1. Introduction
The use of conventional low molecular weight biocides for the treatment of premises with high sanitary standards has a number of significant disadvantages, which can be accompanied by economic costs, as well as potential danger to humans [1-11]. First of all, this is the low durability of the resulting coatings. Generally speaking, solutions of low molecular weight biocides, with which walls are treated, have low adhesion to the surface, and therefore such treatment must be carried out more often. Also, one of the problems is the evolution of bacterial cultures, the emergence of new strains and the growth of resistance of bacterial microorganisms to disinfectants. [12- 14]. As a result, there is a possibility that absolute sterilization will not be achieved when treated with a low molecular weight biocide, which can lead to serious poisoning. In this regard, there is an urgent need to search for new systems that would combine properties such as increased biocide and durability.
Polymers are often considered as a material with the ability to form durable coatings [15- 17]. Biocidal compositions based on water-soluble positively charged interpolyelectrolyte complexes (IPEC) are of potential interest for practical use. Such complexes, firstly, contain a large number of positively charged groups, due to which the polycations in the complex impart biocidal properties. Also, charged areas can impart good adhesion to hydrophilic surfaces, such as glass. [18, 19]. In addition, positively charged water-soluble IPECs will contain charge-compensated regions, which will improve adhesion on hydrophobic surfaces. However, it is known that, in general, polycations have lower biocidal activity compared to low molecular weight bioactive compounds [
20]. In this regard, it is proposed to modify IPEC by including an additional low-molecular-weight biocidal agent, for example, metal nano particles or metal oxides. Among the metals, nano-Ag, nano-Au, nano-Cu, nano-Se are often considered as an additive [21, 22]. Silver, as well as its oxide, have proven themselves especially widely.
The bactericidal properties of silver and its derivatives have been repeatedly discussed in the literature and have found application in the creation of wound dressings, plasters, bandages or as an antibacterial and anticancer agents etc. [23- 26]. However, most studies where nanoparticles are included in compositions as an antibacterial agent consider the use of an inert polymer matrix. [24, 27, 28]. Therefore, biocidal compositions based on silver (I) oxide nanoparticles and interpolyelectrolyte complexes as a stabilizer are of potential interest for practical use, since such a polymer matrix contains both areas with free charged groups and hydrophobic ones areas to improve adhesion to hydrophobic surfaces. Moreover, the use of silver (I) oxide in this case is explained by the availability and simplicity of its chemical preparation, as well as the absence of interaction with the polyanion, which makes it possible to achieve a uniform distribution of the resulting nanoparticles in the volume of the material.
Previously, we have already studied the formation and properties of water-soluble IPECs based on pH-independent polydiallyldimethylammonium chloride (PDADMAC), the biocidal properties of which are widely known [29, 30], and sodium polystyrene sulfonate (PSS) [18, 31]. The partial neutralization of PDADMAC did not reasonably effect the charge of the resulted IPEC. The obtained IPEC has shown excellent resistance towards dissociation and phase separation in water-salt media formed by various salts. It means that such complexes have been shown to be stable in solutions of high ionic strength, which may allow modification with low molecular weight biocides that provide ions in solution. It was shown that a coating formed by the individual polycation could be easily removed by wash-off with water, but the the modification of PDADMAC with PSS results in formation of the coating with good resistance to wash-off. The gradual washing off of coatings from the complexes from the surface will allow the biocidal activity of such a coating to be preserved for a long time - with each cycle of washing off the IPEC layer, the biocidal layer will be regenerated [
19].
In this work, IPEC was modified by including a low molecular weight biocide - Ag2O nanoparticles. The formation of coatings from a ternary IPEC/Ag2O composite on a glass surface was studied, and the mechanical and biocidal properties of the resulting coatings were also studied.
2. Materials and Methods
2.1. Materials
Silver oxide Ag2O was purchased from SilverSalt (St. Petersburg, Russia), poly-(diallyldimethylammonium) chloride (PDADMAC) with weight average mass Mw 450 000 and poly-(styrenesulfonate) sodium salt (PSS) were purchased from Sigma-Aldrich (USA). All chemicals were used without additional purification. The bidistilled water was used in all experiments.
Borosilicate glass microspheres (BSM) with average diameter 5 µm from Duke Scientific (USA), glass slides (GS) and glass cover slips from ThermoFisher (USA) were used as test glass surfaces.
IPEC Preparation
The formation of IPEC was performed by the addition of the 9.6 mL of 2wt.% PDADMAC solution in bidistilled water to 86.4 mL of 0.034 wt.% of PSS solution under stirring conditions to provide homogenous distribution of the macromolecules. The homogeneity of the solution was controlled by turbidimetry [
32]. As a result, a complex with a molar ratio of monomer units [PSS]/ [PDADMAC] = 0.12 was obtained. After this, the complex was concentrated on a vacuum rotary concentrator to 2 wt.% solution.
IPEC/Ag2O Preparation
The 0.4 mg of Ag2O powder was added to 2 mL of 2 wt.% of IPEC. The obtained inhomogeneous system separated into Ag2O precipitate and IPEC solution. Then the composition was homogenized in a sonic bath with power 500 W for 10 min. The resulted turbid dispersion did not contain eye-visible micro- and macroparticles of silver oxide.
PDADMAC and IPECs Coatings Preparation
Coatings from the polycation and its complexes were obtained using the following method. The pre-cleaned glass substrate was dipped into a 2 wt% polymer solution, kept for 2 minutes, and then washed with bidistilled water for 2 minutes to remove excess polymer.
Polymer coatings for microbiological studies were prepared in the following way. A 20 mg/mL aliquot of the polymer solution was applied to the substrate so that the entire surface was covered with the solution, then the substrate was air dried.
BSM covering with PDADMAC and IPECs procedure
2 mg of borosilicate glass microspheres were suspended in 1 ml of methanol and then immediately centrifuged. Next, the BSM were resuspended in 1 ml of 1 M KOH and were precipitated again in a centrifuge. After this, the BSM were washed 5 times with distilled water.
Polymer-coated microspheres were prepared as described below. A suspension of 0.5 mg/mL BSM and 0.0675 wt.% PDADMAC was vortexed for 5 minutes. After that, the polymer-coated BSMs were rinsed 2 times with distilled water to remove residual unbound polymer. Microspheres coated with IPEC were obtained using the method described above.
2.2. Methods
H The hydrodynamic diameters were measured by dynamic light-scattering (DLS) using Brookhaven ZetaPlus (Brookhaven, USA) equipment with software provided by manufacturer.
The electrophoretic mobility (EPM) of liposomes and their complexes was controlled by Brookhaven ZetaPlus (Brookhaven, USA) equipment with software provided by manufacturer.
