1. Introduction
Spinal metastatic tumors frequently develop due to the primary tumor's spread to the spine through blood or lymphatic metastasis, making them common among advanced malignancies (1). Vertebral body metastases (VBM) are widespread and account for 90% of spinal column lesions detected on imaging in oncological patients (2). The majority of VBM cases, approximately 70%, are found in the thoracic vertebrae. Following this, lumbosacral vertebrae account for 22% of cases, and cervical vertebrae represent 8%. These metastases primarily result from hematogenous spread through Batson's vertebral venous plexus (3). These spinal tumors typically present with multiple lesions affecting various segments of the spine (4).
The clinical presentation of VBM is diverse, including symptoms that range from back pain and reduced mobility to metastatic spinal cord compression (MSCC), which can lead to lasting neurological deficits due to vertebral body collapse or fracture (2). In as many as half of these cases, spinal metastases can lead to persistent pain due to direct tumor infiltration into the bone, pathological fractures, the release of pro-inflammatory cytokines by tumor cells, which enhance osteoclast activity, or the compression of nerve roots and the spinal cord (5–7). Pain and neurological impairment, whether or not accompanied by spinal instability, frequently adversely affect patients' ability to function independently and overall quality of life (4,8).
Regarding the diagnosis of these spinal column lesions, open spine procedures are the gold standard and are valued for providing sufficient tissue samples and high diagnostic accuracy and efficiency. However, they come with invasiveness, tendency to infections and associated higher morbidity rates (9–12). As an alternative, closed biopsy methods, including fine-needle aspiration and image-guided percutaneous spine biopsy, provide alternatives with reduced invasiveness and cost-effectiveness (13,14). Common imaging modalities for percutaneous spine biopsies include fluoroscopy, ultrasound, CT, and MRI (12,14–17). However, improving the accuracy of closed biopsy and cost efficiency to surpass open biopsy remains a critical challenge (18). Emerging fusion techniques, like CBCT-MRI-guided biopsy, promise to enhance accuracy and efficiency (19). Further large-scale studies are needed to validate their efficacy.
Among the evolving treatment modalities, image-guided percutaneous thermal ablation (IPTA) stands out for its minimally invasive nature. IPTA directly delivers heat to the tumor, employing techniques like radiofrequency or microwave energy. This method is resource-efficient and reduces morbidity and mortality compared to traditional procedures (20). IPTA encompasses various techniques, from ethanol ablation and cementoplasty to laser photocoagulation and cryoablation. Each method's unique advantages and challenges contribute to the broader goal of reducing tumor size, consolidating the spine, and managing the symptoms particularly the pain (20,21).
Among these techniques, Radiofrequency ablation (RFA) has demonstrated efficacy in achieving tumor control. RFA employs high-frequency alternating current to generate localized heat within the tumor, resulting in thermal coagulation and tumor cell destruction. This method is precious for patients with secondary spinal tumors, often challenging due to their location and proximity to critical structures (21–23).
IPTA is a promising technique for managing spinal column tumors due to its precision and potential to prevent local tumor progression. Traditional guidance modalities, such as CT, fluoroscopy, and ultrasound, have been crucial in targeting tumors while minimizing damage as much as possible to adjacent critical structures (24,25). However, with the advancements in imaging technology, fusion techniques that combine multiple modalities are being explored (26). For instance, the fusion of CBCT with MRI offers the potential for enhanced visualization and precision during diagnosis of spinal column lesions and utilizing it would likely be beneficial in the lesion’s ablation (19). Such fusion techniques have also proven effective in ablating tumors in other regions, like the liver and kidney (27–29).
Our goal is to enhance the precision and safety of percutaneous thermal ablation for spinal tumors by utilizing the accurate diagnostic and guidance capabilities of CBCT-MRI fusion. This innovative approach aims to optimize therapeutic outcomes, offering an improved standard of care for individuals with secondary spinal tumors.
2. Materials and Methods
2.1. Patient selection and follow-up
The study cohort comprised four patients with various spinal tumors selected for surgery. In the patient selection process, specific criteria were applied. These criteria required that the tumor be located within the spinal column without any associated neurological deficits or direct contact with the spinal cord or nerve roots. This precaution was taken to prevent potential heat-related damage to these critical structures. Patients were also required to present with localized pain without radiation to the legs.
Furthermore, patients who had previously undergone radiation therapy for previous tumors, such as prostate cancer, and had reached the maximum allowable radiation dose were considered eligible for inclusion in the study. Pain levels were assessed using the Visual Analog Scale (VAS) before the surgery and at the third, ninth, twelfth, and eighteenth post-operative months. A follow-up MRI was conducted 6 months post-surgery and another at 12 months to monitor for any signs of tumor recurrence.
