1. Introduction
According to IEA report of 2022, global energy related CO2 emissions has increased by 0.9% resulting in a new peak of over 36.8 Gt. The highest contribution of this rise in CO2 emission is by the electricity and heat generation sector, where the emissions increased by 1.8% equivalent to 261 million metric tons reaching a peak of 14.6Gt [1]. These figures indicate the need for reduction of CO2 emission and for the utilisation of sustainable energy sources. One of the promising and sustainable energy sources to reduce greenhouse gas emissions and attain a low-carbon economy is geothermal energy. According to the International Renewable Energy Agency (IRENA), geothermal energy delivered around 15.96 gigawatts electricity (GWe) in 2021, with this figure anticipated to rise to 18.3 GWe by 2025, showing a rise from 69856 GWh in 2011 to 94,949 GWh in 2020 [2]. When compared to standard heating and cooling systems, the usage of geothermal heating and cooling systems in buildings can result in up to an 85% reduction in carbon emissions. Additionally, geothermal energy has huge potential, with estimates indicating that it may fulfil up to 18% of world electricity demand and satisfy the electricity requirements of approximately 17% of the global population [2]. It is anticipated that geothermal as renewable energy will progressively play larger roles in the energy sector.
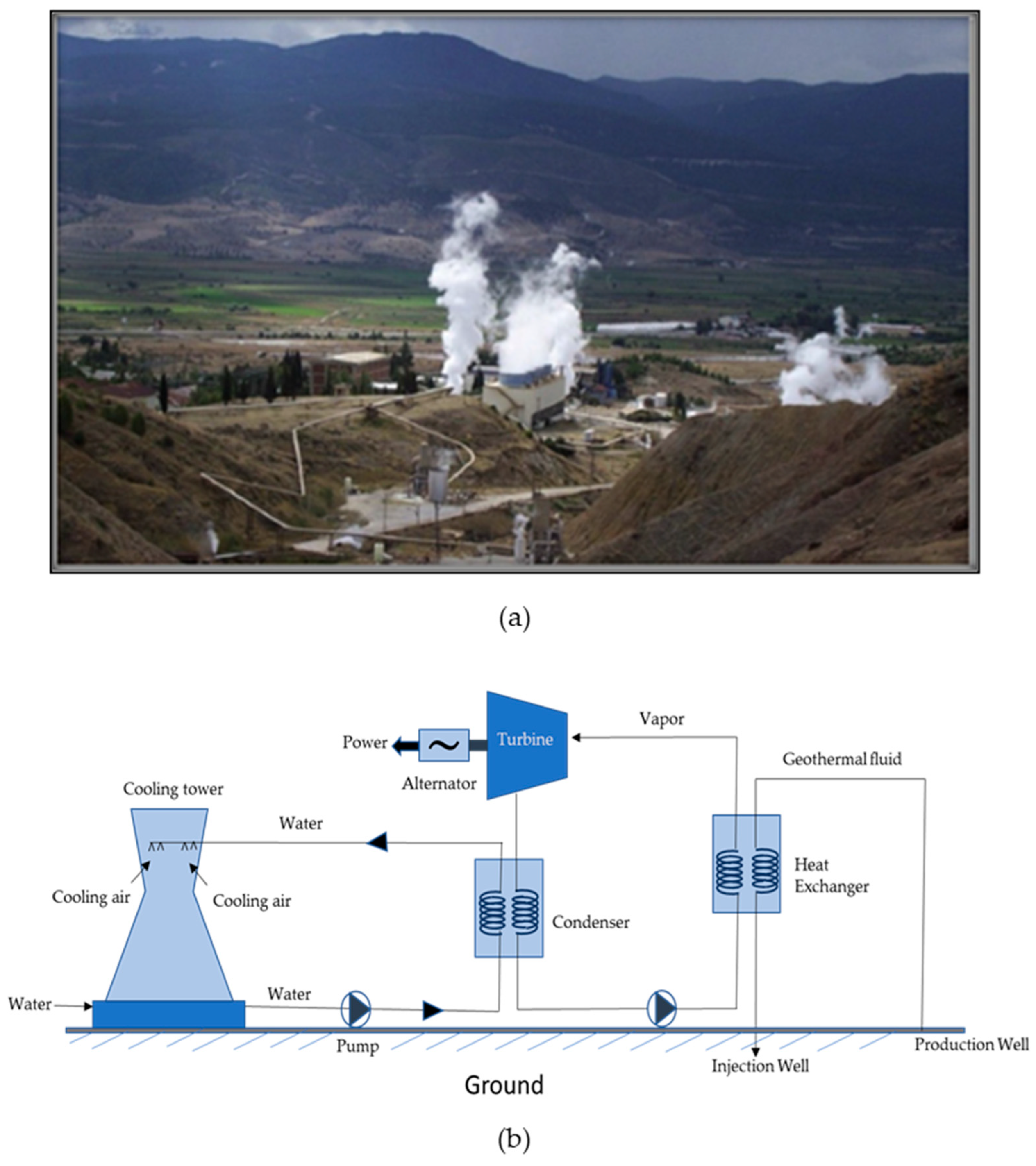
Figure 1.
(a) Kızıldere-I single flash type geothermal powerplant in the BMG reprinted with permission from Elsevier [
3] and (b) schematic demonstrating working of ORC binary geothermal powerplant inspired from [4].
Figure 1.
(a) Kızıldere-I single flash type geothermal powerplant in the BMG reprinted with permission from Elsevier [
3] and (b) schematic demonstrating working of ORC binary geothermal powerplant inspired from [4].
Geothermal energy stands out for its cost-effectiveness and continuous high operational capacity year-round, setting it apart from other renewable energy sources like solar and wind, which are intermittent in nature. Geothermal energy contributes significantly towards the electricity requirements in countries such as Iceland, El Salvador, New Zealand, Kenya and the Philippines [5]. In Iceland, over 90% of the heating demand is satisfied by geothermal sources, with around 1000 geothermal sites [6, 7]. Error! Reference source not found. shows geothermal power plants in Büyük Menderes Graben (BMG) in Western Anatolia and working of ORC binary geothermal plant. Nonetheless, its utilization remains limited in comparison to other sources due to the substantial investment costs associated with surface and subsurface infrastructure and high risk in the first phase, location constraints, requirement of the advanced technology and the expensive maintenance [5, 8-11]. One of the vital components for the efficient utilization of geothermal energy are heat exchangers which facilitate the transfer of thermal energy from the geothermal fluid to a secondary fluid or the working fluid [12, 13]. The operational conditions and the complex composition of geothermal water present an array of challenges in the heat exchangers namely extreme temperatures, corrosive fluid, scaling, and abrasive particles. These challenges not only affect the efficiency of heat transfer but also impact the longevity and maintenance requirements of geothermal heat exchangers. In recent years, the exploration of advanced coatings has emerged as a promising method to mitigate the adverse effects of geothermal environment on heat exchangers. By considering the evaluation of the potential advantages of the coating techniques, limitations and the future directions, this review aims to contribute to the technical advancement of coatings for heat exchangers in geothermal powerplants.
To investigate coatings for geothermal heat exchangers, we used search engines like Google Scholar, Web of Science and Scopus. Although literature specifically focusing on coatings for geothermal heat exchangers is limited, less than 25 articles, published between 2000 and 2023, we found several studies related to coatings on various other geothermal components such as pipelines, condensers, and turbines, some of which have been incorporated into this review. In the past 13 years, there has been no published review paper in the public domain specifically on the topic of coatings for geothermal heat exchangers. One known generic review related to Geothermal Environments has been recently published by Fanicchia
et al. [
14].
1.1. Types of Heat Exchangers
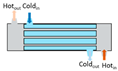
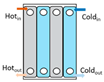
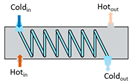
Heat exchangers (HEXs), find applications for temperature-sensitive mediums, renewable energy technologies, and energy recovery systems [
15]. The primary components of HEXs include the fluid streams, their inlet and outlet points, and the heat transfer surface. Depending on the specific type of HEX, additional components like baffles, fins, pipes, and tanks may also be incorporated [
16]. There are various types of HEXs namely, plate type in
Figure 2, shell and tube type, regenerative type, finned tube type, coiled tube type, double pipe type, printed circuit, scraped surface type HEX etc. [
17]. Thermophysical properties of the fluids involved, difference in streams’ temperature, the materials used and the design of the HEX comprise the key factors that influence heat transfer rates and HEX performance [
15].
Heat exchangers can be classified based on the stream phase and arrangement, degree of surface compactness, thermal energy transfer mechanism, and construction [
15]. Choice of an appropriate type of heat exchanger (HEXs) involves consideration of multiple factors owing to the diversity of available types, their thermal configurations, material choices, initial investment costs, and ongoing operational expenses. When addressing a particular process requirement, the following factors must be taken into account: operating pressure, operating temperature, fluid characteristics, flow rates, construction materials, fabrication expenses and maintenance expenditures and susceptibility to fouling, wear and corrosion [
15]. Various types of heat exchangers and their advantages and limitations are mentioned in
Table 1.