Transmission electron microscopy (TEM) imaging was performed with JEM-2100 200 kV electron microscope (JEOL Ltd., Tokyo, Japan). Images were acquired with a US1000XP CCD camera (Gatan, Pleasanton, USA) at near-zero defocus. The samples were prepared by applying a drop of an aqueous solution of the substance to a copper grid covered with type B carbon support film (EMCN, Beijing, China), followed by further drying in an air atmosphere. The samples were examined without preliminary contrasting. The series of images was analyzed using ImageJ software.
Gram-negative
Escherichia coli K12 (
E. coli K12) strain transformed with “pDualrep2” reporter plasmid [
20] was used to study the antimicrobial activity of polymer coatings. The plate with a lawn of
E. coli K12 cells was treated by the glass substrate covered with PDADMAC, IPEC or IPEC/Ag
2O. In this experiment Tryptic Soy Broth (TSB) was used which is favorable for biofilm formation. The plates were incubated at 37°C for 18 hours. Then the plates were scanned by ChemiDoc (Bio-Rad, Hercules, CA, USA).
The estimation of minimal inhibitory concentration (MIC) values was carried out for the Gram-negative Escherichia coli K12 strain in TSB medium. The MICs in TSB medium were determined using a serial dilution assay [
33]. The cell concentration was adjusted to approximately 5 × 10
5 cells/mL. PDADMAC, IPEC, and IPEC/Ag
2O solutions with an initial concentration of 20 mg/mL were used as the test compound. Two-fold serial dilution of the polymers solutions was done in a 96-well microplate (100 L per well). The microplates were covered and incubated at 37 °C with shaking. The OD600 of each well was measured, and the MIC was assigned as the lowest concentration of the tested compound that resulted in no growth after 16–20 h. Bacterial cell growth was measured at 590 nm using a microplate reader (VICTOR X5 Light Plate Reader, PerkinElmer, Waltham, MA, USA).
The surface of a clean glass substrate, as well as polymer coatings, was characterized by determining the contact angle using TRACKERTM Standart Drop Tensiometer (Teclis Scientific, Lyon, France).
Mechanical tests of the coatings
The dynamometric measurements were performed according to the procedure described elsewhere [
34]. The 2 wt.% IPEC solution was applied on the freshly cleaned GS so that the entire surface was covered with the solution. Two minutes later, the IPEC solution was removed and the resulted GS with modified surface was washed with bidistilled water. Another GS was placed upon the resulted coating so that the “sandwich” structure GS-IPEC-GS was formed. After 24 h of drying, the adhesive properties of the IPEC were studied by dynamometry using a tensile testing machine by Metrotest (Moscow, Russia). The values of the stress required to separate the “sandwich” structure were collected.
Optical tweezers measurements
The laser tweezers setup was described elsewhere [
35]. The method of optical tweezers was used to study the adhesion properties of the polymer complex at the microscopic level. The microscale process visualization in the optical trap was carried out using a high-aperture objective lens and a SMOP camera. The position of the traps was controlled by a movable lens and a servo-driven coordinate stage. The interaction of PDADMAC or IPEC with the glass surface was studied by the determination of forces needed to break the polymer-to-surface interfacial complex.
Experiments were carried out in the following way. To make a chamber with a studied suspension of polycationic brushes with BSMs, two coverslips were taken. The coverslips were treated with surface-active substance, then carefully washed with water and treated with hydrogen peroxide. After the coverslips dried, the surface of one of them was covered with the layer of albumin to prevent the adhesion of the particles in suspension to the surface of the coverslip. Further, 45 μl of aqueous suspension of the studied sample were placed in the gap between two coverslips. Prepared chamber was placed on an objective table. Two optical traps were formed within the sample. Complex of PDADMAC or IPEC with the BSM was captured by traps. Then, the distance between the centers of the traps was increasing, creating an external force to break this complex. The maximum optical force value of the trap corresponding to power, at which the break took place.
This force was detected using the force calibration based on the drag force method [
36]. Laser tweezers allow not only to capture various microobjects and manipulate their position in space, but also to carry out quantitative measurements of the forces that act on these microobjects. For this purpose, the calibration of the experimental setup of optical tweezers was made by the following means of viscous friction. As is known, a viscous friction force acting on the spherical particle with radius
a is proportional to the particle’s speed
u and determined by the Stokes law by the relation:
where the parameter
η is the dynamic viscosity of the medium. In order to calibrate the force of the optical tweezers with this force F
vis, a microobject with the determined dimensions was taken and placed in a medium whose dynamic viscosity was also known. Then, an optical trap was formed at the location of this microobject. As soon as the microparticle is trapped, the latter begins to move at a constant speed in the same plane in which the capture took place. The last action was repeated until there was such a value for the speed of the trap at which, at high speeds, microobjects would fly out of the waist of the laser trap. For this speed value, the following condition takes place:
In other words, the force of viscous friction will be equal to the maximum force of optical capture of the microobject with laser tweezers for the selected radiation power in the waist.
An attempt to break the complex is made at the different maximum optical trapping forces (Ftrapmax). By incrementally increasing the laser power, a force that allows the complex to be separated is achieved. Since the viscous friction force is known, using the calibration described above, the binding force of the complex can be determined. In the statistical analyses, the average results of at least five experiments are presented as mean values.
3. Results and Discussion
3.1. IPEC/Ag2O complexes characterization
Interaction of nanoparticles with polyelectrolytes could induce formation of gel structures where nanoparticles act as crosslinkers of the network or nanoparticles could be distributed within macromolecules coils [35, 37, 38]. That is why at the first step we have investigated distribution of the Ag2O particles in the dispersion of IPEC/Ag2O using DLS and TEM measurements. The mean diameter of the complexes determined by DLS was found to be 260 nm. This value corresponded to the size of the bare IPEC without Ag2O inclusions. So, no aggregation of the IPECs induced by formation of bounds with Ag2O particles was detected and ternary complexes of PDADMAC/PSS/Ag2O could be considered as individual IPECs with incorporated nanoparticles of Ag2O.