2.2. Image-Guided Navigation and O-arm System Setup
We adhered to a specific protocol employing the Stealth Station navigation system Spine 8 (SSS8) (Medtronic Sofamor Danek, headquartered in Minneapolis, MN, USA). This protocol commenced with the administration of a gadolinium contrast-enhanced MRI scan of the spinal segment, utilizing the advanced SIGNA™ Voyager 1.5T MRI machine. After ensuring the utmost patient safety and obtaining informed consent, all procedures were conducted under general anesthesia, with patients positioned in the prone orientation on a Maquet carbon operating table. A stringent sterile environment was strictly maintained around the O-arm device using the Sterile Tube Drape O-arm System.
The Spine Stealth Air reference frame (RF) was securely affixed to the spinous process just above the target vertebral body to facilitate precise navigation. The O-arm registration process was achieved within the cranial software integrated into the SSS8 system. Throughout the procedure, the navigation camera consistently ensured clear visibility of both the O-arm trackers and the RF. This continuous integration of the O-arm device with the SSS8 system was established via a robust Ethernet cable connection.
2.3. Imaging Processing and Analysis
The comprehensive imaging procedure was initiated by acquiring two-dimensional O-arm scans in both axial and sagittal planes, precisely confirming the exact target area. Subsequently, a three-dimensional (3D) scan of the carefully selected spinal region was strictly conducted, encompassing a length of 3-4 levels for the lumbar spine and 4-5 levels for the thoracic spine to ensure thorough coverage. The 3D scan was meticulously executed following this rigorous verification process utilizing the state-of-the-art O-arm device. Without delay, these helpful 3D scans were promptly registered and seamlessly transmitted to the SSS8 system, which adhered to the established SSS8 protocol for optimal integration.
A fundamental step was taken within the "Merge Images" menu as the O-arm and MR scans underwent an accurate manual merging process facilitated by utilizing the specialized "Manual Merge" function. The "Verify Merge" option was employed to confirm the seamless merging of the O-arm and MRI scans to ensure these critical scans' utmost accuracy and alignment. Additionally, the O-arm's memory function adeptly identified and retained the scan positions and parking configurations of the O-arm device, contributing to the overall precision of the procedure.