2. Challenges and Effective Solutions
2.1. Challenges
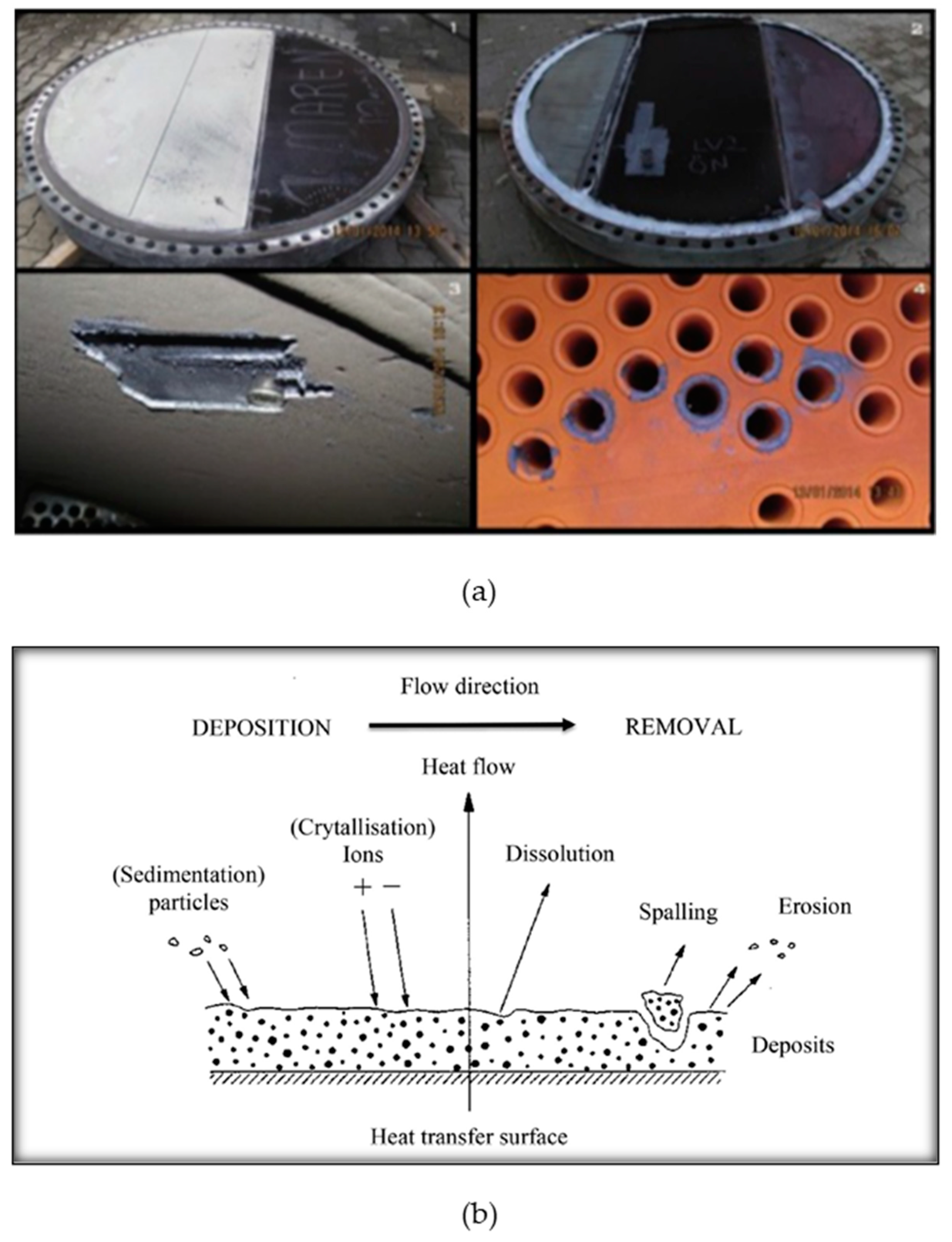
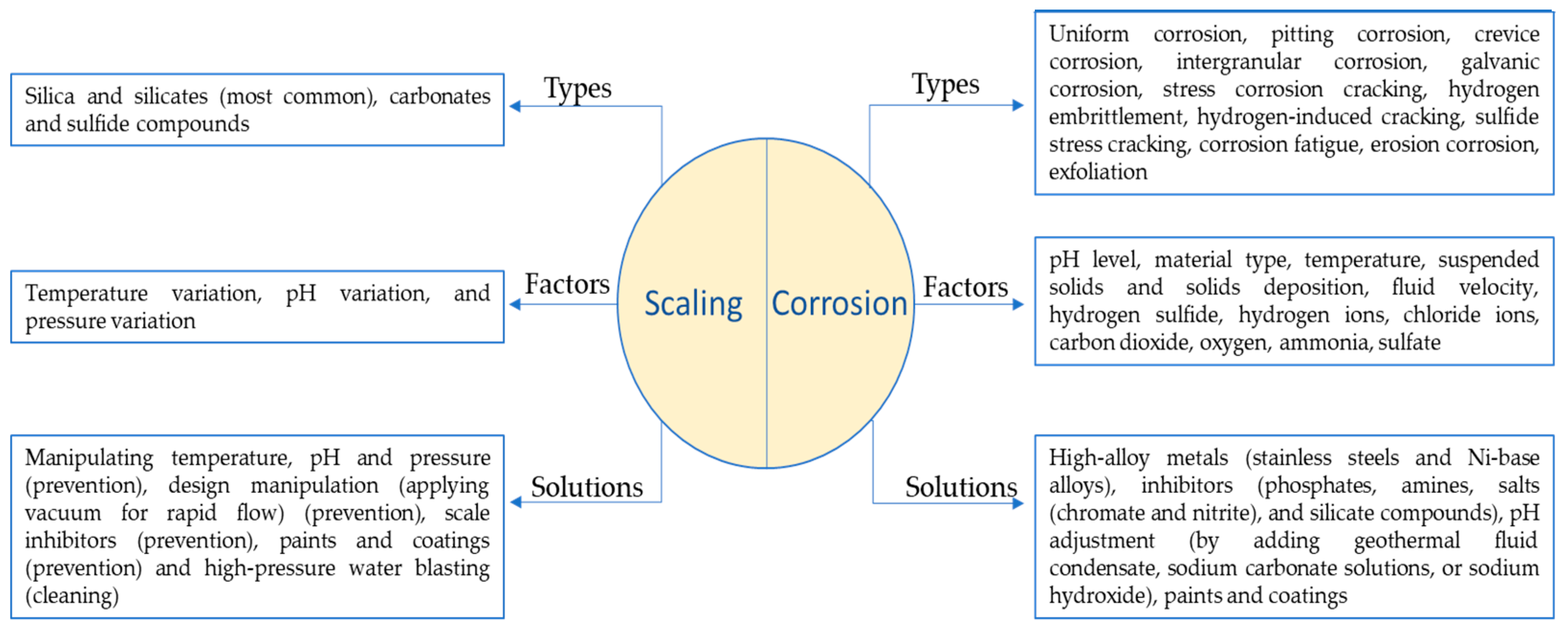
Geothermal environment presents a unique set of challenges, including corrosive nature of geothermal fluids, extreme temperature variations, scaling and fouling, affecting the efficiency and lifetime of geothermal systems. Different forms of corrosion and scaling in geothermal environment are presented in
Figure 4. A detailed discussion about challenges faced by geothermal heat exchangers is given in the following sections.
Fouling refers to the unwanted build-up of materials onto the surfaces involved in heat transfer [20, 21]. This accumulation, in the form of salts or other deposits negatively impacts the performance of operational equipment. The process of fouling is affected by a range of factors such as operational conditions, feed composition, heat exchanger geometry, and surface properties and mechanism of fouling is shown in
Figure 3b[
22]. The most prevalent form of fouling seen is the crystallization fouling caused by deposits like sulphates, carbonate and silicates of calcium. Carbonates and sulphates due to their retrograde solubility with respect to temperature, are dominant in low to medium enthalpy geothermal fluids, whereas silicates in high enthalpy geothermal fluids. In the case of heat exchangers, amorphous silica, aluminosilicate are the common deposits and in some cases stibnite scales have also been reported. These deposits hinder heat transfer due to their low conductivity (0.2-3.0 Wm
-1K
-1), and decrease the effective cross-sectional area within the heat exchanger tube, leading to the reduced efficiency raising concerns due to decreased flow rate and increased pressure drop[
23]. Consequently, this results in increased energy consumption and maintenance costs.
Figure 2.
(a) Scalling and pitting corrosion observed in heat exchanger of Büyük Menderes Graben in geothermal heat exchanger reprinted with permission from Elsevier [
3] and (b) mechanisn of fouling reprinted with permission from Elsevier [
24].
Figure 2.
(a) Scalling and pitting corrosion observed in heat exchanger of Büyük Menderes Graben in geothermal heat exchanger reprinted with permission from Elsevier [
3] and (b) mechanisn of fouling reprinted with permission from Elsevier [
24].
Silica scaling, one of the predominant forms of fouling observed in heat exchangers, arises due to the high concentration of silica in the brine solution of geothermal reservoirs. Two major factors governing the rate of silica deposition is the temperature and the pH of brine. Scale deposition increases with an increase in the pH and a decrease in the temperature, mostly occuring in the cooler parts of the plant. However, antimony and arsenic precipitates are mostly seen in geothermal brines at high temperatures [
3]. A study conducted on the small tubular heat exchangers of Soultz-sous-forêts by Ledésert, B. A., et al. mentioned the occurrence of PbS scaling alongside As and Sb sulphides and halite [
25]. One of the impacts of silica scaling include roughening of the heat exchanger surface. In the Wairakei geothermal power station, roughness generated due to silica scales in a shell and tube heat exchanger resulted in the drop of pressure and flow rate eventually leading to decrease in the efficiency of heat exchanger of the ORC [
20].
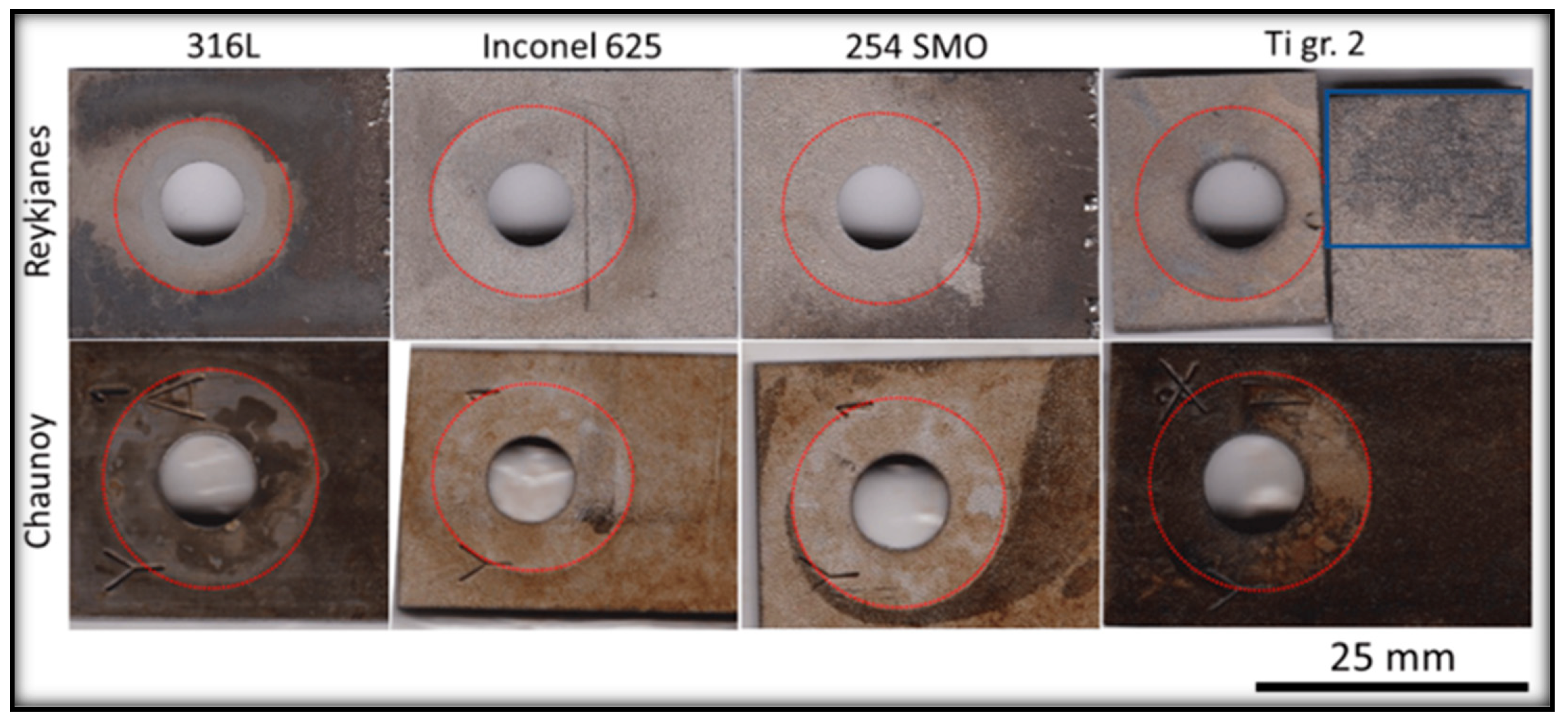
Corrosion is another significant challenge that disrupts the proper functioning of geothermal power plants. Factors such as pH, dissolved oxygen levels, temperature, and the presence of corrosive compounds like chlorides, hydrogen sulphides, and carbon dioxide in the brine play an important role in influencing the corrosion process, consequently leading to the failure of components. The nature of corrosion varies depending on the fluid chemistry of power plants [
11] and differences in the geographical region as given in
Figure 3, depicting dissimilarity in corrosion rate of the identical materials at two different sites. In geothermal environment, depending on the materials and components, different types of corrosion such as uniform, microbial, pitting, galvanic, and crevice corrosion are observed[
26].
In ORC binary plant, corrosion is observed in different heat exchanger components such as tubes and channels due to their contact with the aggressive geothermal fluid. Thus, materials like stainless steel, titanium and duplex steels are preferred over the carbon steel for construction of such components [11, 28]. Faes, W., et al has given a detailed discussion on the failure of heat exchangers due to corrosion [
28]. Practice of subjecting materials to the actual conditions of geothermal environment provides a means to evaluate the response of the materials towards the extreme conditions of the geothermal fluids and to predict the longevity and suitability of these materials. Various studies have relied on this, for instance Davíðsdóttir, S., et al. investigated the local corrosion of 4 different heat exchanger materials (316L, 254 SMO, Inconel 625, Titanium grade 2.) in two different high saline geothermal plants (Reykjanes, Iceland; 200°C and 18 bar vapour, and Chaunoy, France; 94°C and 9.5 bar fluid) [
27]. Their results revealed subsurface cracks and Cu deposits on Ti grade 2 in Reykjanes whereas in Chaunoy, the corrosion products were rich in Fe and S. Erosion was also observed in Ti grade 2 used in both sites. The 254SMO exposed in both sites showed subsurface cracks and in Reykjanes, pitting corrosion was also observed. Two corrosion layers were formed on the 316L exposed to the Reykjanes and local corrosion were observed in Chaunoy. The differences observed are mainly due to pH difference in both the plants. The corrosion products were of Fe, O and Cr and a trace of Al in Chaunoy. The Inconel 625 showed no evidence of corrosion on the surface exposed in Reykjanes but showed subsurface cracks after exposure [
27].