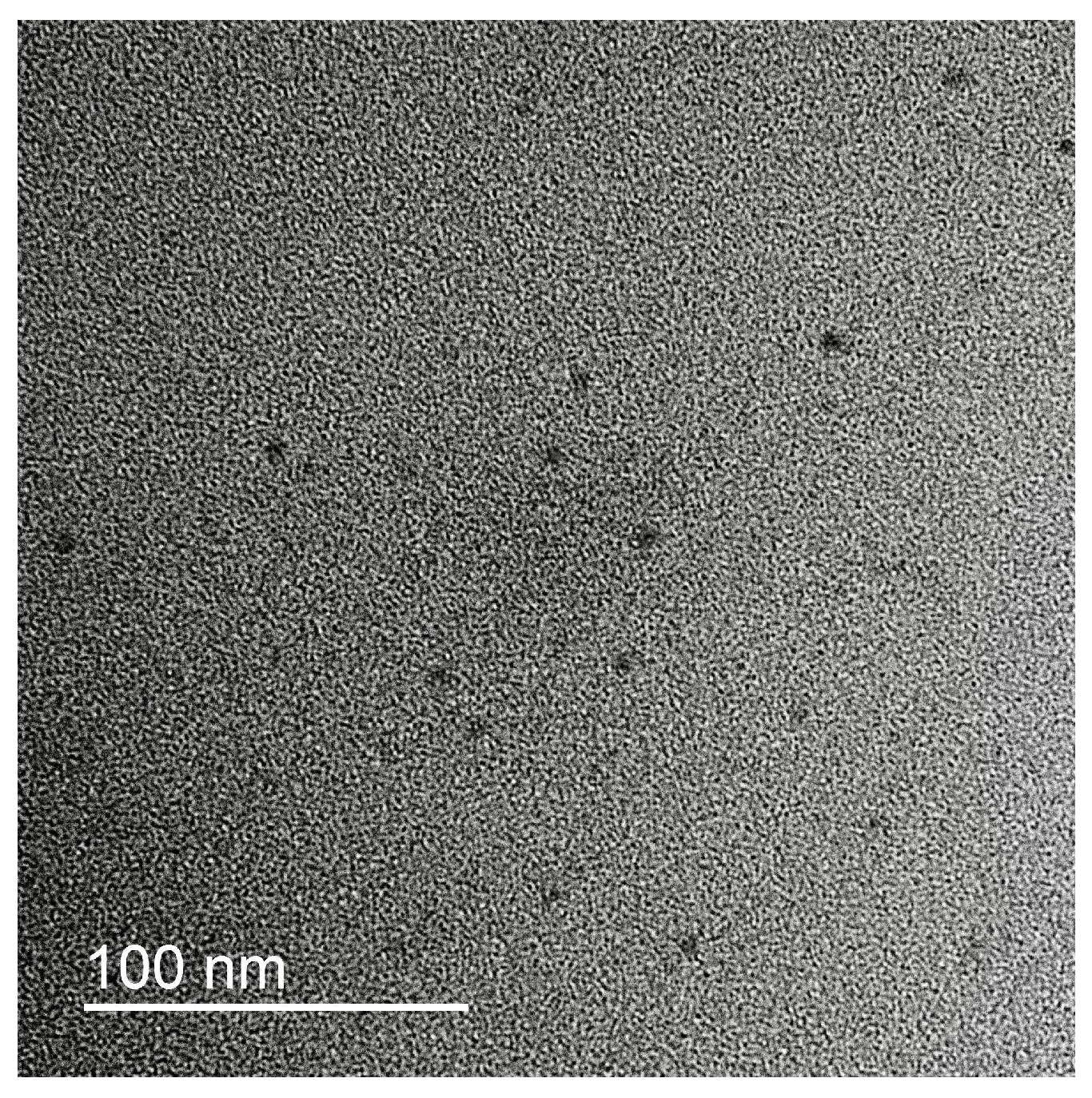
In order to analyze the particle size of silver oxide particles in the complex and their intra-complex distribution, the TEM images were obtained. Typical microphotograph is presented on the
Figure 1. Individual contrast Ag
2O nanoparticles with mean diameter 5.5+/-0.5 nm could be detected on the image. Amorphous chains of the polyelectrolytes in the IPEC could not be detected without additional contrast.
3.2. Biocidal properties of IPEC/Ag2O
The antibacterial properties of IPEC/Ag
2O were investigated on agar plate with TSB with a lawn of Gram-negative E. coli K12 strain. The cover slips with identical shape and area coated with PDADMAC, IPEC and IPEC/Ag
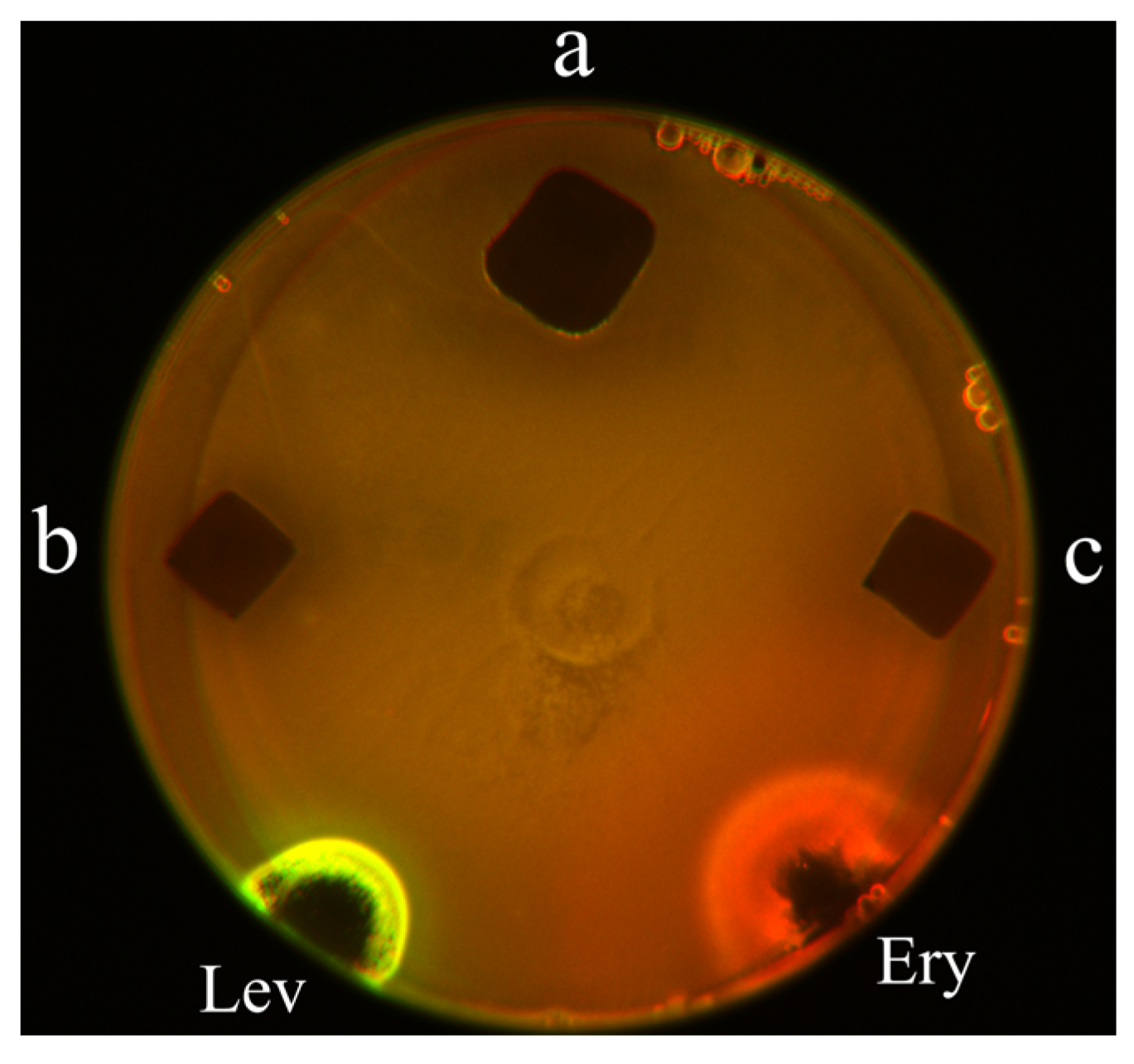
2O were deposited on the test surface in Petri dish so that the polymeric layer was in direct contact with the bacterias. In
Figure 2, the areas of inhibition zones for PDADMAC (a), IPEC (b) and IPEC/Ag
2O (c) are presented. All samples have demonstrated antibacterial activity - dark areas reflect death of E.coli. The slightly larger area for PDADMAC sample could be attributed to the more effective migration of polycationic macromolecules from the coating in agar in comparison to migration from IPEC and IPEC/Ag
2O coatings. The differences in diffusion of polymer chains are due to hydrophobization of the IPECs by PSS molecules and thus the coatings on the glass are more stable regarding diffusion of PDADMAC and PSS molecules from the coating layer to agar. This result is in good agreement with the data upon the resistance of the polymer coatings from pure polycation and IPECs towards wash-off with water [
18].