Furthermore, the navigating instruments were registered with different colors on the Patient Reference, including essential components such as the Passive Planar Blunt Probe, SureTrak II Large Clamp, and SureTrak II Small Passive Tracker Orange. The SureTrak II Large Clamp was attached to the Johnson and Johnson 8G Vertebroplasty Needle. At the same time, the SureTrack II Small Passive Tracker Orange was adeptly linked to the SureTrack II Large Clamp, ensuring thorough coordination and alignment throughout the process.
2.4. Biopsy and Coagulation Procedure Workflow
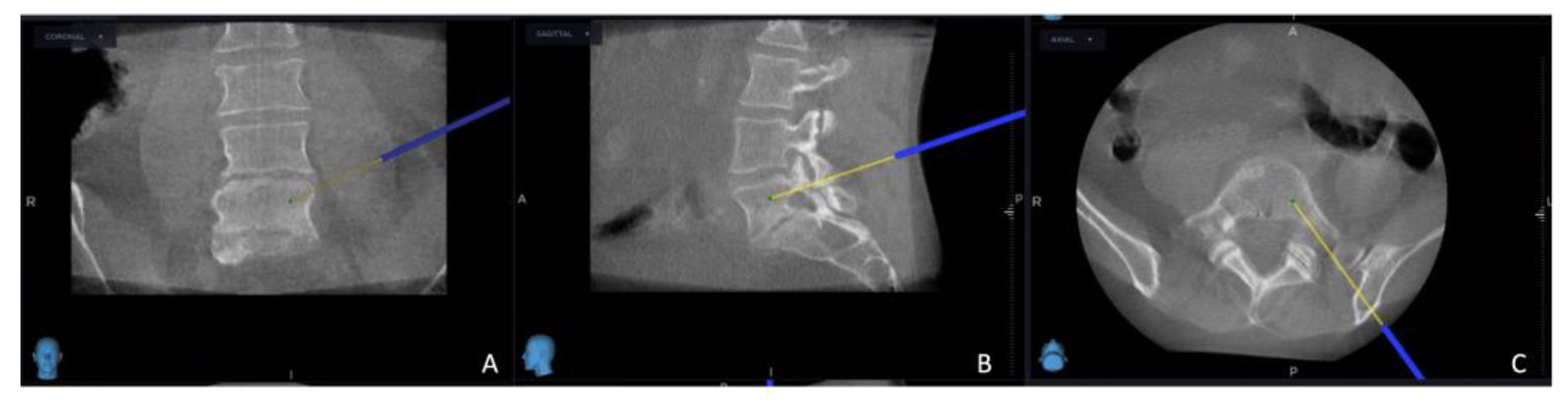
The intricate procedural sequence embarked upon with the precise determination of the initial entry point, meticulously navigated with the aid of the O-arm scan (
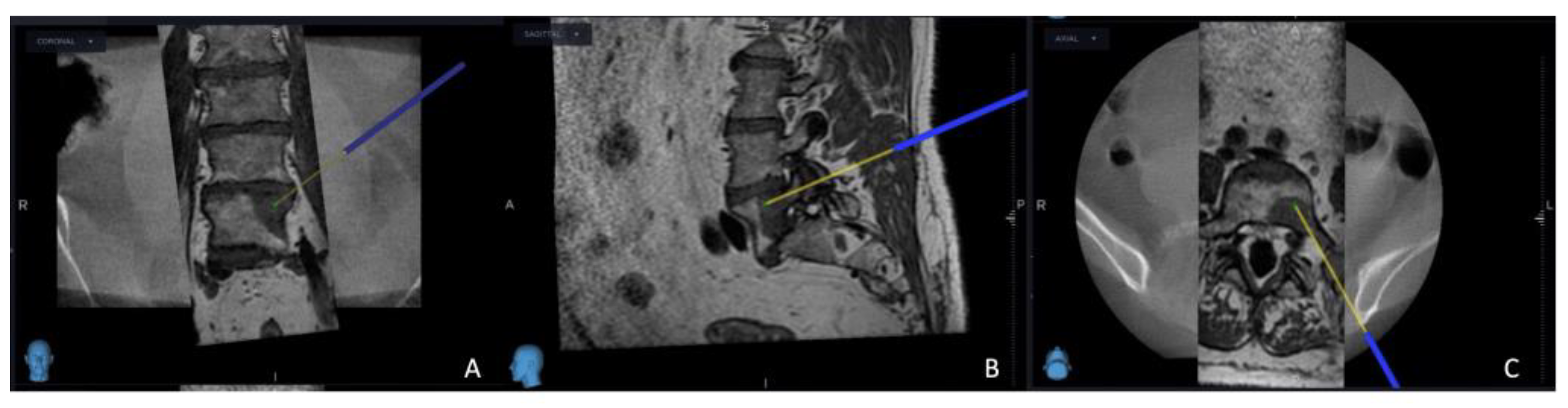
Figure 1). Following this pivotal step, the subsequent stage involved the establishment of the targeted point for the upcoming sampling procedure guided by an MRI scan (
Figure 2). The surgical roadmap for the needle biopsy procedure was delineated through the utilization of the instrument projection function This was followed by a precise 5 mm skin incision, paving the way for the needle biopsy procedure using the Johnson and Johnson 8G Vertebroplasty Needle.
The exact positioning of the needle was methodically verified by cross-referencing its location on both the O-arm and MRI scans. Concurrently, the thermostat, a crucial safety component of the procedure, was carefully introduced in close proximity to the nerve root; additionally, the electrode (Medtronic Sofamor Danek, Memphis, TN, USA) was carefully positioned, resulting in the establishment of the electrode primer location.
As the procedure advanced, a repeat O-arm scan was performed on the same spinal segment, with the main goal of verifying the biopsy's precise location, ensuring the accuracy of the electrode placement, and confirming the thermostat's position. This repeat O-arm scan was subsequently integrated with the needle biopsy plan selected from the MRI scan using the same previous "Merge Image" function. This fusion of imaging data served as the guiding framework for the precise execution of the procedure.
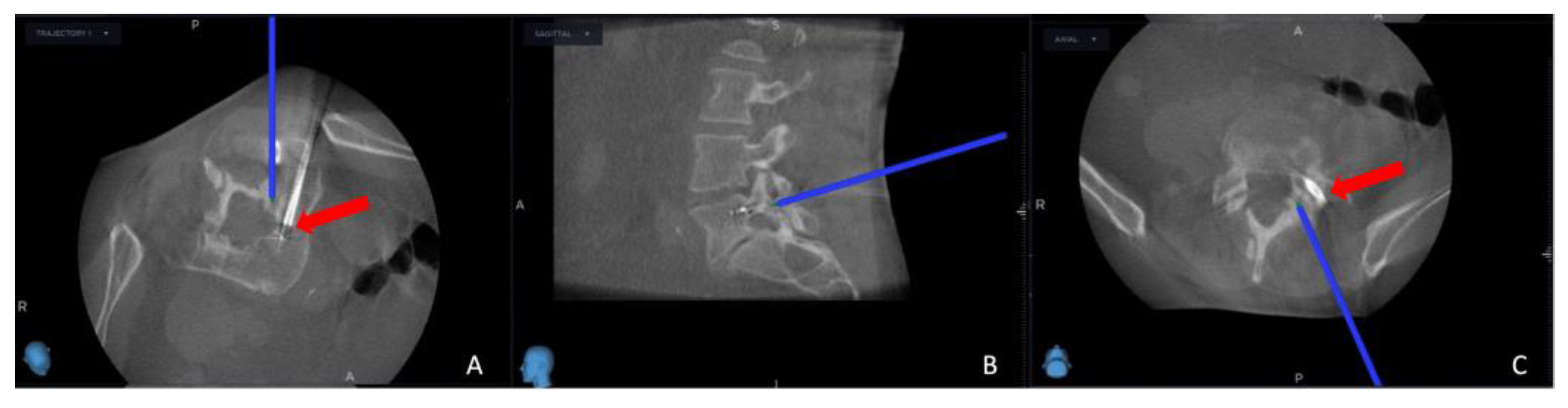
Successful needle biopsy sampling was confirmed if the needle biopsy channel's position in the second O-arm scan matched the plan selected in the MRI scan, and the sample was sent to the pathology department for tumor typing. Subsequently, the electrode's and the thermostat's placement were verified by cross-referencing the registered instrument with the merged image (
Figure 3). Thermocoagulation was set at 70 °C and lasted for 7 minutes, and the process halted upon reaching a thermostat temperature of 39.5°C. Finally, the Spine Stealth Air reference frame was removed. In cases deemed necessary, Percutaneous Vertebroplasty (PVP) was performed.
