1. Introduction
The ongoing challenge of global warming is a pressing environmental issue of significant concern. In response to the need of reducing CO
2 emissions, the development of carbon capture and storage (CCS) technologies has emerged as a promising alternative [
1]. CCS includes diverse methodologies such as absorption, adsorption, membrane-based systems, and cryogenic separation [
2]. In this context, the application of porous materials as adsorbents is gaining substantial attraction due to its advantages, including low energy requirements for adsorbent regeneration, high adsorption capacity and selectivity for CO
2.
Numerous adsorbents have been proposed and developed, such as activated carbon [
3], zeolites [
4], mesoporous silicas [
5], lamellar double hydroxides [
6], metal-organic frameworks [
7], graphene oxide [
8], magnesium oxide-based adsorbents [
9]. Among these materials, mesoporous silicas have emerged as particularly noteworthy candidates, due to their well-defined pore structures, high surface areas, and chemical surface composition, suitable for functionalization with reactive species for CO
2 chemisorption. In particular, amine-modified MCM-41 is effective in capturing CO
2, with reported sorption capacities between 0.87 and 2.41 mmol/g under different adsorption conditions [
10,
11,
12,
13,
14,
15,
16,
17,
18].
Nowadays, a persistent challenge involves improving the efficiency of existing adsorbents while reducing production costs. Achieving this balance is critical for the widespread use of CO
2 adsorption technologies at an industrial scale. In this regard, considerable attention has been given to template agent removal procedures. Thermal treatments are proven to be both fast and effective in removing surfactants, as reported in most of the current literature that analyses MCM-41 silicas as CO
2 adsorbents [
10,
11,
12,
13]. However, they also produce the condensation of silanol groups into siloxane bonds when applied at temperatures above 270 °C with a detrimental effect on the functionalization capability of the material [
19]. Moreover, the combustion of the organic template also leads to CO
2 emission, which is contrary to the potential application of these adsorbent materials [
19]. An alternative consists in the use of washing methodologies for template removal. Conversely, employing solvents may offer the advantage of retaining these essential groups and avoiding CO
2 emissions. On the other hand, an inefficient extraction might lead to incomplete template removal, resulting in materials with a diminished surface area and restricted accessibility to the silanol groups. In addition, it is necessary to consider the environmental impact of chemical solvents employed in the washing procedure.
Once the porosity of the MCM-41 silicas has been released, surface modification becomes necessary to enhance their efficacy as carbon dioxide adsorbents. Among the different molecules used for this purpose, amines stand out, given their highly selective CO
2 uptake [
20]. The 3-aminopropyltrimethoxysilane (APTS) is broadly used, due to its low cost and relatively small size, for its incorporation into the MCM-41 through two methodologies: dry-grafting [
10,
12,
14,
15,
16] and wet-grafting [
11,
13]. It has been proven that wet conditions promote materials with higher CO
2 adsorption capacities. This was attributed to the role of water in the generation of silanol groups on the silica surface during the grafting process, which serve as new active sites for the anchoring of 3-aminopropyl groups [
17].
In this work, the use of low-cost industrial sources of silicon and surfactants for the synthesis of MCM-41 mesoporous silica was explored. Different methodologies for template removal were applied to maximize surface area while safeguarding the surface-bound silanol groups, minimizing energy consumption, and using environmentally friendly reagents. The materials were surface modified by grafting with APTS to enhance CO2 adsorption properties and their retention was evaluated as a function of temperature by in situ Fourier transform infrared spectra (FTIR).
2. Experimental
2.1. Materials
The reactants used in this study were tetraethyl orthosilicate (TEOS 98%, Aldrich), industrial sodium silicate solution (ISS, 27.35 wt% SiO2 and 8.30 wt% Na2O, Mejorsil), hexadecyltrimethylammonium bromide (CTAB 96%, Sigma-Aldrich), industrial N-hexadecyltrimethylammonium chloride (CTAC, 48-50 wt% CTAC, 30-35 wt% EtOH and less than 1 wt% N,N-dimethylhexadecylamine (DMHA), Meranol), and sulfuric acid (H2SO4, 98%, Anedra), ammonia (25%, Merck), absolute ethyl alcohol (99.5%, Soria Analytical) and 3-aminopropyltrimethoxysilane (Sigma-Aldrich).
2.2. Synthesis and functionalization
Different sources of silicon and structure-directing agents were explored. The samples were identified considering the silicon source, surfactant, and the thermal treatment used to remove the template.
The MCM-41 synthesized from ISS and CTAB or CTAC industrial solution was obtained following the methodology proposed by Edler [
21]. In a typical preparation, CTAB or CTAC was dissolved in deionized water under stirring at 30 °C until a clear solution was obtained. Then, ISS was added dropwise to the mixture under vigorous stirring. After 15 min under stirring the pH was adjusted using a solution of H
2SO
4 (10 wt%). The composition of the obtained gel was 1 SiO
2: 0.29 Na
2O: 0.25-0.26 CTAB or CTAC: <0.006 DMHA: 1.09-1.27 EtOH: 155.47-156.12 H
2O. The mixture was then transferred to a Teflon-lined stainless-steel autoclave and kept in an oven at 100 °C for 4 h. After that, the solid was filtered, washed with deionized water, and dried at 100 °C for 24 h. The samples were called S-B and S-C respectively.
A reference sample was prepared by using TEOS and CTAB [
22]. The molar composition was 1 TEOS: 0.3 CTAB: 11 NH
4OH: 58 EtOH: 144 H
2O. The medium synthesis temperature was 30 °C. The complete dissolution of CTAB was ensured and subsequently, under stirring, the silicon source was added dropwise under stirring at 30 °C for 2 h. The material was recovered by filtration and washed with ethanol and then with distilled water. Finally, excess moisture was removed by drying it at 100 °C for 24 h. The sample was called T-B.
The surfactant was removed using different thermal treatments in air: A long treatment (LT, 540 °C for 10 h, heating rate 1 °C/min) or a short treatment (ST, 510 °C for 2 h, 5 °C/min). Samples were called S-B-LT, S-B-ST, S-C-LT, S-C-ST, T-B-LT, and T-B-ST, respectively.
To avoid the condensation of silanol groups induced by thermal treatments [
23], a washing process with ethanol (i.e., solvent extraction) under acidic conditions at 70 °C for 40 min was used to remove the template [
24]. This procedure was repeated 4 times, mixing the sample previously extracted with a fresh solvent solution. Finally, the solid was dried at 100 °C for 24 h. The samples were identified by the -W suffix.