In addition, MICs were estimated using Gram-negative E. coli K-12 in TSB media (
Table 1). While PDADMAC and IPEC have demonstrated similar biocidal activity the incorporation of Ag
2O nanoparticles into IPEC resulted in 4-times decrease of MIC from 0.4 mg/mL to 0.1 mg/mL reflecting increase of antibacterial activity of the composition. It seems that modification of 12 mol.% of cationic units with PSS does not dramatically change the antibacterial activity of PDADMAC. According to the structure of the interpolyelectrolyte complexes discussed in literature the IPECs formed at specific molar ratio of polycations and polyanions do not form the complex where all charged units of the polyelectrolyte that is added in deficiency to opposite charged polyelectrolyte form salt bonds. In fact due to non-complementary structure of polymers the number of salt bonds is smaller than could be direct calculated from molar ratio of anionic to cationic units [
39]. Thus the real number of unbound PDADMAC units that ensure biocidal action is more than 90%.
3.3. Shelf-life of IPEC/Ag2O complexes
Storage of the dispersion during several days resulted in precipitation of IPEC/Ag
2O complexes. So, the questions about colloid stability, possible sediment aging or evolution of complexes during storage arise. First, the colloid stability of the freshly prepared dispersion was studied during 12 hours by means of DLS. No change in size of the complexes was detected. Then the sample after 14 days of shelf life was analyzed. The incubated After two weeks of incubation at room temperature the formation of the sediment of IPEC/Ag
2O was observed.Vigorous shaking of this sediment resulted in redistribution of the IPEC/Ag
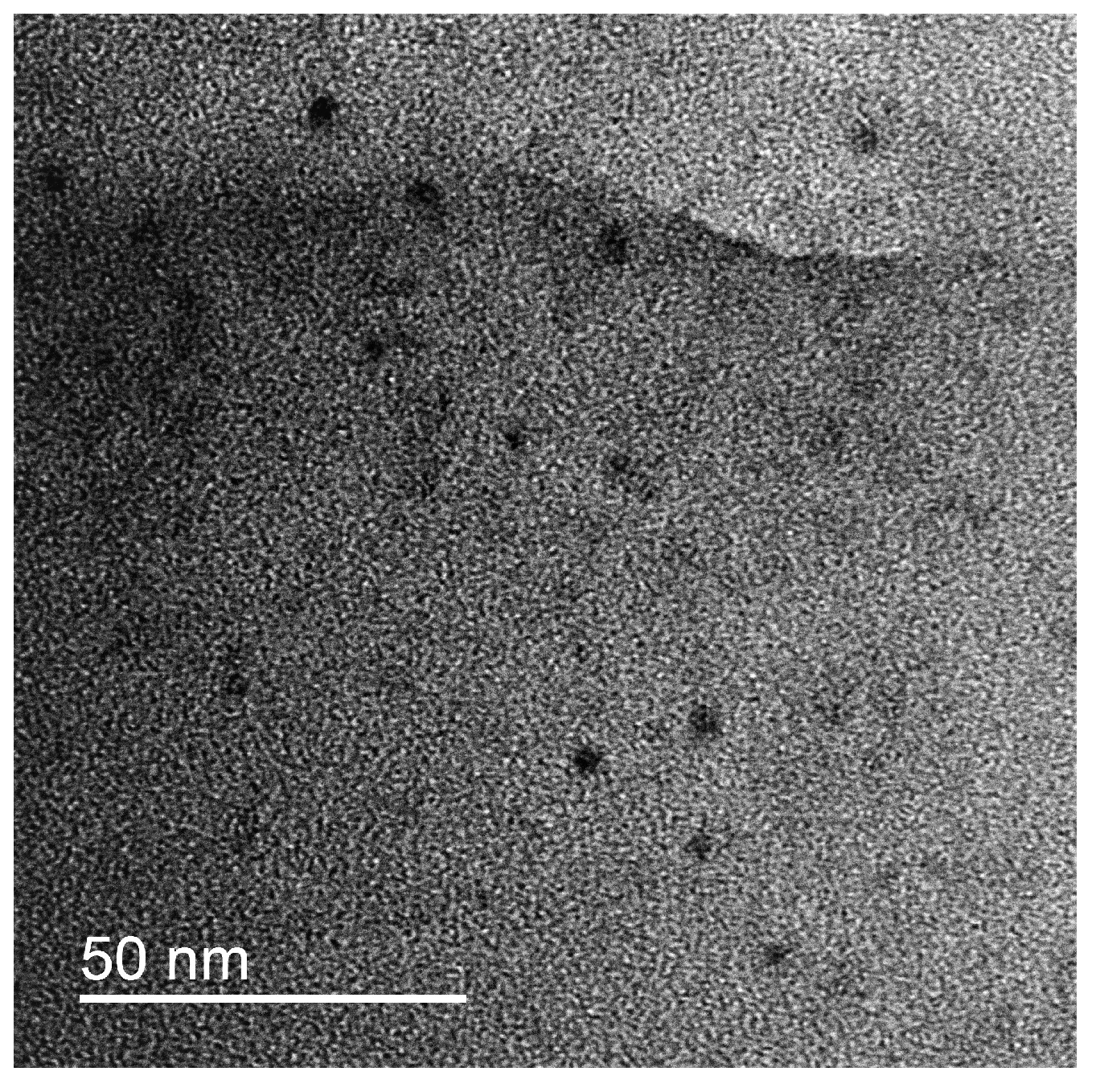
2O complexes and formation of uniform turbid dispersion similar to the freshly prepared one. The redispersed IPEC/Ag2O complexes were investigated using DLS and TEM. The mean diameter of the complexes in the water dispersion was found to remain 260 nm. The TEM microphotograph of the complex is presented on
Figure 3. The average size of the individual Ag
2O nanoparticles was found to be 6.0+/-5 nm. So, no evolution of nanoparticles or complexes took place during storage.
