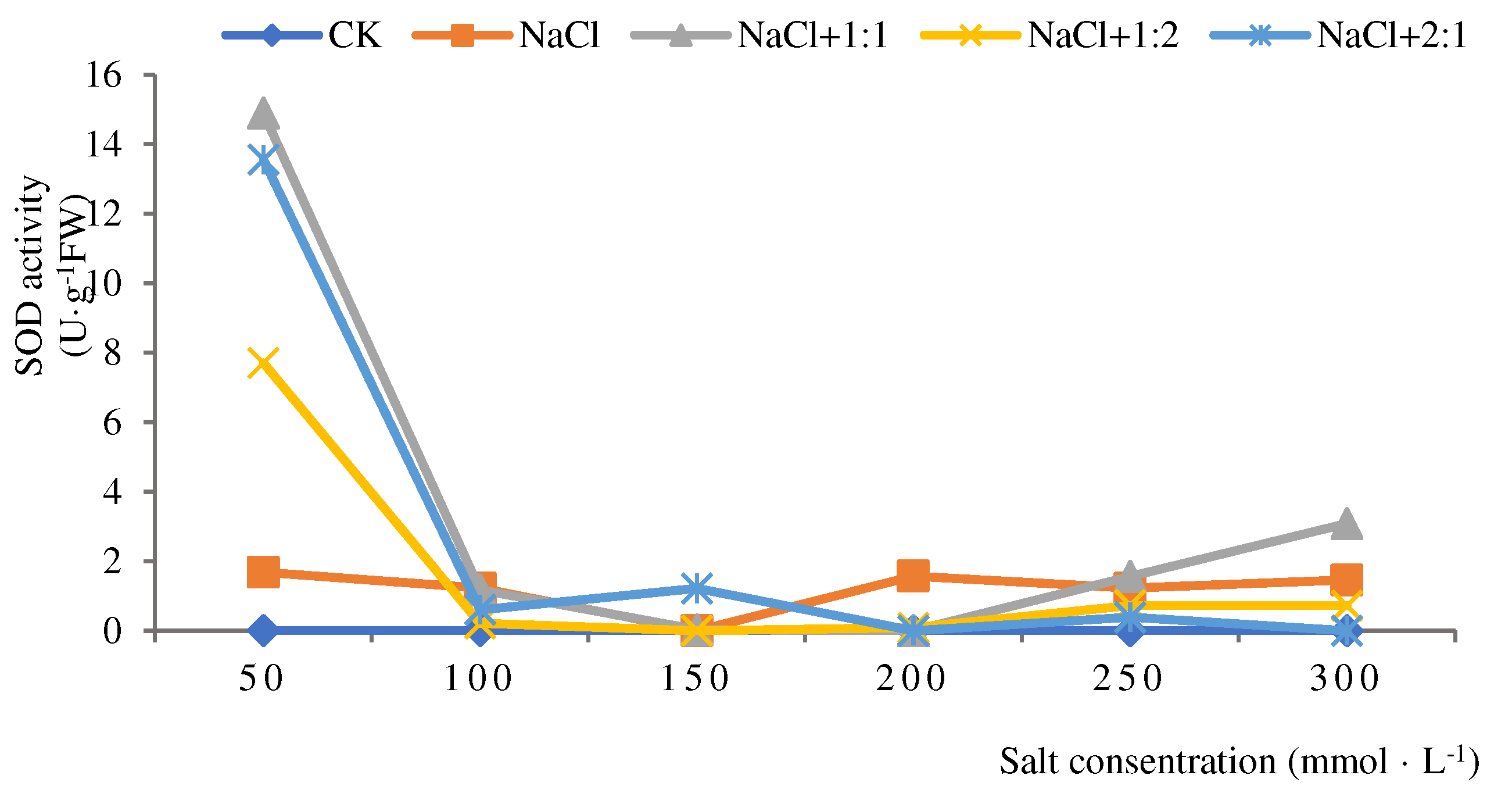
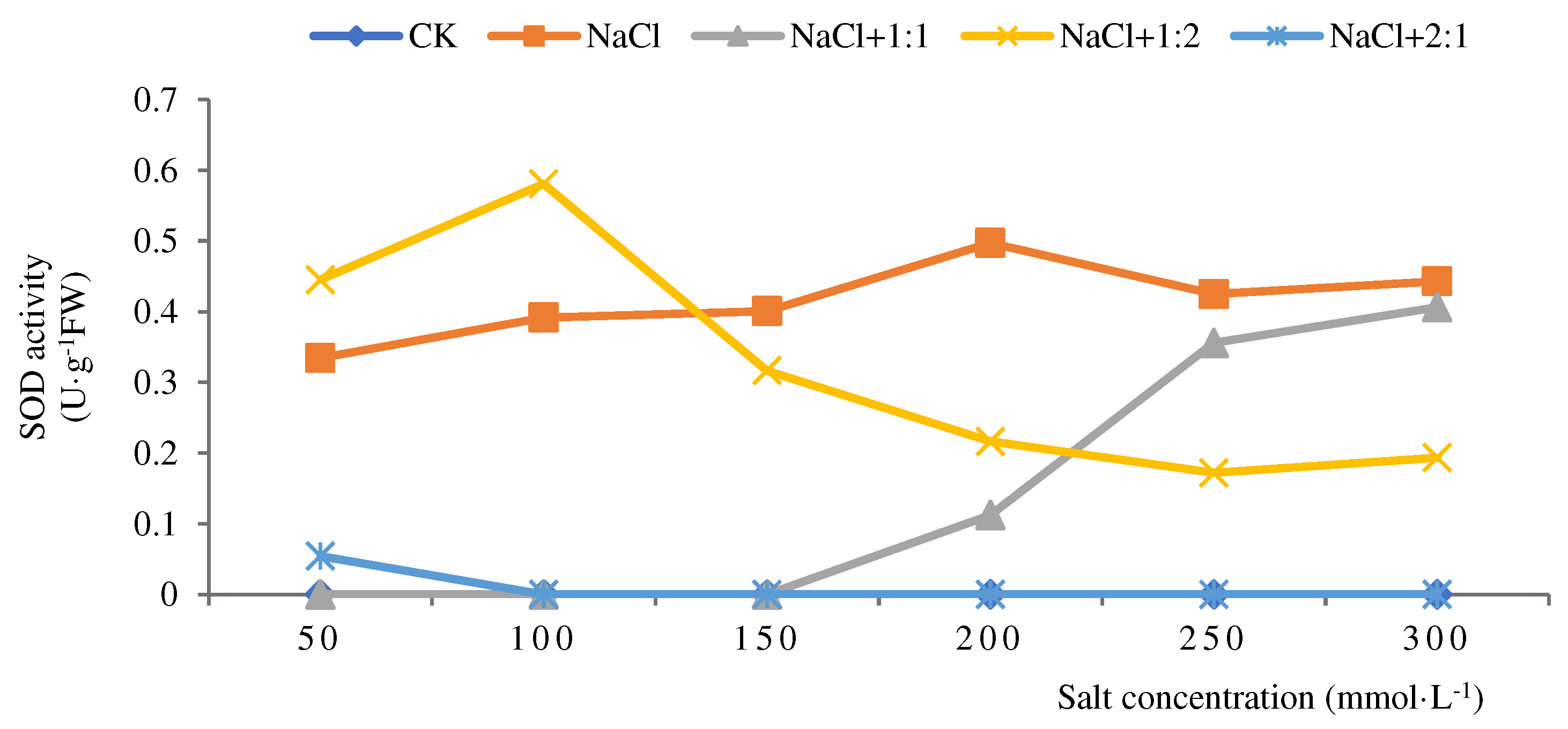
2.2.1. SOD Activity Analysis at 14 Days
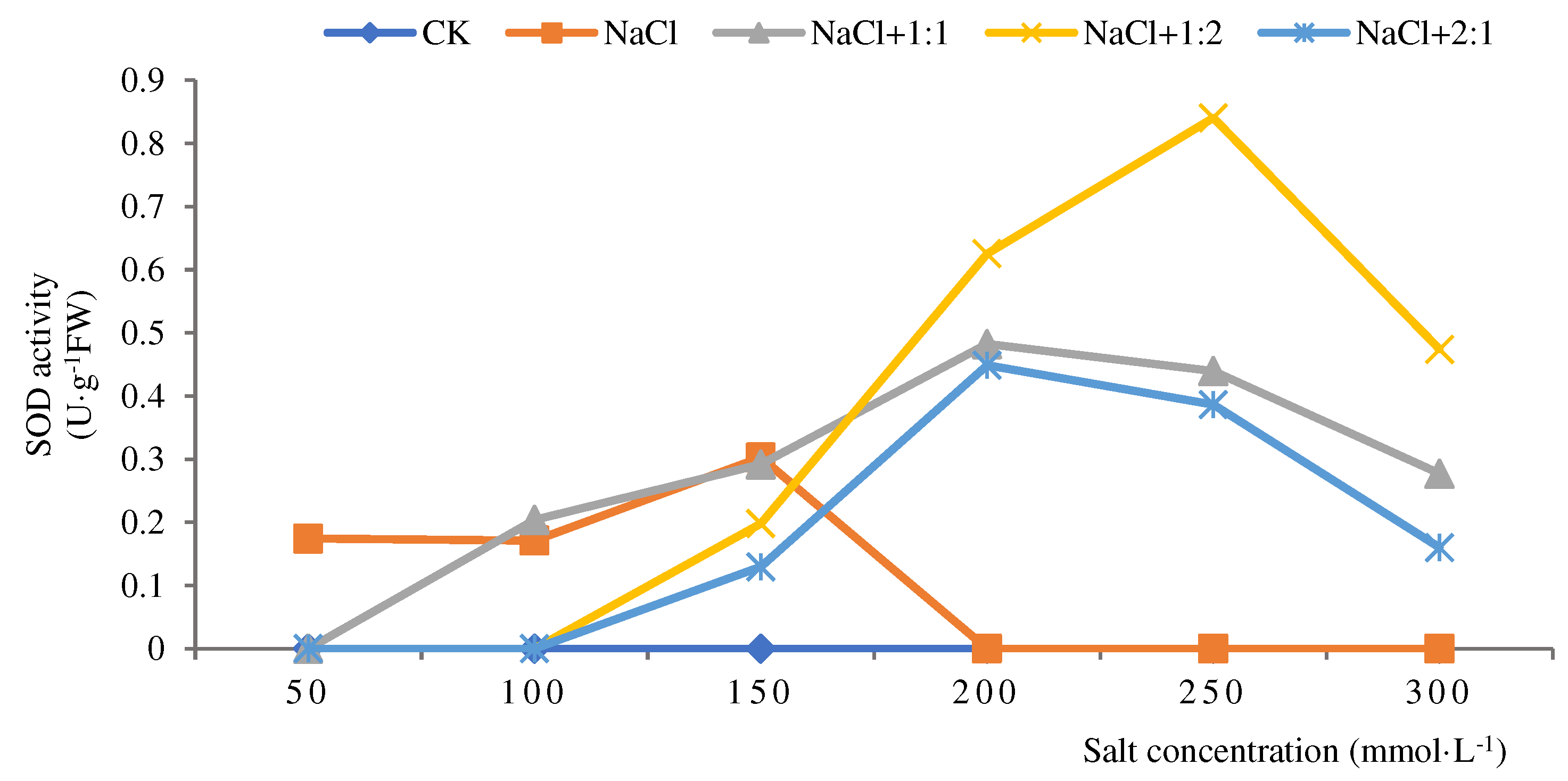
2.2.2. SOD Activity Analysis at 28 Days
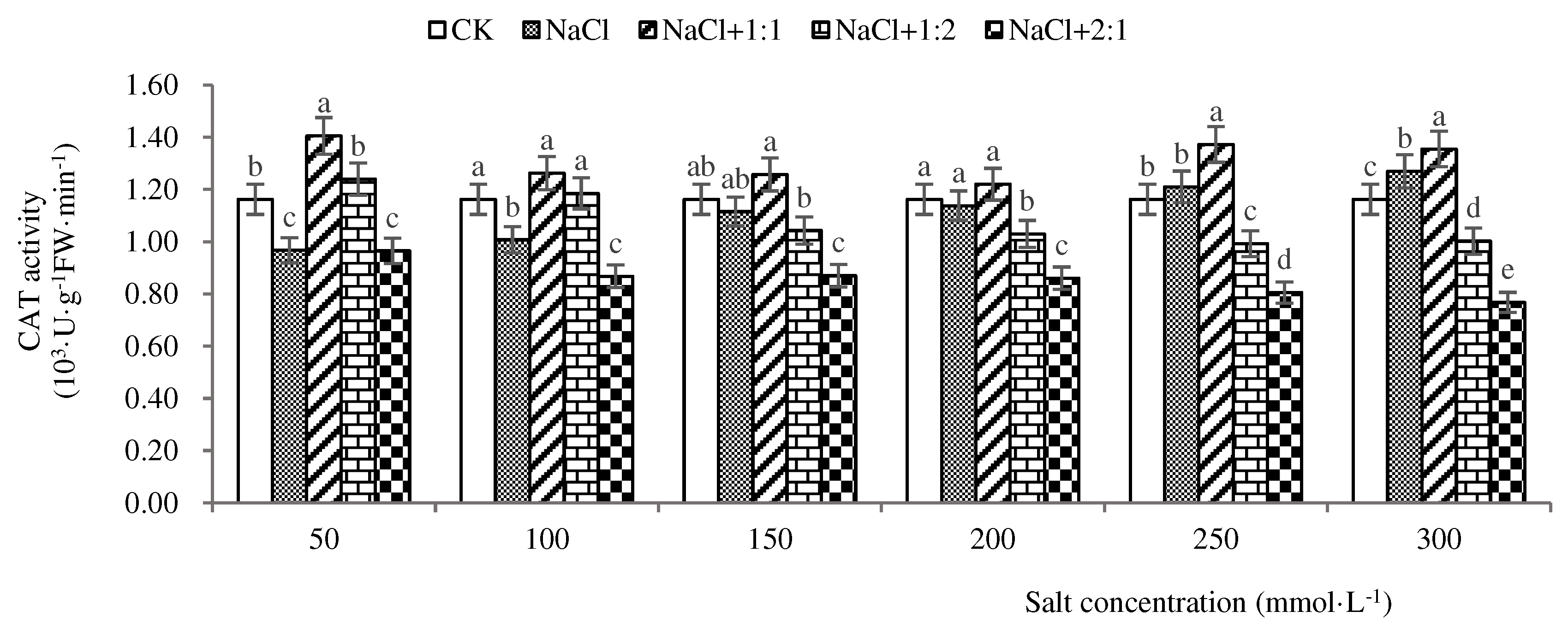
2.3. CAT Activity Analysis
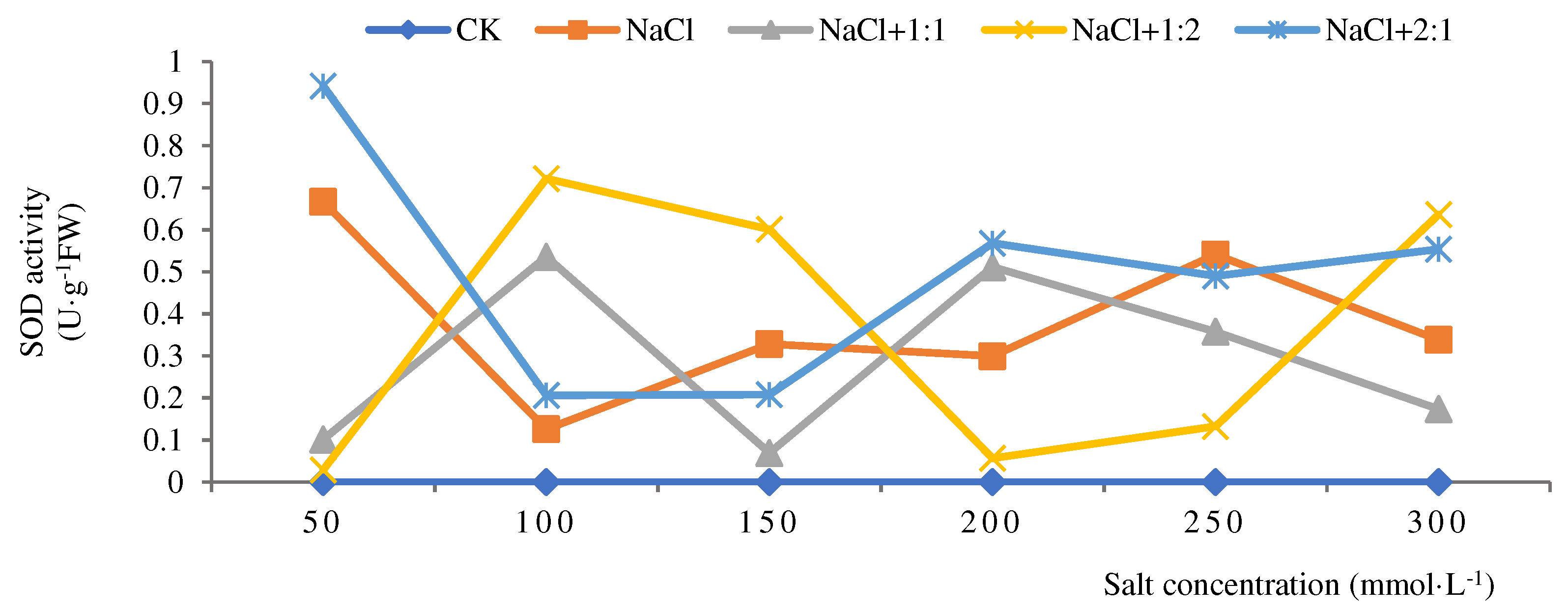
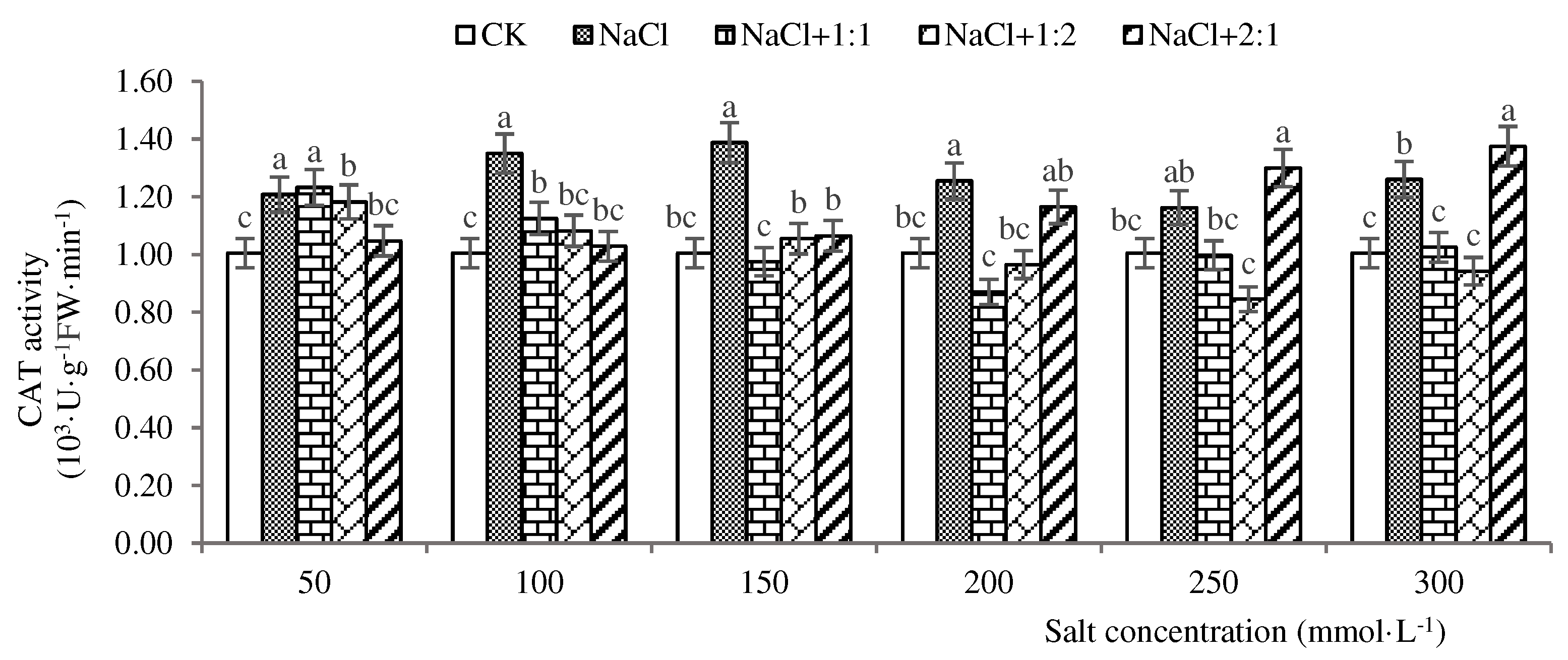
2.3.1. CAT Activity Analysis at 14 d
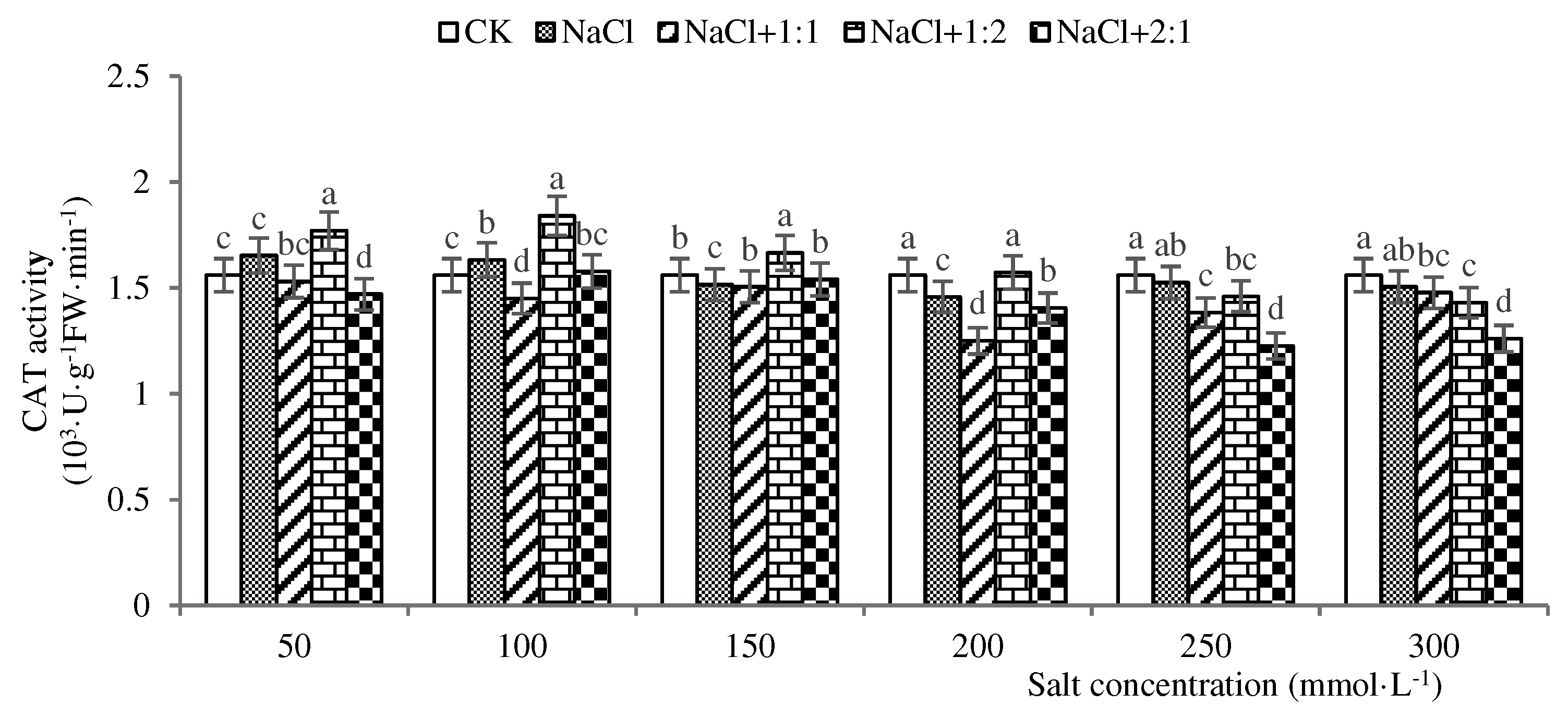
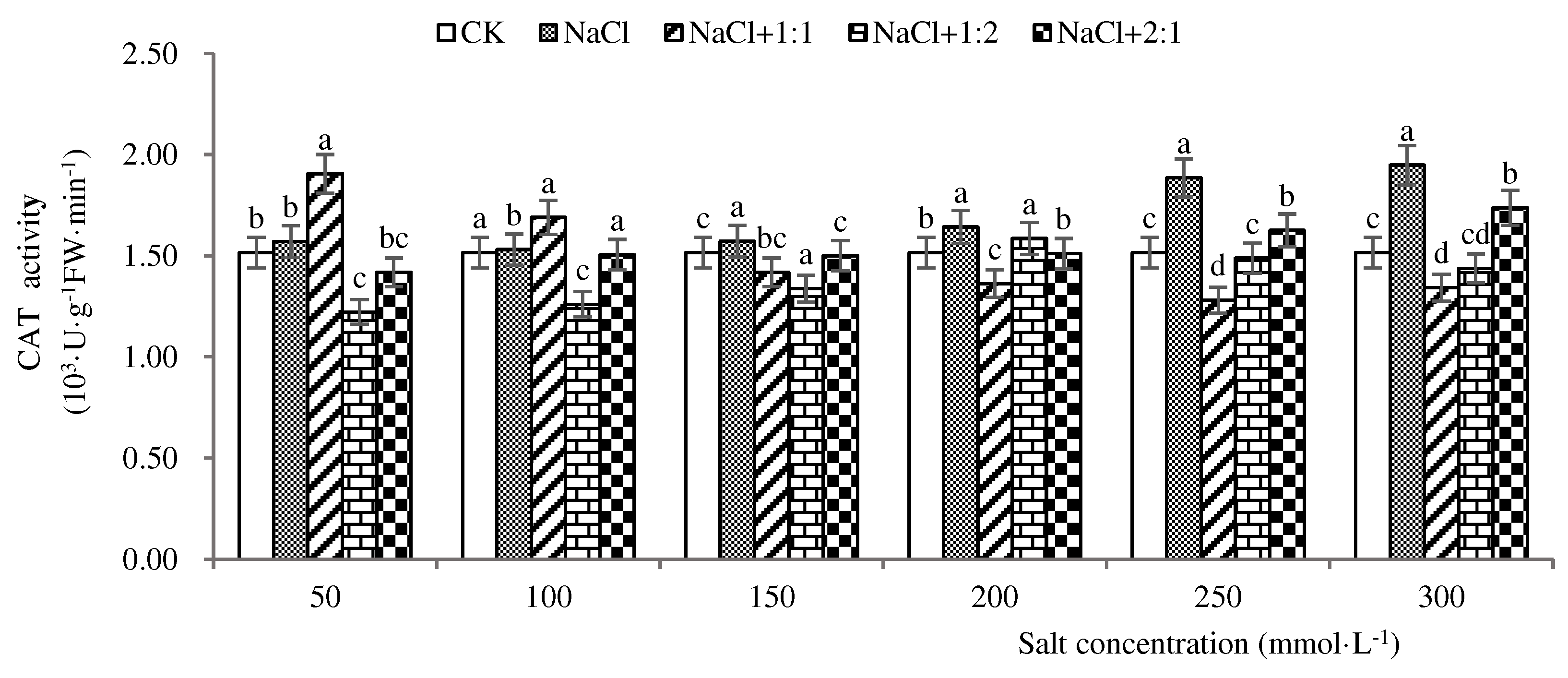
2.3.2. CAT Activity Analysis at 28 Days
3. Discussion
3.1. Effect of Endophytic Bacteria on SOD Activity of Soybean Seedlings under Salt Stress
3.2. Effect of Endophytic Bacteria on CAT Activity of Soybean Seedlings under Salt Stress
3.3. Discussion on Salt Tolerance of Endophytic Bacteria Species
4. Materials and Methods
4.1. Test Materials and Culture