3. Results
Four patients were enrolled diagnosed with secondary spinal column tumors in T9, T12, L4, and L5. Among these patients, three had a history of non-Hodgkin lymphoma (two with diffuse large B-cell lymphoma and one with follicular lymphoma), while one had prostate cancer (
Table 1). Initially, we conducted PET-CT FDG imaging to detect the lesions. However, due to limited resolution and sensitivity, we subsequently performed MRI investigations for all patients.
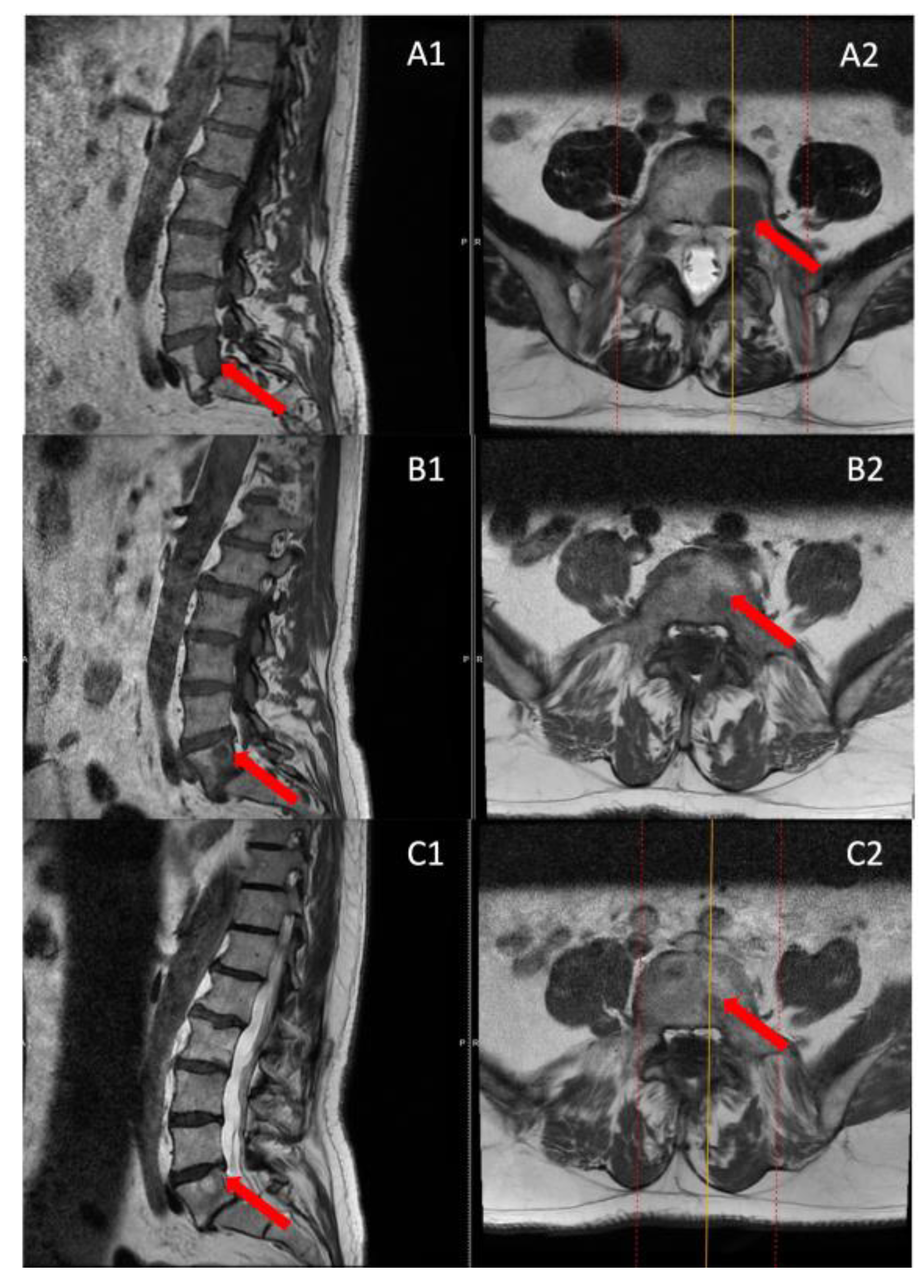
Interestingly, the tumor was visible on CT in only one patient, but MRI provided more accurate tumor dimensions (
Table 2). MRI successfully revealed the tumors in all four patients (
Figure 4). These patients presented with localized pain that did not radiate to the legs.