Once the surfactant was removed, the materials were functionalized with APTS by the grafting method reported in reference [
13]. The MCM-41 was added to anhydrous ethanol under stirring for 10 min. Then, distilled water was added under continuous stirring for 30 min. The APTS was added dropwise at 70 °C. The mixture was stirred and refluxed for 10 h. The APTS-modified material was obtained after washing with anhydrous ethanol and drying at 80 °C for 12 h. Samples were called S-B-ST-g, T-B-ST-g, S-C-ST-g, and S-C-W-g.
2.3. Characterization
The small angle X-ray diffraction patterns (SAXS) were obtained using XEUSS 1.0 equipment with a Pilatus 100K detector with Cu Kα radiation (λ = 1.5405 Å). Scanning electron microscopy (SEM) images were acquired on an Inspect S50 microscope operated at 2 kV. The samples were sputtered with a thin film of gold. Transmission electron microscopy (TEM) was done using a Tecnai F20 G2 microscope operated at 200 kV. FTIR spectra were obtained in a FTIR Nicolet iS5 spectrophotometer with an iD5 ATR accessory.
The N
2 adsorption-desorption isotherms at 77 K were measured on Micromeritics ASAP 2020 apparatus. Prior to the analysis, the samples were degassed at 100 °C for 12 h under a vacuum of 10
-2 mmHg. The specific surface area was obtained from the gas adsorption isotherms by applying the Brunauer-Emmett-Teller theory (S
BET) and non-local density functional theory (S
DFT). The total pore volume (V
P) was estimated using the Gurvich rule at a relative pressure of 0.953 [
25]. The pore size distribution (PSD) analysis was performed using the NLDFT model applied to cylindrical pores and an oxide surface.
2.4. CO2 adsorption testing
The CO2 adsorption/desorption isotherms were measured in Quantachrome Autosorb iQ model automatic sorption equipment with its control and data analysis software (ASiQwin 6.0). The samples were introduced into a 12 mm diameter quartz cell provided for the chemisorption mode. The isotherms were fitted using the Langmuir isotherm to determine the moles of CO2 sorbed in a monolayer covering the surface. CO2 isotherms were obtained at 25 °C in the pressure range from 10 to 800 Torr.
Diffuse reflectance infrared spectroscopy (DRIFTS) measurements were performed on a Thermo Fisher Scientific Nicolet 6700 with a low-temperature high sensitivity Thermo Fischer MCT-A detector. This equipment was assembled with a sealed cell that allowed the treatment of samples with gases at a controlled temperature. Spectra were taken after dosing 5 Torr of CO2 and then in vacuum at different temperatures up to 100 °C.
3. Results and discussion
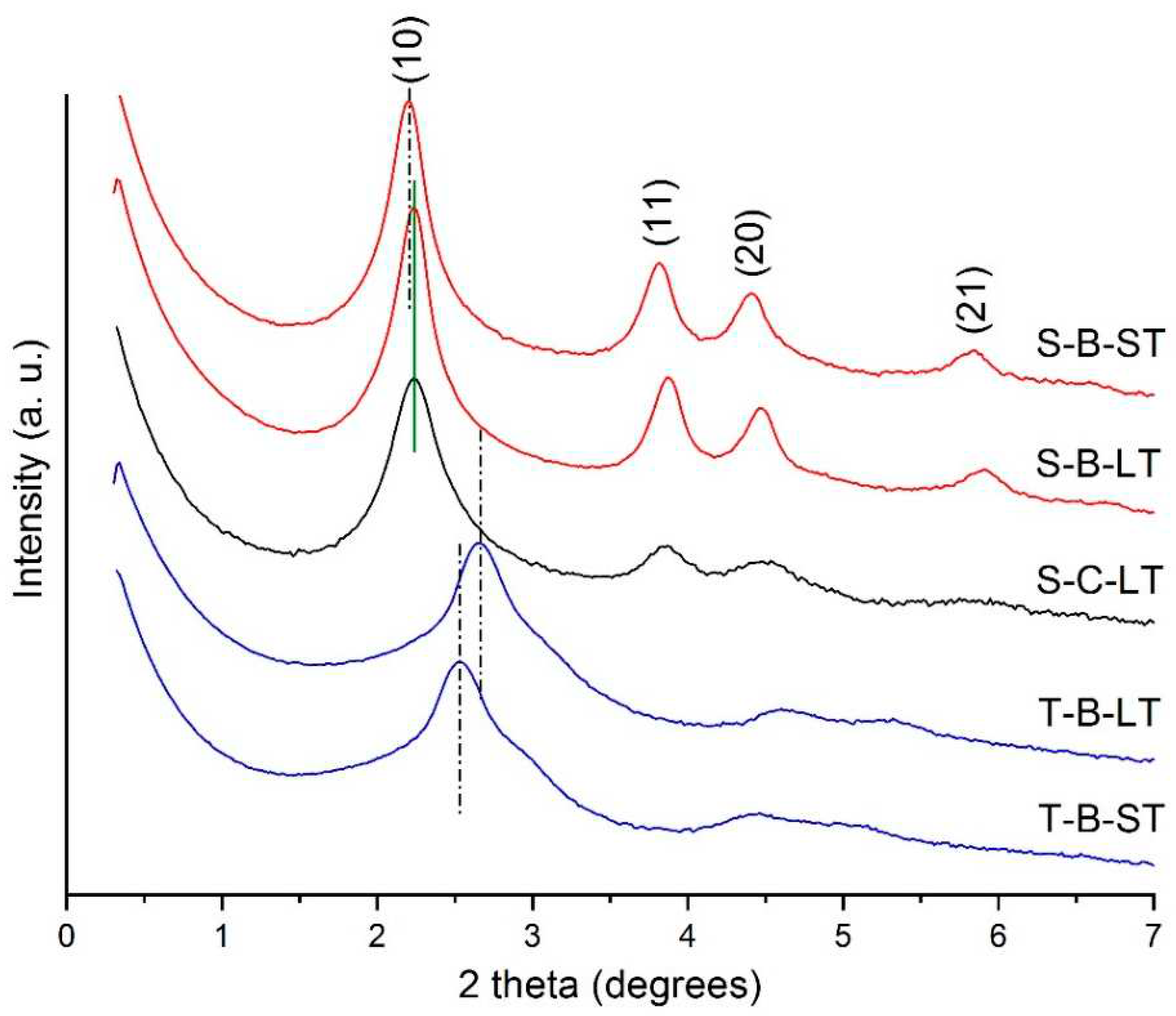
The SAXS diffraction patterns shown in
Figure 1 are consistent with the expected 2D hexagonal structure (plane group p6m) and are indexed accordingly. The samples synthesized with ISS exhibited a higher degree of order. Among them, the one obtained using CTAC presented broadened reflections. It can be observed that longer thermal treatment at higher temperature (LT) produced a slight decrease in the interpore distance from 4.64 to 4.57 nm for S samples, and from 4.02 to 3.83 nm for T samples. The number of observed reflections indicated high-quality samples in the bulk analysis, confirming a well-ordered hexagonal structure.