Therefore, we can conclude that the sedimentation of IPEC/Ag2O complexes is due not to the aggregation of particles, but to the action of gravitational forces. As well, distribution of the nanoparticles in IPEC matrix remains uniform and no aggregation or change of the morphology of nanoparticles takes place.
3.4. Formation of polymer coatings on glass surface
The formation of adsorption layers of PDADMAC, IPEC and IPEC/Ag2O on glass surface was studied by measuring of EPM of suspension of BSM with added polymer solutions.
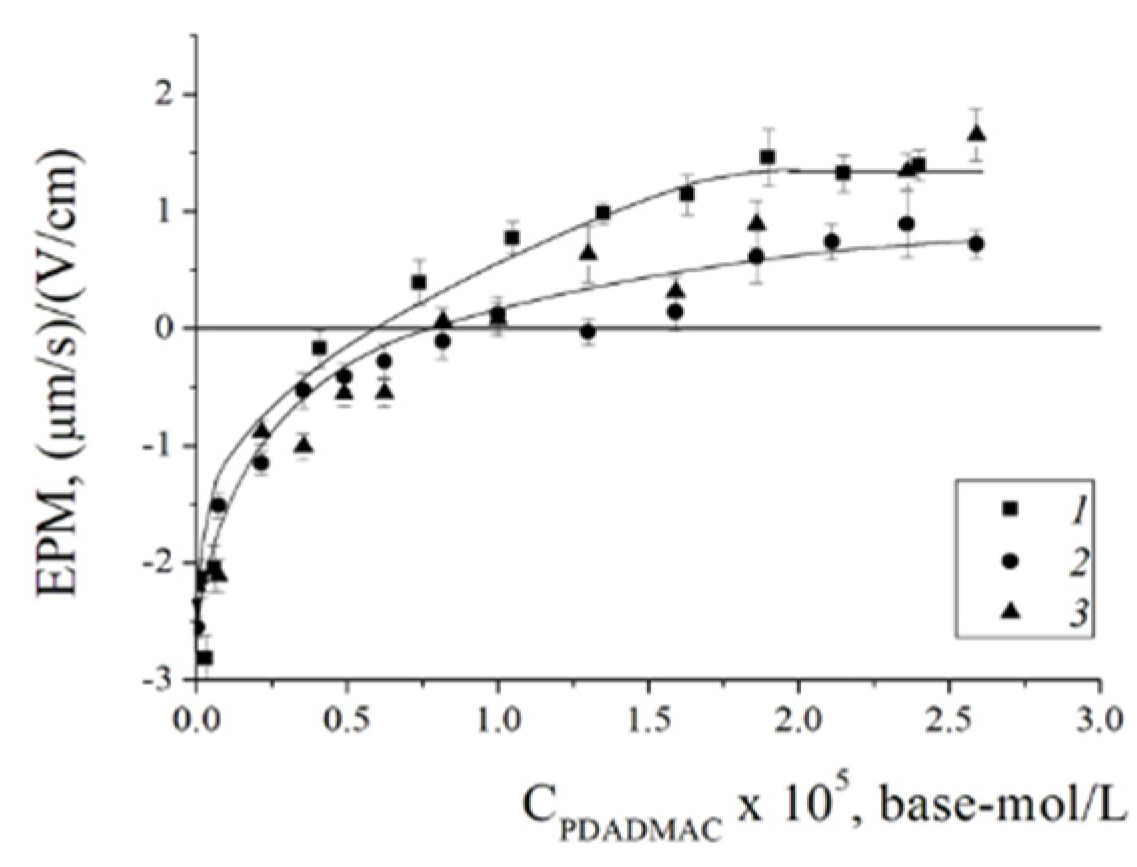
The titration curve is presented in
Figure 4. The addition of PDADMAC to the suspension of BSM resulted in an increase in the EPM values from -3 (mm/s)/(V/cm) to +1.3 (mm/s)/(V/cm) due to formation of the electrostatic complexes and charge compensation (Fig.4 curve 1). The point with an electroneutral EPM at PDADMAC concentration 6e-6 base-mol/L corresponds to complete surface charge neutralization. While reaching the positive charge plato of the titration curve reflects the termination of the PDADMAC adsorption due to electrostatic repulsion between polycation and recharged BSMs. Such behavior of the system is typical for the adsorption of polycations on anionic colloid particles [40-44]. Substitution of PDADMAC with IPEC resulted in shift of the titration curve to higher concentrations of PDADMAC required to neurtalize the surface charge of BSM- 7.5e-6 base-mol/L of PDADMAC in IPEC. This is due to fact that PSS neutralize 12% of charged units of PDADMAC that are responsible for binding with negatively charged BSM surface and its recharging. Also, it is important to stress that 12% shift of the neutralization point for IPEC in compare to pure PDADMAC reflects that all PSS molecules remained in adsorbed IPEC and were not eliminated from the complex by competitive interaction with anionic surface [45, 46]. The incorporation of Ag
2O nanoparticles into the complex did not lead to a change in the behavior of adsorption of the ternary IPEC/Ag
2O complex on BSM compared to the binary IPEC - curves 2 and 3 in Figure 5 coincide.
Thus, to study the adhesive properties of coatings further, we will consider only PDADMAC and binary IPECs.
3.5. Mechanical properties of IPEC/Ag2O coatings
The adhesion of the PDADMAC and IPEC coatings on the glass surface were studied with dynamometry. Cohesive forces of the coatings were studied by estimation of the peak stress values required to the break polymer “bridges” between two glass slides in lateral direction while adhesive forces of the coatings were estimated in perpendicular direction of the application of the stress. The values of the peak stress are presented in the
Table 2.