Figure 4.
Different forms of corrosion and scaling in geothermal environment.
Figure 4.
Different forms of corrosion and scaling in geothermal environment.
2.2. Effective Solutions
The issues outlined above limit the effectiveness of the heat exchanger, raise capital, operating, and maintenance expenses, and also give rise to safety concerns. As a result, ensuring optimal heat exchanger performance has prompted extensive research into developing effective strategies for tackling fouling and corrosion. Research has shown that the design phase of the heat exchangers plays a crucial role in mitigating fouling [
29]. This involves considering factors such as shape, geometry, operating conditions, and ease of cleaning. Various studies have utilized chemical and mechanical techniques to mitigate the corrosion and fouling of components in geothermal environment. Chemical inhibitors both organic and inorganic, with poly functionality such as phosphates, phosphonates and carboxylates are widely used in heat exchangers of complex geometries for mitigating fouling [30-32]. Scheiber, J., et al. [
33] studied the scaling inhibition in Soultz-sous-Forêts by phosphonates-based inhibitor against the Sr and Ba deposits. However, these inhibitors negatively impact the environment and their stability is temperature dependent [
3]. Furthermore, the applications of these inhibitors developed for ionic solids becomes challenging when dealing with covalent compounds like silica which affects the efficiency and selectivity of them [
34]. Cho, Y. and B.-G. Choi have used electronic anti-fouling [EAF] technique in order to mitigate fouling in heat exchangers [
35]. Mechanical techniques, such as laser machining and electrical discharge machining (EDM), are employed for creating anti-fouling surfaces, but they tend to be relatively costly and less efficient. Both EDM and magnetron sputtering have been applied to create C-films on Cu substrates, resulting in elevated water contact angles [
36]. A simple and relatively cheap method of fabricating superhydrophobic surface by CaCO
3 self-assembled coatings modified by sodium stearate in simulated geothermal water has been investigated by Wang, G. G., et al. These coatings were found to have a contact angle of 158.9 °C after 48h of immersion in geothermal water [
37]. Research has explored the substitution of CO
2 for acid additions and found it as effective to regulate pH and manage scale inhibition [
34].
Even though Ti alloys and Stainless steels are used in various components of geothermal plants, due to the passive layer providing resistance to corrosion and scaling but the interaction of silicate, silica, and calcite present in the solution with the surface oxide layer of them resulting in the increased adhesion of these compounds reducing the efficiency. These also react with the chloride ions causing corrosion of the components [
38]. In addition, compared to carbon steel these are expensive, for instance, stainless steel, AISI 316 costs €3480 and AISI 306 costs €2900 per tonnes compared to carbon steel which costs €900 per tonnes [
39]. Hence, it could be more cost-effective to apply a protective coating to carbon steel, rendering it suitable for use in geothermal heat exchangers, rather than opting for more expensive stainless steels. And of all the employed methods, surface modification through coatings emerges as an exceptionally efficient and economically viable approach to address challenges within geothermal heat exchangers, for example,
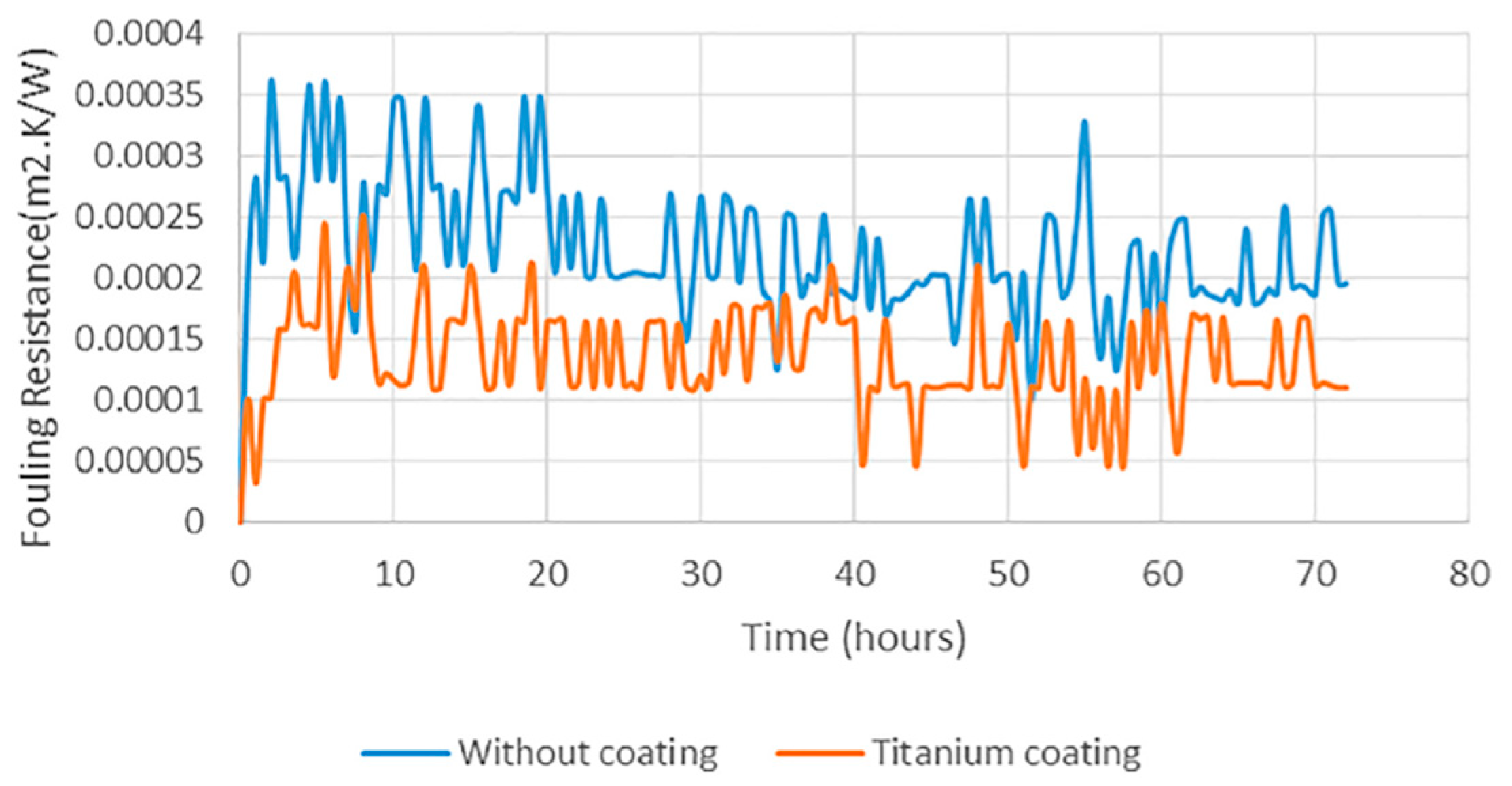
Figure 5 provides a clear illustration of the impact of coatings on fouling rates, highlighting the significance of coatings. It is therefore found to be necessary to review the coating techniques and the coatings which are used for the geothermal environment.
In recent times, high entropy alloys have gained significant attention for applications in geothermal environments. A study led by Thorhallsson, A. I., et al at the Hellisheidi geothermal site explored the use of CoCrFeNiMo(0.85) and Al(0.5)CoCrFeNi high entropy alloy coatings through laser melt deposition on the C-steel[S3257R] and stainless steel[316L]. Notably, the former alloy exhibited superior erosion-corrosion resistance compared to the latter in the geothermal environment [
44]. Geambazu, L. E., et al. found that the electro-spark deposited CoCrFeNiMo(0.85) performed well in a geothermal environment, and the rate of corrosion of the coated 316L stainless steel substrate in 3.5 wt% NaCl was 0.00016 mm/years [
45]. Oppong Boakye, G., et al. conducted examinations into CoCrFeNiMo
x (x = 20% and 27%) coated surfaces, employing laser cladding, high-velocity oxy-fuel (HVOF), and electro-spark deposition techniques, for potential application in geothermal environments [
40].
3. Coating Technologies for Geothermal Heat Exchangers
There are different coating methods that can be used to develop coatings especially for geothermal heat exchangers, aiming to enhance the resistance to corrosion, erosion, scaling and fouling. The choice of coating material and method is determined by the specific requirements that are affected by geothermal fluid composition, temperature, pressure and type of geothermal heat exchangers. Potential coating techniques for geothermal heat exchangers are mentioned in the following sections.
3.1. Coating Methods
3.1.1. Thermal Spray
Thermal spray stands as a long-established technique utilized for depositing an extensive array of materials onto diverse components, serving various applications such as biomedical, electronics, and aerospace sectors [
41]. Thermally sprayed coatings, characterized by their ease of application, compact structures, and strong adhesive strength, are used for protection against corrosion, wear, erosion, thermal degradation, and oxidation. In comparison to other coating methods, thermal spraying offers several benefits including high production efficiency, durability, and cost-effectiveness [
42]. Commonly employed thermal spray methods include plasma spray, high-velocity oxy-fuel (HVOF), flame spray, and arc-spray deposition. In thermal spray deposition process, the coating material is either fully or partially melted by a heat source and propelled by gases towards the substrate. On impacting the substrate or previously deposited particles, the molten or semi-molten consumable spreads, cools and forms ‘pancake-shaped’ splats. These splats build up and form the coating. The deposited coating is impacted by kinetic and thermal energy profiles of feedstock droplets traveling towards the substrate during deposition [
43].
Plasma spray and HVOF processes are favoured over other thermal spray techniques due to their superior coating quality and versatility in terms of feedstock. Both these methods allow the use of feed (coating materials) in either powder or liquid/suspension form [41, 43]. Liquid feedstock is sometimes preferred over powder feedstock due to various reasons. For instance; a) in powder feedstock particles smaller than 10μm in size cannot be used as they lack the necessary momentum to be fed into the system; b) nozzle clogging can result from the accumulation of dry powder feedstock [
41]. As a result, coating materials that are suspended or dissolved in a solvent are sometimes preferred over powder feedstock. However, obtaining such feedstock commercially can be challenging.