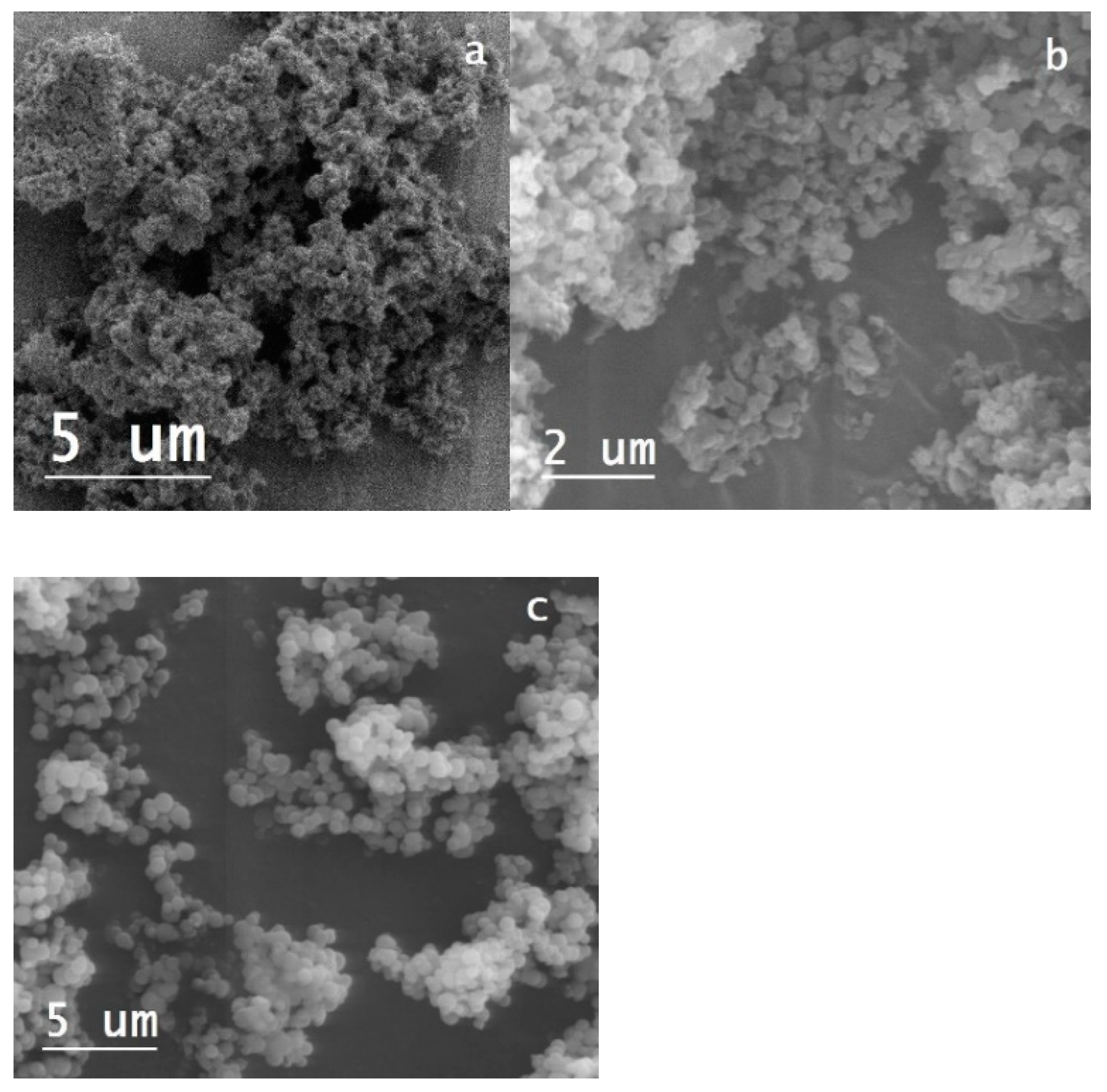
The SEM images (
Figure 2) showed morphological differences depending on the silicon source. Samples made using TEOS had a spherical shape, while those synthesized with sodium silicate presented a more irregular geometry and a smaller particle size.
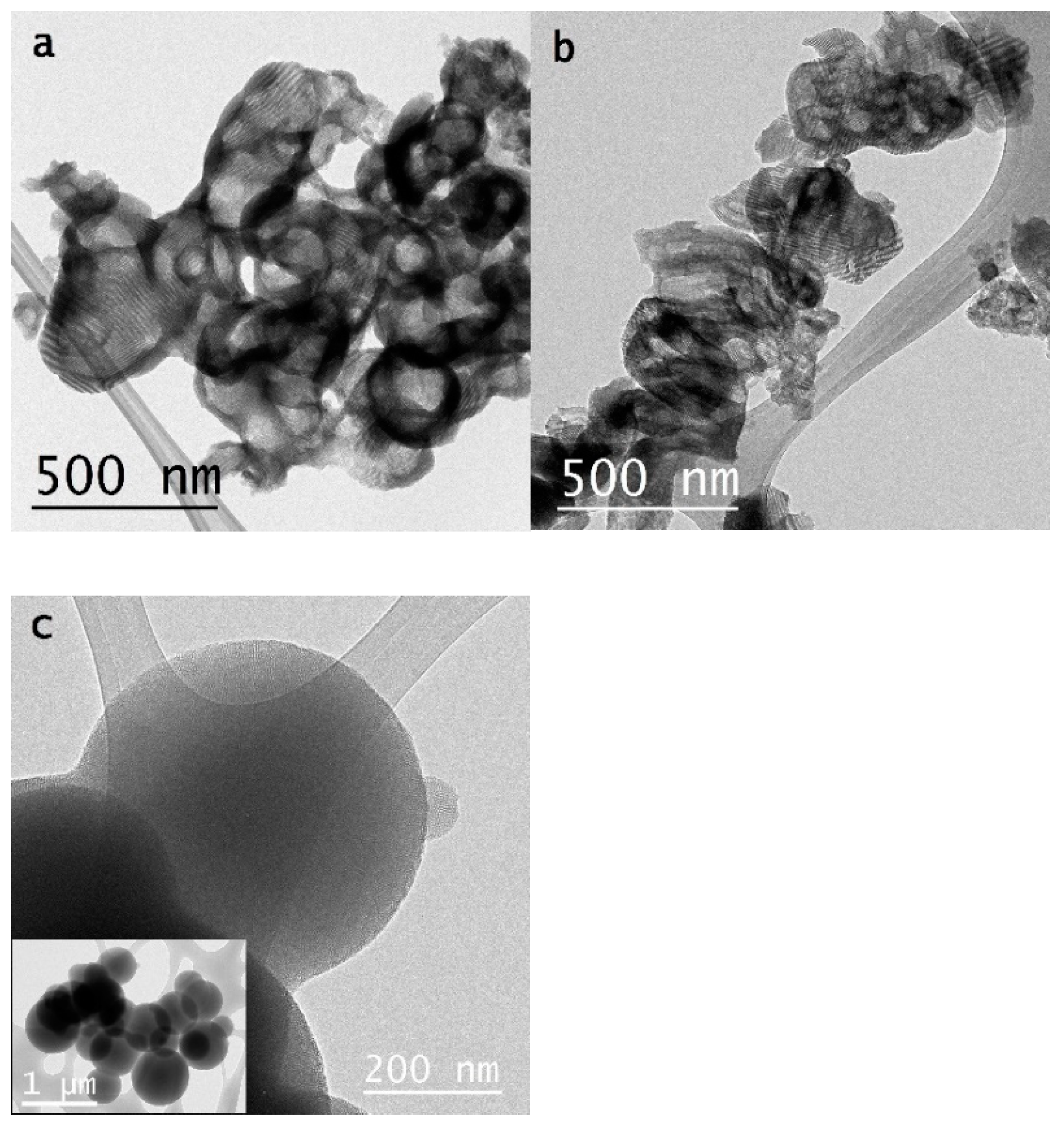
In
Figure 3 are shown representative images of each sample. Different morphologies are observed. Samples obtained using ISS tend to present porous structures organized at different spatial scales. S-C samples presented particles with a bowl shape and shorter mesochannels compared to samples S-B. Mesochannels in S-C materials are oriented more randomly than those in S-B samples due to their length. Samples T-B have a spherical shape with a broad particle size distribution and radial mesochannels.
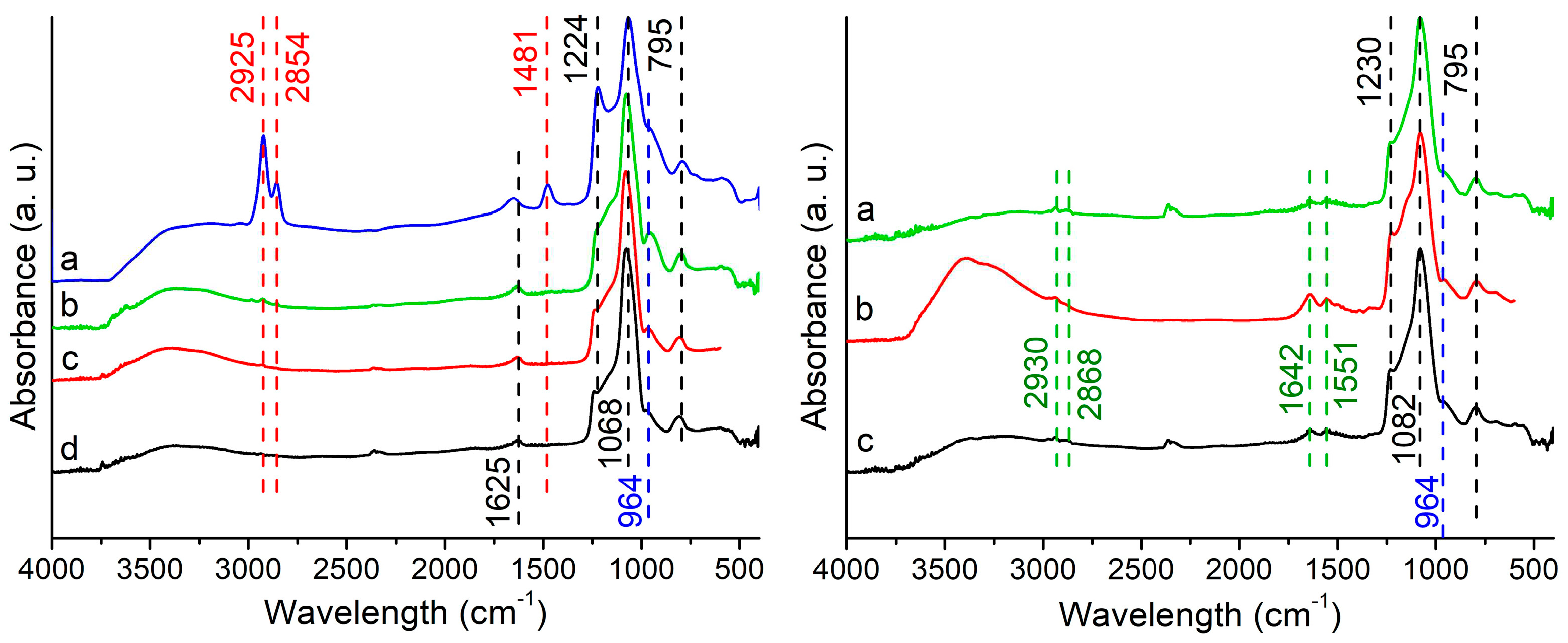
Before textural analysis, the surfactant elimination was verified by FTIR. Spectra of the S-C sample before and after removal treatments are shown in
Figure 4 (left). Similar results (not shown) were obtained for S-B and T-B samples.
The spectra of as-made samples showed bands attributed to surfactant at 2854 cm
-1, 2925 cm
-1, and 1480 cm
-1 [
26,
27]. These bands are assigned to C-H asymmetric stretching of alkyl chains of CTA cation and symmetric and asymmetric stretching C-H scissoring vibration of the CH
3-N
+ group. The absence of these bands after treatments indicated the removal of the template. Other bands observed between 1224 cm
-1 and 795 cm
-1 are assigned to different Si-O modes [
28,
29]. The preservation of silanol groups was confirmed by the band at 964 cm
-1 (blue line), which corresponds to the stretching vibrations of the surface Si-O- groups [
28,
30,
31,
32]. Bands observed at 1625 cm
-1 and 3400 cm
-1 are assigned to OH bending vibrations and -OH units of adsorbed water and silanol groups [
28].
From the point of view of preservation of silanol groups, solvent extraction and ST treatment seem to be more efficient than the longer one. The incorporation of APTS molecules was confirmed (Fig. 4, right) by the appearance of the bands at 2925 cm-1 and 2854 cm-1 (C-H bonds), and 1642 cm-1 (N-H deformation in RNH3+).