Patients diagnosed with lumbar metastases presented with localized back pain, as indicated by VAS scores of 7/10 and 6/10. However, they reported complete pain relief after three months. Over nine months, twelve months, and eighteen months, the first patient remained pain-free, while the second patient reported VAS scores of 3/10 at nine months and twelve months (
Table 3).
Patients with thoracic metastases underwent thermal ablation and percutaneous vertebroplasty (PVP). They reported initial VAS scores of 8/10 and 7/10. After three months, their pain scores decreased significantly to 3/10 and 1/10, respectively. Subsequently, the first patient reported a VAS score of 4/10 at nine months and 3/10 at twelve and eighteen months. Conversely, the second patient experienced no pain during the follow-up period (
Table 3).
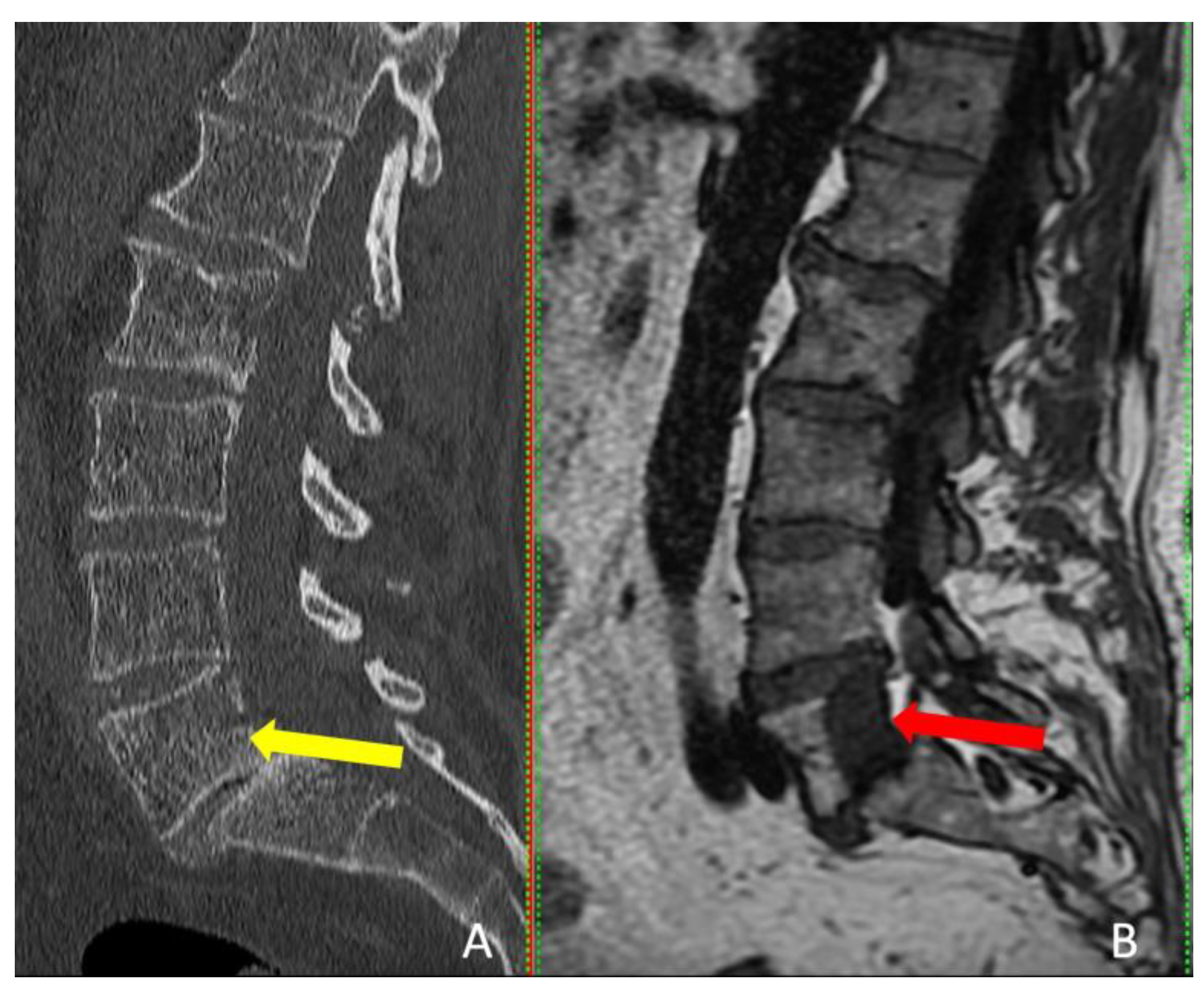
The follow-up MRI scans conducted at both the 6th and 12th months post-surgery revealed no indications of tumor recurrence in any of the patients, and a reduction in the size of the entire lesion was observed compared to pre-surgery (
Figure 5).