Liquid feedstock involves submicron or nanosized particles suspended within a liquid medium leading to multiscale features in deposited coatings [
44]. Both plasma spray and HVOF spray processes utilise thermo-chemical interactions (in the case of solution precursor feedstock) and thermo-physical interactions between the feedstock and plasma. However, coatings deposited through each method exhibit distinct characteristics depending on the difference in generated heat and velocity. Coatings deposited using HVOF method tend to be denser and smoother compared to plasma spray coatings. Conversely, plasma spray generates more heat, facilitating melting of high temperature consumables such as zirconia or alumina [43, 45]. In addition to the deposition methods, coating quality is also influenced by other spray parameters namely the heat source, feedstock flowrate, standoff distance (SOD), suspension medium, fuel type (for HVOF), and injection mode (radial or axial) [43, 46, 47]. Buzaianu et al. [
48] employed HVOF technique to deposit Ni
20Cr
10Al
2Y coatings to improve erosion corrosion properties of carbon steel for geothermal environments. In another study, Zhang et al. [
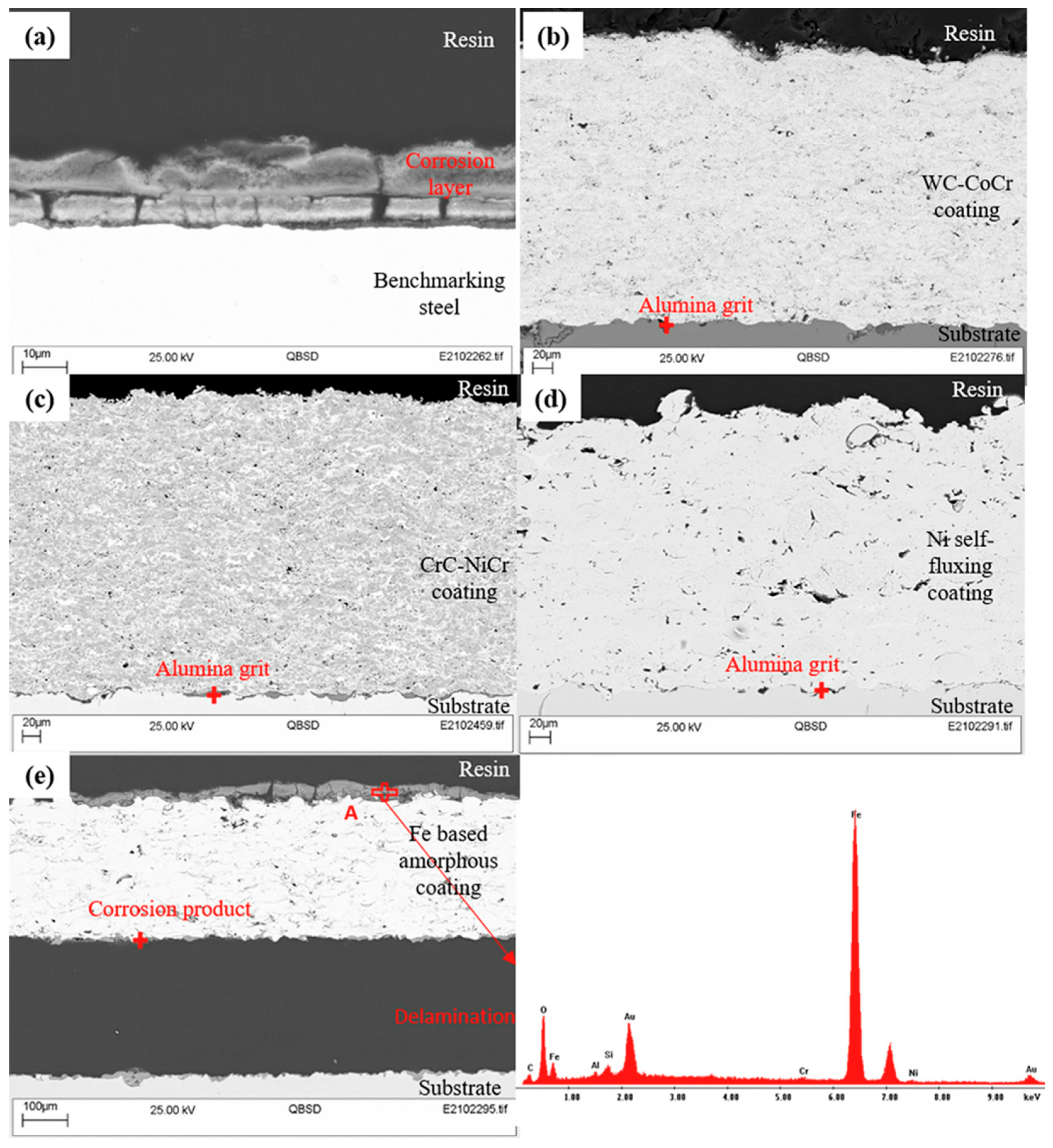
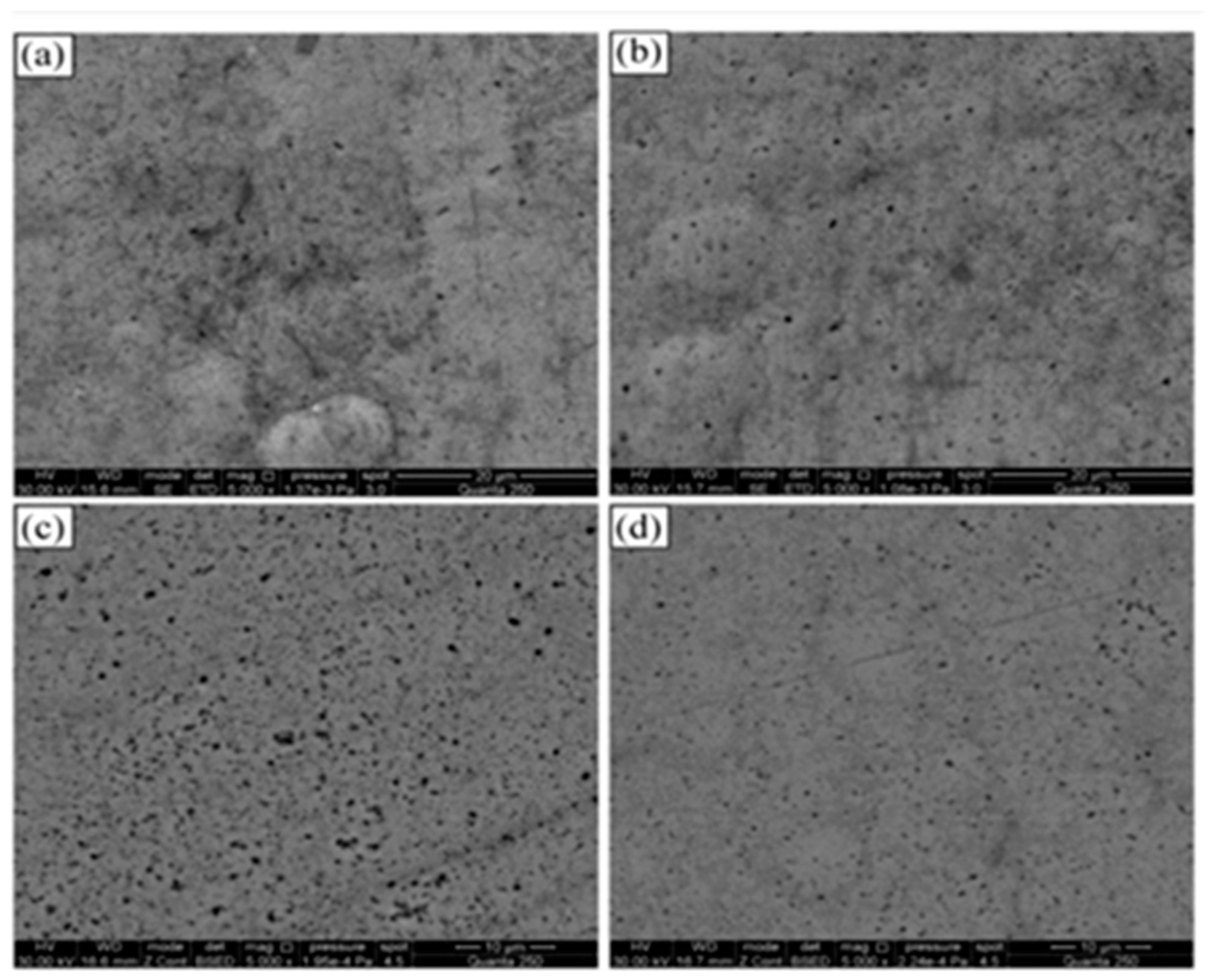
42] utilised liquid feedstock based HVOF to deposit various cermet (WC-CoCr and CrC-NiCr) and other alloys (Ni self-fluxing, and Fe-based amorphous), and examined their erosion-corrosion performance in geothermal environments. The SEM images of these coatings after 24 h immersion in a simulated geothermal environment are shown in
Figure 4., and there was no sign of corrosion product formation for WC-CoCr and CrC-NiCr cermet, and Ni self-fluxing coatings due to the uniform microstructures of coatings with a low volume fraction of fine pores and non-interconnected voids. Fe-based amorphous coatings demonstrated the presence of interconnected defects, including cracks and voids, which favoured the permeation of electrolyte into these defects. Thus, these coatings showed accelerated corrosion and corrosion-induced delamination of the coating. Mittal et al. [
43] deposited TiO
2 coatings using suspension-based plasma spray (SPS) and suspension-based HVOF spray (S-HVOF) to explore the effects of standoff distance (for plasma spray) and fuel gas (for HVOF spray) on coating properties and microstructures. It was shown that increase in the standoff distance leads to a reduction in the amount of suspension-transported material in the coating. Moreover, it was also established that the coating formation is dependent on the pre-heating and deposition temperatures.
Figure 6.
SEM and EDX analyses on polished cross-sectional surface of test coupons after 24-h erosion–corrosion testing in simulated geothermal fluid: (a) benchmarking steel, (b) WC-CoCr, (c) CrC-NiCr, (d) self-fluxing, and (e) Fe-based amorphous reproduced from ref. [
49] under Creative Commons CC BY license, Copyright © 2023, The Author(s).
Figure 6.
SEM and EDX analyses on polished cross-sectional surface of test coupons after 24-h erosion–corrosion testing in simulated geothermal fluid: (a) benchmarking steel, (b) WC-CoCr, (c) CrC-NiCr, (d) self-fluxing, and (e) Fe-based amorphous reproduced from ref. [
49] under Creative Commons CC BY license, Copyright © 2023, The Author(s).
3.1.2. Chemical Vapor Deposition (CVD)
The process of CVD, (
Figure 7) entails the disintegration and/or chemical reaction of gaseous reactants within an activated environment (such as heat, plasma, or light) to yield dense thin films with elevated purity and performance [
24]. Fundamental chemical reaction types within the realm of CVD encompass pyrolysis (thermal decomposition), oxidation, reduction, hydrolysis, formation of nitrides and carbides, synthesis reactions, disproportionation, and chemical transport. In more complex scenarios, a combination of these reaction types might be implicated to generate a specific final product. The deposition rate and characteristics of the deposited film are dictated by deposition parameters such as temperature, pressure, reactor geometry, input concentrations, gas flow rates, and operational principles [
50]. CVD process can produce thin-films inclusive of a vast array of elements and compounds. These materials encompass inorganic, organometallic, and organic reactants as starting substances. Thermally activated CVD process is a conventional CVD approach which utilises thermal energy for initiating chemical reactions and is most commonly employed for depositing thin films of transition metal nitrides. Based on the pressure range under which deposition occurs, thermally activated CVD can be categorised into atmospheric pressure CVD (APCVD), low pressure CVD (LPCVD) having a pressure range of 0.01–1.33 kPa, or ultra-high vacuum CVD (UHVCVD) comprising a pressure of <10
−4 kPa [24, 50]. The sole distinction between APCVD and LPCVD is that the reduced pressure modifies the rate-controlling step during the deposition reaction. Thus, LPCVD processes are generally limited by the rate of surface reaction, whereas APCVD processes have the restriction of mass transport or diffusion rates. One of the drawbacks of CVD is that the gaseous by-products produced in this process are usually quite toxic [
51]. CVD epitaxy, atomic layer deposition (ALD), plasma-enhanced CVD (PECVD), photo-enhanced CVD (PHCVD) laser-assisted or laser-induced CVD (LACVD), metal-organic CVD (MOCVD) and electron enhanced CVD include some other CVD methods [50, 52]. Wang et al. [
53] demonstrated the effectiveness of graphene coatings deposited through CVD technique in promoting the dropwise condensation effect of water. The graphene coatings were deposited by LP-CVD and AP-CVD and they exhibited an enhanced heat transfer and better chemical stability compared to contemporary coatings produced using other techniques. These coatings could be a potential candidate to be applied in a geothermal environment.