4.2. Sequencing and Phylogenetic Analysis
4.3. Activation of Endophytic Bacteria and Preparation of Bacterial Suspension
4.4. Pretreatment and Experimental Treatment of Soybean Seedling Seeds
4.5. SOD Activity Detection
4.6. CAT Activity Detection
4.7. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Xue, E.Y.; Lu, W.C.; Cui, G.W.; Li, Y.M.; Han, T.F.; Wang, S.D. Breeding and feeding quality analysis of a new soybean strain deficient in Kunitz tryps in inhibitor. Acta Prataculturae Sinica 2020, 29, 91–98. [Google Scholar]
- De Carvalho Oliveira, R.A.; De Andrade, A.S.; Imparato, D.O.; Lima, J. Dalmolin R. Analysis of Arabidopsis thaliana redox gene network indicates evolutionary expansion of class iii peroxidase in plants. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Cha, T.; Zhong, X.B.; Zhou, Q.Z.; He, M.D.; Wang, G.F.; You, J.H.; Wang, Z.Q.; Tang, G.X. Development status of China’s soybean industry and strategies of revitalizing. Soybean Sci. 2018, 37, 458–463. [Google Scholar]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC microbiol. 2012, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Li, Y.T.; An, D.D.; Zhang, R. Research progress on effects of endophytes on plant drought resistance. Biotechnol.Bull. 2015, 31, 23–29. [Google Scholar]
- Zarea, M.J.; Karimi, N.; Goltapeh, E.M.; Ghalavand, A. Effect of cropping systems and Arbuscular mycorrhizal fungi on soil microbial activity and root nodule nitrogenase. J. Saudi Soc. Agric. Sci. 2011, 10, 109–120. [Google Scholar] [CrossRef]
- Vardharajula, S.; Zulfikar, A.S.; Grover, M. Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar] [CrossRef]
