1. Introduction
Dacomitinib is a selective, adenosine triphosphate–competitive, irreversible, small-molecule inhibitor of the ErbB human epidermal growth factor receptor (HER) family of receptor tyrosine kinases, including epidermal growth factor receptor (EGFR) or HER1, HER2, HER4 and their oncogenic variants (i.e. EGFR with exon 19 deletions or exon 21 L858R mutation) [
1]. When used as a first-line treatment in patients with EGFR mutation–positive non-small cell lung cancer (NSCLC), dacomitinib was found to statistically significantly improve progression-free survival [
2] and overall survival [
3,
4] versus gefitinib, a first-generation EGFR tyrosine kinase inhibitor, in a randomized, open-label, phase 3 trial (ARCHER 1050). On the basis of the results from ARCHER 1050, dacomitinib was approved for the first-line treatment of patients with metastatic NSCLC containing EGFR exon 19 deletion or exon 21 L858R substitution [
5].
Cancer patients frequently take acid-reducing agents (ARA) to alleviate symptoms of gastroesophageal disease, thereby raising the potential for a common but underappreciated drug-drug interaction (DDI) that could decrease the exposure of anticancer medication and result in subsequent failure of therapy. Many approved orally administered, small-molecule, tyrosine kinase inhibitor (TKI) drugs are weak bases that exhibit pH-dependent solubility [
6]. Consequently, the oral bioavailability of these drugs may be significantly influenced when co-administered with ARAs.
H2 Receptor Antagonists (H2RA) are ARAs which competitively block histamine H2 receptors and interfere with one of three pathways for proton pump activation, resulting in a substantial reduction in acid secretion but is less potent than proton pump inhibitors (PPI) [
7,
8]. The increase in gastric pH may limit the absorption of TKI drugs that require an acidic environment for optimal dissolution, which in turn can lead to decreased plasma exposure [
9]. The aqueous solubility of dacomitinib is pH dependent, with highest solubility observed at acidic pH. Studies were conducted to assess whether the absorption of dacomitinib may be affected by ARAs that increase stomach pH [
10,
11,
12]. A clinical study in healthy volunteers showed 7 days of continuous dosing with rabeprazole 40 mg, a PPI, reduced dacomitinib C
max and AUC0-96 by 51% and 39% respectively, following a single 45 mg dose of dacomitinib [
10]. Furthermore, T
max was delayed from 5-6 hours (dacomitinib alone) to 12 hours (dacomitinib co-administered with PPI) [
10]. Thus, this study demonstrated that there was a significant difference in exposure of dacomitinib during treatment with PPI [
10]. On the other hand, treatment with 20 mL of locally acting antacid (Maalox® Maximum Strength, 400 mg/5 mL) did not cause clinically relevant changes dacomitinib concentrations [
12]. Based on these findings, it is recommended that concomitant use of PPI with dacomitinib should be avoided, and locally acting antacids and H2RA can be taken instead. [
12]. Since H2RA PK mirrors its pharmacodynamics, it is a more ideal alternative to PPI for dose staggering. It has a shorter duration of effect (10-12 hours) and relatively shorter half-life of 2.5-3.5 hours [
13]. Lung cancer is one of five cancer types with the highest prevalence of ARA use for indications like gastroesophageal reflux disease (GERD), esophagitis, or peptic ulcers [
14]. Per AAFP guidelines, the first line treatment for GERD is trial of H2RA for 8 weeks, then switch to PPI [
15].
Although H2RA in lieu of PPIs are recommended with dacomitinib, the impact of H2RA on dacomitinib exposure has not been clinically studied. Early evaluation of all targeted agents in the context of oncology drug development is often limited by the need to make rapid decisions based on a small number of patients. However, a multipronged approach, using modeling and simulation approaches as well as clinical pharmacokinetic (PK) data from early-phase trials, could be useful to evaluate drug exposure. Therefore, we conducted a retrospective analysis using a linear mixed effects model to assess the effect of H2RA on dacomitinib exposure by pooling data from eleven clinical trials, and further evaluated if the staggered H2RA coadministration would affect dacomitinib absorption using physiologically based pharmacokinetic (PBPK) modeling with the data from the two studies (NCT01702506, NCT01796327).
Here, the results of the retrospective analysis and PBPK modeling to investigate the effect of H2RA on dacomitinib exposure are presented.
2. Materials and Methods
2.1. Linear mixed effects model analysis
The retrospective analysis is based on pooled data from 11 patient studies in advanced NSCLC or with other various solid tumors (NCT00225121, NCT00548093, NCT00553254, NCT00783328, NCT01360554, NCT00728468, NCT00818441, NCT00768664, NCT00769067, NCT01465802, and NCT01774721). It is a cross-over within patient comparison on patients who have data for trough concentrations of dacomitinib with concomitant H2RA and without concomitant H2RA. H2RA included in this study were famotidine, ranitidine, cimetidine, and nizatidine.
The reference group was defined as dacomitinib without coadministration with an H2RA with dacomitinib Ctrough,ss value collected prior to any reported H2RA use or at least 14 days after the last reported date of H2RA use. The test group was defined as dacomitinib coadministered with an H2RA continuously for at least 3 days prior to the dacomitinib Ctrough,ss collection.
The data had patients on 4 dose levels of dacomitinib including 15 mg, 30 mg, 45 mg, and 60 mg. The concentrations were dose normalized to 45 mg. Comparisons were made for trough concentrations for dacomitinib parent, metabolite, and active moiety. The metabolite had similar in vitro pharmacologic activity as dacomitinib [
16]. The active moiety of dacomitinib was calculated using molar mass of dacomitinib parent and metabolite (Total Active moiety (ng/mL) = dacomitinib (ng/mL) + metabolite (ng/mL) * 469.4[MW dacomitinib]/455.9[MW metabolite]). The C
trough,ss was defined as an observed concentration collected at nominal predose (0 hour) time point on Day 1 of Cycle 2 through Cycle 10 with at least 14 days of continuous dacomitinib dosing at one dose level.
R version 3.4.1 (R Foundation for Statistical Computing. Vienna, Austria) was used for all data manipulation, analysis steps (using the lme() function of the nlme package in R), graphics, and table creation.
A linear mixed effects model was used to perform the within patient comparison of dacomitinib parent, metabolite, and active moiety Ctrough,ss, as described by:
Log (yi jk) = µ + θ1 · x1 jk + θ2 · TAFD + ηj + εi jk
Where
yi jk = dacomitinib parent, metabolite, and active moiety Ctrough,ss for the ith group (test), the jth patient and the kth within patient observation.