4. Discussion
This research highlights the critical importance of utilizing a fusion of CBCT and MRI techniques to enhance the effectiveness of percutaneous thermal ablation for spinal column tumors. In a follow-up period of 18 months involving four patients, we observed a general reduction in pain levels, improving their quality of life. The study indicates that ablation guided by fused images can be a viable treatment option for inoperable spinal column tumors resistant to radiation therapy and chemotherapy. Remarkably, no complications or adverse effects were noted in patients throughout the extended follow-up period.
The nerve root and spinal column handled the heat from the radiofrequency ablation procedure without any issues. They did not exceed 39 °C, thanks to the double-checking of electrode placement via a second O-arm scan. While all participants appeared to benefit from the treatment, achieving complete symptom relief for an extended period was impossible in two cases. Additionally, no instances of local tumor recurrence were detected in any of the control MRI post-operatively.
Various treatment modalities have been explored to manage spinal column tumors, aiming to alleviate pain, restore or preserve neurological function, and improve the overall quality of life. Traditionally, surgical resection is the most common modality, aiming to remove the tumor mass and alleviate associated symptoms (30). However, many surgeons of that era recognized that the surgical procedure had limited potential for restoring neurological function. Some even suggested that surgery's primary objective should shift from rescuing neurological function to providing effective pain management (31–34). The surgery's invasive nature, potential complications, and the tumor's location can sometimes limit its applicability (30,35).
In cases where the tumor is located close to the spinal canal or spinal cord, surgical intervention may not be a viable option. It is further aggravated by factors such as tumor-related vertebral fractures, loss of bone due to osteoporosis, and comorbidities that alter the likelihood of surgical stabilization of the spine (25). If left untreated, spinal column tumors can cause severe pain and potentially progress to neurological impairment. RFA has become an effective technique for destroying primary and secondary malignant tumors, especially in organs such as the liver, kidney, brain, pancreas, lung, breast, thyroid, parathyroid, and bone tissue. Percutaneous vertebroplasty can be used in cases of metastatic tumors to strengthen and palliate damaged vertebral bodies (36).
To accurately detect and target tumors to undergo ablation, it is essential to address the limitations of each imaging technique. While CT scans are often recommended for hard to reach lesions due to their high-resolution capabilities, which are particularly useful for visualizing the sides of vertebrae and thus minimizing the risk to neural elements (37), MRI excels in soft-tissue and bone marrow contrast and certain lesions such as non-Hodgkin's lymphoma, can only be visualized using MRI (14,38,39). A study by Al-Smadi et al. involving 18 patients with spinal column tumors revealed that the tumors were either not visible or their dimensions were underestimated when relying solely on CT scans, as opposed to MRI (19).
IPTA hinges on accurately delivering thermal energy to the tumor site. Any deviation can compromise the efficacy of the treatment or, worse, damage critical structures. By employing the fusion technique, clinicians can harness the strengths of both modalities and navigate the intricate spinal anatomy with unparalleled accuracy, ensuring that the tumor is ablated effectively while preserving the spinal cord and nerve roots.
The benefits of image fusion are not confined to spinal interventions. Studies on percutaneous thermal ablation of liver and kidney tumors have shown that fusion techniques improve targeting accuracy, minimize complications, and enhance therapeutic outcomes (27). Using FI to guide ablation procedures makes the operators more confident because it helps visualize the lesions and nearby structures better, making ablation more accurate, doable, and safe by showing where lesions are and how they relate to nearby organs (40,41).
While our study demonstrates the promising potential of CBCT-MRI fusion in managing spinal column tumors, we acknowledge several limitations that should be considered. Firstly, the patient number in this study was relatively small, limiting our findings' generalizability. Nonetheless, we benefited from an 18-month follow-up duration, offering valuable and encouraging glimpses into the enduring sustainability and credibility of therapeutic results achieved through the CBCT-MRI percutaneous thermal ablation.
Our research underscores the critical need for innovative approaches in managing spinal column tumors. The fusion of CBCT with MRI offers a unique opportunity to achieve therapeutic goals in a field where accurate tumor targeting is essential. As we strive for minimally invasive yet effective treatments, such fusion techniques hold immense promise in shaping the future of spinal oncology interventions.
5. Conclusions
In conclusion, our study emphasizes the CBCT-MRI fusion technique's significance in guiding the percutaneous thermal ablation of spinal column tumors. This fusion technique can effectively elevate the related symptoms to these tumors. The combined strength of these imaging modalities guarantees unattainable precision with either modality in isolation.
As the medical community strives for minimally invasive yet effective treatments, such fusion techniques will undoubtedly play a central role in shaping the future of spinal oncological interventions. Future studies with larger cohorts and longer follow-up periods are warranted to validate our findings further and refine the fusion technique for broader clinical applications.
Author Contributions
Conceptualization, S.A., and Á.V.; methodology, all authors.; investigation and follow-up, all authors; data curation, S.A., and I.K.; writing—original draft preparation, S.A. and M.W.A.-S; writing—review and editing, S.A., and M.W.A.-S.; supervision, Á.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Péterfy Sándor Utcai Hospital-Clinic and National Institute of Traumatology, Medical Library (Dr. Manninger Jeno ̋ Hospital), 1081, Budapest. The approval number is 02/2022, and the approval date is 2 February 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study to do the procedures and to publish this paper.