3.1.3. Physical Vapor Deposition (PVD)
The process of PVD involves deposition of thin layers of various materials onto a substrate in the presence of a vacuum, serving to either enhance the material's functionality or to confer thermal and/or chemical stability [
54]. Metal oxides are vaporised through physical means like cathodic arc deposition, electron beam PVD (EB-PVD), evaporative deposition, pulsed laser deposition, sputter deposition, or sublimation [
55]. PVD coatings are applied to enhance surface properties such as hardness, wear resistance, corrosion resistance [
54] and protection against fouling [
24]. This process has the advantage of tailoring composition with high precision and permitting thin films to be deposited at lower temperatures compared to other techniques such as CVD, which is important when the substrate being coated with is a temperature sensitive material [
55]. EB-PVD, a high vacuum thermal coating process, is considered to be a simple and relatively cheaper technique as compared to some other deposition processes, including ionic sputtering of a target source, ionic bombardment of the particles by ion beam assisted deposition (IBAD) and pulsed laser evaporation technique [
56]. Other advantages of EB-PVD include high deposition purity, enlarged coating area, precise film thickness, in-situ growth monitoring, and smoothness control [
57]. However, limitations of PVD processes include requirement of a large vacuum chamber making it a high-cost process, the sensitivity of deposited materials to the orientation of substrate resulting in epitaxial stresses and strains which leads to variations in film properties across a large substrate making it incompatible for large-scale production. PVD methods also have a relatively slower deposition rate compared to CVD [52, 58]. Oon et al. [
24] employed PVD magnetron sputtering to deposit titanium coating (selected because of its high corrosion resistance and surface adhesion) on a stainless steel, SS316L heat exchanger. Ti-coated SS316L demonstrated a decrease in calcium carbonate related fouling caused by the deposition of 201 mg/l of deposits whereas the uncoated specimen displayed 269 mg/l of deposits on its surface. Moreover, Ti coating also revealed an enhancement in the average heat transfer coefficient with an increase of Reynold number (Re, the ratio of inertial to viscous forces within a fluid). This was observed at Re 3803 and Re 15,212. In another research, Ali et al. [
59] established that combining EB-PVD coating and modification of brine parameters is a promising approach for altering the degree of wettability of copper surface, leading to the enhancement in heat transfer efficiency (via improvement in critical heat flux and fluid dynamics) of plate heat exchangers. These authors produced copper films on a copper substrate through EB-PVD and showed that the thickness of the film/coating, pH of the liquid (water) and the surface roughness caused by the deposited film affect the wettability behaviour. It was demonstrated that increase in the pH value led to a reduction in average contact angle (ACA) of water. This was attributed to the enhancement in surface energy of the substrate due to the deposited film. This had led to a reduction in both surface micro-roughness and formation of air-pockets between the liquid and substrate interface. Moreover, increase in the film thickness also resulted in a decrease of surface roughness of the copper surfaces thus leading to a deviation in the surface wettability from hydrophobic nature to hydrophilic nature.
3.1.4. Liquid Phase Deposition (LPD)
The LPD is a wet-chemical method based in the controlled hydrolysis of metallic fluoro-complexes. This aqueous technique is utilized for the deposition of oxide films and it demonstrates various advantageous such as the utilization of low-energy-cost equipment and partially crystalline products at ambient temperatures [60-64]. Additionally, it allows for good control over deposition rates and crystal orientations [
61]. LPD has found significant application with materials like TiO
2 [61, 65-69], SiO
2 [69, 70] etc. A study by Liu et al. [
71] demonstrated a significant reduction in fouling resistance (increase of the resistance to the flow of heat (m
2KW
-1) due to fouling) of LPD TiO
2-FPS coated stainless steel plates in comparison to uncoated stainless steel samples. The LPD TiO
2-FPS coating exhibited an asymptotic fouling resistance of 1.2×10
-5 m
2KW
-1, whereas the untreated SS plates maintain a higher fouling resistance of 3.3 × 10
-5 m
2KW
-1 for an experimental duration of around 12 days. Moreover, the LPD TiO
2-FPS coating plates demonstrated a threefold extension in the fouling induction period, lasting for approximately 10 days compared to the untreated SS plates. This was attributed to the flow rate of fluid. It was concluded that higher flow rates result in higher heat transfer coefficient thus reducing fouling. In another work [
69], LPD TiO
2 coatings on stainless steel substrate were investigated for anti-fouling performance in hot-dry-rock (HDR) geothermal water, containing 7 g/L of total dissolved solids, at 423K. These coatings displayed a fouling resistance of 2.20 x 10
-4 m
2KW
-1 and the heat transfer coefficient obtained was 900 Wm
-2K
-1. These results were obtained after an experimental period of around six days. Moreover, the authors observed that at an experimental time of 20 hours, the heat transfer coefficient was 955 Wm
-2K
-1 which was similar to that observed in sol-gel TiO
2 coatings deposited under same conditions. However, the fouling resistance of LPD TiO
2 coatings was 30% higher than the sol-gel TiO
2 coatings due to higher heat transfer coefficient of LPD coatings.
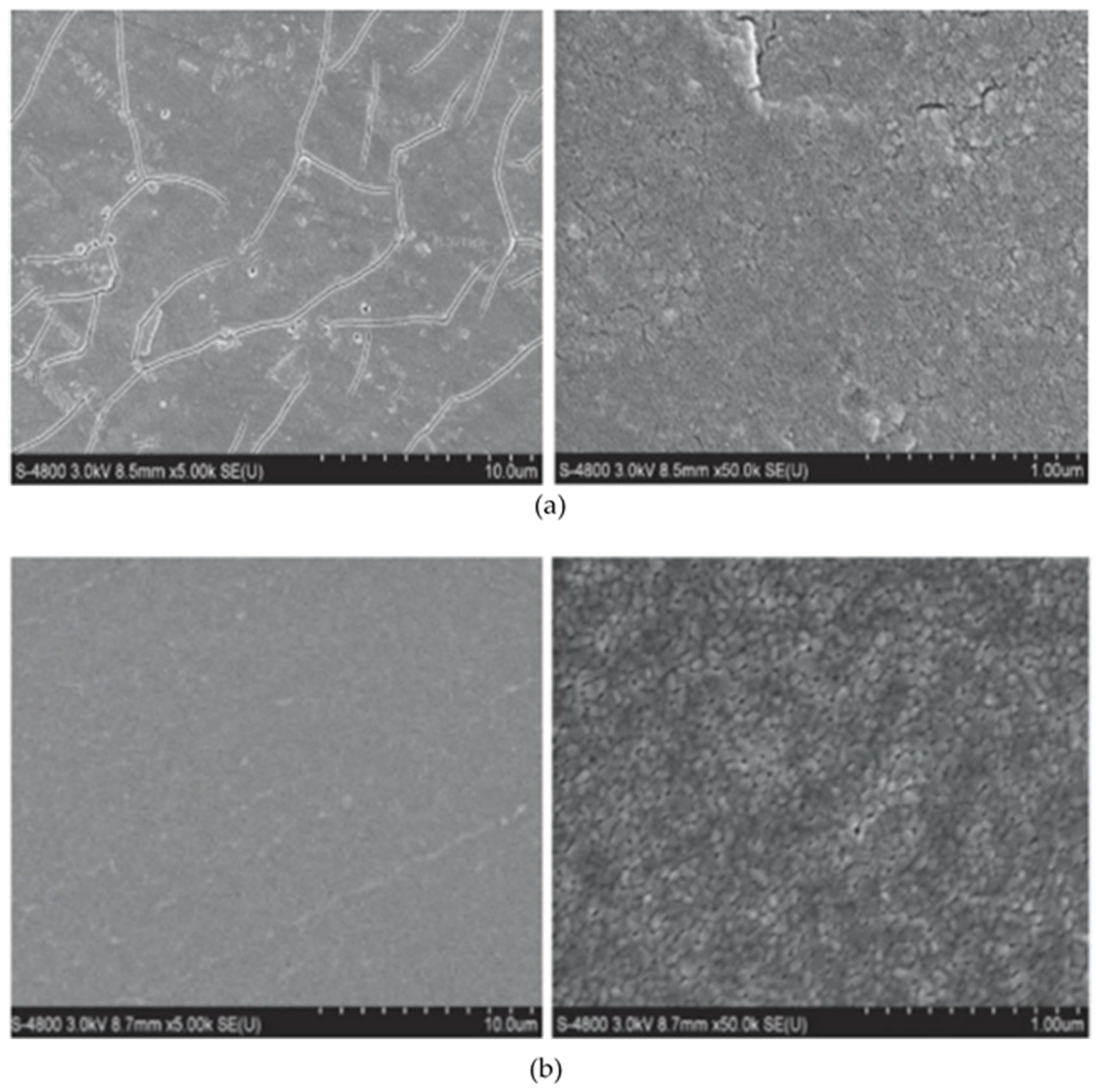
Figure 8.
SEM micrographs of TiO2 coating on SS 304 a) by LPD and b) sol-gel deposition reprinted with permission from Elsevier [
69].
Figure 8.
SEM micrographs of TiO2 coating on SS 304 a) by LPD and b) sol-gel deposition reprinted with permission from Elsevier [
69].
3.1.5. Sol-Gel Method
Sol-gel method is a versatile approach for the formulation of inorganic or hybrid coatings, with applicability through various procedures like dipping, spraying, or spinning [
72]. The sol-gel method offers numerous advantages, including low deposition temperature, production of homogenous coatings, good control over both metal concentration and coating thickness and permits the addition of reducing and oxidizing agents in small quantities [
73]. Additionally, this method requires simpler coating equipment [
71]. Liu et al. [
69] deposited sol-gel TiO
2, SiO
2 and SiO
2-FPS coatings on stainless steel (AISI 304) tubes and examined their anti-fouling and anti-corrosion behaviour in a simulated HDR geothermal water at around 423 K. The findings indicated that the sol-gel TiO
2 coatings exhibited excellent anti-fouling characteristics when exposed to simulated geothermal water with a calcium bicarbonate composition. Meanwhile, the sol-gel SiO
2 and SiO
2-FPS coatings demonstrated superior antifouling and anti-corrosion properties when tested in moderately corrosive HDR geothermal water with a total dissolved solids (TDS) concentration of approximately 7g/L. In comparison to SS304 tubes, sol-gel TiO
2 coated heat exchanger tubes exhibited a fouling resistance reduction of more than 48%. Whereas, sol-gel SiO
2 and SiO
2-FPS demonstrated a fouling resistance reduction of 30% and 58%, respectively, in their study. Moreover, electrochemical corrosion tests conducted on these coatings, and SS304 specimens for 14 days reveal that both coatings, compared to SS304, can reduce the corrosion rate by 60.1% and 85.2%. Schulz et al. [
74] and Nofz et al. [
75] highlighted the protective nature of Al
2O
3 sol-gel coatings in environments characterized by high-temperature flue gas and the presence of NaCl, respectively. Since, geothermal environments often exhibit elevated temperatures and salinity levels, Bäßler et al. [
72] explored the potential application of Al
2O
3 sol-gel coating for safeguarding martensitic steels in such geothermal conditions and concluded that these coatings offered a bright future in providing corrosion protection in geothermal environments with a neutral pH. Liu et al. [
71] performed experiments to observe anti-fouling on silica and titania coated plate heat exchangers in 80
oC simulated geothermal water. They coated stainless steel (AISI 304) substrates with TiO
2, TiO
2-FPS, SiO
2 and SiO
2-FPS coatings, and from their findings, they concluded that SiO
2 and SiO
2-FPS sol-gel coatings were not appropriate for anti-fouling in geothermal water. However, combination of these coatings with mechanical cleaning results in good fouling and corrosion resistance in geothermal water. Additionally, corrosion was observed on both TiO
2 and TiO
2-FPS coatings and the fouling induction period for these coatings only extended to 50 hours after which no fouling resistance was perceived. The authors concluded that fouling deposition can be reduced by utilisation of surface engineering technology on heat exchanger plates.
