Submitted:
26 October 2023
Posted:
27 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
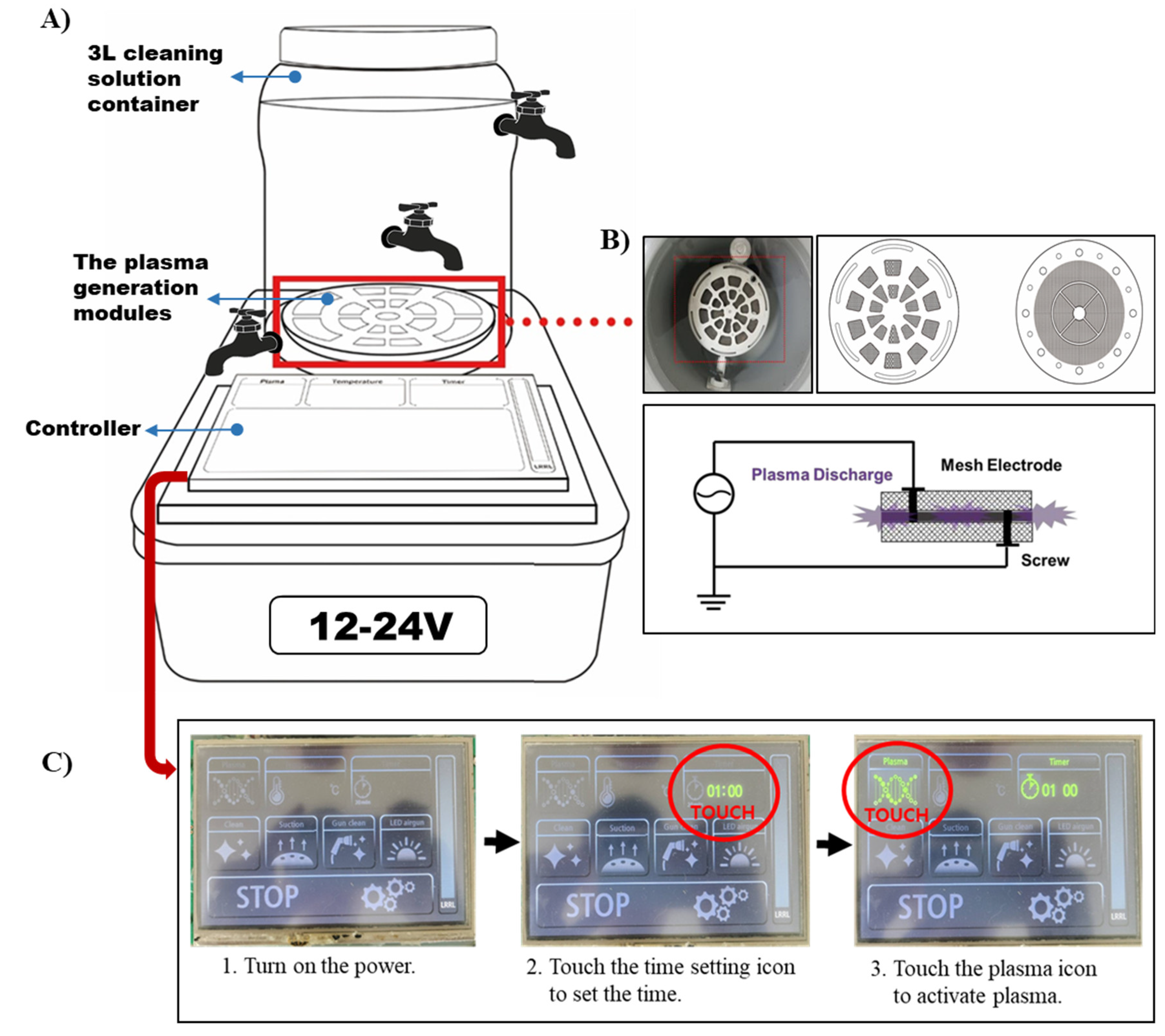
2.1. PAW Generating System and PAW Processing
2.2. PAW Sample Preparation Conditions
2.3. Analytical Methods
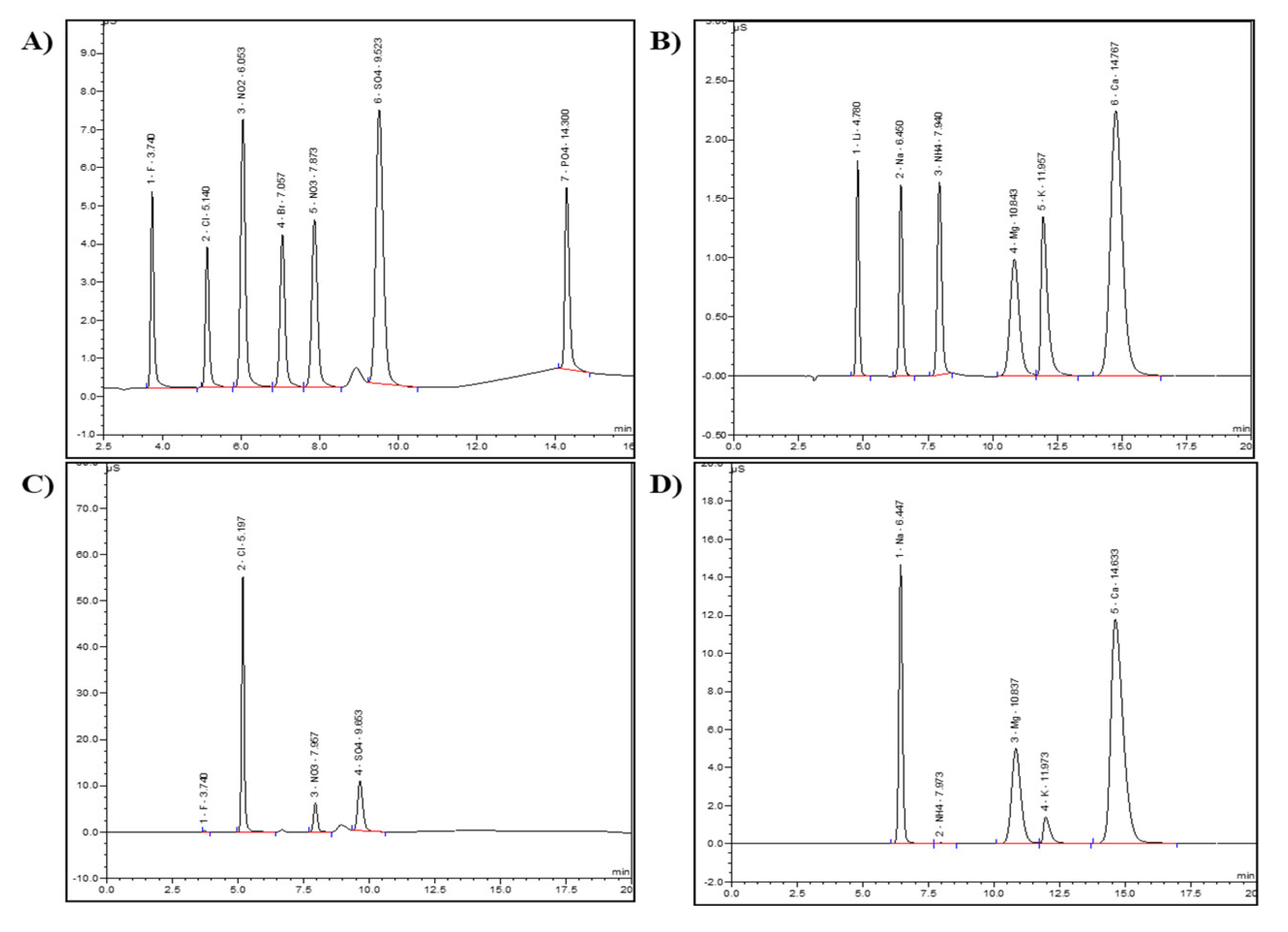
2.3.1. Ion Chromatography (IC)
2.3.2. Residual Chlorine Analyzer
2.4. Evaluation of Antibacterial Efficacy
2.4.1. Microorganisms and Materials
2.4.2. Antibacterial Activity
3. Results
3.1. Inorganic Anions and Cations Composition in PAW
| Sampling | Treatment Time | Sampling Position |
Retention Time | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F- | Cl- | NO3- | SO42- | HOCl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | (min) | (min) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (ppm) | |
| 1 | 20min | baseline | 9.20 | 0.18 | 2.48 | 3.52 | 10.46 | 0.05 | 20.21 | 5.79 | 10.23 | 0.00 | |
| 2 | sample① Top |
0 | 9.11 | 0.13 | 2.43 | 4.41 | 18.30 | 0.06 | 19.24 | 5.70 | 10.25 | 1.03 | |
| 3 | 30 | 9.16 | 0.16 | 2.43 | 4.48 | 20.08 | 0.06 | 19.96 | 5.97 | 10.91 | 1.11 | ||
| 4 | 60 | 9.16 | 0.13 | 2.44 | 4.51 | 20.73 | 0.06 | 19.79 | 5.82 | 10.50 | 1.17 | ||
| 5 | baseline | 7.46 | 0.15 | 2.33 | 3.86 | 15.17 | 0.05 | 19.35 | 6.46 | 9.59 | 0.09 | ||
| 6 | sample② Middle |
0 | 7.45 | 0.16 | 2.32 | 4.02 | 18.37 | 0.05 | 18.54 | 6.44 | 9.67 | 1.21 | |
| 7 | 30 | 7.45 | 0.18 | 2.32 | 4.05 | 19.15 | 0.05 | 18.18 | 6.31 | 9.36 | 1.20 | ||
| 8 | 60 | 7.41 | 0.17 | 2.31 | 4.04 | 19.33 | 0.05 | 18.46 | 6.39 | 9.53 | 1.00 | ||
| 9 | baseline | 7.34 | 0.14 | 2.28 | 3.89 | 15.00 | 0.06 | 19.27 | 6.96 | 10.06 | 0.05 | ||
| 10 | sample③ Bottom |
0 | 7.06 | 0.17 | 2.20 | 3.84 | 16.99 | 0.05 | 17.31 | 6.54 | 9.25 | 1.07 | |
| 11 | 30 | 7.28 | 0.16 | 2.27 | 3.99 | 18.40 | 0.05 | 17.90 | 6.77 | 9.58 | 1.12 | ||