Data Availability Statement
All data produced or examined in this study have been included in this published article.
Acknowledgments
We want to express our sincere gratitude to the Department of Neurosurgery and Neurotraumatology staff at Péterfy Hospital—Manninger Jeno ̋ National Traumatology Institution in Budapest, Hungary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang J, Fang Z, Lang N, Yuan H, Su MY, Baldi P. A multi-resolution approach for spinal metastasis detection using deep Siamese neural networks. Comput Biol Med. 2017 May 1;84:137–46. [CrossRef]
- Murali N, Turmezei T, Bhatti S, Patel P, Marshall T, Smith T. What is the effectiveness of radiofrequency ablation in the management of patients with spinal metastases? A systematic review and meta-analysis. J Orthop Surg Res. 2021 Nov 6;16(1):659. [CrossRef]
- Colonna S, Bianconi A, Cofano F, Prior A, Di Perna G, Palmieri G, et al. Radiofrequency Ablation in Vertebral Body Metastasis with and without Percutaneous Cement Augmentation: A Systematic Review Addressing the Need for SPINE Stability Evaluation. Diagnostics. 2023 Jan;13(6):1164. [CrossRef]
- Roser S, Maharaj MM, Taylor MA, Kuru R, Hansen MA, Ferch R. Vertebrectomy in metastatic spinal tumours: A 10 year, single-centre review of outcomes and survival. J Clin Neurosci. 2019 Oct;68:218–23. [CrossRef]
- Urch, C. The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med. 2004 May;18(4):267–74. [CrossRef]
- Munk PL, Rashid F, Heran MK, Papirny M, Liu DM, Malfair D, et al. Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. J Vasc Interv Radiol. 2009 Jul;20(7):903–11. [CrossRef]
- Giammalva GR, Costanzo R, Paolini F, Benigno UE, Porzio M, Brunasso L, et al. Management of Spinal Bone Metastases With Radiofrequency Ablation, Vertebral Reinforcement and Transpedicular Fixation: A Retrospective Single-Center Case Series. Front Oncol. 2021;11:818760. [CrossRef]
- Kim JM, Losina E, Bono CM, Schoenfeld AJ, Collins JE, Katz JN, et al. Clinical outcome of metastatic spinal cord compression treated with surgical excision ± radiation versus radiation therapy alone: a systematic review of literature. Spine (Phila Pa 1976). 2012 Jan 1;37(1):78–84. [CrossRef]
- Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996 May;78(5):656–63.
- Errani C, Traina F, Perna F, Calamelli C, Faldini C. Current concepts in the biopsy of musculoskeletal tumors. ScientificWorldJournal. 2013;2013:538152. [CrossRef]
- Rougraff BT, Aboulafia A, Biermann JS, Healey J. Biopsy of soft tissue masses: evidence-based medicine for the musculoskeletal tumor society. Clin Orthop Relat Res. 2009 Nov;467(11):2783–91. [CrossRef]
- Liu JC, Chiou HJ, Chen WM, Chou YH, Chen TH, Chen W, et al. Sonographically guided core needle biopsy of soft tissue neoplasms. J Clin Ultrasound. 2004;32(6):294–8. [CrossRef]
- Stringham DR, Hadjipavlou A, Dzioba RB, Lander P. Percutaneous transpedicular biopsy of the spine. Spine (Phila Pa 1976). 1994 Sep 1;19(17):1985–91.
- Carrino JA, Khurana B, Ready JE, Silverman SG, Winalski CS. Magnetic resonance imaging-guided percutaneous biopsy of musculoskeletal lesions. J Bone Joint Surg Am. 2007 Oct;89(10):2179–87. [CrossRef]
- López JI, Del Cura JL, Zabala R, Bilbao FJ. Usefulness and limitations of ultrasound-guided core biopsy in the diagnosis of musculoskeletal tumours. APMIS. 2005 May;113(5):353–60. [CrossRef]
- Möller S, Kothe R, Wiesner L, Werner M, Rüther W, Delling G. Fluoroscopy-guided transpedicular trocar biopsy of the spine--results, review, and technical notes. Acta Orthop Belg. 2001 Dec;67(5):488–99.