3.1.6. Electrochemical Method
Electroless plating is a type of surface treatment in which a thin layer of metals, salts or other compound is plated without the use of external power. This electrochemical deposition occurs as a result of a redox reaction between the metal and the reducing agent for e.g., hypophosphite, leading to the reduction and subsequent deposition of metallic ions on to the surface [76-80]. The wide application of electroless plating is due to the uniformity regardless of the shapes and size of the surface, low porosity and roughness, high adhesion of these coatings to the substrate and the excellent corrosion, wear and abrasion and fouling resistance [80, 81]. Cheng, Y., et al. [
82] investigated the anti-fouling properties of Ni-P electroless plating on low-carbon steel under various operating conditions. The immersion tests in boiling water and cooling using tap water containing Ca ions, revealed that the coated low-carbon steel exhibited less surface fouling deposition compared to the uncoated stainless steel substrate. However there is no mention of whether tap water have been used for the immersion testing Furthermore, after 20 hours of immersion, they observed loose and discontinuous fouling on the coated surface, in contrast to the uncoated stainless steel. Notably, the rate of fouling appeared to increase with a higher proportion of nanocrystalline phase in the electroless plating; surfaces with 92 mass % of nanocrystalline phase were less resistant to fouling than those with 17 mass % and 5 mass %. From this observation they concluded that the structural inhomogeneity of nanostructures; the presence of number of grain boundaries in the nanocrystalline structure, promotes the formation of numerous electrochemical cells resulting in acceleration of corrosion and fouling when compared to amorphous structures. However, it's important to note that the study did not address the heat transfer performance of the plated surfaces. The same authors conducted a subsequent study [
83] on electroless Ni-Cu-P plating on AISI 1015, with coating thickness within the range of 12-16 µm. They observed that the adhesion strength between the coating and the substrate exhibited an increase corresponding to higher copper concentrations, ranging from 2.97 wt.% to 13.78 wt.%. In addition, all the coatings displayed effective anti-fouling properties when compared to the uncoated stainless steel. The water contact angle exceeded 90° for all samples containing copper. Notably, the contact angle exhibited an initial increase with the rise in CuSO
4 content up to 0.4 g/L, followed by a decrease.
Conversely, surface energy displayed an increasing trend, reaching a maximum at 26.56 MJ/m², but a further increase in copper concentration resulted in decreased surface energy. The coating with the highest copper content demonstrated the least resistance to fouling. The study made conclusive evidence that the addition and amount of Cu has a significance in the fouling deposition rate, contact angle and surface energy of the coated surface. The authors also investigated the introduction of varying concentrations of PTFE particles (4, 8, 12, 16 ml/L, denoted as Samples 1, 2, 3, and 4) into electroless Ni-Cu-P coatings on mild steel. The surface morphology of electroless Ni-Cu-P-PTFE after 2h of deposition is given in
Figure 9, showing the homogenous distribution of PTFE particle (black spots) in the matrix. They observed that, when compared to the uncoated mild steel surface, the anti-fouling rate was notably reduced on the coated surface. Moreover, the contact angle and surface free energy were found to be dependent on the PTFE concentration. Specifically, the surface free energy decreased with increasing PTFE particle content, ranking as Sample 3 < 4 < 2 < 1, while the contact angle displayed a reverse trend as expected. Furthermore, fouling experiments revealed that the surface with the lowest surface energy exhibited the weakest adhesion of deposits. However, it is worth noting that the addition of PTFE particles had no effect on surface roughness, but it lead to a reduction in the surface's microhardness [
84].In the study conducted by Oppong Boakye, G., et al., on the Electroless Ni-P + PTFE composite duplex coatings, they investigated for the wear and abrasion resistance. The study revealed that the coating with a medium PTFE content of 10 g/L exhibited superior wear and abrasion resistance. Notably, the coating with medium PTFE achieved the highest water contact angle of 102.6°, compared to coatings with low and high PTFE contents. The findings suggest that this specific coating composition, with its enhanced properties, holds potential for application in heat exchangers [
85].
3.1.7. Weld Overlay
Weld overlay, also known as weld cladding, involves welding a material onto the base metal to provide protection against corrosion, erosion, and high temperatures. This process utilizes techniques such as arc welding and electron beam welding, laser cladding [86-88]. In order to enhance the corrosion-erosion of the turbine-rotor weld overlaying of Inconel 625 has been adapted in [
89]. Tayactac, R. G. and E. B. Ang conducted a comprehensive review of various alloys for their suitability as Corrosion Resistant Alloy (CRA) cladding in wellhead piping systems operating in geothermal environments. Their analysis revealed that alloy 625 emerges as a highly advantageous choice for CRA cladding in geothermal power plants [
90]. Currently, there is no published research article in public domain mentioning the utilization of weld overlay in geothermal heat exchangers.
A comparison of advantages and disadvantages of various coating technique is summarsied in Error! Reference source not found.. It should be noted that not many research articles are published focusing on the coatings for geothermal heat exchangers. But the sectors where coatings are used to protect their components against harsh conditions might be useful for geothermal environment.
Table 2.
Advantages and disadvantages of different coating techniques [24, 42, 51, 52, 55-57, 60-64, 69, 73, 90-96].
Table 2.
Advantages and disadvantages of different coating techniques [24, 42, 51, 52, 55-57, 60-64, 69, 73, 90-96].
| Techniques |
Advantages |
Disadvantages |
| Thermal Spray |
High production efficiency, durability and cost-effectiveness |
High temperature results in decomposition, rapid cooling results in amorphous coatings, line-of-sight process |
| PVD |
Ease of tailoring composition with high precision, thin films deposited at lower temperatures |
Sensitivity of deposited materials to the orientation of substrate, comparatively slower deposition rate to CVD, line-of-sight process, requires vacuum |
| CVD |
Deposition of thin-films, high manufacturing yield, non-line-of-sight process |
Deposition at higher temperatures, production of toxic gaseous by-products, need of vacuum systems or glove boxes, expensive.
|
| LPD |
Deposition at room temperatures, does not require vacuum systems and sensitive reagents, low energy and production cost, deposition of substrates with large surface area and complex geometries, good control over deposition rate and crystal orientations, non-line-of-sight process. |
Long reaction time, post-treatment required at high temperatures to obtain high crystallinity. |
| Electrochemical [Electroless Plating] |
Uniformity, low porosity and roughness, strong adhesion to the substrate, adaptability to complex geometries, high corrosion and wear resistance, non-line-of-sight process |
Expensive, environmental concerns, temperature sensitivity of the structure, requirement of complex pre-treatment, only suitable for some materials |
| Chemical [Sol-Gel] |
High quality coating, low operational temperature, producibility of materials with large surface areas, non-line-of-sight process. |
Long processing time, residues contain hydroxyl or carbon groups, time-consuming process, use of expensive chemicals |
| Weld Overlay |
Cost-effective, superior properties to base materials, dense coating, high technology readiness level, commercially available |
Complexity of the process, maintenance requirement, line of sight process |
3.2. Coating Materials and Performance
As coatings are vital for the prolonged service life and enhancement of heat exchanger efficiency, the research on coatings for heat exchanger has gained significant attraction among researchers of geothermal sector. It is therefore imperative to understand the commonly employed coatings that are used in heat exchangers and their performance and the type of technology that have been used to apply these coatings. Table 4 demonstrates various coatings used in geothermal environment along with their deposition techniques and impacts.
Coating
material |
Substrate |
Coating method |
Impact on HX |
Performance |
Reference |
CNTs in PTFE base
polymer coatings |
Brass |
Applied to the surface
and baked |
Dropwise condensation promoted, HTC decreased for the MWNTs in Polymer, Superhydrophobicity created |
Wettability – 170 ± 2.6° (3%)and 169 ± 2.1°(5%) for MWNT contents
|
[97] |
PPS sealed
Ni-Al coating |
Mild
C-Steel |
Flame sprayed Ni-Al + Dipped and heated in oven and air and subsequent cooling [HEX] |
Corrosion and oxidation protection |
Thickness - 0.09mm (Ni-Al layer) and 0.01 to 0.05 mm (PPS sealant)
|
[98] |
| SiC filled polymer |
Cold
rolled steel |
|
Silica scaling and corrosion protection |
Thickness - 0.75mm
|
[99] |
PTFE on PPS as
anti-oxidant |
1008 C steel |
|
Fouling and corrosion |
Thickness - 75 to 100mm
|
[100] |
Carbon fibre
reinforced PPS |
1008 C steel |
Dip coating
|
Corrosion protection |
Thickness - 60mm, 110mm,160mm,170mm
|
[101] |
Montmorillonite
[MMT] filled PPS
nanocomposite |
C -Steel |
Dip Coating [Geothermal well head] |
Corrosion resistant |
Thickness - 150mm on a Zn-Ph primed C- steel
|
[102] |
ZrO2-TiO2
nanocomposite |
Austenitic stainless steel AISI 304 |
LPD |
Corrosion resistant |
-
|
[103] |
Zn-Graphite
Composite Coating |
Steel 304 |
Brushing [pipelines] |
Anti-fouling |
-
|
[104] |
| SiO2, SiO2-FPS and TiO2 |
stainless steel (304) |
sol-gel |
Anti-fouling and anti-corrosion |
Wettability - 38.4 ± 4.0° (TiO2), 19.5 ± 1.1°(SiO2) and 105.3 ± 3.4°(SiO2-FPS) |
[69] |
| TiO2 |
stainless steel (304) |
LPD |
Anti-fouling and anti-corrosion |
Wettability - 10.4 ± 0.9° |
[69] |
| TiO2, TiO2-FPS |
stainless steel (304) |
LPD |
Anti-fouling |
Wettability - 63.7 ± 7.9° (TiO2) and 117.1 ± 2.6° (TiO2-FPS) |
[71] |
| SiO2, SiO2-FPS, TiO2 and TiO2-FPS |
stainless steel (304) |
sol-gel |
Anti-fouling |
Wettability - 79.3 ± 0.9° (TiO2), 120.8 ± 1.4° (TiO2-FPS), 68.9 ± 2.4°(SiO2) and 122.7 ± 0.5°(SiO2-FPS) |
[71] |
| TiO2 |
C-steel |
SPS |
- |
Wettability - 17.58° (Ti-50) and 19.47° (Ti-80) |
[43] |
| TiO2 |
C-steel |
S-HVOF |
- |
Wettability - 115.77° (Ti-H) and 105.8° (Ti-P) |
[43] |
| cermet (WC-CoCr and CrC-NiCr ), Ni-self fluxing and Fe based amorphous coatings |
low alloy steel (34CrNiMo6) |
HVOF |
- |
Roughness, Ra - 4.3 ± 0.5 µm (WC-CoCr), 3.5 ± 0.7 µm (CrC-NiCr), 6.4 ± 0.4 µm (Ni-flux coatings), 8.5 ± 0.4 µm (Fe-based amorphous coatings); Thickness – 341 ± 9.9 µm (WC-CoCr), 316.6 ± 7.9 µm (CrC-NiCr), 285.6 ± 13.9 µm (Ni-flux coatings), 281.4 ± 12.9 µm (Fe-based amorphous coatings) |
[42] |
| PTFE blended PPS |
C-steel |
dip coating |
Silica scaling |
- |
[105] |
| SiC-PPS ACA-PPS, PTFE-PPS and Zn-Ph (primer) |
stainless steel |
fill-drain-baking |
Anti-fouling and anti-corrosion |
Thickness - 300 - 330 µm (liners) and 8 - 60 µm (Zn-Ph primer) |
[106] |
4. Challenges and Future Direction
In geothermal power plants, paints and coatings could be a feasible and economical solution to mitigate various issues, including corrosion, scaling, erosion, and fouling. This is advantageous compared to the expensive replacement of power plant components. However, no universal coating method or material is suitable for all geothermal heat exchangers, as the selection and design of a coating system depend on the geothermal fluid chemistry influenced by geographical location and heat exchanger configuration. Despite promising advantages, coatings for geothermal heat exchangers face challenges associated with extreme conditions, corrosive environments, cost, and scalability. Designed coatings must withstand extreme thermal gradients, aggressive fluids, and mechanical stress by maintaining their integrity and functionality. Key factors influencing coating performance and durability include coating adhesion, (uniform) coating thickness, porosity, and surface energy. These factors vary with the coating deposition method, emphasizing the value of carefully considering coating methods in accordance with the specific application requirements.