| 12 | 60 | 7.30 | 0.17 | 2.27 | 4.01 | 18.75 | 0.05 | 17.98 | 6.78 | 9.63 | 1.11 | ||
| 13 | 10min | baseline | 7.59 | 0.16 | 2.27 | 3.40 | 13.38 | 0.04 | 17.41 | 7.71 | 9.02 | 0.11 | |
| 14 | sample① Top | 0 | 7.59 | 0.22 | 2.27 | 3.50 | 16.22 | 0.05 | 17.16 | 7.75 | 9.13 | 0.78 | |
| 15 | 30 | 7.62 | 0.20 | 2.28 | 3.51 | 17.02 | 0.05 | 17.23 | 7.74 | 9.09 | 0.66 | ||
| 16 | 60 | 7.57 | 0.26 | 2.30 | 3.50 | 17.33 | 0.05 | 16.85 | 7.65 | 8.95 | 0.60 | ||
| 17 | sample② Middle | 0 | 7.65 | 0.20 | 2.28 | 3.55 | 17.66 | 0.05 | 16.92 | 7.69 | 8.98 | 0.71 | |
| 18 | 30 | 7.66 | 0.20 | 2.30 | 3.56 | 17.74 | 0.05 | 16.98 | 7.63 | 8.95 | 0.72 | ||
| 19 | 60 | 7.60 | 0.17 | 2.28 | 3.54 | 17.76 | 0.05 | 16.96 | 7.63 | 8.96 | 0.66 | ||
| 20 | sample③ Bottom | 0 | 7.69 | 0.17 | 2.30 | 3.52 | 17.71 | 0.05 | 17.05 | 7.57 | 8.88 | 0.59 | |
| 21 | 30 | 7.72 | 0.18 | 2.29 | 3.53 | 17.77 | 0.05 | 16.99 | 7.64 | 9.00 | 0.57 | ||
| 22 | 60 | 7.67 | 0.17 | 2.28 | 3.51 | 17.73 | 0.05 | 17.17 | 7.64 | 9.00 | 0.65 | ||
| 23 | 1min | baseline | 7.49 | 0.23 | 2.28 | 3.44 | 13.31 | 0.05 | 19.08 | 7.84 | 9.37 | 0.02 | |
| 24 | sample① Top | 0 | 7.21 | 0.25 | 2.05 | 3.18 | 16.41 | 0.06 | 18.39 | 7.48 | 8.80 | 0.21 | |
| 25 | 30 | 7.20 | 0.22 | 2.16 | 3.18 | 16.32 | 0.06 | 18.83 | 7.67 | 9.12 | 0.11 | ||
| 26 | 60 | 7.19 | 0.19 | 2.15 | 3.18 | 16.33 | 0.06 | 18.62 | 7.60 | 8.98 | 0.11 | ||
| 27 | sample② Middle | 0 | 6.37 | 0.08 | 1.89 | 3.14 | 15.03 | 0.04 | 14.00 | 7.44 | 7.85 | 0.23 | |
| 28 | 30 | 6.74 | 0.11 | 1.94 | 3.14 | 15.70 | 0.05 | 13.77 | 7.58 | 8.59 | 0.15 | ||
| 29 | 60 | 6.42 | 0.09 | 1.99 | 3.22 | 16.20 | 0.05 | 13.83 | 7.65 | 7.77 | 0.11 | ||
| 30 | sample③ Bottom | 0 | 6.31 | 0.09 | 1.92 | 3.15 | 16.06 | 0.05 | 13.84 | 7.65 | 7.90 | 0.11 | |
| 31 | 30 | 6.26 | 0.09 | 1.93 | 3.15 | 16.02 | 0.05 | 13.76 | 7.66 | 7.88 | 0.09 | ||