An indication of the high reactivity of the functionalized material against CO
2 adsorption was evidenced by the presence of the band around 1555 cm
-1 (C=O stretch), which is attributed to carbamate formed by the exposure to air [
33,
34,
35]. The symmetric and asymmetric stretching modes of CO
2- could be seen around 2349 cm
-1, 1386 cm
-1, and 1430 cm
-1 [
20,
36].
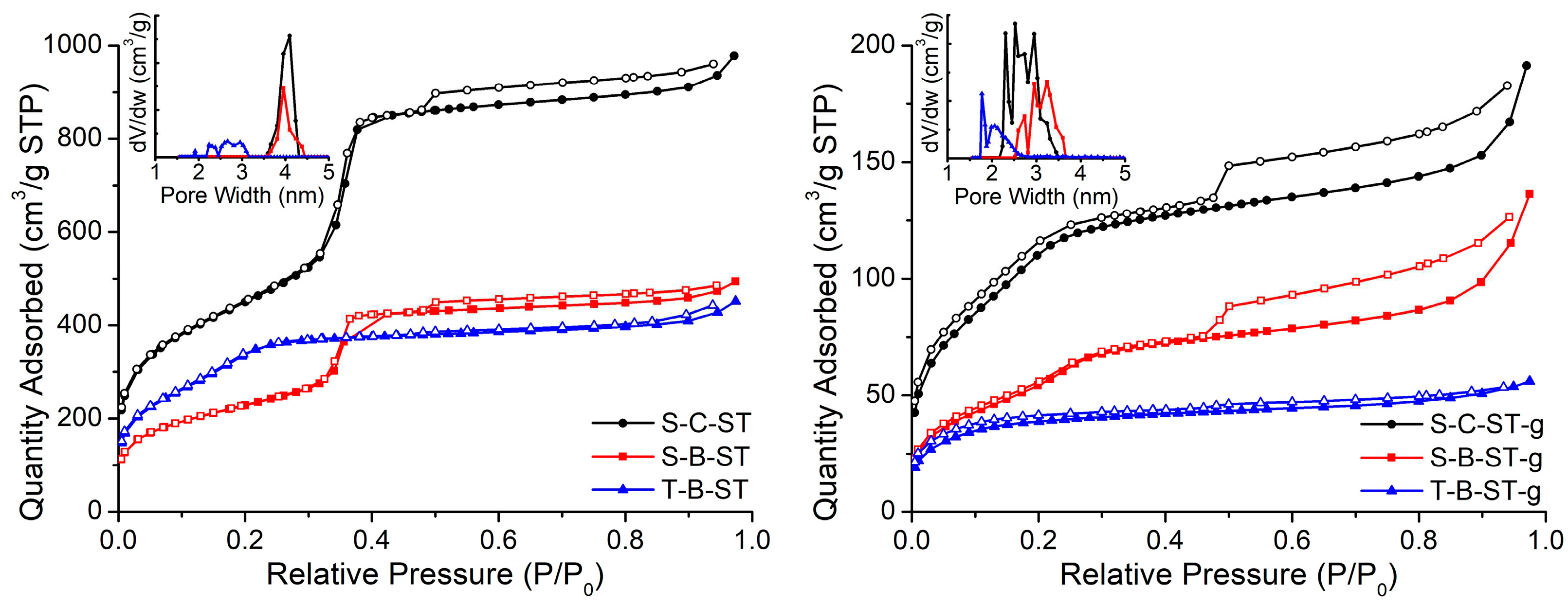
Nitrogen adsorption and desorption isotherms and the PSD for the synthesized materials subjected to ST treatment and for their functionalized counterparts are shown in
Figure 5. The main textural parameters obtained from different methods are compiled in
Table 1, with the sample S-C-ST showing the best textural properties.
Nitrogen isotherms from S-C-ST and S-B-ST can be classified as type IV according to the IUPAC classification, which is characteristic of mesoporous materials. When examining the curves, two different hysteresis loops can be seen. The first one corresponds to H1 type and appears at a relative pressure of around 0.35. This can be attributed to a narrow pore size distribution of the open-ended tubular pores from MCM-41 material framework [
25]. The second one extends over the relative pressure range of 0.46-0.95. This hysteresis loop can be associated with the existence of the porosity observed at a bigger scale, as revealed in the TEM images. On the other hand, T-B-ST exhibits a type I-like isotherm, as reported elsewhere [
37,
38]. Samples S-C-ST and S-B-ST presented a narrow PSD with a pore diameter of about 4 nm.
After amine modification, the textural parameters decreased significantly. Samples synthesized using ISS (S-C-ST-g and S-B-ST-g) presented isotherms similar to type I, preserving the H4 hysteresis loop. This might suggest a reduction in the MCM-41 channel diameter. The second loop seems to be preserved. However, T-B-ST-f exhibited a full type I isotherm commonly attributed to microporous materials, after amine modification, which agrees with the shift of PSD to lower values. In addition, amine-modified samples did not display fully reversible isotherms over the complete relative pressure range. A similar behavior occurs in activated carbons with the only presence of micropores, where the solid wall potential has a great influence on physical adsorption [
39].
Estimating the surface area of adsorbents with mesopores in the range of 20 to 40 Å, such as MCM-41 and MCM-48, using the BET approach can pose challenges. In this case, pore filling takes place at pressures very near the range where the formation of monolayer-multilayer structures on the pore walls occurs. This phenomenon is a consequence of the growing potential of pore walls during the condensation process [
40]. The presence of these multilayer structures leads to a significant overestimation of the monolayer capacity when conducting BET analysis. As a response to this challenge, we chose to present the surface area reported by the NLDFT model. These models account for the increasing potential of the walls concerning nitrogen adsorption as pore size decreases, thereby yielding more dependable and accurate surface area results.