µ = mean dacomitinib parent, metabolite, and active moiety Ctrough,ss (natural log scale) for the reference group.
θ1 = H2RA effect as the mean dacomitinib parent, metabolite, and active moiety Ctrough,ss difference (natural log scale) between test (x1i = 1) and reference (x1i = 0).
θ2 = time effect for the test dacomitinib parent, metabolite, and active moiety Ctrough,ss.
TAFD = time of observed dacomitinib parent, metabolite, and active moiety Ctrough,ss after first dose in hours.
ηj = inter-patient random effect.
εi jk = intra-patient random error.
The H2RA effect was estimated by adjusted least square means for test and reference groups, compared by estimating the ratio of adjusted geometric means (test/reference) and the 90% CI for the ratio. Summary statistics were exponentiated to back-transformed values on the observed scale.
2.2. PBPK model development and simulation
To evaluate the effect of changes in gastric pH on dacomitinib absorption, compartmental modeling and simulation were performed using GastroplusTM version 9.7 (Simulation Plus, Inc., Lancaster, CA). An oral absorption model of dacomitinib was developed based on physic- and bio-chemical properties, in vitro experimental data, and clinical PK results. The PK parameters for dacomitinib were derived by fitting the PK profiles of IV administered doses from the study (NCT01796327, a phase 1, single dose, fixed sequence study to estimate the absolute bioavailability of dacomitinib by comparing oral to intravenous administration in healthy volunteers) to estimate the systemic clearance and volume of distribution. A 2- compartmental model was used to fit the IV profiles from this study (NCT01796327). The base model that utilized the default physiological conditions of the software was used to simulate the plasma profiles for 45 mg oral dose in healthy volunteers from the same study (NCT01796327). Visual comparison of the predicted to the observed PK profiles resulted in a decision to modify the oral absorption model to capture the initial phase of dacomitinib absorption. The modified model was developed by optimizing the absorption scale factors (ASF) model based on the clinical PK data to reflect the prolonged Tmax of the plasma profile since all the systemic PK parameters were fixed after fitting the IV profile. The optimization steps resulted in well captured the observed Cmax and Tmax.
The model was validated using the mean plasma profiles of dacomitinib under 3 different conditions or treatments (fasted, fed, and antacid treatment using a PPI of rabeprazole 40 mg administered QD for 7 days) following oral administration of 45 mg dose to healthy volunteers in the study (NCT01702506, an open-label, randomized, single-dose, 2-sequence, and 3-period crossover Phase 1 study to investigate the effect of food and the effect of increased gastric pH achieved by treatment with a PPI on the PK behavior of dacomitinib in healthy adult subjects).
The gastric pH in the model was modified to allow evaluation of values ranging from pH 1.0 to 6.0 during PK simulations. Parameter sensitivity analyses (PSA) were conducted in PBPK model to evaluate the impact of changing pH from 1.0 to 5.0 on the absorption of dacomitinib.
3. Results
3.1. Linear mixed effects model analysis results
As
Table 1 shown, across the 11 studies (1450 enrolled patients treated with dacomitinib), there were 1001 patients with available dacomitinib C
trough,ss meeting the criteria defined in
Section 3.1, and 86 total patients reported use of an H2RA. The within-patient H2RA-Dacomitinib C
trough,ss analysis population consisted of 16 total patients with 57 dacomitinib C
trough,ss in the reference or test group. Of these 16 patients, 12 had metabolite and active moiety concentrations in the analysis population with 46 metabolite and active moiety C
trough,ss in the reference or test group.
As presented in
Table 2, the geometric mean (geometric coefficient of variation (CV%)) dacomitinib parent C
trough,ss (dose normalized to 45 mg) for the reference group was 57.56 ng/mL (8.4%) based on 35 observations. The geometric mean (geometric CV%) dacomitinib C
trough,ss (dose normalized to 45 mg) for the test group was 53.2 ng/mL (18.7%) based on 22 observations. The geometric mean (geometric CV%) C
trough,ss (dose normalized to 45 mg) of dacomitinib metabolite and active moiety for the reference group based on 29 observations were 6.41 ng/mL (47.8%) and 66.45 ng/mL (7.7%), respectively. The geometric mean (geometric CV%) C
trough,ss (dose normalized to 45 mg) of dacomitinib metabolite and active moiety for the test group based on 17 observations were 8.81 ng/mL (41.6%) and 72.23 ng/mL (5.7%), respectively.
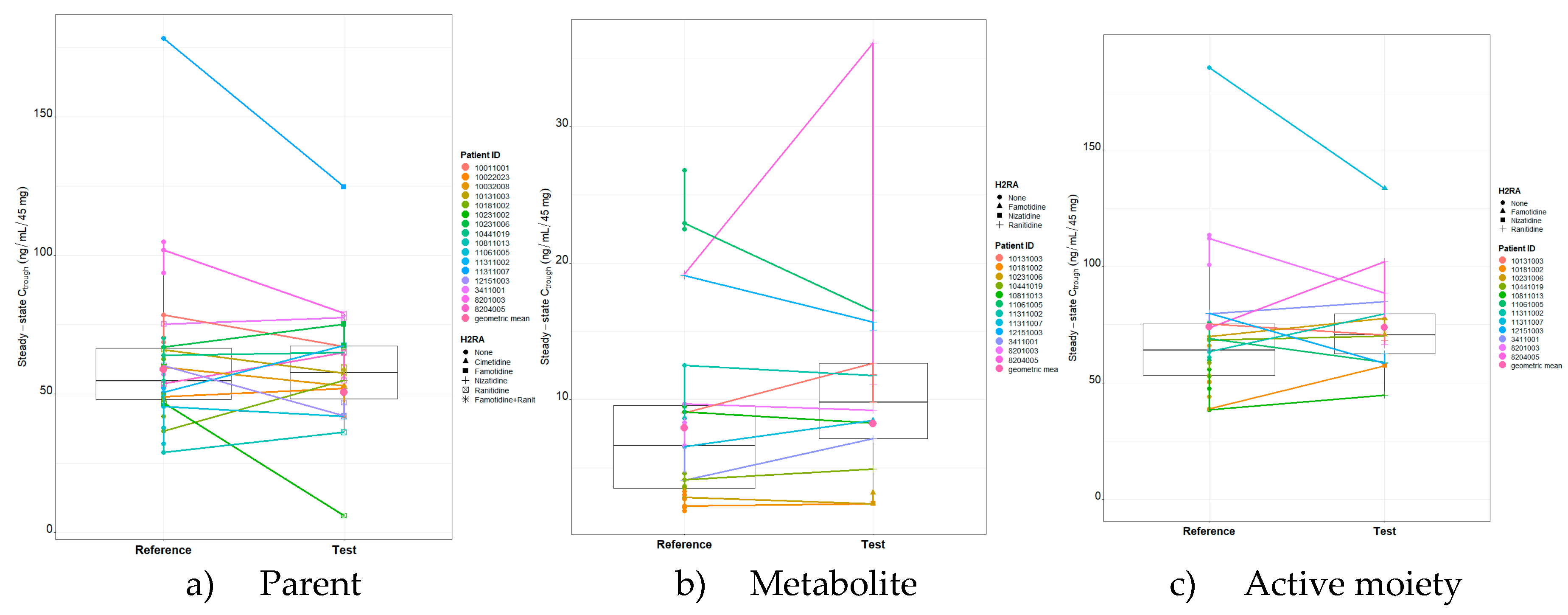
The effect of concomitant H2RA use (test) on dacomitinib parent, metabolite, and active moiety Ctrough,ss was evaluated by a linear mixed effects model and the test group was not statistically significant compared to the reference group (p=0.1245, 0.6611, and 0.9668). Time was also evaluated in the model and was not significant (p=0.6628, 0.9558 and 0.7304).