| 32 | 60 | 6.30 | 0.11 | 1.94 | 3.18 | 16.31 | 0.05 | 13.62 | 7.61 | 7.76 | 0.15 |
| Sampling | Treatment Time | Sampling Position |
Retention Time | Na+ | NH4+ | K+ | Mg2+ | Ca2+ | F- | Cl- | NO3- | SO42- | HOCl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | (min) | (min) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (ppm) | |
| 1 | 20min | baseline | 6.92 | 0.17 | 2.22 | 3.33 | 14.61 | 0.05 | 18.25 | 7.34 | 8.66 | 0.00 | |
| 2 | sample① Top |
0 | 6.95 | 0.15 | 2.22 | 3.45 | 16.12 | 0.04 | 15.46 | 7.20 | 8.37 | 2.10 | |
| 3 | 30 | 6.94 | 0.13 | 2.22 | 3.49 | 16.59 | 0.05 | 15.67 | 7.35 | 8.63 | 2.56 | ||
| 4 | 60 | 6.93 | 0.13 | 2.20 | 3.50 | 16.78 | 0.05 | 16.17 | 7.42 | 8.81 | 2.19 | ||
| 5 | baseline | 6.77 | 0.14 | 2.32 | 3.14 | 14.35 | 0.05 | 17.93 | 7.44 | 8.69 | 0.10 | ||
| 6 | sample② Middle |
0 | 6.53 | 0.13 | 2.22 | 3.19 | 15.32 | 0.04 | 14.95 | 7.20 | 8.46 | 2.64 | |
| 7 | 30 | 6.74 | 0.19 | 2.30 | 3.32 | 16.40 | 0.05 | 15.32 | 7.37 | 8.65 | 2.62 | ||
| 8 | 60 | 6.77 | 0.16 | 2.31 | 3.37 | 16.79 | 0.05 | 15.55 | 7.38 | 8.67 | 2.22 | ||
| 9 | baseline | 7.27 | 0.25 | 2.29 | 4.61 | 12.17 | 0.05 | 17.10 | 7.13 | 8.39 | 0.04 | ||
| 10 | sample③ Bottom |
0 | 7.04 | 0.20 | 2.27 | 3.43 | 14.61 | 0.05 | 16.00 | 7.46 | 8.96 | 2.00 | |
| 11 | 30 | 7.15 | 0.29 | 2.29 | 3.37 | 15.64 | 0.04 | 15.16 | 7.38 | 8.70 | 2.62 | ||
| 12 | 60 | 7.15 | 0.28 | 2.32 | 3.38 | 15.95 | 0.04 | 15.28 | 7.40 | 8.61 | 2.32 | ||
| 13 | 10min | baseline | 7.49 | 0.23 | 2.28 | 3.44 | 13.31 | 0.05 | 19.08 | 7.84 | 9.37 | 0.02 | |
| 14 | sample① Top | 0 | 6.38 | 0.35 | 1.98 | 2.83 | 13.10 | 0.05 | 17.11 | 7.78 | 9.40 | 1.54 | |
| 15 | 30 | 7.40 | 0.25 | 2.27 | 3.45 | 16.70 | 0.05 | 17.38 | 7.82 | 9.39 | 1.43 | ||
| 16 | 60 | 7.40 | 0.34 | 2.03 | 3.38 | 16.98 | 0.05 | 17.35 | 7.72 | 9.31 | 1.14 | ||
