1. Introduction
Shorebirds are iconic examples of the partitioning of food resources through use of contrasted morphological structures (e.g., bills, legs) to capture prey. Shorebirds forage mainly on the land-water borders of fresh, brackish, or marine waters where they feed mostly on invertebrates, but vegetal material are also ingested by many species, and can constitute a predominant part of their diet during certain periods of the year (
e.g., in
Calidris pugnax [
1]. Differences in bill lengths provide shorebird species with the opportunity to catch food particles at various sediment depths. Similarly, contrasted leg lengths allow species to forage at distinct water depths. Despite these morphological differences, shorebirds appear to use a small number of stereotyped food acquisition behaviors (
e.g., [
2,
3]. A seminal interspecific comparative study introduced a classification of these foraging-feeding species-specific stereotyped behaviors [
4]. This classification was subsequently used in numerous studies investigating the diet or the food acquisition behaviors of shorebirds (e.g., [
5,
6,
7,
8,
9,
10,
11,
12,
13].
Here we propose to revisit this classification, by defining a functional and integrative analysis of behaviors associated with food acquisition in shorebirds. More precisely, based on our filmed observations, food acquisition can be divided in three successive stages: foraging, feeding, and swallowing. The foraging stage concerns the different behaviors associated to food detection and capture: locomotion behaviors (how do birds move in suitable foraging habitats) and capture behaviors (how do birds locate and catch food particles. The feeding stage encompasses the behaviors used to possibly stun or kill the food particle: handling behaviors (how do birds manipulate the food particle to extract edible parts) and transport behaviors (how do birds bring it to the pharynx). In the swallowing stage, the edible part of the food particle enters the digestive tractus. By doing this deconstruction of food acquisition, we assume that the evolution of morphological structures associated with the partitioning of food resources in shorebirds may covariate with the selection of behaviors specific to each of these three stages, as predicted by the concept of neuroethological morphology [
14].
This association is rather obvious for capture behaviors: the pecking behavior of a golden plover (
Pluvialis apricaria) that hunts prey by sight on the surface of the substrate is very different from the probing behavior of a long-billed snipe (
Gallinago gallinago) that locates its prey by touch deep into the sediments [
2]. But our hypothesis is that the two following stages of food acquisition can also be affected by the structure of the beak: the opening of the valves of shellfish by oystercatchers (
Haematopus ostralegus) and the transport of their flesh out of the shell towards their buccal cavities all along their long, robust beaks [
15] engenders constraints that are very different from those faced by red phalaropes (
Pahaloropus fulicarius) handling very small preys that birds enclose in drops of water to move them along their narrow beaks to their buccal cavities [
16,
17]. The solidly bony structure of the decurved bills of curlews obliterates the lumen for much of its length and brings about great reduction in relative size of the tongue. The bird is obliged to remove a worm from the mud before it can transport it back to the mouth and swallow it; this is achieved by head jerking, coordinated with opening and shutting of the jaws. By contrast, snipes (
Gallinago gallinago) and woodcocks (
Scolopax rusticola), which have the lumen open and long tongues, are able to transport a worm along the bill while it is still inserted in the substrate, by a combination of a rapid series of retractions of the rhynchokinetic upper jaw tip and tongue action [
2,
18]. Godwits (
Limosa spp.), although showing some bill reinforcement and tongue reduction, are often able to do the same [
2,
18].
Here we approach in a more quantitative way the stereotyped behaviors involved in each of the three stages of food acquisition, by analyzing video sequences of
ca. two dozen species of Western Palearctic shorebirds, during migration or wintering. This procedure makes it possible to standardize and quantify observations of stereotyped behaviors, and to summarize them in a comprehensive repertoire that is as complete as possible. In addition to introducing three stages in the study of shorebird food acquisition, we will revisit the existing state of the art. Descriptions of several stereotyped behaviors involved in the foraging stage in shorebirds do exist in the literature (
e.g., [
2,
3], and Barbosa & Moreno [
4] provided the outline of a first classification recently rearranged by Angarita-Baez & Carlos [
13]. Whereas we do not doubt the heuristic importance of these descriptions and classification, here we want to reassess and quantify the foraging stage by using our video analysis method. Besides, we improve the descriptions of the feeding and the swallowing stages that are not explicitly addressed in the literature (but see [
13,
16,
17,
19,
20] and especially [
21] for behaviors associated to food transport from the beak to the pharynx).
Overall, our study provides insights on the interspecific variations and on the evolution of food acquisition behaviors in shorebirds. We look for stereotyped behaviors for each species during each of the three stages of food acquisition and investigate the relationships between these behaviors. We also provide a glimpse on the development of these behaviors, both by putting these relationships into a phylogenetic context using the dated phylogeny of Cerny & Natale [
22] and by exploring the ontogeny of these behaviors.