- Zhao, L.F.; Xv, Y.J.; Lai, X.H.; Chang, J.L.; Ou, Q.F.; Meng, J.Q.; Peng, D.H. Effects of endophytic bacteria 252 and 254 on peroxidase (POD) and catalase (CAT) activities of wheat seedlings under salt stress. Chin. J. Appl. Ecol. 2017, 28, 2984–2992. [Google Scholar]
- Yang, R.P.; Bao, Z.X.; Chen, G.R.; Li, Y. Research Progress on Drought Resistance of soybean seedlings. Crop J. 2018, 28, 8–12. [Google Scholar]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Qi, H. Exogenous spermidine enhances chilling tolerance of tomato (Solanum lycopersicum L.) seedlings via involvement in polyamines metabolism and physiological parameter levels. Acta Physiol. Plant. 2015, 37, 1–15. [Google Scholar] [CrossRef]
- Jiang, X.W.; Li, H.Q.; Wang, J.H. Physiological response of Scutellaria baicalensis seed germination and seedling to exogenous ascorbic acid under salt stress. Plant Physiol. J. 2015, 51, 166–170. [Google Scholar]
- Dinneny, J.R. Traversing organizational scales in plant salt-stress responses. Curr. Opin. plant biol. 2015, 23, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.Y.; Wu, Y.H.; Tian, X.L. Recent advances and issues on the endophyte. Chin. J. Ecol. 2019, 2, 86–91. [Google Scholar]
- Elisa, K.; Hassan, M.; Asaph, A. Plant–microbe interactions in the rhizosphere via a circular metabolic economy. The Plant Cell 2022, 34, 3168–3182. [Google Scholar]
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and engineering beneficial plant-microbe interactions: plant growth promotion in energy crops. Plant biotechnol. J. 2014, 12, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Feng, R.Z.; Shi, S.L. Mitigation effect of NO on growth inhibition and oxidative damage of alfalfa roots under salt stress. Acta Ecol. Sinica 2015, 35, 3606–3614. [Google Scholar]