| 17 | sample② Middle | 0 | 7.35 | 0.26 | 2.03 | 3.16 | 15.88 | 0.05 | 17.38 | 7.66 | 8.96 | 1.49 | |
| 18 | 30 | 7.21 | 0.29 | 2.23 | 3.14 | 15.96 | 0.06 | 17.42 | 7.72 | 8.98 | 1.36 | ||
| 19 | 60 | 7.22 | 0.23 | 2.12 | 3.15 | 16.07 | 0.06 | 17.18 | 7.53 | 8.85 | 1.20 | ||
| 20 | sample③ Bottom | 0 | 7.20 | 0.29 | 2.15 | 3.18 | 16.22 | 0.06 | 17.42 | 7.42 | 8.64 | 1.03 | |
| 21 | 30 | 7.22 | 0.28 | 2.16 | 3.18 | 16.21 | 0.06 | 17.29 | 7.64 | 9.01 | 1.31 | ||
| 22 | 60 | 7.14 | 0.27 | 2.15 | 3.15 | 16.08 | 0.06 | 17.15 | 7.54 | 8.82 | 1.21 | ||
| 23 | 1min | baseline | 6.35 | 0.07 | 1.89 | 3.03 | 12.43 | 0.04 | 13.45 | 7.50 | 7.64 | 0.10 | |
| 24 | sample① Top | 0 | 6.48 | 0.19 | 1.96 | 3.19 | 16.48 | 0.06 | 14.09 | 7.52 | 7.99 | 0.14 | |
| 25 | 30 | 6.15 | 0.16 | 1.88 | 3.07 | 15.92 | 0.05 | 13.76 | 7.61 | 7.84 | 0.24 | ||
| 26 | 60 | 6.32 | 0.17 | 1.94 | 3.18 | 16.42 | 0.05 | 13.71 | 7.64 | 7.84 | 0.20 | ||
| 27 | sample② Middle | 0 | 6.32 | 0.12 | 1.93 | 3.15 | 16.33 | 0.05 | 13.84 | 7.55 | 7.78 | 0.23 | |
| 28 | 30 | 6.32 | 0.12 | 1.94 | 3.18 | 16.46 | 0.05 | 13.67 | 7.63 | 7.78 | 0.29 | ||
| 29 | 60 | 5.50 | 0.15 | 1.68 | 2.65 | 13.80 | 0.05 | 13.91 | 7.71 | 7.98 | 0.20 | ||
| 30 | sample③ Bottom | 0 | 5.44 | 0.17 | 1.70 | 2.60 | 13.50 | 0.05 | 13.78 | 7.61 | 7.87 | 0.15 | |
| 31 | 30 | 5.64 | 0.14 | 1.77 | 2.72 | 14.18 | 0.05 | 13.82 | 7.69 | 7.98 | 0.18 | ||
| 32 | 60 | 6.36 | 0.20 | 1.94 | 3.20 | 16.54 | 0.05 | 13.72 | 7.62 | 7.81 | 0.19 |
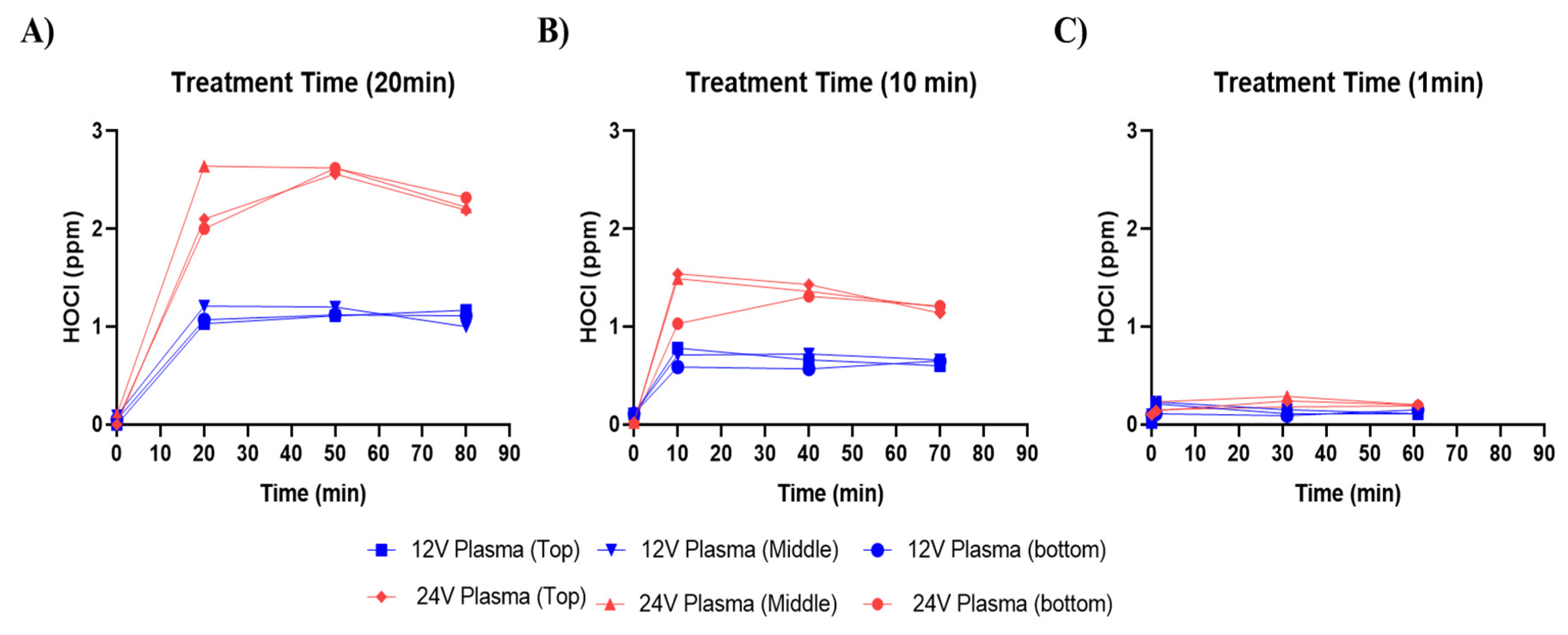
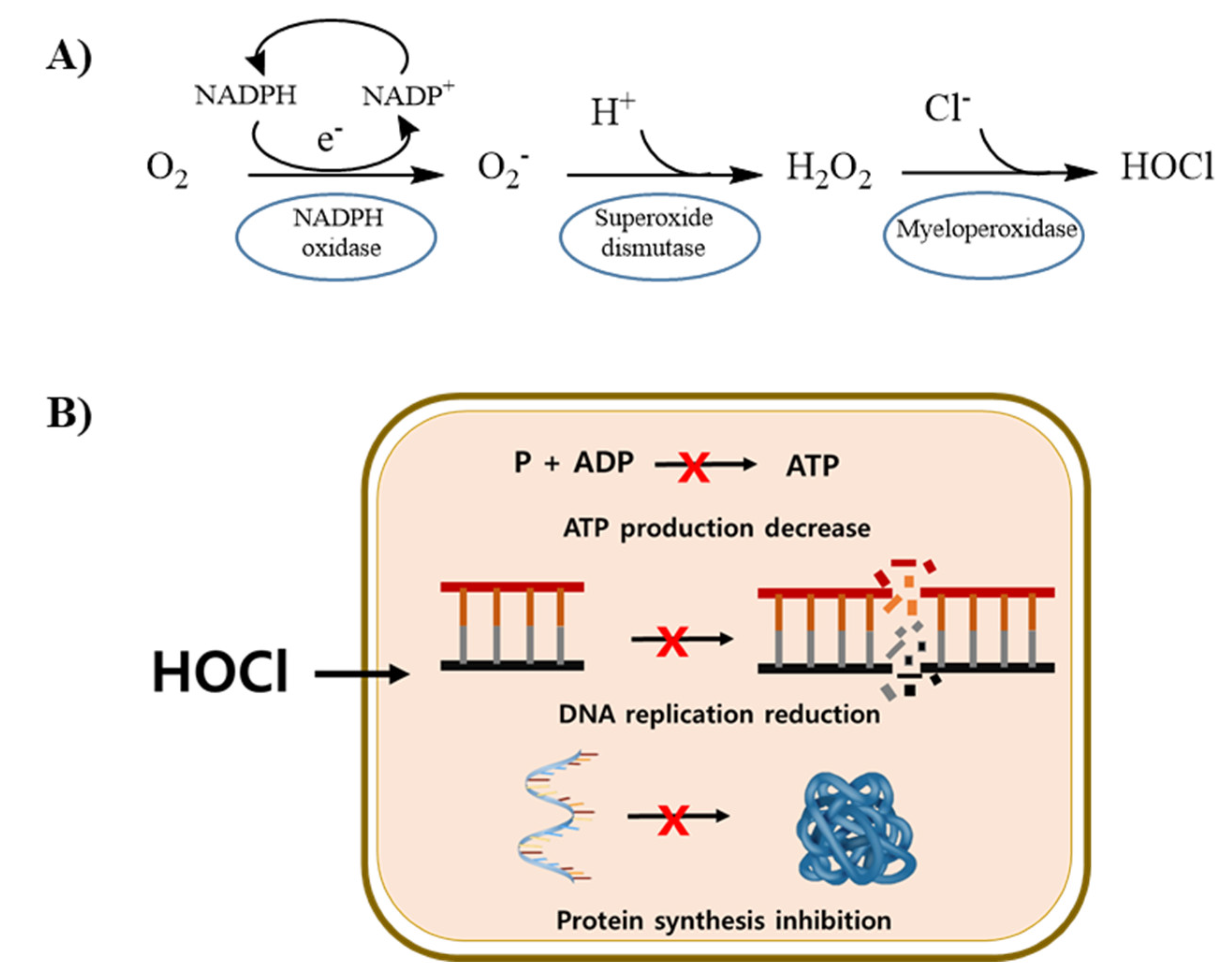
3.2. Hypochlorous Acid (HOCl) Concentration in PAW
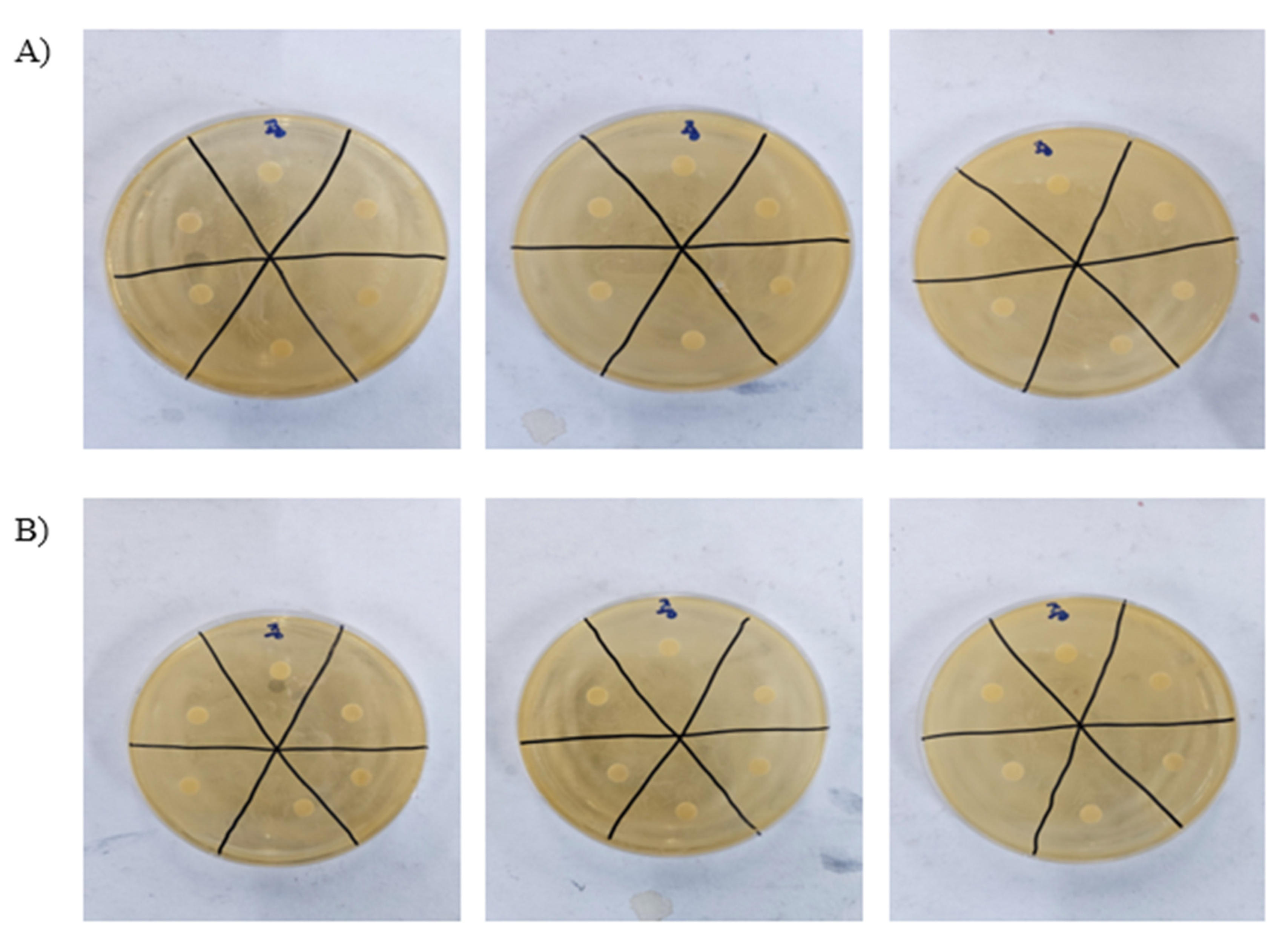
3.3. Antibacterial Activity of PAW