4. Discussion
Our data set is restricted to shorebird species living in Western Palearctic. We sampled behaviors of 26 species, which represent the majority of shorebirds that breed, migrate and/or overwinter in this area. For most species, we monitored the behaviors of at least 10 adult individuals using video sequences during at least 1 minute each. Given the methodology used, including repeated observations in space and time, we have a good coverage of food acquisition behaviors for the studied species, although the attribution of one or the other behavior of our repertoire to a given species may be missing. In this sense, our repertoire is a first step towards a more complete final product. Besides, it is possible that our subset of shorebirds uses other behaviors than those that we present here. Literature data indeed indicate that some
Calidris species graze on biofilm,
i.e., a surface layer (~0.01–2 mm) of organic detritus, unicellulars, benthic invertebrates, and sediments bound together by extracellular polymeric substances secreted by diatoms and bacteria [
28,
29]. We did not have the opportunity to observe this behavior, although the dunlin (
Calidris alpina), which is recorded in our dataset was observed practicing it in North America. This might be due to the fact that
C. alpina is a polytypic species, with 10 subspecies described [
30], and the individuals on which biofilm grazing behavior was observed belong to a subspecies (
C. a. pacifica) [
28] that differs from the subspecies which overwinters in the Western Palearctic. Finally, we do not pretend that we have recorded all possible behaviors used by shorebirds. Beside biofilm grazing, we can expect that species with extravagant bills like the aptly named spoon-billed sandpiper (
Calidris pygmaea), which has a dorso-ventrally flattened beak, or the wrybill (
Anarhynchus frontalis) with its thin elongate (for a plover) and especially right bended beak would use unusual behaviors to acquire food [
31]. Moreover, the Tuamotu sandpiper (
Prosobonia parvirostris), which is the only living representative of an almost extinct clade of South Pacific sandpipers, is reputed to be nectarivorous [
31]. The surprising diet specialization of this insular bird suggests the use of particular behaviors of food acquisition.
4.1. Postcranial system and locomotor behavior
In all vertebrates [
32], any foraging and feeding behaviors is the result of integrative motor actions of various skeletal and muscular systems (
e.g., fixed-action-pattern
sensu Tinbergen and Lorenz in the 1930th [
33,
34,
35] or modal-action-pattern [
36]). During the last decade, the functional integration between the cranial and postcranial muscular and skeletal systems for determining the characteristics of the foraging and feeding behaviors has been largely emphasized in a large diversity of vertebrates including aquatic birds [
37,
38,
39]. But such integration has not yet been studied in Aves as shorebirds living at the limit of both aquatic and terrestrial environments, although food resources with highly different ecological, physiological, morphological and behavioral traits are available in various habitats (e.g., prey living at the water surface, on sediments, and within sediments at various depths). Heiss et al. [
38] did not discussed the shorebirds in their exhaustive comparative analysis of Sauropsida because they primarily considered foraging and feeding behaviors targeting freely moving aquatic preys (e.g., filtration, skimming, suction, and ram feeding). Our data demonstrate that the foraging stage of food acquisition in all studied shorebirds relies on the combination of two motor patterns integrated with various sensory controls: locomotion and capture. The salient point is that during evolution successfully foraging strategies involve either coupled or decoupled postcranial and cranial motor patterns to achieve food capture. In our integrative approach to determine the foraging behaviors, we thus propose a major alternative method to the classification of Barbosa and Moreno [
4] in which both patterns are mixed. Indeed, a strong difference was recorded in shorebirds that use three major locomotor behaviors: (i) stop-run-stop, (ii) continuous walking, and (iii) swimming. The stop-run-stop behavior is always associated with a visual detection of the prey and a pecking capture behavior (
Figure 1). Birds using the continuous walking or swimming locomotion behavior can also detect their preys by sight, but they most often do not interrupt their walk or swim: the prey is picked up by the beak at the surface of the sediment or of the water while the bird remains in motion. Locomotion is thus interrupted in the first case, whereas it is a continuous process in the other cases. This is a key finding because it implies that birds using the stop-run-stop locomotion behavior decouple the appendicular postcranial musculoskeletal system involved in locomotion from the cranial and axial postcranial musculoskeletal systems involved in capture. This decoupling occurs in the basal clades of the sub-order Charadri (plovers, lapwing) in our data set that are considered as the most ancient shorebirds according to the dated phylogeny of Cerny & Natale [
22]. On the contrary, in birds that use continuous locomotion behaviors (walking or swimming), there is no decoupling between the different musculoskeletal systems. It is interesting to notice that this absence of decoupling concerns all Scolopaci, but also those recent Charadri in our dataset (avocet, stilt, and oystercatcher) that use continuous walking or swimming locomotion behaviors.