Table 3 summarizes the results of the statistical model for the within patient comparison of dacomitinib parent, metabolite, and active moiety C
trough,ss using the pooled data from the H2RA-dacomitinib C
trough,ss analysis population. The adjusted geometric mean C
trough,ss of dacomitinib parent, metabolite, and active moiety for the reference group was 58.79 ng/mL, 7.96 ng/mL and 73.94 ng/mL, while the adjusted geometric mean C
trough,ss for the test group was 50.49 ng/mL, 8.27 ng/mL and 73.77 ng/mL, respectively. The corresponding geometric mean ratio (test/reference), expressed as a percentage, is 85.88% with a 90% CI of 72.9 to 101.1, 103.82% with a 90% CI of 89.9 to 119.8, and 99.77% with a 90% CI of 90.9 to 109.5, respectively.
Table 3.
Statistical Comparison of Dacomitinib Parent, Metabolite and Active Moiety Ctrough,ss (Test versus Reference).
Table 3.
Statistical Comparison of Dacomitinib Parent, Metabolite and Active Moiety Ctrough,ss (Test versus Reference).
| |
Adjusted Geometric Mean Ctrough,ss
|
Geometric Mean Ratio |
|
| |
Reference |
Test |
(Test/Reference) |
90% CI |
| Parent |
58.79 |
50.49 |
85.88 |
72.9; 101.1 |
| Metabolite |
7.96 |
8.27 |
103.82 |
(89.9; 119.8) |
| Active moiety |
73.94 |
73.77 |
99.77 |
(90.9; 109.5) |
Figure 1.
Presents dacomitinib parent, metabolite, and active moiety Ctrough,ss matchstick boxplot by patient and the H2RA medication used.
Figure 1.
Presents dacomitinib parent, metabolite, and active moiety Ctrough,ss matchstick boxplot by patient and the H2RA medication used.
3.2. PBPK results
PBPK model for dacomitinib was developed and optimized based on both in vitro and clinical data. Input parameters of the dacomitinib model were summarized in
Table 4. The predicted versus clinically observed PK results were summarized.
Figure 2,
Figure 3, and