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cortese, E.; Settimi, A.G.; Pettenuzzo, S.; Cappellin, L.; Galenda, A.; Famengo, A.; Dabala, M.; Antoni, V.; Navazio, L. Plasma-Activated Water Triggers Rapid and Sustained Cytosolic Ca(2+) Elevations in Arabidopsis thaliana. Plants 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Keener, K.M. Cold plasma: background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Gao, Y.; Francis, K.; Zhang, X. Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res. Int. 2022, 157, 111246. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.D.G.; Chiappim, W.; Milhan, N.V.M.; Botan Neto, B.; Pessoa, R.; Koga-Ito, C.Y. Effect of the pH on the Antibacterial Potential and Cytotoxicity of Different Plasma-Activated Liquids. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Nema, S.K. A comparative study of dielectric barrier discharge plasma device and plasma jet to generate plasma activated water and post-discharge trapping of reactive species. Phys. Plasmas 2022, 29. [Google Scholar] [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Gott, R.P.; Engeling, K.W.; Olson, J.; Franco, C. Plasma activated water: a study of gas type, electrode material, and power supply selection and the impact on the final frontier. Phys. Chem. Chem. Phys. 2023, 25, 5130–5145. [Google Scholar] [CrossRef]
- Berardinelli, A.; Pasquali, F.; Cevoli, C.; Trevisani, M.; Ragni, L.; Mancusi, R.; Manfreda, G. Sanitisation of fresh-cut celery and radicchio by gas plasma treatments in water medium. Postharvest. Biol. Technol. 2016, 111, 297–304. [Google Scholar] [CrossRef]
- Neretti, G.; Taglioli, M.; Colonna, G.; Borghi, C.A. Characterization of a dielectric barrier discharge in contact with liquid and producing a plasma activated water. Plasma Sources Sci. Technol. 2016, 26. [Google Scholar] [CrossRef]