- Radhakrishnan, R.; Khan, A.L.; Lee, I.J. Endophytic fungal pre-treatments of seeds alleviates salinity stress effects in soybean plants. J. Microbiol. 2013, 51, 850–857. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Ahmad, N.; Waqas, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Exophiala sp. LHL08 reprograms Cucumis sativus to higher growth under abiotic stresses. Physiol. plant. 2011, 143, 329–343. [Google Scholar] [CrossRef]
- Elsey-Quirk, T.; Middleton, B.A.; Proffitt, C.E. Seed flotation and germination of salt marsh plants: the effects of stratification, salinity, and/or inundation regime. Aquat. Bot. 2009, 91, 40–46. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends plant sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules, 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Pereg, L.; McMillan, M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 2015, 80, 349–358. [Google Scholar] [CrossRef]
- Wu, Q.; Feng, H.Q.; Li, H.Y.; et al. Effect of Puccinia striiformis west infection on cyanide-resistant respiration and metabolism of reactive oxygen in wheat, Acta Phytopathol. Sinica 2006, 36, 49–56. [Google Scholar]
- Han, K.; Tian, Z.Y.; Liu, K.; Zhang, J.Y.; Chang, Y.Y.; Guo, Y.Q. Effect of endophytic bacteria with ACC deaminase activity in Kosteletzkya pentacarpos on wheat salt tolerance. Plant Physiol. J. 2015, 51, 212–220. [Google Scholar]
- Zhang, Y.Q. Study on induced resistance of R. rugosa ‘Plena’. Dissertation for the Degree of Master. HaErbin: Northeast Forestry University,2013.