3.1. CO2 adsorption performance
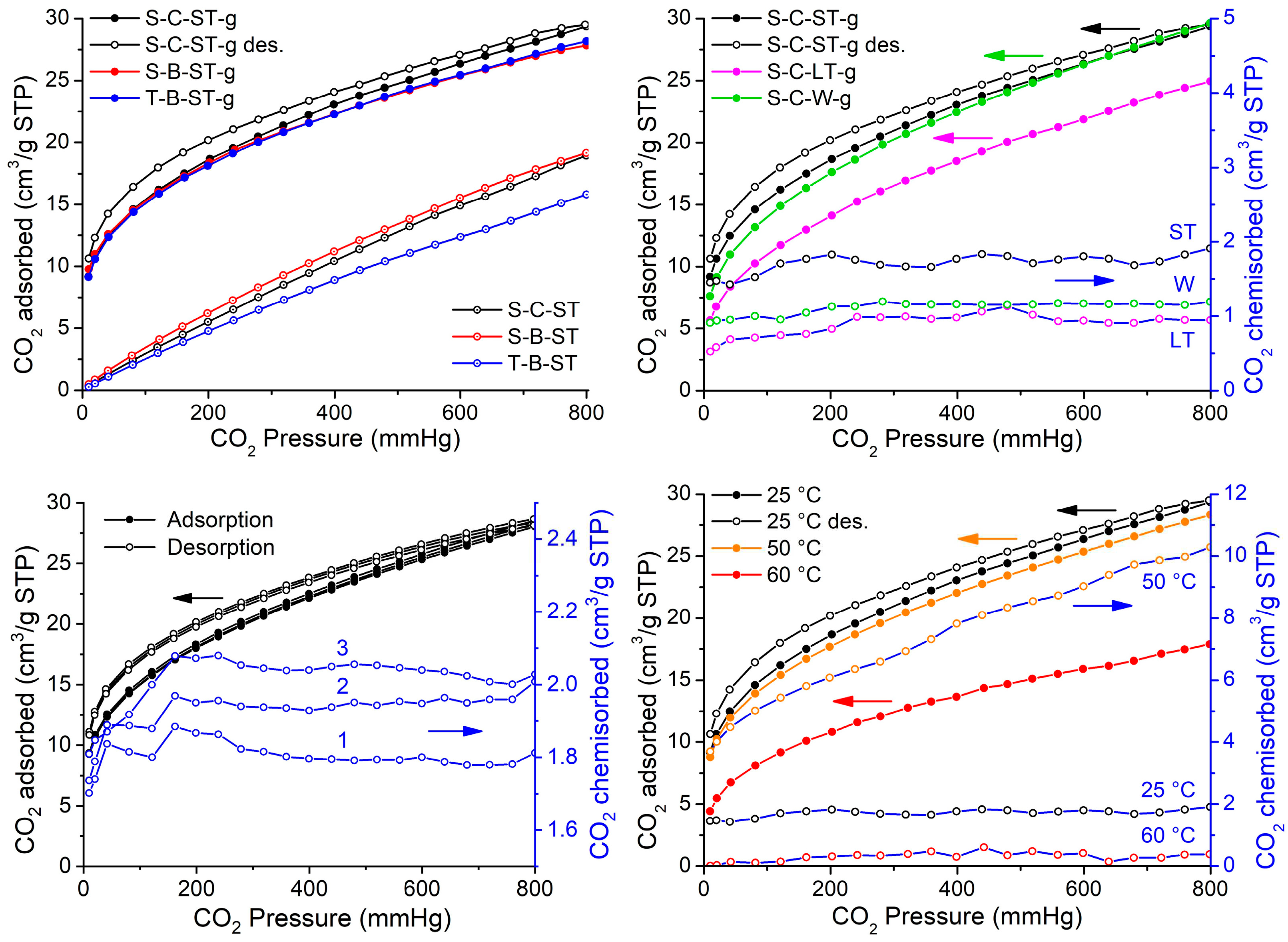
The CO
2 sorption isotherms are shown in
Figure 6. In the left upper panel are compared the results for different sources of silicon and surfactant for ST samples. The isotherms are similar to those of type I of the IUPAC classification [
41], in which the sorption of CO
2 on the surface can be described without the formation of a multilayer for the pressure range up to 800 mmHg. They can be fitted by the Langmuir isotherm, indicating the monolayer adsorption. The physisorption isotherms (not shown) are nearly identical to the sorption ones, suggesting that the adsorption is almost completely of a physical nature. However, all samples exhibited nonreversible isotherms, which might indicate some degree of CO
2 chemisorption. The samples functionalized significantly increased the sorption of CO
2 [
42]. The adsorbed volumes are comparable to those found in the literature (see
Table 2). All modified samples exhibited similar adsorption curves, with S-C-ST-g material demonstrating a slightly better performance at higher pressures. This might be due to its superior textural properties.
Table 2 summarizes the results for our materials and similar ones reported in the literature. When available, it can be seen that there is a great variation in the conditions of the studies and the preparation of reported samples. Our studies presented a slightly better performance than those carried out at similar conditions of pressure and temperature [
10,
12,
14,
15]. Other studies performed under dynamic conditions led to higher adsorption values [
11,
13]. In all cases, textural properties decreased after amine modification.
In the right upper panel are compared the effects of the method used to remove the surfactant on the CO
2 sorption isotherms. The long treatment has a deleterious effect on the CO
2 adsorption performance. This might be attributed to a diminished concentration of silanol groups on the surface. Interestingly, the S-C-W-g sample has a very similar performance to the S-C-ST-g, with considerable economic and environmental benefits. The better performance of -ST and -W samples can be rationalized based on the stronger Si-OH signal observed in FTIR and the employed wet grafting methodology, which induced the formation of new silanol groups helping to create new active sites for aminopropyl anchoring [
17].
Figure 6 (left lower panel) shows the sorption isotherms of three consecutive cycles for the S-C-ST-g sample. The adsorption and desorption are comparable. A progressive small difference in chemisorption with cycles is observed, which would reflect a greater number of adsorption sites. This result may be attributed to the progressive removal of surface residue from the surface occupied by active sites for adsorption, added to the fact that the binding of the amine responsible for this chemical adsorption is strongly anchored to the surface of the MCM-41, which is consistent with the existing literature [
43].
Upon exposing the sample S-C-ST-g to adsorption at different temperatures (
Figure 6, right lower panel), it is observed that between 25 and 50 °C, the combined adsorption decreases slightly, while the chemisorption increases fivefold. As the adsorption temperature is raised to 60 °C, the combined adsorption decreases remarkably, and chemical adsorption is reduced almost to zero. The adsorption energy decreases with increasing temperature; however, it appears that there is a competition between this physical phenomenon and the phenomenon of chemical adsorption, the latter being favored in the range of 25-50 °C.
3.2. FTIR with in situ CO2 adsorption
FTIR measurements were carried out to assess CO
2 retention at different temperatures,
Figure 7. The amine-modified samples (S-C-ST-g, S-B-ST-g, and T-B-ST-g) were exposed to a controlled atmosphere of CO
2 (
Figure 7 left), and then the spectra were acquired under vacuum at different temperatures. The spectra corresponding to 50 °C and 100 °C are shown in
Figure 7 (right).