- Georgescu, N.; Apostol, L.; Gherendi, F. Inactivation of Salmonella enterica serovar Typhimurium on egg surface, by direct and indirect treatments with cold atmospheric plasma. Food Control 2017, 76, 52–61. [Google Scholar] [CrossRef]
- Rathore, V.; Patel, D.; Butani, S.; Nema, S.K. Investigation of physicochemical properties of plasma activated water and its bactericidal efficacy. Plasma Chem. Plasma Process. 2021, 41, 871–902. [Google Scholar] [CrossRef]
- Jang, Y.; Bok, J.; Ahn, D.K.; Kim, C.K.; Kang, J.S. Human Trial for the Effect of Plasma-Activated Water Spray on Vaginal Cleaning in Patients with Bacterial Vaginosis. Med Sci (Basel) 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Ma, S.-H.; Hong, Y.C.; Choi, M.C. Effects of pulsed and continuous wave discharges of underwater plasma on Escherichia coli. Sep. Purif. Technol. 2018, 193, 351–357. [Google Scholar] [CrossRef]
- Hong, Y.C.; Park, H.J.; Lee, B.J.; Kang, W.-S.; Uhm, H.S. Plasma formation using a capillary discharge in water and its application to the sterilization of E. coli. Phys. Plasmas 2010, 17. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, Y.S.; You, Y.S.; Huh, J.Y.; Kim, K.; Hong, Y.C.; Kim, C.H. Antimicrobial effects of microwave plasma-activated water with skin protective effect for novel disinfectants in pandemic era. Sci. Rep. 2022, 12, 5968. [Google Scholar] [CrossRef] [PubMed]
- Perinban, S.; Orsat, V.; Lyew, D.; Raghavan, V. Effect of plasma activated water on Escherichia coli disinfection and quality of kale and spinach. Food Chem. 2022, 397, 133793. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kang, E.K.; Jeon, A.; An, W.; Shin, H.A.; Kim, Y.J.; Om, A.S. Vaginal cleansing effect using plasma-activated water(PAW) spray method in patients with vaginitis (suspected). J. Rehabil. Welf. Eng. Assisitve Technol. 2023, 17, 18–28. [Google Scholar]