Figure 4 illustrate that the optimized model well described the PK profiles of dacomitinib under different conditions.
Table 5,
Table 6 and
Table 7 show all predicted to observed ratios for AUC
inf and C
max reported were within 2-fold of the observed values, 0.857-1.07 for AUCinf and 0.940-1.01 for C
max.
Table 4.
Key Physicochemical, PK Inputs, and ASF Model Used in the Models.
Table 4.
Key Physicochemical, PK Inputs, and ASF Model Used in the Models.
| Parameter |
Dacomitinib |
Reference |
| Molecular weight |
469.94 |
Measured |
| Log P |
5.3 (neutral),
1.8 (cationic) |
Measured |
| pKa |
5.03 (base) |
Measured |
| pH-solubility profile |
10.0 (pH 1.28)
3.7 (pH 4.71)
0.34 (pH 5.09)
0.23 (pH 5.17)
0.16 (pH 5.3)
0.006 (pH 6.13)
0.001 (pH 6.94) |
Measured |
| Effective human permeability (cm/s) |
1.18x104
|
Measured |
| Permeability source |
Caco-2 |
Measured |
| Precipitation time (s) |
900 |
GastroPlus™ 9.7 default value |
| Particle size (radius) |
58 μm |
Measured |
| Disposition model parameters |
CL = 36.1 L/h
Vc=21.9 L/kg
V2=19.018 L/kg
K12=0.033 (1/h)
K21=0.038 (1/h) |
Measured |
| ASF model |
stomach =0
duodenum = 0.020
jejunum 1= 0.081
jejunum 2 = 0.152
Ileum 1 = 0.029
Ileum 2 = 10.00
Ileum 3 = 40.00
Caecum = 120.00
Asc Colon = 20.00 |
Defined |
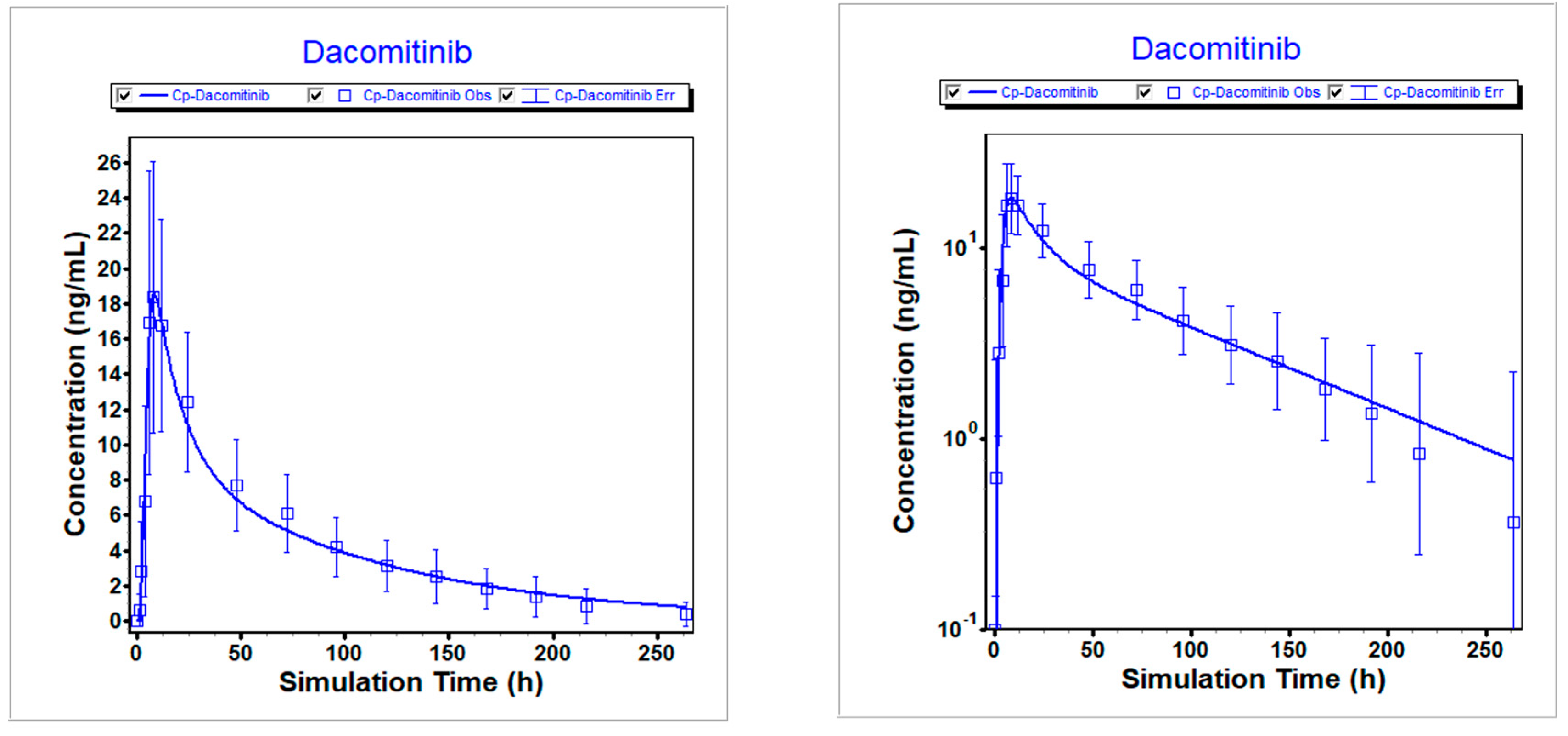
Figure 2.
Simulated vs. observed PK profiles following 45 mg dacomitinib in fasted state. Left is linear scale; Right is logarithmic scale.
Figure 2.
Simulated vs. observed PK profiles following 45 mg dacomitinib in fasted state. Left is linear scale; Right is logarithmic scale.
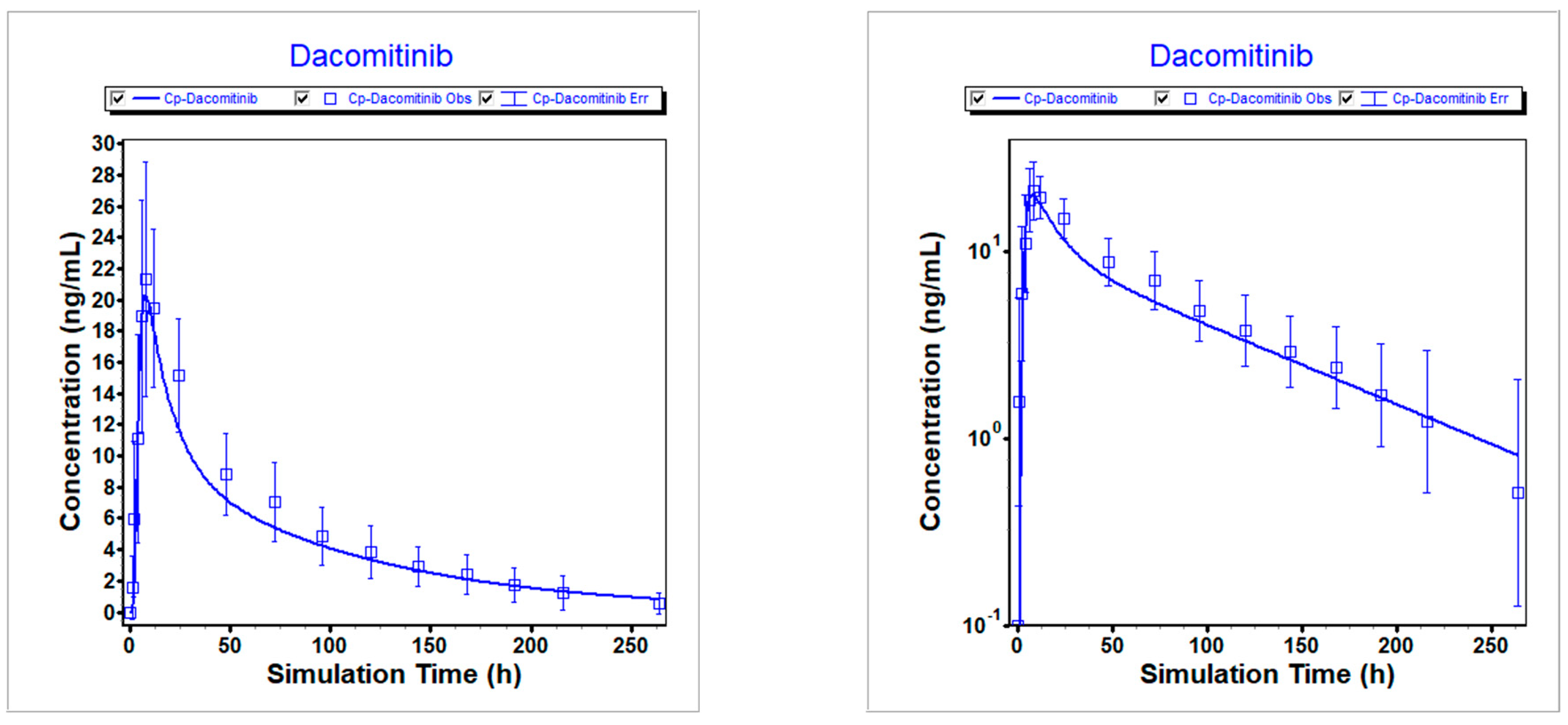
Figure 3.
Simulated vs. observed PK profiles following 45 mg dacomitinib in fed state. Left is linear scale; Right is logarithmic scale.
Figure 3.
Simulated vs. observed PK profiles following 45 mg dacomitinib in fed state. Left is linear scale; Right is logarithmic scale.