- Khan, A.L.; Waqas, M.; Khan, A.R.; Hussain, J.; Kang, S.M.; Gilani, S.A.; Hamayun, M.; Shin, J.H.; Kamran, M.; Al-Harrasi, A.; Yun, B.W.; Adnan, M.; Lee, I.J. Fungal endophyte Penicillium janthinellum LK5 improves growth of ABA-deficient tomato under salinity. World J. Microbiol. Biotechnol. 2013, 29, 2133–2144. [Google Scholar] [CrossRef]
- Li, F.L.; Guan, L.L.; Hu, F.R. Physiological Response of Different Resistant Cultivars of Lilium Orietal Hybrid after Inoculation with Botrytis cinerea. J. Northeast Forest. Univ. 2020, 48, 107–113. [Google Scholar]
- Zhang, R.; Lv, J.; Mi, Q.S.; Wang, S.G. Effects of salicylic acid on antioxidant enzymes in rice seedlings under chilling stress. J. Southwest Agric. Univ. (Natural Sci.) 2016, 01, 29–32. [Google Scholar]
- Pi, E.; Xu, J.; Li, H.H.; Fan, W.; Zhu, C.M.; Zhang, T.Y.; Jiang, J.C.; He, L.T.; Lu, H.F.; Wang, H.Z.; Poovaiah, B.W.; Du, L.Q. Enhanced salt tolerance of rhizobia-inoculated soybean correlates with decreased phosphorylation of the transcription factor GmMYB183 and altered flavonoid biosynthesis. Mol. Cell.proteomics 2019, 18, 2225–2243. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Mao, S.X.; Yan, S.Z.; Lu, C.M. Effects of endophytes DLJ1 (Pseudomonas fluorescens)and SZ5(Bacillus cereus)on the resistance, yield and quality of pepper plants under the stress of Meloidogyne incognita. Plant Protection 2020, 46, 96–102. [Google Scholar]
- Saravanakumar, D.; Kavino, M.; Raguchander, T.; Subbian, P.; Samiyappan, R. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta physiol. Plant. 2011, 33, 203–209. [Google Scholar] [CrossRef]
- Patricio, J.B.; Nitza, G.I.; Jacquelinne, J.A.; Mora, M.L.; Crowley, D.E.; Jorquera, M.A. Formulation of bacterial consortia from avocado ( Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. Appl. Soil Ecol. 2016, 102, 80–91. [Google Scholar]
- Singh, R.P.; Jha, P.N. Mitigation of salt stress in wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorghum bicolor. Acta physiol. Plant. 2016, 38, 110–116. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Lan, Y.Y.; Chen, Y.Y.; Wang, S.F.; Xin, Y.R. Effects of different concentrations of Bacillus mycoides on fruit cracking, yield and quality of Honey pomelo. Mod. Agric. 2017, 11, 30–31. [Google Scholar]
- Wang, H.X.; Liu, H.; Yang, G.P.; Zhang, X.; Li, Z.; Zhang, Y. Effects of Bacillus amyloliquefaciens YM6 on Physiological and Biochemical Characteristics of Maize under Salt Stress. Acta Agric. Boreali-occidentalis Sinica, 2020, 29, 436–443. [Google Scholar]
- Sun, J.G.; Hu, H.Y.; Liu, J.; Chen, Q.; Gao, M.; Xu, J.; Zhou, Y.Q. Growth Promotion Potential and Distribution Features of Nitrogen-Fixing Bacteria in Field Environments. Scientia Agric. Sinica 2012, 45, 1532–1544. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