- Hwang, Y.; Jeon, H.; Wang, G.Y.; Kim, H.K.; Kim, J.-H.; Ahn, D.K.; Choi, J.S.; Jang, Y. Design and Medical Effects of a Vaginal Cleaning Device Generating Plasma-Activated Water with Antimicrobial Activity on Bacterial Vaginosis. Plasma 2020, 3, 204–213. [Google Scholar] [CrossRef]
- Marnach, M.L.; Wygant, J.N.; Casey, P.M. Evaluation and Management of Vaginitis. Mayo Clin. Proc. 2022, 97, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.j.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2015, 6, 1528. [Google Scholar] [CrossRef]
- Cerca, N.; Jefferson, K.K.; Oliveira, R.; Pier, G.B.; Azeredo, J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect. Immun. 2006, 74, 4849–4855. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Thompson, A.; Sobue, T.; Kashleva, H.; Xu, H.; Vasilakos, J.; Dongari-Bagtzoglou, A. Candida albicans biofilms do not trigger reactive oxygen species and evade neutrophil killing. J. Infect. Dis. 2012, 206, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Qadrie, Z.L.; Jacob, B.; Anandan, R.; Rajkapoor, B.; Ulla, M.R. Anti-bacterial activity of ethanolic extract of Indoneesiella echioides (L) nees. evaluated by the filter paper disc method. Pak. J. Pharm. Sci. 2009, 22. [Google Scholar]
- Denda, M.; Katagiri, C.; Hirao, T.; Maruyama, N.; Takahashi, M. Some magnesium salts and a mixture of magnesium and calcium salts accelerate skin barrier recovery. Arch. Dermatol. Res. 1999, 291, 560–563. [Google Scholar] [CrossRef]
- Schempp, C.M.; Dittmar, H.C.; Hummler, D.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C.; Schulte-Mönting, J. Magnesium ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase, HLA-DR, B7 molecules, and cytokines. J. Invest. Dermatol. 