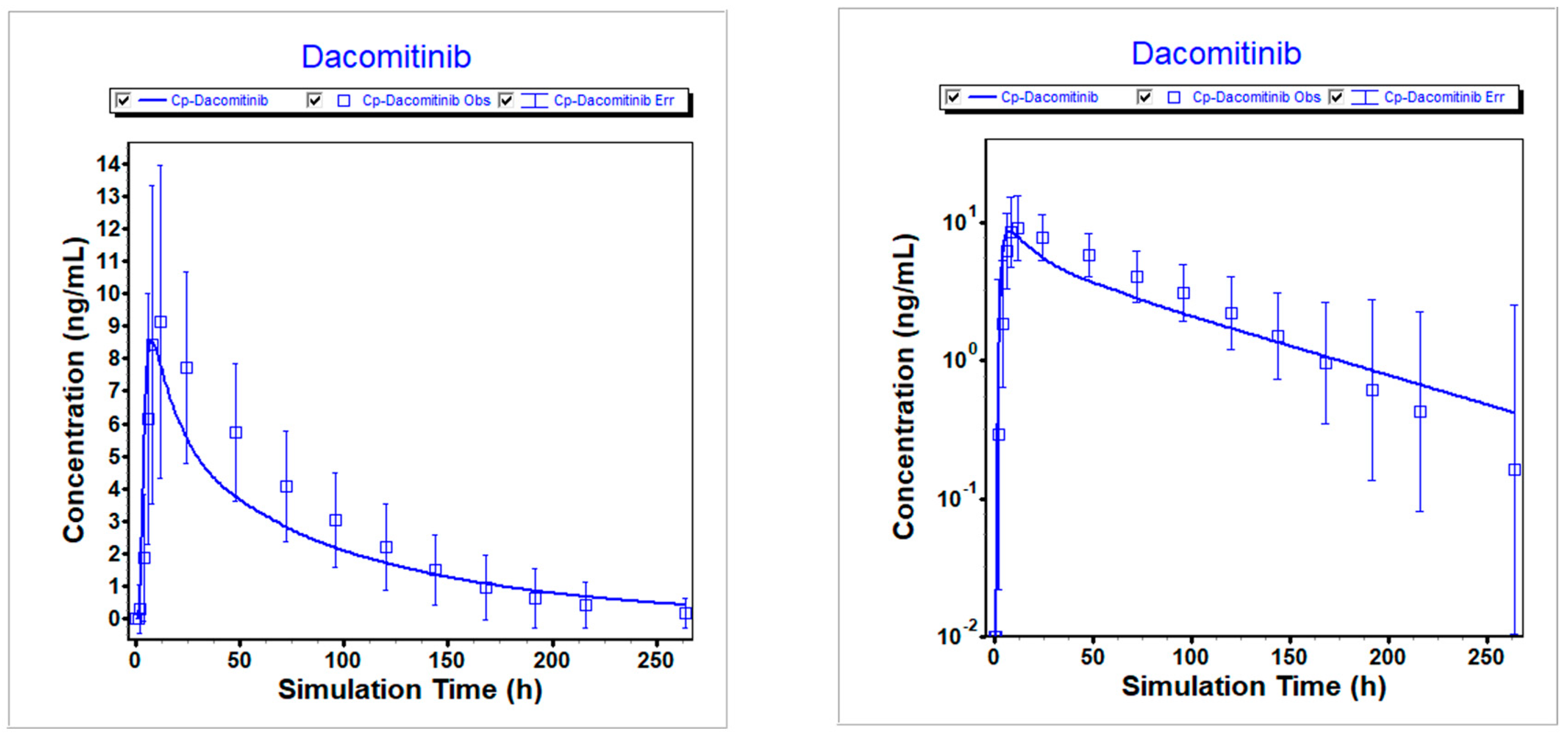
Figure 4.
Simulated vs. observed PK profiles following 45 mg dacomitinib with PPI (pH=6). Left is linear scale; Right is logarithmic scale.
Figure 4.
Simulated vs. observed PK profiles following 45 mg dacomitinib with PPI (pH=6). Left is linear scale; Right is logarithmic scale.
Table 5.
Comparison of simulated and observed exposures following 45 mg dacomitinib in fasted state.
Table 5.
Comparison of simulated and observed exposures following 45 mg dacomitinib in fasted state.
| Item |
Cmax (ng/mL) |
AUCinf (ng*h/mL) |
Ratio of Cmax
|
Ratio of AUCinf |
| Predicted |
18.526 |
1175.2 |
1.01 |
1.00 |
| Observed |
18.41 |
1171 |
|
|
Table 6.
Comparison of simulated and observed exposures following 45 mg dacomitinib in fed state.
Table 6.
Comparison of simulated and observed exposures following 45 mg dacomitinib in fed state.
| Item |
Cmax (ng/mL) |
AUCinf (ng*h/mL) |
Ratio of Cmax
|
Ratio of AUCinf |
| Predicted |
20.317 |
1246.3 |
0.952 |
1.07 |
| Observed |
21.35 |
1163 |
|
|
Table 7.
Comparison of simulated and observed exposures following 45 mg dacomitinib with PPI (pH=5).
Table 7.
Comparison of simulated and observed exposures following 45 mg dacomitinib with PPI (pH=5).