Lateral stress is caused by cohesive forces, while perpendicular stress is mainly caused by adhesive forces. It should be noted that adhesive forces with lower values of stress should be considered as determining the properties of coatings. Thus, it can be assumed that coatings formed from a polycation and an interpolyelectrolyte complex have similar adhesive and cohesive properties. The explanation could be as follows. IPEC has amphiphilic structure where charged units provide hydrophilic properties and areas of bound PSS and PDADMAC units are responsible for hydrophobic areas. Adsorption from the water media on the glass surface results in formation of the interfacial complex with cationic units that are in molar excess expanded to the water media forming loops and tails. IPEC has dynamic internal structure where macromolecules are capable to move along the opposite charged chains. Hydrophobic fragments of the IPEC are expected to be situated closer to the surface to minimize the surface tension. As a result the outer layer of the IPEC represents itself almost individual PDADMAC. Hence, the peak stress values obtained in dynamometry experiments could be attributed to break of the contacts between coating and upper glass slide that interact with top layer of the adsorbed PDADMAC and IPEC. However, these results demonstrate that formation of the IPEC, i.e. hydrophobization of the PDADMAC, does not result in loss of the adhesive properties of the forming coating.
In order to support the assumption above the contact angles of water on the coatings were studied. The freshly cleaned glass had contact angle 30°, formation of coating from PDADMAC resulted in slight hydrophilization of the surface- the contact angle was found to be 20°. The formation of coatings from either IPEC or IPEC/Ag2O did not resulted in change of contact angle in compare to PDADMAC coating reflecting that the top layers of the coatings have similar chemistry. Hence, PSS macromolecules do not present on the surface of the coatings otherwise the hydrophobization of the layer and the increase of the contact angle should be observed.
Therefore, in order to evaluate the real adhesion properties of interpolyelectrolyte complexes on a glass surface, it is necessary to use a method capable of recording the properties of individual macromolecules.
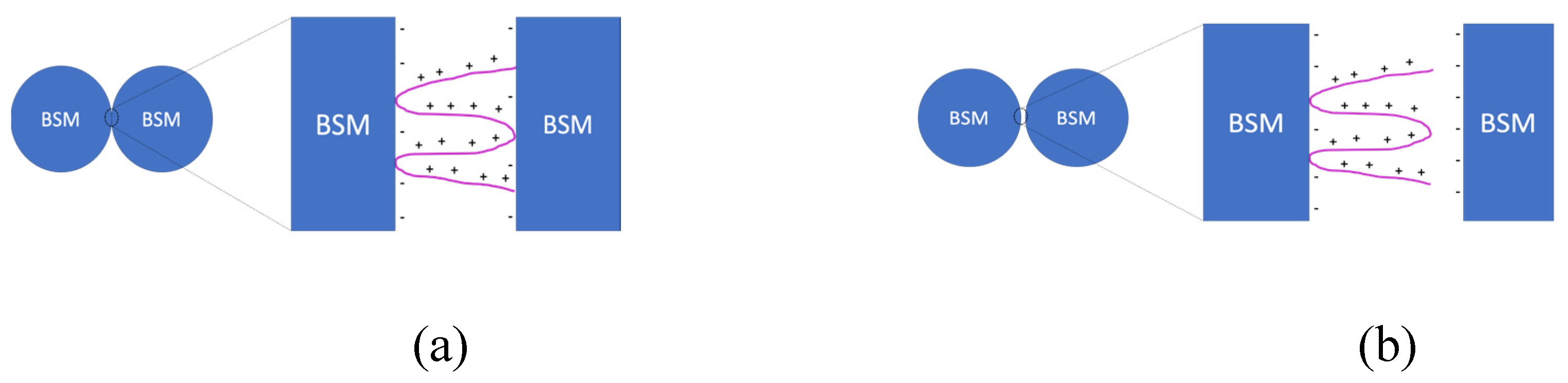
3.1. Mechanical properties of IPEC/Ag2O coatings on submicron-scale
The use of optical tweezers makes it possible to estimate the force required to detach the interpolyelectrolyte complex from the glass microsphere surface. For this purpose, some borosilicate microspheres coated with the polymer and the interpolyelectrolyte complex were obtained. The coated microspheres were brought into contact with unmodified microspheres using optical tweezers, resulting in a complex of two microspheres linked through the polymer/interpolyelectrolyte complex. Next, a force was applied to the resulting complex to separate two microspheres (Fdet), in other words, tearing the polymer off the surface of one of the microspheres. Thus, the resulting value characterizes the adhesive force of the polycation/interpolyelectrolyte complex. The experimental design is shown in Figure 5.
The optical tweezers study revealed that the force required for the detachment of two microspheres connected via PDADMAC “bridge” is (37±3) pN. This force value could be attributed to the characteristic of the adhesive force of the individual polycation macromolecule on the glass surface. Modification of the PDADMAC with PSS forming IPEC resulted in an increase of the force required to break the connection between the two BSMs connected via IPEC “bridge”. This value overcomes 118 pN - the limiting value of the experimental setup.
5. Conclusions
Since cationic polymers have already proven themselves to be effective antibacterial chemicals [30, 47-49], for which, unlike classical antibiotics, microorganisms do not develop resistance, the key problem for systems based on them remains the production of coatings with good adhesion to the surface being treated. Traditionally, in practice, the surfaces being treated are quite heterogeneous and may contain both hydrophilic and hydrophobic areas, which can arise as a result of contamination or damage to the original surfaces. Therefore, among polymer biocides, amphiphilic macromolecules are of the greatest interest, since they have an affinity for surfaces with different hydrophobicity. Interpolyelectrolyte complexes can be considered as a class of amphiphilic copolymers, where free charged groups form hydrophilic regions, and regions of salt bonds between the polycation and polyanion form hydrophobic blocks. The production of IPEC has an advantage over the production of traditional amphiphilic copolymers by directed synthesis, since it has greater variability of components and compositions, as well as the possibility of using mass-available commercial polymers. In this work, we focused on the study of a water-soluble interpolyelectrolyte complex based on PDADMAС and PSS, for which the ability to form effective coatings on both hydrophilic glass surfaces and hydrophobic polycarbonate surfaces was previously demonstrated [
18]. Adsorption of the IPEC on the glass surface is governed by electrostatic forces as well as adsorption of PDADMAC. The forming coatings contain free PDADMAC cationic chain fragments expanded to external to the treated surface layer ensuring biocidal activity. Investigation of adhesive and cohesive forces in the resulting revealed that the stability of the coating is determined primarily by adhesive interactions. At the macro level, it is not possible to reliably identify the role of IPEC formation on adhesion. On the other hand, the use of the optical tweezers method makes it possible to analyze macromolecules in submicron scale and to evaluate the multiple increase in adhesive forces when forming a coating from IPEC compared to coatings from PDADMAC.
The introduction of 1 wt.% silver oxide nanoparticles into IPEC by ultrasonic dispersion of Ag2O powder in solution of the IPEC resulted in formation stable system IPEC/Ag2O with uniformly distributed 5-6 nm in diameter nanoparticles in polymer matrix. Formation of such ternary complex made it possible to increase the antibacterial properties of the initial IPEC by 4 times against E. coli K12. At the same time, the introduced Ag2O nanoparticles did not affect the nature of adsorption of the complex on glass microspheres.