2000, 115, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Hennings, H.; Michael, D.; Cheng, C.; Steinert, P.; Holbrook, K.; Yuspa, S.H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 1980, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Bikle, D.D.; Hincenbergs, M.; Elias, P.M. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J. Cell. Physiol. 1988, 134, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yuspa, S.H.; Kilkenny, A.E.; Steinert, P.M.; Roop, D.R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J. Cell Biol. 1989, 109, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jun, J.E.; Choi, E.H.; Ahn, S.K.; Lee, S.H. Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin. Exp. Dermatol. 2010, 35, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, K.; Kakiuchi, T.; Suzuki, M.; Sakurai, H.; Tokudome, Y. The ion balance of Shotokuseki extract promotes filaggrin fragmentation and increases amino acid production and pyrrolidone carboxylic acid content in three-dimensional cultured human epidermis. Nat. Prod. Bioprospecting 2022, 12, 37. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Overhage, J. Surviving Reactive Chlorine Stress: Responses of Gram-Negative Bacteria to Hypochlorous Acid. Microorganisms 2020, 8, 1220. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Wholey, W.Y.; Jakob, U. Bacterial responses to reactive chlorine species. Annu. Rev. Microbiol. 2013, 67, 141–160. [Google Scholar] [CrossRef]
- Reid, G. Probiotic and Prebiotic Applications for Vaginal Health. J. AOAC Int. 2019, 95, 31–34. [Google Scholar] [CrossRef]
| Position | Power | Treatment time (min) |
Retention time (min) |
|
|---|---|---|---|---|
| Distilled water | Top, middle, bottom | 12V, 24V | 1, 10, and 20 | 0, 30, and 60 |
| Tap water |
| Microorganism | Strain | |
| Gram-positive | Lactobacillus reuteri | KCTC 3594 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