| Item |
Cmax (ng/mL) |
AUCinf (ng*h/mL) |
Ratio of Cmax
|
Ratio of AUCinf |
| Predicted |
8.576 |
613.23 |
0.940 |
0.857 |
| Observed |
9.125 |
715.96 |
|
|
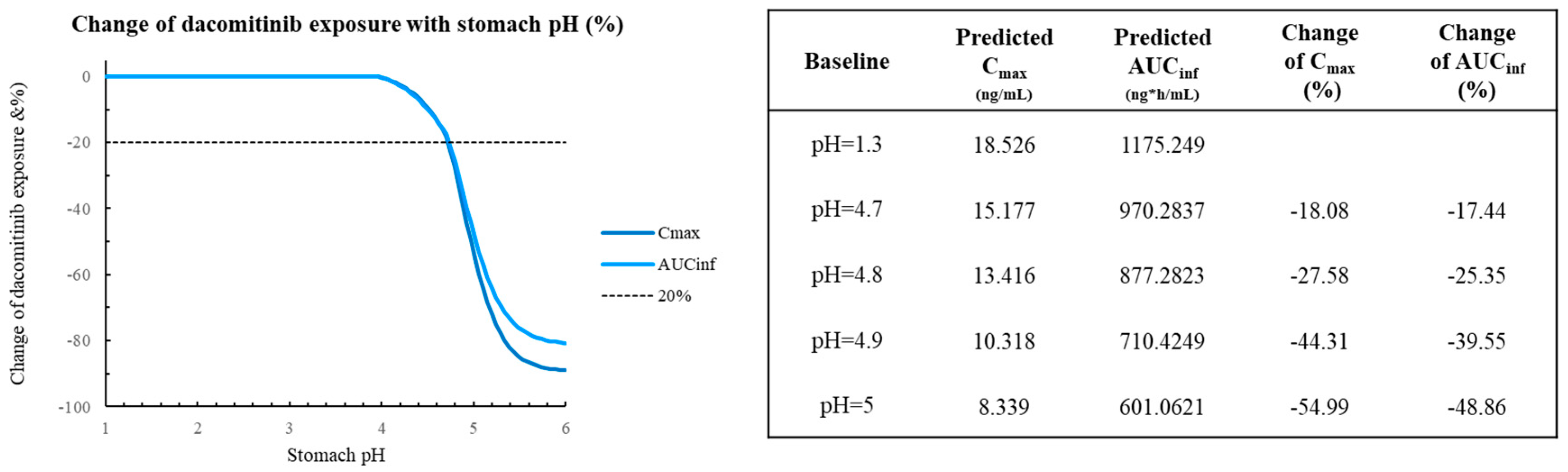
Figure 5 depicts the PSA on 45 mg dacomitinib AUC
inf and C
max for the pH range 1-5 (range 1-2 represents normal stomach, 3-5 represents stomach after H2RA) [
17]. The results showed that the AUC
inf and C
max did not dramatically change for dacomitinib from pH 1 to 4.7, although at the extreme situation with pH=5, the change of predicted AUC
inf is -48.86% and -54.99% for C
max. The simulation results suggest that varying the stomach pH (1-5) would not apparently affect the absorption of dacocitinib.
4. Discussion
A within-patient comparison of dacomitinib Ctrough,ss of parent, metabolite and active moiety between dacomitinib without concomitant H2RA use (reference) and dacomitinib co-administered with an H2RA medication (test) was retrospectively conducted to evaluate the effect of H2RA use on the dacomitinib PK. Our within-patient analysis allows the comparison with smaller sample size and minimized the random noise.
Data was pooled from 11 studies in patients with lung cancer, where H2RA use was permitted during the treatment. A total of 16 patients met the pre-defined criteria of having data for trough concentrations of dacomitinib with concomitant H2RA and without concomitant H2RA for inclusion in the analysis. H2RA included in this analysis were famotidine, ranitidine, cimetidine, and nizatidine. The adjusted geometric means of dacomitinib C
trough,ss of dacomitinib parent, metabolite, and active moiety following co-administration with any of H2RA medications was approximately 86%, 104% and 100%, relative to that following dacomitinib administration without an H2RA, respectively. The value of the 90% CI for the ratio included 100%, suggesting no statistical difference between the reference and test groups. Our results indicated that there is no statistically significant effect of an H2RA on the plasma concentrations of dacomitinib, which is unlike that PPI showed effect on dacomitinib PK. Coadministration of dacomitinib with multiple doses of rabeprazole in the dedicated healthy volunteer study (NCT01702506) which was designed to represent the worst case where the maximum effect of acid suppression by PPI on dacomitinib absorption decreased dacomitinib AUC by 39% [
12].
PBPK modeling has been increasingly used by drug developers to address the DDI effects of ARAs since it directly links PK with gastrointestinal physiological parameters, compound and formulation properties [
18,
19,
20,
21]. We also explored the utility of PBPK modeling to assess the impact of H2RA on dacomitinib exposure to mimic the gastrointestinal pH changes in the presence of H2RAs.
An oral absorption model using PBPK was constructed to predict the effect of H2RAs on its oral absorption. The model was constructed and verified using in vitro and clinical data from the DDI study with rabeprazole 40 mg on dacomitinib PK (NCT01702506) and the absolute bioavailability study by comparing oral to intravenous administration in healthy volunteers (NCT01796327). The model predicted reasonably well the mean plasma concentration of the 45 mg dose in healthy volunteers in the aforementioned clinical trials. After the verification step, the model was used to predict the PK profiles of dacomitinib under different situations (fasted, fed and with the treatment of PPIs, gastric pH=6).
The resulted PK profiles from the simulations were compared to the results of the observed profiles from the DDI study with rabeprazole 40 mg on dacomitinib PK (NCT01702506). All predicted to observed ratios for AUC
inf and C
max reported were within ranges of 0.857-1.07 for AUCinf and 0.940-1.01 for C
max. These data suggest that the PBPK model appropriately describes the pharmacokinetic profile of dacomitinib and has good prediction accuracy. Sensitivity analysis was conducted by changing gastric pH to explore its impact on dacomitinib exposures. The results showed that the exposures did not dramatically change for dacomitinib from pH 1 to 4.8, although at the extreme situation with pH=5 which is unlikely or transient after dosing with H2RA [
39,
40,
41], the change of predicted AUCinf is -48.86% and -54.99% for C
max.
Li J et al. has reported that even with potential lower exposure (37% decrease) after PPI co-administration, there is no difference in efficacy as showed PFS and OS in patients with EGFR positive NSCLC were not associated with PPI use. [
25]. Similar findings have been reported for other TKIs. The phase III study in 485 patients with NSCLC on erlotinib as second- or third-line therapy showed that no significant differences in PFS or OS with ARA therapy (either PPI or H2RA therapy) [
26,
27]. Van De Sijpe, G. et al. has concluded no evidence of a negative impact of concomitant PPI/H2RA on outcome in mRCC patients treated with first line pazopanib, measured as PFS, OS, and tumor response, could be found in the patient series [
28,
29,
30].
By contrast, PPIs block hydrogen potassium ATPase, which is the final common step in acid secretion [
31]. H2RAs cause a substantial reduction in acid secretion, but they do not completely and irreversibly inhibit acid production [
31]. H2RAs, namely reversible inhibition of the histamine (H2) receptor on the acid-secreting parietal cell of the stomach, have very similar mechanisms of action. Unlike the prolonged effect of PPIs, H2RAs result in short-lived inhibition of acid secretion; the onset of inhibition occurs after about 4 hours and maximal inhibition after about 8 h, with return of acid secretion after about 12 hours [
32]. In consequence, compared to PPIs, there is typically a lower degree of reduced bioavailability and systemic exposure with H2RAs for oral kinase inhibitors, consistent with the weaker efficacy of H2RAs [
33,
34,
35,
36,
37]. Therefore, the effect of H2RA on survival outcomes is not expected to be significant, especially considering the administration of H2RAs with dacomitinib are recommended to be scheduled several hours apart [
12].