In all cases, the band attributed to the CO
2 gas phase at 2345 cm
-1 [
36] is observed under the 5 Torr of CO
2 atmosphere. Additional bands are visible in the range 1700-1400 cm
-1 [
20,
34,
35,
44], which could be assigned to the species formed from CO
2 chemisorption on -NH
2 groups. Bands at 1500 cm
-1 and 1625 cm
-1 are associated with -NH
3+ deformation [
35]. In the as-made samples (not shown) we did not observe bands in this range. It can be concluded that without aminopropyl groups there is no CO
2 chemisorption on MCM-41 surface. It is corroborated that CO
2 is retained at least up to 50 °C, in agreement with adsorption measurements.
In summary, the response of the functionalized materials shows sensitivity to CO2 adsorption at very low pressures. This adsorption is retained after high vacuum evacuation at 25 °C. Furthermore, upon heating the sample to 100 °C, the bands corresponding to chemisorbed CO2 disappear.
5. Conclusions
Inexpensive MCM-41 materials were synthesized successfully using different sources of silicon and surfactants of industrial origin. All of them presented a high specific surface area with different structural characteristics and textural properties.
The template was removed by using thermal treatments in an air atmosphere or a washing process. Preservation of silanol groups proved to be more effective under the chemical method or mild thermal treatments with the advantage of its lower cost and reduced environmental impact.
We modified the surface reactivity against CO2 by introducing APTS through grafting. All amino-functionalized materials, including the new inexpensive ones, showed a similar performance as CO2 adsorbents, being comparable to those reported in the literature. The wet grafting methodology favored the regeneration of silanol groups creating new active sites for aminopropyl anchoring. The better performance of -ST and -W samples can be rationalized on this basis.
Our studies indicate that adsorbed CO2 is retained at least up to 50 °C, and the presence of aminopropyl groups is a key issue in CO2 chemisorption on the MCM-41 surface. There is also an amount of chemisorbed gas at very low pressures that could indicate the potential of these materials for their use in CO2 storage.
Author Contributions
Conceptualization, G.D.A., A.M.P., G.P.B. and M.S.M.; methodology, G.D.A., A.M.P., G.P.B. and M.S.M; investigation, G.D.A., A.M.P., G.P.B., C.M.P and M.S.M.; writing-original draft preparation, G.D.A., A.M.P., G.P.B., C.M.P and M.S.M.; writing-review and editing, G.D.A., A.M.P., G.P.B., C.M.P and M.S.M.; project administration, A.M.P. and M.S.M; funding acquisition, A.M.P. and M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the ANPCyT (PICT 2020-03966), UTN (PID 8621TC) and CONICET (PIP 11220200100768CO).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Paula Troyón for SEM image acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shu, D.Y.; Deutz, S.; Winter, B.A.; Baumgärtner, N.; Leenders, L.; Bardow, A. The Role of Carbon Capture and Storage to Achieve Net-Zero Energy Systems: Trade-Offs between Economics and the Environment. Renewable and Sustainable Energy Reviews 2023, 178. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property Impacts on Carbon Capture and Storage (CCS) Processes: A Review. Energy Conversion and Management 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Vorokhta, M.; Nováková, J.; Dopita, M.; Khalakhan, I.; Kopecký, V.; Švábová, M. Activated Three-Dimensionally Ordered Micromesoporous Carbons for CO2 Capture. Materials Today Sustainability 2023, 24, 100509. [Google Scholar] [CrossRef]
- Cavallo, M.; Dosa, M.; Porcaro, N.G.; Bonino, F.; Piumetti, M.; Crocella, V. Shaped Natural and Synthetic Zeolites for CO2 Capture in a Wide Temperature Range. Journal of CO2 Utilization 2023, 67, 102335. [Google Scholar] [CrossRef]
- Ma, B.; Lin, R.; He, H.; Wu, Q.; Chen, S. Rapid Synthesis of Solid Amine Composites Based on Short Mesochannel SBA-15 for CO2 Capture. Composites Part B: Engineering 2020, 185, 107782. [Google Scholar] [CrossRef]
- Rocha, C.; Soria, M.A.; Madeira, L.M. Doping of Hydrotalcite-Based Sorbents with Different Interlayer Anions for CO2 Capture. Separation and Purification Technology 2020, 235, 116140. [Google Scholar] [CrossRef]
- Elsabawy, K.M.; Fallatah, A.M. Synthesis of Newly Wings like Structure Non-Crystalline Ni++-1,3,5-Tribenzyl-1,3,5-Triazine-2,4,6-(1H,3H,5H)-Trione Coordinated MOFs for CO2-Capture. Journal of Molecular Structure 2019, 1177, 255–259. [Google Scholar] [CrossRef]
- Shen, Z.; Song, Y.; Yin, C.; Luo, X.; Wang, Y.; Li, X. Construction of Hierarchically Porous 3D Graphene-like Carbon Material by B, N Co-Doping for Enhanced CO2 Capture. Microporous and Mesoporous Materials 2021, 322, 111158. [Google Scholar] [CrossRef]
- Alkadhem, A.M.; Elgzoly, M.A.A.; Onaizi, S.A. Novel Amine-Functionalized Magnesium Oxide Adsorbents for CO2 Capture at Ambient Conditions. Journal of Environmental Chemical Engineering 2020, 8, 103968. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, J.; Wang, J.; Zhou, L.; Liu, H. CO2 Capture by the Amine-Modified Mesoporous Materials. Acta Physico-Chimica Sinica 2007, 23, 801–806. [Google Scholar] [CrossRef]
- Rao, N.; Wang, M.; Shang, Z.; Hou, Y.; Fan, G.; Li, J. CO2 Adsorption by Amine-Functionalized MCM-41: A Comparison between Impregnation and Grafting Modification Methods. Energy and Fuels 2018, 32, 670–677. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-Modified MCM-41 Mesoporous Silica for Carbon Dioxide Capture. Microporous and Mesoporous Materials 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Guo, Q. Development of Hybrid Amine-Functionalized MCM-41 Sorbents for CO2 Capture. Chemical Engineering Journal 2015, 260, 573–581. [Google Scholar] [CrossRef]
- Sanz, R.; Calleja, G.; Arencibia, A.; Sanz-Pérez, E.S. CO2 Capture with Pore-Expanded MCM-41 Silica Modified with Amino Groups by Double Functionalization. Microporous and Mesoporous Materials 2015, 209, 165–171. [Google Scholar] [CrossRef]