Besides, the developed coating must be compatible with the substrate to ensure good adhesion, efficient heat transfer, and minimized (galvanic) corrosion between the coating and substrate. Coatings must also be compatible with a broad spectrum of geothermal fluids, preventing adverse reactions between the coating and geothermal fluid. Scalability is another challenge that needs to be addressed, as some coating methods are size- and/or shape-restricted. For instance, thermal spray is a line-of-sight process unsuitable for coating complex geometries in geothermal infrastructure. Similarly, heavy components of the heat exchangers cannot be coated using CVD as there is a specific weight limit that a quartz tube can sustain during deposition. Since coating development can be expensive, balancing performance benefits and the associated costs is also challenging. In today’s day and time, considering the environmental sustainability of coating materials, developmental methods, and the end of the coating lifecycle is crucial in diminishing the detrimental environmental impacts.
Addressing these challenges with the help of ongoing research and innovation, developing durable, efficient, and sustainable coating solutions for geothermal applications is possible. There is not much literature related to coating development for geothermal heat exchangers. Still, the knowledge can be acquired from other sectors where coating systems are used for harsh environments, such as hydrothermal power and biomass power productions, which could provide a potential solution for the protection and performance enhancement of geothermal heat exchangers. Implementing multifunctional coatings with novel ceramics, nanomaterials, and composites can mitigate corrosion, scaling, and fouling issues and improve heat transfer efficiency. Innovative coatings with self-healing properties and integrated sensors could be a promising solution for early detection problems, monitoring real-time conditions, and autonomous repairing. This would also help in reducing the O&M costs. Apart from these advancements, the industry-specific standardisation and regulations for geothermal coatings would also help their widespread implementation.
In short, the prospect for coatings in geothermal heat exchangers is very promising, which, with the collective efforts of researchers, industry stakeholders, and policymakers, would help geothermal power plants integrated with unconventional coatings become a sustainable and reliable source of clean and renewable energy.
Author Contributions
Conceptualization S.P.; methodology S.P, A.B.N.J, N.G.M, G.M; investigation, A.B.N.J, N.G.M.; data curation A.B.N.J, N.G.M; writing— A.B.N.J, N.G.M, G.M ; writing—review and editing, A.B.N.J, N.G.M, G.M, D.M; supervision S.P; project administration D.M, S.P, N.K. All authors have read and agreed to the published version of the manuscript.” Please turn to the Credit taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the project: GeoHex-advanced material for cost-efficient and en-hanced heat exchange performance for geothermal application (Grant agreement 851917).
Acknowledgments
We want to acknowledge the contribution of the GeoHex consortium to this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Energy Agency, IEA, CO2 Emissions in 2022. 2022 [cited 2023; Available from: https://iea.blob.core.windows.net/assets/3c8fa115-35c4-4474-b237-1b00424c8844/CO2Emissionsin2022.pdf].
- IRENA, IGA, ''Global geothermal market and technology assessment'' Internation Renewable Energy Agency, Abudhabi; International Geothermal Association, The Hague, 2023.
- Haklıdır, F.S.T. and T.Ö. Balaban, A review of mineral precipitation and effective scale inhibition methods at geothermal power plants in West Anatolia (Turkey). Geothermics, 2019. 80: p. 103-118. [CrossRef]
- Manzella, A. Geothermal energy. in EPJ Web of Conferences. 2017. EDP Sciences.
- Kumar, L., et al., Technological Advancements and Challenges of Geothermal Energy Systems: A Comprehensive Review. Energies, 2022. 15(23). [CrossRef]
- Gunnlaugsson, E., et al., Problems in geothermal operation–scaling and corrosion. Goethermal Training Program, United Nations University, 2014: p. 1-18.
- IRENA. Geothermal. [cited 2023; Available from: https://www.irena.org/Energy-Transition/Technology/Geothermal-energy.
- Stefansson, V., Investment cost for geothermal power plants. Geothermics, 2002. 31(2): p. 263-272. [CrossRef]
- Dr Corinna Abesser, D.A.W., Geothermal Energy, UK Parliament Post. 2022.
- Gurgenci, H., et al. Challenges for geothermal energy utilisation. in Thirty-third workshop on geothermal reservoir engineering, Stanford University, SGP-TR-185. 2008.
- Spadacini, C., L. Xodo, and M. Quaia, Geothermal energy exploitation with Organic Rankine Cycle technologies, in Organic Rankine Cycle (ORC) Power Systems. 2017, Elsevier. p. 473-525.
- Valdimarsson, P., Geothermal power plant cycles and main components. Short course on geothermal drilling, resource development and power plants, 2011. 24.
- Frick, S., et al., Geochemical and process engineering challenges for geothermal power generation. Chemie Ingenieur Technik, 2011. 83(12): p. 2093-2104. [CrossRef]
- Fanicchia, F. and S.N. Karlsdottir, Research and Development on Coatings and Paints for Geothermal Environments: A Review. Advanced Materials Technologies, 2023: p. 2202031. [CrossRef]
- Ghalandari, M., et al., Applications of intelligent methods in various types of heat exchangers: a review. Journal of Thermal Analysis and Calorimetry, 2021. 145(4): p. 1837-1848. [CrossRef]
- Ma, Y., G. Xie, and K. Hooman, Review of printed circuit heat exchangers and its applications in solar thermal energy. Renewable and Sustainable Energy Reviews, 2022. 155. [CrossRef]
- Wang, B., et al., Heat exchanger network retrofit with heat exchanger and material type selection: A review and a novel method. Renewable and Sustainable Energy Reviews, 2021. 138. [CrossRef]
- Kapustenko, P., et al., Accounting for local features of fouling formation on PHE heat transfer surface. Frontiers of Chemical Science and Engineering, 2018. 12(4): p. 619-629. [CrossRef]
- Shah, R.K. and D.P. Sekulic, Fundamentals of heat exchanger design. 2003: John Wiley & Sons.
- Zarrouk, S.J., B.C. Woodhurst, and C. Morris, Silica scaling in geothermal heat exchangers and its impact on pressure drop and performance: Wairakei binary plant, New Zealand. Geothermics, 2014. 51: p. 445-459. [CrossRef]
- Kazi, S., et al., Study of mineral fouling mitigation on heat exchanger surface. Desalination, 2015. 367: p. 248-254. [CrossRef]
- He, Z., et al., Experimental study on the anti-fouling effects of EDM machined hierarchical micro/nano structure for heat transfer surface. Applied Thermal Engineering, 2019. 162: p. 114248. [CrossRef]
- Malayeri, M., A. Al-Janabi, and H. Müller-Steinhagen, Application of nano-modified surfaces for fouling mitigation. International journal of energy research, 2009. 33(13): p. 1101-1113. [CrossRef]
- Oon, C.S., et al., Heat transfer and fouling deposition investigation on the titanium coated heat exchanger surface. Powder Technology, 2020. 373: p. 671-680. [CrossRef]
- Ledésert, B.A., et al., Scaling in a geothermal heat exchanger at soultz-sous-forêts (Upper Rhine Graben, France): A XRD and SEM-EDS characterization of sulphide precipitates. Geosciences, 2021. 11(7): p. 271. [CrossRef]
- Iberl, P., N. Alt, and E. Schluecker, Evaluation of corrosion of materials for application in geothermal systems in Central Europe. Materials and Corrosion, 2015. 66(8): p. 733-755. [CrossRef]
- Davíðsdóttir, S., et al., Study of Corrosion Resistance Properties of Heat Exchanger Metals in Two Different Geothermal Environments. Geosciences, 2021. 11(12): p. 498. [CrossRef]
- Faes, W., et al., Corrosion and corrosion prevention in heat exchangers. Corrosion reviews, 2019. 37(2): p. 131-155. [CrossRef]
- Müller-Steinhagen, H., M. Malayeri, and A. Watkinson, Heat exchanger fouling: mitigation and cleaning strategies. 2011, Taylor & Francis. p. 189-196. [CrossRef]
- Gill, J.S. New Inhibitors for silica and calcium carbonate control in geothermal. in International Workshop on Mineral Scaling. 2011.
- Gallup, D.L. and E. Barcelon, Investigations of organic inhibitors for silica scale control from geothermal brines–II. Geothermics, 2005. 34(6): p. 756-771. [CrossRef]
- Gallup, D.L., Investigations of organic inhibitors for silica scale control in geothermal brines. Geothermics, 2002. 31(4): p. 415-430. [CrossRef]
- Scheiber, J., et al. Application of a scaling inhibitor system at the geothermal power plant in Soultz-sous-Forêts: laboratory and on-site studies. in Proceedings of the European Geothermal Congress. 2013.
- Stapleton, M. and O. Weres. Recent developments in geothermal scale control. in Paper Presented at the International Workshop on Mineral Scaling. 2011.
- Cho, Y. and B.-G. Choi, Validation of an electronic anti-fouling technology in a single-tube heat exchanger. International Journal of Heat and Mass Transfer, 1999. 42(8): p. 1491-1499. [CrossRef]
- He, Z., et al., Preparation of anti-fouling heat transfer surface by magnetron sputtering aC film on electrical discharge machining Cu surface. Surface and coatings technology, 2019. 369: p. 44-51. [CrossRef]
- Wang, G.G., et al., Self-assembled biomimetic superhydrophobic CaCO3 coating inspired from fouling mineralization in geothermal water. Langmuir, 2011. 27(20): p. 12275-12279. [CrossRef]
- He, Z., et al., Hierarchical micro/nano structure surface fabricated by electrical discharge machining for anti-fouling application. Journal of Materials Research and Technology, 2019. 8(5): p. 3878-3890. [CrossRef]
- Cadelano, G., et al., Evaluation of the effect of anti-corrosion coatings on the thermal resistance of ground heat exchangers for shallow geothermal applications. Energies, 2021. 14(9): p. 2586. [CrossRef]
- Oppong Boakye, G., et al., Microstructural Properties and Wear Resistance of Fe-Cr-Co-Ni-Mo-Based High Entropy Alloy Coatings Deposited with Different Coating Techniques. Applied Sciences, 2022. 12(6): p. 3156. [CrossRef]
- Joshi, S.V. and G. Sivakumar, Hybrid Processing with Powders and Solutions: A Novel Approach to Deposit Composite Coatings. Journal of Thermal Spray Technology, 2015. 24(7): p. 1166-1186. [CrossRef]
- Zhang, F., et al., Feasibility Study of High-Velocity Oxy-fuel (HVOF) Sprayed Cermet and Alloy Coatings for Geothermal Applications. Journal of Thermal Spray Technology, 2023. 32(2-3): p. 339-351. [CrossRef]
- Mittal, G., et al., Development of Suspension-Based Plasma and HVOF Spray TiO2 Coatings, in Thermal Spray 2021: Proceedings from the International Thermal Spray Conference. 2021. p. 489-492.
- Bolelli, G., et al., Tribology of NiCrAlY+Al2O3 composite coatings by plasma spraying with hybrid feeding of dry powder+suspension. Wear, 2015. 344-345: p. 69-85.
- Murray, J.W., et al., Microstructure and wear behaviour of powder and suspension hybrid Al2O3–YSZ coatings. Ceramics International, 2018. 44(7): p. 8498-8504. [CrossRef]
- Gopal, V., et al., Performance of Hybrid Powder-Suspension Axial Plasma Sprayed Al(2)O(3)-YSZ Coatings in Bovine Serum Solution. Materials (Basel), 2019. 12(12).
- Kiilakoski, J., et al., Characterization of Powder-Precursor HVOF-Sprayed Al2O3-YSZ/ZrO2 Coatings. Journal of Thermal Spray Technology, 2018. 28(1-2): p. 98-107. [CrossRef]
- Buzaianu, A., et al., Structural Properties Ni20Cr10Al2Y Coatings for Geothermal Conditions, in The 2nd International Research Conference on Sustainable Energy, Engineering, Materials and Environment. 2018.