Our research has some limitations. For the linear mixed effects model analysis by pooling 11 clinical studies retrospectively, patients were treated as outpatients and were considered to take a H2RA when this was mentioned in the electronic medical record. However, we lack information on the dosing administration and dose of H2RA medication. Moreover, we do not have information about the respective timings of H2RA and dacomitinib administration. As the potential to suppress the acidity of the stomach differs between the different H2RAs, and between different doses, this could be relevant. For the PBPK modeling, in terms of simulation of the pH effect, the gastric pH was kept constant in our PBPK predictions, which is a general practice that most PBPK models are taking when predicting pH-dependent DDIs [
38,
39]. But numerous studies have shown that gastric pH undergoes a dynamic change after ARA administration [
40,
41,
42]. Thus, incorporating a dynamic pH change during the simulation may help the model to achieve a better quantitative prediction on the impact of gastric pH change. In addition, in our study, we assume that H2RAs only changed the gastric pH, but not other parameters. However, certain studies suggested that H2RAs may delay gastric emptying, which may also contribute to the change of absorption [
22,
23]. Meanwhile, it should be noted that Dong Z’s study in the application of PBPK to predict gastric pH dependent DDI for weak base drugs suggested there were gaps for PBPK models to correctly predict change of AUC or C
max in the presence of ARA [
34]. Afterwards, our PBPK model may be refined further when more data is available.
5. Conclusions
The developed models demonstrate the PK of dacomitinib, and simulated results are well aligned with the clinical observations. Co-administration of an H2RA with dacomitinib is not expected to have any statistical or clinically meaningful effect on dacomitinib exposure, especially considering the administration of H2RAs with dacomitinib are recommended to be scheduled several hours apart.
Author Contributions
Formal analysis, J.L., S.L., and A.H.; Supervision, W.T.; Validation, J.L., S.L., and A.H; Writing, J.L. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pfizer Inc.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this analysis. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual anonymized participant data. See
https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Acknowledgments
The authors thank the patients who participated in the mentioned studies and their families, the investigators, nurses, and site staff.
Conflicts of Interest
All authors are employees of Pfizer Inc, except Swan Lin who was previously employee of Pfizer and Anthony Huynh as an intern from Skaggs School of Pharmacy and Pharmaceutical Sciences when conducting this project.
References
- Lung Cancer. American Cancer Society. https://www.cancer.org/cancer/lung-cancer.html. Accessed 20 FEB 2020.
- TARCEVA® (erlotinib) tablets, US Prescribing Information [Online]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf (accessed 21 FEB 2020).
- Sullivan I, Planchard D (2016) Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line. Front Med (Lausanne) 3:76. [CrossRef]
- Mok TS CY, Zhou X, Lee KH, Nakagawa K, Niho S, Chawla A, Rosell R, Corral J, Migliorino MR, Pluzanski A, Noonan K, Tang Y, Pastel M, Wilner KD, Wu YL (2020) Updated Overall Survival in a Randomized Study Comparing Dacomitinib with Gefitinib as First-Line Treatment in Patients with Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. Drugs. [CrossRef]
- Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomized, open-label, phase 3 trial. Lancet Oncol 18 (11):1454-1466. [CrossRef]
- Budha NR, Benet LZ, Ware JA (2013) Response to "Drug interactions produced by proton pump inhibitors: not simply a pH effect". Clin Pharmacol Ther 93 (2):151. [CrossRef]
- Welage L. S. (2005). Overview of pharmacologic agents for acid suppression in critically ill patients. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists, 62(10 Suppl 2), S4–S10. https://doi.org/10.1093/ajhp/62.10_Supplement_2.S4. [CrossRef]
- Smelick GS, Heffron TP, Chu L, Dean B, West DA, Duvall SL, Lum BL, Budha N, Holden SN, Benet LZ, Frymoyer A, Dresser MJ, Ware JA (2013) Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm 10 (11):4055-4062. doi:10.1021/mp400403s. [CrossRef]
- Numico G, Fusco V, Franco P, Roila F (2017) Proton Pump Inhibitors in cancer patients: How useful they are? A review of the most common indications for their use. Crit Rev Oncol Hematol 111:144-151. doi:10.1016/j.critrevonc.2017.01.014. [CrossRef]
- Ruiz-Garcia A, Masters JC, Mendes da Costa L, LaBadie RR, Liang Y, Ni G, Ellery CA, Boutros T, Goldberg Z, Bello CL. Effect of food or proton pump inhibitor treatment on the bioavailability of dacomitinib in healthy volunteers. J Clin Pharmacol. 2016 Feb;56(2):223-30. doi: 10.1002/jcph.588. Epub 2015 Oct 9. PMID: 26179237. [CrossRef]
- Jänne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, Guo F, Wong S, Liang J, Letrent S, Millham R, Taylor I, Eckhardt SG, Schellens JH. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res. 2011 Mar 1;17(5):1131-9. doi: 10.1158/1078-0432.CCR-10-1220. Epub 2011 Jan 10. PMID: 21220471; PMCID: PMC3048920. [CrossRef]
- VIZIMPRO® (dacomitinib) tablet: US Prescribing Information [Online]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211288s000lbl.pdf (accessed 23 September 2021).
- PEPCID® (famotidine) tablet: US Prescribing Information [Online]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019462s037lbl.pdf (accessed 23 September 2021).
- Smelick, G. S., Heffron, T. P., Chu, L., Dean, B., West, D. A., Duvall, S. L., Lum, B. L., Budha, N., Holden, S. N., Benet, L. Z., Frymoyer, A., Dresser, M. J., & Ware, J. A. (2013). Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Molecular pharmaceutics, 10(11), 4055–4062. https://doi.org/10.1021/mp400403s. [CrossRef]
- DeVault, K. R., & Castell, D. O. (1995). Guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Practice Parameters Committee of the American College of Gastroenterology. Archives of internal medicine, 155(20), 2165–2173.
- Piscitelli J, Chen J, LaBadie RR, Salageanu J, Chung CH, Tan W. The Effect of Hepatic Impairment on the Pharmacokinetics of Dacomitinib. Clin Drug Investig. 2022 Mar;42(3):221-235. doi: 10.1007/s40261-022-01125-x. Epub 2022 Feb 23. PMID: 35195881; PMCID: PMC8930943. [CrossRef]
- Dong Z, Li J, Wu F, Zhao P, Lee SC, Zhang L, Seo P, Zhang L. Application of Physiologically Based Pharmacokinetic Modeling to Predict Gastric pH-Dependent Drug-Drug Interactions for Weak Base Drugs. CPT Pharmacometrics Syst Pharmacol. 2020 Aug;9(8):456-465. doi: 10.1002/psp4.12541. Epub 2020 Jul 31. PMID: 32633893; PMCID: PMC7438815. [CrossRef]
- Wagner, C. et al. Predicting the effect of cytochrome P450 inhibitors on substrate drugs: analysis of physiologically based pharmacokinetic modeling submissions to the US Food and Drug Administration. Clin. Pharmacokinet. 54, 117–127 (2015).