Thus, the formation of ternary IPEC/Ag2O complexes makes it possible to obtain coatings with increased antibacterial action and improved adhesive characteristics.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, AVS; methodology, VAP, NMS, AAF; validation, DAL, NMS, SAV; formal analysis, DAL, AAF, AVS.; investigation, VAP, OSP, VIM, AVM, NMS; resources, DAL, AAF, AVS; data curation, VAP; writing—original draft preparation, VAP and AVS; writing—review and editing, DAL, NMS.; visualization, AVM; supervision, AVS; project administration, AVS; funding acquisition, AVS All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the project “Modern Problems of Chemistry and Physical Chemistry of Macromolecular Compounds” (state budget no. АААА-А21-121011990022-4).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data available on request .
Acknowledgments
TEM studies were carried out at the Shared Research Facility “Electron microscopy in life sciences” at Moscow State University (Unique Equipment “Three-dimensional electron microscopy and spectroscopy”). Determination of contact angle was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract No. 075-03-2023-642). Biological assays were funded by the Russian Science Foundation (Grant No. 21-64-00006).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tarashi, S.; Siadat, S.D.; Fateh, A. Nontuberculous Mycobacterial Resistance to Antibiotics and Disinfectants: Challenges Still Ahead. Biomed Res Int. 2022, 2022, 8168750. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control. 2023, 12, 32. [Google Scholar] [CrossRef]
- Protano, C.; Cammalleri, V.; Romano Spica, V.; Valeriani, F.; Vitali, M. Hospital environment as a reservoir for cross transmission: cleaning and disinfection procedures. Ann. Ig. 2019, 31, 436–448. [Google Scholar]
- Slaughter, R.J.; Watts, M.; Vale, J.A.; Grieve, J.R., Schep. The clinical toxicology of sodium hypochlorite. Clin Toxicol. 2019, 57, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. North. Am. 2021, 35, 575–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, L.; Guo, A.; Zhang, X.; Liu, W.; Ruan, Y. Formation of Multispecies Biofilms and Their Resistance to Disinfectants in Food Processing Environments: A Review. J. Food Prot. 2021, 84, 2071–2083. [Google Scholar] [CrossRef]
- Mc Carlie, S.; Boucher, C.E.; Bragg, R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updat. 2020, 48, 100672. [Google Scholar] [CrossRef]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere. 2022, 308, 136404. [Google Scholar] [CrossRef]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to biocides used in food processing environments. Food Microbiol. 2021, 97, 103758. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Sickbert-Bennett, E.E.; Kanamori, H.; Anderson, D. Prevention Epicenters Program. Continuous room decontamination technologies. Am. J. Infect. Control. 2019, 47S, A72–A78. [Google Scholar] [CrossRef]
- Arzani, F.A.; Dos Santos, J.H.Z. Biocides and techniques for their encapsulation: a review. Soft Matter. 2022, 18, 5340–5358. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, J.M.; Fu, Yu.; Mei, Zh.; Schäffer, A.; Dou, Q.; Amelung, W.; Elsner, M.; Adu-Gyamfi, J.; Heng, L.; Virta, M.; Jiang, X.; Smidt, H.; Topp, E.; Wang, F. Antibiotic resistance genes in food production systems support One Health opinions. Curr. Opin. Environ. Sci. 2023, 34, 100492. [Google Scholar] [CrossRef]
- Ben, Yu.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, Ch. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review, Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review, Int. J. Environ. Res. Public Health, 2021, 18(4) 2014. 18(4).
- Pinho, A.C.; Piedade, A.P. Polymeric Coatings with Antimicrobial Activity: A Short Review. Polymers 2020, 12, 2469. [Google Scholar] [CrossRef] [PubMed]
- Weththimuni, M.L.; Chobba, M.B.; Sacchi, D.; Messaoud, M.; Licchelli, M. Durable Polymer Coatings: A Comparative Study of PDMS-Based Nanocomposites as Protective Coatings for Stone Materials. Chemistry 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Duan, J.; Wang, J. Bioinspired Durable Antibacterial and Antifouling Coatings Based on Borneol Fluorinated Polymers: Demonstrating Direct Evidence of Antiadhesion, ACS Appl. Mater. Interfaces 2021, 13(28), 33417–33426. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Senchikhin, I.N.; Bolshakova, A.V.; Sybachin, A.V. Modification of Polydiallyldimethylammonium Chloride with Sodium Polystyrenesulfonate Dramatically Changes the Resistance of Polymer-Based Coatings towards Wash-Off from Both Hydrophilic and Hydrophobic Surfaces. Polymers 2022, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Pigareva, V.A.; Bolshakova, A.V.; Marina, V.I.; Sybachin, A.V. Water-Soluble Interpolyelectrolyte Complex Based on Poly(diallyldimethylammonium chloride) and Sodium Polyacrylate as a Component for Creating Stable Biocidal Coatings, Colloid J. 2023, 85, 433–441.
- Pigareva, V.A.; Stepanova, D.A.; Bolshakova, A.V.; Marina, V.I.; Osterman, I.A.; Sybachin, A.V. Hyperbranched Kaustamin as an antibacterial for surface treatment, Mend. Comm. 2022, 32(4), 561–563. [Google Scholar] [CrossRef]
- Kaczor, P.; Bazan, P.; Kuciel, S. Bioactive Polyoxymethylene Composites: Mechanical and Antibacterial Characterization. Materials 2023, 16, 5718. [Google Scholar] [CrossRef]
- Hidayat, M.I.; Adlim, M.; Suhartono, S.; Hayati, Z.; Bakar, N.H.H.A. Comparison of antibacterial properties between chitosan stabilized silver and zinc oxide nanoparticles immobilized on white silica beads, S. Afr. J. Chem. Eng., 2023, 45, 111–119. [Google Scholar] [CrossRef]
- Dhir, S.; Dutt, R.; Singh, R.P.; Chauhan, M.; Virmani, T.; Kumar, G.; Alhalmi, A.; Aleissa, M.S.; Rudayni, H.A.; Al-Zahrani, M. Amomum subulatum Fruit Extract Mediated Green Synthesis of Silver and Copper Oxide Nanoparticles: Synthesis, Characterization, Antibacterial and Anticancer Activities, Processes 2023, 11, 2698.
- Alharthi, A.F.; Gouda, M.; Khalaf, M.M.; Elmushyakhi, A.; Abou Taleb, M.F.; Abd El-Lateef, H.M. Cellulose-Acetate-Based Films Modified with Ag2O and ZnS as Nanocomposites for Highly Controlling Biological Behavior for Wound Healing Applications. Materials 2023, 16, 777. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, G.E.; Göktürk, I.; Ovezova, M.; Yılmaz, F.; Kılıç, S.; Denizli, A. Antimicrobial Nanomaterials: A Review, Hygiene, 2023, 3, 269-290.