- Zhang, F., et al., Feasibility Study of High-Velocity Oxy-fuel (HVOF) Sprayed Cermet and Alloy Coatings for Geothermal Applications. Journal of Thermal Spray Technology, 2023. 32(2-3): p. 339-351. [CrossRef]
- Kern, W. and K.K. Schuegraf, Deposition technologies and applications: Introduction and overview, in Handbook of Thin Film Deposition Processes and Techniques. 2001, Elsevier. p. 11-43.
- Manawi, Y.M., et al., A Review of Carbon Nanomaterials' Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials (Basel), 2018. 11(5). [CrossRef]
- Kafizas, A., C.J. Carmalt, and I.P. Parkin, CVD and precursor chemistry of transition metal nitrides. Coordination Chemistry Reviews, 2013. 257(13-14): p. 2073-2119. [CrossRef]
- Preston, D.J., et al., Scalable graphene coatings for enhanced condensation heat transfer. Nano Lett, 2015. 15(5): p. 2902-9. [CrossRef]
- Mishra, R. and R.S. Ningthoujam, High-Temperature Ceramics, in Materials Under Extreme Conditions. 2017. p. 377-409.
- Li, Z. and K. Aik Khor, Preparation and Properties of Coatings and Thin Films on Metal Implants, in Encyclopedia of Biomedical Engineering. 2019. p. 203-212.
- Ali, N., et al., Deposition of Stainless Steel Thin Films: An Electron Beam Physical Vapour Deposition Approach. Materials (Basel), 2019. 12(4). [CrossRef]
- Arunkumar, P., et al., Deposition rate dependent phase/mechanical property evolution in zirconia and ceria-zirconia thin film by EB-PVD technique. Journal of Alloys and Compounds, 2018. 765: p. 418-427. [CrossRef]
- Makhlouf, A., Current and advanced coating technologies for industrial applications, in Nanocoatings and ultra-thin films. 2011, Elsevier. p. 3-23.
- Ali, N., J.A. Teixeira, and A. Addali, Effect of Water Temperature, pH Value, and Film Thickness on the Wettability Behaviour of Copper Surfaces Coated with Copper Using EB-PVD Technique. Journal of Nano Research, 2019. 60: p. 124-141. [CrossRef]
- Park, H., K.Y. Kim, and W. Choi, Photoelectrochemical approach for metal corrosion prevention using a semiconductor photoanode. The Journal of Physical Chemistry B, 2002. 106(18): p. 4775-4781. [CrossRef]
- Liu, Y., C. Xu, and Z. Feng, Characteristics and anticorrosion performance of Fe-doped TiO 2 films by liquid phase deposition method. Applied Surface Science, 2014. 314: p. 392-399. [CrossRef]
- Deki, S., et al., Titanium (IV) oxide thin films prepared from aqueous solution. Chemistry Letters, 1996. 25(6): p. 433-434. [CrossRef]
- Fujita, R., et al., Corrosion resistant TiO2 film formed on magnesium by liquid phase deposition treatment. Electrochimica Acta, 2011. 56(20): p. 7180-7188. [CrossRef]
- Lee, M.-K., et al., Fluorine passivation of titanium oxide films on ITO/glass grown by liquid phase deposition for electrochroism. Journal of the Electrochemical Society, 2011. 158(8): p. D511. [CrossRef]
- Arnelli, A., R.A.Y.S. Rahayu, and Y. Astuti, Synthesis, Characterization, and Antibacterial Activity Test of Geothermal Silica/AgNO3 Thin Film. Molekul, 2023. 18(2). [CrossRef]
- Gutiérrez-Tauste, D., et al., Alternative fluoride scavengers to produce TiO 2 films by the liquid phase deposition (LPD) technique. Journal of Materials Chemistry, 2006. 16(23): p. 2249-2255. [CrossRef]
- Yu, J., et al., Preparation, characterization and photocatalytic activity of in situ Fe-doped TiO2 thin films. Thin Solid Films, 2006. 496(2): p. 273-280.
- Tu, Y.-F., et al., Preparation of Fe-doped TiO2 nanotube arrays and their photocatalytic activities under visible light. Materials Research Bulletin, 2010. 45(2): p. 224-229. [CrossRef]
- Song, J., et al., Antifouling and anticorrosion behaviors of modified heat transfer surfaces with coatings in simulated hot-dry-rock geothermal water. Applied Thermal Engineering, 2018. 132: p. 740-759. [CrossRef]
- Valiulis, A.V. and P. Silickas, Liquid phase deposition methods monitoring techniques influence for solid substrates and thin metal oxide films properties. Journal of Achievements in materials and manufacturing engineering, 2007. 24(1): p. 188-192.
- Zhang, F., M. Liu, and W. Zhou, Inhibition of Fouling With Titania and Silica Coatings on Plate Heat Exchanger in 80℃ Simulated Geothermal Water. Heat Exchanger Fouling and Cleaning, 2017. 8: p. 183-190.
- Aristia, G., et al., Short-term exposure tests of ɣ-Al2O3 Sol-gel coating on X20Cr13 in artificial geothermal waters with different pH. Geothermics, 2021. 96. [CrossRef]
- Li, W., et al., Physical and optical properties of sol-gel nano-silver doped silica film on glass substrate as a function of heat-treatment temperature. Journal of applied physics, 2003. 93(12): p. 9553-9561. [CrossRef]
- Schulz, W., et al., Corrosion of uncoated and alumina coated steel X20CrMoV12-1 in H2O–CO2–O2 and air at 600°C. Corrosion Science, 2013. 68: p. 44-50.
- Nofz, M., et al., Microstructural origin of time-dependent changes in alumina sol–gel-coated Inconel 718 exposed to NaCl solution. Journal of Sol-Gel Science and Technology, 2015. 75(1): p. 6-16. [CrossRef]
- Muench, F., Electroless plating of metal nanomaterials. ChemElectroChem, 2021. 8(16): p. 2993-3012. [CrossRef]
- Loto, C., Electroless nickel plating–a review. 2016, Springer.
- Zhang, H., et al., Review on electroless plating Ni–P coatings for improving surface performance of steel. Surface Review and Letters, 2014. 21(04): p. 1430002. [CrossRef]
- Ren, L., et al., Study on heat transfer performance and anti-fouling mechanism of ternary Ni-WP coating. Applied Sciences, 2020. 10(11): p. 3905.
- Sharma, A., C.-S. Cheon, and J.P. Jung, Recent progress in electroless plating of copper. J. Microelectron. Packag. Soc, 2016. 23(4): p. 1-6. [CrossRef]
- Boakye, G.O., et al., The effect of polytetrafluoroethylene (PTFE) particles on microstructural and tribological properties of electroless Ni-P+ PTFE duplex coatings developed for geothermal applications. Coatings, 2021. 11(6): p. 670. [CrossRef]
- Cheng, Y., et al., Effect of the microstructure on the anti-fouling property of the electroless Ni–P coating. Materials letters, 2008. 62(27): p. 4283-4285. [CrossRef]
- Cheng, Y., et al., Effect of copper addition on the properties of electroless Ni-Cu-P coating on heat transfer surface. The International Journal of Advanced Manufacturing Technology, 2015. 76: p. 2209-2215. [CrossRef]
- Cheng, Y., et al., Experimental study on the anti-fouling effects of Ni–Cu–P-PTFE deposit surface of heat exchangers. Applied thermal engineering, 2014. 68(1-2): p. 20-25. [CrossRef]
- Krishnan, K.H., et al., An overall aspect of electroless Ni-P depositions—A review article. Metallurgical and materials transactions A, 2006. 37: p. 1917-1926. [CrossRef]
- Rozmus-Górnikowska, M., M. Blicharski, and J. Kusiński, Influence of weld overlaying methods on microstructure and chemical composition of Inconel 625 boiler pipe coatings. Kovove Materialy, 2014. 52(3): p. 1-7. [CrossRef]
- Rao, N.V., G.M. Reddy, and S. Nagarjuna, Weld overlay cladding of high strength low alloy steel with austenitic stainless steel–structure and properties. Materials & Design, 2011. 32(4): p. 2496-2506. [CrossRef]
- Volpi, A. and G. Serra. Weld overlay of highly corrosion resistant nickel chromium molybdenum alloys, UNS N06059, on low alloy equipment operating at high temperature. in Pressure Technology. 2018. American Society of Mechanical Engineers.
- Saito, S. Technologies for High Performance and Reliability of Geothermal Power Plant. in Proceedings World Geothermal Congress. 2010.
- Tayactac, R.G. and E.B. Ang. A Review of Corrosion Resistance Alloy Weld Overlay Cladding Material for Geothermal Applications. in Materials Science Forum. 2021. Trans Tech Publ. [CrossRef]
- Yoon, S.Y., S.-E. Choi, and J.S. Lee, Liquid Phase Deposition of Silica on the Hexagonally Close-Packed Monolayer of Silica Spheres. Journal of Nanomaterials, 2013. 2013: p. 1-7. [CrossRef]
- Kishimoto, H., et al., Photocatalytic activity of titanium oxide prepared by liquid phase deposition (LPD). Journal of Materials Chemistry, 1998. 8(9): p. 2019-2024. [CrossRef]
- Lu, P. and B. Ding, Nano-modification of textile surfaces using layer-by-layer deposition methods, in Surface Modification of Textiles. 2009. p. 214-237.
- Danks, A.E., S.R. Hall, and Z. Schnepp, The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Materials Horizons, 2016. 3(2): p. 91-112.
- Bokov, D., et al., Nanomaterial by Sol-Gel Method: Synthesis and Application. Advances in Materials Science and Engineering, 2021. 2021: p. 1-21. [CrossRef]
- Modan, E.M. and A.G. PlĂIaȘU, Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. The Annals of “Dunarea de Jos” University of Galati. Fascicle IX, Metallurgy and Materials Science, 2020. 43(1): p. 53-60. [CrossRef]
- Cheng, K., et al. Fine-tuned polymer Nano-composite coatings for use in geothermal plants. in Smart Materials, Adaptive Structures and Intelligent Systems. 2011.
- Sugama, T., Polyphenylenesulphide-sealed Ni–Al coatings for protecting steel from corrosion and oxidation in geothermal environments. Journal of materials science, 1998. 33: p. 3791-3803. [CrossRef]
- Sugama, T., Interfaces between geothermal brine-induced scales and SiC-filled polymer linings. Geothermics, 1998. 27(4): p. 387-400.
- Sugama, T., Antioxidants for retarding hydrothermal oxidation of polyphenylenesulphide coatings in geothermal environments. Materials Letters, 2000. 43(4): p. 185-191.
- Sugama, T. and K. Gawlik, Carbon fibre-reinforced poly (phenylenesulphide) composite coatings. Polymers and Polymer Composites, 2001. 9(6): p. 377-384. [CrossRef]
- Sugama, T., Polyphenylenesulphide/montomorillonite clay nanocomposite coatings: Their efficacy in protecting steel against corrosion. Materials Letters, 2006. 60(21-22): p. 2700-2706.
- Cai, Y., et al., Anticorrosion and scale behaviors of nanostructured ZrO2–TiO2 coatings in simulated geothermal water. Industrial & Engineering Chemistry Research, 2016. 55(44): p. 11480-11494.
- Wang, G., et al., Zinc-graphite composite coating for anti-fouling application. Materials Letters, 2011. 65(19-20): p. 3095-3097. [CrossRef]
- Sugama, T. and K. Gawlik, Anti-silica fouling coatings in geothermal environments. Materials Letters, 2002. 57(3): p. 666-673. [CrossRef]
- Sugama, T., D. Elling, and K. Gawlik, Poly (phenylenesulphide)-based coatings for carbon steel heat exchanger tubes in geothermal environments. Journal of materials science, 2002. 37: p. 4871-4880.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).