- Loganathan, S.; Tikmani, M.; Ghoshal, A.K. Novel Pore-Expanded MCM-41 for CO2 Capture: Synthesis and Characterization. Langmuir 2013, 29, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.Y.; Chao, K.J.; Cheng, H.H.; Tan, C.S. Adsorption of CO2 onto Amine-Grafted Mesoporous Silicas. Separation and Purification Technology 2009, 70, 87–95. [Google Scholar] [CrossRef]
- Anyanwu, J.T.; Wang, Y.; Yang, R.T. CO2 Capture (Including Direct Air Capture) and Natural Gas Desulfurization of Amine-Grafted Hierarchical Bimodal Silica. Chemical Engineering Journal 2022, 427, 131561. [Google Scholar] [CrossRef]
- Jin, X.; Ge, J.; Zhang, L.; Wu, Z.; Zhu, L.; Xiong, M. Synthesis of Hierarchically Ordered Porous Silica Materials for CO2 Capture: The Role of Pore Structure and Functionalized Amine. Inorganics 2022, 10, 87. [Google Scholar] [CrossRef]
- Ghaedi, H.; Zhao, M. Review on Template Removal Techniques for Synthesis of Mesoporous Silica Materials. Energy and Fuels 2022, 36, 2424–2446. [Google Scholar] [CrossRef]
- Bacsik, Z.; Atluri, R.; Garcia-Bennett, A.E.; Hedin, N. Temperature-Induced Uptake of CO2 and Formation of Carbamates in Mesocaged Silica Modified with n-Propylamines. Langmuir 2010, 26, 10013–10024. [Google Scholar] [CrossRef]
- Edler, K.J.; White, J.W. Further Improvements in the Long-Range Order of MCM-41 Materials. Chemistry of Materials 1997, 9, 1226–1233. [Google Scholar] [CrossRef]
- Grün, M.; Unger, K.K.; Matsumoto, A.; Tsutsumi, K. Novel Pathways for the Preparation of Mesoporous MCM-41 Materials: Control of Porosity and Morphology. Microporous and Mesoporous Materials 1999, 27, 207–216. [Google Scholar] [CrossRef]
- Araujo, A.S.; Jaroniec, M. Thermogravimetric Monitoring of the MCM-41 Synthesis. Thermochimica Acta 2000, 363, 175–180. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltran, M.; Gómez-Siurana, A.; Martinez, I.; Berenguer, D. Template Removal in MCM-41 Type Materials by Solvent Extraction: Influence of the Treatment on the Textural Properties of the Material and the Effect on Its Behaviour as Catalyst for Reducing Tobacco Smoking Toxicity. Chemical Engineering Research and Design 2011, 89, 2330–2343. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press, 2014; ISBN 9780080970356. [Google Scholar]
- Su, G.; Yang, C.; Zhu, J.J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Banjare, R.K.; Banjare, M.K.; Panda, S. Effect of Acetonitrile on the Colloidal Behavior of Conventional Cationic Surfactants: A Combined Conductivity, Surface Tension, Fluorescence and FTIR Study. Journal of Solution Chemistry 2020, 49, 34–51. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO Nanoparticles Encapsulated inside Al-MCM-41 Mesoporous Materials via Direct Synthetic Route. Scientific Reports 2014 4:1 2014, 4, 1–9. [Google Scholar] [CrossRef]
- La-Salvia, N.; Lovón-Quintana, J.J.; Lovón, A.S.P.; Valença, G.P. Influence of Aluminum Addition in the Framework of MCM-41 Mesoporous Molecular Sieve Synthesized by Non-Hydrothermal Method in an Alkali-Free System. Materials Research 2017, 20, 1461–1469. [Google Scholar] [CrossRef]
- Li, L. Le; Sun, H.; Fang, C.J.; Xu, J.; Jin, J.Y.; Yan, C.H. Optical Sensors Based on Functionalized Mesoporous Silica SBA-15 for the Detection of Multianalytes (H+ and Cu2+) in Water. Journal of Materials Chemistry 2007, 17, 4492–4498. [Google Scholar] [CrossRef]
- Ganji, S.; Mutyala, S.; Neeli, C.K.P.; Rao, K.S.R.; Burri, D.R. Selective Hydrogenation of the CC Bond of α,β-Unsaturated Carbonyl Compounds over PdNPs–SBA-15 in a Water Medium. RSC Advances 2013, 3, 11533–11538. [Google Scholar] [CrossRef]
- Medeiros de Paula, G.; do Nascimento Rocha de Paula, L.; Freire Rodrigues, M.G. Production of MCM-41 and SBA-15 Hybrid Silicas from Industrial Waste. Silicon 2022, 14, 439–447. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High Efficiency Nanocomposite Sorbents for CO2 Capture Based on Amine-Functionalized Mesoporous Capsules. Energy & Environmental Science 2011, 4, 444–452. [Google Scholar] [CrossRef]
- Knöfel, C.; Martin, C.; Hornebecq, V.; Llewellyn, P.L. Study of Carbon Dioxide Adsorption on Mesoporous Aminopropylsilane- Functionalized Silica and Titania Combining Microcalorimetry and in Situ Infrared Spectroscopy. Journal of Physical Chemistry C 2009, 113, 21726–21734. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; 3rd Ed.; John Wiley & Sons, Ltd: Chichester, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Danon, A.; Stair, P.C.; Weitz, E. FTIR Study of CO2 Adsorption on Amine-Grafted SBA-15: Elucidation of Adsorbed Species. Journal of Physical Chemistry C 2011, 115, 11540–11549. [Google Scholar] [CrossRef]
- Schmidt, R.; Stöcker, M.; Hansen, E.; Akporiaye, D.; Ellestad, O.H. MCM-41: A Model System for Adsorption Studies on Mesoporous Materials. Microporous Materials 1995, 3, 443–448. [Google Scholar] [CrossRef]
- Wloch, J.; Rozwadowski, M.; Lezanska, M.; Erdmann, K. Analysis of the Pore Structure of the MCM-41 Materials. Applied Surface Science 2002, 191, 368–374. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer Netherlands: Dordrecht, 2004; Vol. 16, ISBN 978-90-481-6633-6. [Google Scholar]
- Kruk, M.; Jaroniec, M.; Sayari, A. Adsorption Study of Surface and Structural Properties of MCM-41 Materials of Different Pore Sizes. Journal of Physical Chemistry B 1997, 101, 583–589. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure and Applied Chemistry 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Builes, S.; Vega, L.F. Understanding CO 2 Capture in Amine-Functionalized MCM-41 by Molecular Simulation. Journal of Physical Chemistry C 2012, 116, 3017–3024. [Google Scholar] [CrossRef]
- Chanapattharapol, K.C.; Krachuamram, S.; Youngme, S. Study of CO2 Adsorption on Iron Oxide Doped MCM-41. Microporous and Mesoporous Materials 2017, 245, 8–15. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons, Ltd: Hoboken, 2005; ISBN 0-471-39362-2. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).