- Rimondi E, Staals EL, Errani C, Bianchi G, Casadei R, Alberghini M, et al. Percutaneous CT-guided biopsy of the spine: results of 430 biopsies. Eur Spine J. 2008 Jul;17(7):975–81. [CrossRef]
- Nourbakhsh A, Grady JJ, Garges KJ. Percutaneous spine biopsy: a meta-analysis. J Bone Joint Surg Am. 2008 Aug;90(8):1722–5. [CrossRef]
- Al-Smadi MW, Kozma I, Aslan S, Bölöni B, Viola Á. Percutaneous Superimposed O-Arm-MRI-Navigated Biopsy for Spinal Column Pathologies. Diagnostics. 2023 Jan;13(13):2252. [CrossRef]
- Gangi, A. Percutaneous Spinal Tumor Management. Neuroradiol J. 2009 Sep 1;22(1_suppl):131–9. [CrossRef]
- Tomasian A, Hillen TJ, Chang RO, Jennings JW. Simultaneous Bipedicular Radiofrequency Ablation Combined with Vertebral Augmentation for Local Tumor Control of Spinal Metastases. AJNR Am J Neuroradiol. 2018 Sep;39(9):1768–73. [CrossRef]
- Tomasian A, Jennings JW. Percutaneous Interventional Techniques for Treatment of Spinal Metastases. Semin Intervent Radiol. 2020 Jun;37(2):192–8. [CrossRef]
- Urbisci T, Thomas B, Tran N. SURG-28. RADIOFREQUENCY ABLATION AS AN ADJUNCT FOR PAIN AND TUMOR CONTROL IN SPINAL METASTATIC DISEASE. Neuro Oncol. 2019 Nov;21(Suppl 6):vi245.
- Tsoumakidou G, Koch G, Caudrelier J, Garnon J, Cazzato RL, Edalat F, et al. Image-Guided Spinal Ablation: A Review. Cardiovasc Intervent Radiol. 2016 Sep 1;39(9):1229–38. [CrossRef]
- Grönemeyer DHW, Schirp S, Gevargez A. Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J. 2002;8(1):33–9.
- Racadio JM, Babic D, Homan R, Rampton JW, Patel MN, Racadio JM, et al. Live 3D guidance in the interventional radiology suite. AJR Am J Roentgenol. 2007 Dec;189(6):W357-364. [CrossRef]
- Carriero S, Della Pepa G, Monfardini L, Vitale R, Rossi D, Masperi A, et al. Role of Fusion Imaging in Image-Guided Thermal Ablations. Diagnostics (Basel). 2021 Mar 19;11(3):549. [CrossRef]
- Biondetti P, Ascenti V, Shehab A, Ierardi AM, Carriero S, Lanza C, et al. Percutaneous Microwave Ablation of Hepatocellular Carcinoma with “Double Fusion” Technique: Technical Note and Single-Center Preliminary Experience. Diagnostics. 2023 Jan;13(14):2349. [CrossRef]
- Ierardi AM, Carnevale A, Stellato E, De Lorenzis E, Uccelli L, Dionigi G, et al. Cone Beam Computed Tomography Image Fusion with Cross Sectional Images for Percutaneous Renal Tumor Ablation: Preliminary Data. Technol Cancer Res Treat. 2023 Mar 29;22:15330338231154994. [CrossRef]
- Akgun B, Ergun AC, Ozercan IH, Kok S. Intradural Extramedullary Epidermoid Cyst at the Conus Medullaris Level with Thoracic Syringomyelia: A Case Report. Acta Medica (Hradec Kralove). 2019;62(1):39–42.
- Arseni CN, Simionescu MD, Horwath L. Tumors of the spine. A follow-up study of 350 patients with neurosurgical considerations. Acta Psychiatr Scand. 1959;34(4):398–410. [CrossRef]
- Black, P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979 Dec;5(6):726–46.
- Larson S, Wetzel N, Brochner R, Ruge D. The surgical treatment of metastatic epidural tumors. Q Bull Northwest Univ Med Sch. 1961;35(1):42–4.
- Perese, DM. Treatment of metastatic extradural spinal cord tumors; a series of 30 cases. Cancer. 1958;11(1):214–21.
- Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9(2):188–96. [CrossRef]
- Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976). 2000 Apr 15;25(8):923–8.
- Renfrew DL, Whitten CG, Wiese JA, el-Khoury GY, Harris KG. CT-guided percutaneous transpedicular biopsy of the spine. Radiology. 1991 Aug;180(2):574–6. [CrossRef]
- Liu M, Sequeiros RB, Xu Y, He X, Zhu T, Li L, et al. MRI-guided percutaneous transpedicular biopsy of thoracic and lumbar spine using a 0.23t scanner with optical instrument tracking. Journal of Magnetic Resonance Imaging. 2015;42(6):1740–6. [CrossRef]
- Kerimaa P, Marttila A, Hyvönen P, Ojala R, Lappi-Blanco E, Tervonen O, et al. MRI-guided biopsy and fine needle aspiration biopsy (FNAB) in the diagnosis of musculoskeletal lesions. Eur J Radiol. 2013 Dec;82(12):2328–33. [CrossRef]
- Citone M, Fanelli F, Falcone G, Mondaini F, Cozzi D, Miele V. A closer look to the new frontier of artificial intelligence in the percutaneous treatment of primary lesions of the liver. Med Oncol. 2020 May 18;37(6):55. [CrossRef]
- Huang Q, Zeng Q, Long Y, Tan L, Zheng R, Xu E, et al. Fusion imaging techniques and contrast-enhanced ultrasound for thermal ablation of hepatocellular carcinoma - A prospective randomized controlled trial. Int J Hyperthermia. 2019;36(1):1207–15. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).