- Corrêa, J. M.; Mori, M.; Sanches, H. L.; da Cruz, A. D.; Poiate, E.; Poiate, I.A. Silver nanoparticles in dental biomaterials. Int. J. Biomater., 2015, 2015, 485275. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging, J. King Saud Univ. Sci. 2016, 28(4), 273–279. [Google Scholar] [CrossRef]
- Smirnova, V.V.; Chausov, D.N.; Serov, D.A.; Kozlov, V.A.; Ivashkin, P.I.; Pishchalnikov, R.Y.; Uvarov, O.V.; Vedunova, M.V.; Semenova, A.A.; Lisitsyn, A.B.; et al. A Novel Biodegradable Composite Polymer Material Based on PLGA and Silver Oxide Nanoparticles with Unique Physicochemical Properties and Biocompatibility with Mammalian Cells. Materials 2021, 14, 6915. [Google Scholar] [CrossRef] [PubMed]
- Dirain, C.O.; Silva, R.C.; Antonelli, P.J. Prevention of biofilm formation by polyquaternary polymer. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 57–162. [Google Scholar] [CrossRef]
- Dos Santos, R. L. O.; Sarra, G.; Lincopan, N.; Petri, D.F.S.; Aliaga, J.; Marques, M.M.; Dias, R.B.; Coto, N.P.; Sugaya, N.N.; Paula, C.R. Preparation, Antimicrobial Properties, and Cytotoxicity of Acrylic Resins Containing Poly(diallyldimethylammonium chloride), Int. J. Prosthodont. 2021, 34(5), 635–641. [Google Scholar] [CrossRef] [PubMed]
- Pigareva, V.A.; Bolshakova, A.V.; Sybachin, A.V. Atomic Force Microscopy of Antibacterial Coatings Produced from Polycation and Its Water-Soluble Complex with Polyanion. Moscow Univ. Chem. Bull. 2023, 78, 132–135. [Google Scholar] [CrossRef]
- Izumrudov, V.A.; Paraschuk, V.V.; Sybachin, A.V. Controlled phase separations in solutions of polyelectrolyte complexes: Potential for gene delivery. J. Drug Deliv. Sci. Technol. 2006, 16, 267–274. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Pigareva, V.A.; Marina, V.I.; Bolshakova, A.V.; Berkovich, A.K.; Kuznetsova, O.A.; Semenova, A.A.; Yushina, Y.K.; Bataeva, D.S.; Grudistova, M.A.; Sybachin, A.V. Biocidal Coatings against Gram-Positive Bacteria from Linear and Branched Polycations: The Decisive Role of the Diffusion Coefficients of Macromolecules. Coatings 2023, 13, 1076. [Google Scholar] [CrossRef]
- Kusaia, V.S.; Kozhunova, E.Y.; Stepanova, D.A.; Pigareva, V.A.; Sybachin, A.V.; Zezin, S.B.; Bolshakova, A.V.; Shchelkunov, N.M.; Vavaev, E.S.; Lyubin, E.V.; et al. Synthesis of Magneto-Controllable Polymer Nanocarrier Based on Poly(N-isopropylacrylamide-co-acrylic Acid) for Doxorubicin Immobilization. Polymers 2022, 14, 5440. [Google Scholar] [CrossRef]
- Neuman, K.C.; Block, S. M. Optical Trapping. Rev. Sci. Instrum. 2004, 75, 2787. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int J Biol Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Jain, S.; Bhanjana, G.; Heydarifard, S.; Dilbaghi, N.; Nazhad, M.M.; Kumar, V.; Kim, K.H.; Kumar, S. Enhanced antibacterial profile of nanoparticle impregnated cellulose foam filter paper for drinking water filtration. Carbohyd. Polym. 2018, 202, 219–226. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Müller, A.H.E.; Schacher, F.H. Micellar interpolyelectrolyte complexes. Chem. Soc. Rev. 2012, 41, 6888–6901. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavov, A.A.; Efimova, A.A.; Sybachin, A.V.; Izumrudov, V.A.; Samoshin, V.V.; Potemkin, I.I. Stability of anionic liposome-cationic polymer complexes in water-salt media. Colloid J. 2011, 73, 430–435. [Google Scholar] [CrossRef]
- Panova, I.; Drobyazko, A.; Spiridonov, V.; Sybachin, A.; Kydralieva, K.; Jorobekova, S.; Yaroslavov, Y. Humics-based inter-polyelectrolyte complexes for antierosion protection of soil: Model investigation. Land Degrad. Dev. 2019, 30, 337–347. [Google Scholar] [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; G. Rubio, R.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids Interfaces 2020, 4, 33.
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Adsorption of Polyanion onto Large Alpha Alumina Beads with Variably Charged Surface. Adv. Phys. Chem., 2014, 2014, 460942. [Google Scholar] [CrossRef]
- Kohay, H.; Bilkis, I.I.; Mishael, Y.G. Effect of polycation charge density on polymer conformation at the clay surface and consequently on pharmaceutical binding. J. Colloid Interface Sci. 2019, 552, 517–527. [Google Scholar] [CrossRef]
- Izumrudov, V. A.; Mussabayeva, B. K.; Kassymova, Z. S.; Klivenko, A. N.; Orazzhanova, L. K. Interpolyelectrolyte complexes: advances and prospects of application. Russ.Chem. Rev. 2019, 88, 1046–1062. [Google Scholar] [CrossRef]
- Sofronova, A.A.; Evstafyeva, D.B.; Izumrudov, V.A.; Muronetz, V.I.; Semenyuk, P.I. Protein-polyelectrolyte complexes: Molecular dynamics simulations and experimental study. Polymer, 2017; 113, 39–45. [Google Scholar]
- de Carvalho, G.R.; Kudaka, A.M.; Netto, R.A.; Delarmelina, C.; Duarte, M.C.T.; Lona, L.M.F. Antiviral and antibacterial activity of sodium alginate/poly(diallyldimethylammonium chloride) polyelectrolyte film for packaging applications. Int. J. Biol. Macromol. 2023, 244, 125388. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, A.G. Natural and synthetic polymeric antimicrobials with quaternary ammonium moieties: a review. Environ. Chem. Lett. 2021, 19, 3009–3022. [Google Scholar] [CrossRef]
- Misin, V.M.; Zezin, A.A.; Klimov, D.I.; Sybachin, A.V.; Yaroslavov, A.A. Biocidal Polymer Formulations and Coatings. Polym. Sci. Ser. B 2021, 63, 459–469. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).