- Wagner, C., Pan, Y., Hsu, V., Sinha, V. & Zhao, P. Predicting the effect of CYP3A inducers on the pharmacokinetics of substrate drugs using physiologically based pharmacokinetic (PBPK) modeling: an analysis of PBPK submissions to the US FDA. Clin. Pharmacokinet. 55, 475–483 (2016).
- Dodd S, Kollipara S, Sanchez-Felix M, et al. Prediction of ARA/PPI drug-drug interactions at the drug discovery and development interface. J Pharm Sci.2019;108(1):87-101.
- Mitra A, Kesisoglou F, Beauchamp M, Zhu W, Chiti F, Wu Y. Using absorption simulation and gastric pH modulated dog model for formulation development to overcome achlorhydria effect. Mol Pharm. 2011;8(6):2216-2223.
- Rasmussen, L., Oster-Jørgensen, E., Qvist, N. & Pedersen, S.A. The effects of omeprazole on intragastric pH, intestinal motility, and gastric emptying rate. Scand. J. Gastroenterol. 34, 671–675 (2009).
- Parkman, H.P. et al. Effect of gastric acid suppressants on human gastric motility. Gut 42, 243–250 (1998).
- Lu T, Fraczkiewicz G, Salphati L, Budha N, Dalziel G, Smelick GS, Morrissey KM, Davis JD, Jin JY, Ware JA. Combining "Bottom-up" and "Top-down" Approaches to Assess the Impact of Food and Gastric pH on Pictilisib (GDC-0941) Pharmacokinetics. CPT Pharmacometrics Syst Pharmacol. 2017 Nov;6(11):747-755. doi: 10.1002/psp4.12228. Epub 2017 Oct 17. PMID: 28748626; PMCID: PMC5702897. [CrossRef]
- Li J, Nickens D, Wilner K, Tan W. Evaluation of the Effect of Proton Pump Inhibitors on the Efficacy of Dacomitinib and Gefitinib in Patients with Advanced Non-Small Cell Lung Cancer and EGFR-Activating Mutations. Oncol Ther. 2021 Dec;9(2):525-539. doi: 10.1007/s40487-021-00156-2. Epub 2021 Jun 13. PMID: 34120312; PMCID: PMC8593125. [CrossRef]
- Hilton JF, Tu D, Seymour L, et al. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer. 2013; 82(1): 136–142. [PubMed: 23910908.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012; 13(3):239– 246. [PubMed: 22285168].
- Van De Sijpe, G., Beuselinck, B., Van Nieuwenhuyse, T. et al. Impact of concomitant acid suppressive therapy on pazopanib efficacy and dose reductions in patients with metastatic renal cell carcinoma. Eur J Clin Pharmacol 76, 1273–1280 (2020). https://doi.org/10.1007/s00228-020-02902-3. [CrossRef]
- McAlister RK, Aston J, Pollack M, Du L, Koyama T, Chism DD (2018) Effect of concomitant pH-elevating medications with pazopanib on progression-free survival and overall survival in patients with metastatic renal cell carcinoma. Oncologist 23(6):686–692. https://doi.org/10.1634/theoncologist.2017-0578. [CrossRef]
- Mir O, Touati N, Lia M, Litiere S, Le Cesne A, Sleijfer S, Blay JY, Leahy M, Young R, Mathijssen RHJ, Van Erp NP, Gelderblom H, Van der Graaf WT, Gronchi A (2019) Impact of concomitant administration of gastric acid-suppressive agents and pazopanib on outcomes in soft-tissue sarcoma patients treated within the EORTC 62043/62072 trials. Clin Cancer Res 25(5):1479–1485. https://doi.org/10.1158/1078-0432.Ccr-18-2748. [CrossRef]
- Wolfe MM, Soll AH. The physiology of gastric acid secretion. N Engl J Med. 1988 Dec 29;319(26):1707-15. doi: 10.1056/NEJM198812293192605. PMID: 3060722. [CrossRef]
- Sachs, G., et al. (2010). "Novel approaches to inhibition of gastric acid secretion." Curr Gastroenterol Rep 12(6): 437-447.
- Bosulif (bosutinib) [prescribing information]. New York, NY: Pfizer, Inc; 2016. Available at: http:// labeling.pfizer.com/ShowLabeling.aspx?id=884 [Accessed April 19, 2016].
- Sprycel (dasatinib) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2015. Available at: http://packageinserts.bms.com/pi/pi_sprycel.pdf [Accessed April 19, 2016].
- Ibrance (palbociclib) [prescribing information]. New York, NY: Pfizer, Inc; 2016. Available at: labeling.pfizer.com/ShowLabeling.aspx?id=2191 [Accessed April 19, 2016].
- Tarceva (erlotinib) [prescribing information]. Northbrook, IL: OSI Pharmaceuticals, LLC; 2015. Available at: http://www.gene.com/download/pdf/tarceva_prescribing.pdf [Accessed April 19, 2016].
- DeVault KR, Castell DO. American College of G. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005; 100(1):190–200. [PubMed: 15654800].
- Cristofoletti, R., Patel, N. & Dressman, J. B. Assessment of bioequivalence of weak base formulations under various dosing conditions using physiologically based pharmacokinetic simulations in virtual populations. Case examples: ketoconazole and posaconazole. J. Pharm. Sci. 106, 560–569 (2017). [CrossRef]
- Parrott, N.J., Yu, L.J., Takano, R., Nakamura, M. & Morcos, P.N. Physiologically based absorption modeling to explore the impact of food and gastric pH changes on the pharmacokinetics of alectinib. AAPS J. 18, 1464–1474 (2016). [CrossRef]
- Prichard, P.J. et al. Effect of daily oral omeprazole on 24 hour intragastric acidity. Br. Med. J. (Clin. Res. Ed.) 287, 1378–1379 (1983). [CrossRef]
- Tolman, K.G. et al. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J. Clin. Gastroenterol. 24, 65–70 (1997). [CrossRef]
- Bruley des Varannes, S. et al. Comparison of lansoprazole with omeprazole on 24-hour intragastric pH, acid secretion and serum gastrin in healthy volunteers. Aliment Pharmacol. Ther. 8, 309–314 (1994).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).