4.2. Cranial system and food capture
Pecking is considered as the ancestral capture behavior in birds, from which probing and sweeping are derived by progressive modification of the beak length, shape, and internal structure according to the seminal works of Zweers and colleague [
40,
41,
42,
43]. All the five species of our data set that are in the basal clades of the sub-order Charadri (plovers, lapwing) according to the dated phylogeny of Cerny & Natale [
22] indeed use pecking as the only capture behavior. The three species of Scolopaci using only pecking have each a particular feeding habitat or diet. Purple sandpipers (
Calidris maritima) feed on preys that they peck from hard substrates on rocky coasts and breakwaters. Red phalaropes (
Phalaropus fulicarius) capture invertebrates that they peck on the surface or in shallow water. Eurasian whimbrels (
Numenius phaeopus) feed on crabs that they capture on sandy soils. These three species have in common that they capture their prey on sight, just like the five species belonging to the basal clades of Charadri. Another argument indicating that pecking behavior is indeed an ancestral character is provided by the ontogeny of foraging. Preliminary observations indicate that pulli of sandpipers, shanks, stilts and avocets use pecking exclusively to capture their prey, whereas adults mix pecking and either probing or sweeping capture behaviors. In oystercatchers and snipes, adults capture small preys and present them to pulli, which peck the prey at the tip of the adult’s beak.
As mentioned by Zweers and colleagues [
40,
41,
42,
43], the transition from pecking to other capture behaviors involves the use of touch sense. Mechanoreceptors located in the bill skin that convert pressures and vibrations into electric signals allow the location of prey by direct touch and even by remote touching [
44,
45]. In shorebirds, increased sensitivity is obtained by enlarging the dermal area available for mechanoreceptor positioning. Extra space is gained by a dermis invading the bone, in the form of depressions of the premaxilla and mandible [
41]. The number of these depressions, also called sensory pits has been used by certain authors to try to quantify the more or less prober character of different species of shorebirds. But this approach seems unreliable, because (1) there seems to be a very large variation, at least geographically in the number of sensory pits for a given species, (2) there are currently no data on the density of mechanoreceptors in these depressions, and (3) it seems that in at least one case the depressions are present on the beak, but the mechanoreceptors are no longer active. In their classification of food acquisition behaviors, Barbosa & Moreno [
4] used the work of Hoerschelmann [
46] to infer the number of sensory pits located in the distal portions of maxillae and mandibles of several shorebird species. However, the number of sensory pits reported by Hoerschelmann [
46] on shorebirds probably collected in Germany differs considerably from those figured in Bolze [
47] or more recently published by Lin [
48] on shorebirds from Australia and New Zealand. Lin’s Master’s thesis presents the number of sensory pits of 14 shorebird species corroborated by photographs of beak skeleton with the sensory pits located there, for the ruddy turnstone (
Arenaria interpres), the common greenshank (
Tringa nebularia) and the bar tailed godwit (
Limosa lapponica). In all cases the number of pits is much higher than those mentioned by Hoerschelmann [
46] for the same species. At this stage it is not possible to determine whether this difference comes from a different geographical origin of the birds, from a possible temporal variation, or from differences in the definition of sensory pits. We therefore think that it is better to establish a standardized measurement methodology before using the number of sensory pits as indicators of a bird’s tendency to efficiently practice probing behavior.
To be rigorous, however, it will be necessary to investigate which types of mechanoreceptors are present in the pits, and what are their respective densities. Four distinct morphological types of mechanoreceptors are ordered according to size and complexity: free nerve endings, Merkel cells (often arranged into Merkel corpuscles), Grandry corpuscles, and Herbst corpuscles. All are involved with different facets of mechanoreception: the majority is rapidly adapting, while Merkel cells detect amplitude, Grandry corpuscles velocity and Herbst corpuscles acceleration components in mechanical stimuli [
41,
49]. Accordingly, the identification and the quantification of the mechanoreceptors in the pits are keys to assess the species-specific efficiency of probing behavior. The presence of numerous sensory pits on the beak of the ruddy turnstone (
Arenaria interpres) is intriguing as birds belonging to this species do not use probing as a capture behavior. This particularity encourages Carla du Toit [
50] to consider these sensory pits as a plesiomorphic character in this species. Turnstones would have lost the use of touch but kept the corresponding beak structure. This potential loss of function of the touch organ would have occurred relatively recently ([
22], and further investigations of
A. interpres could be carried out to check if these birds lack the hypertrophy in the regions of the brain associated with the processing of tactile information from the beak.
Many species (11) in our data set use a mixed pecking-probing strategy, which means shifting from sight to touch organ for prey detection. Eye closure seems a good indicator of the preeminent use of touch sense over visual stimuli. All species using probing behaviors close their eyes even before eye submersion, with the exception of snipes (
Gallinago gallinago and
Lymnocryptes minimus) that close their eyes at submersion, and the oystercatcher (
Haematopus ostralegus) that keeps its eyes open underwater. Careful examination of the transport stage indicates that in species using ballistic transport, individuals close their eyes when the prey encounters the tongue at the end of the transport process (
Figure 11C,D). We suggest that at this time the touch stimuli become super important so that the prey is oriented in a good way to enter the pharynx. At this point, all of the bird’s attention would be focused on the stimuli coming from the mechanoreceptors, causing the eyes to be "turned off."
The use of visual vs. touch stimuli during capture in shorebirds can look somewhat controversial. It is clear that many probing individuals do not randomly probe the substrate, but rather use visual stimuli to guide their research during foraging in such a way that their locomotion behavior is oriented. Many visual cues are left by buried preys on the surface of the substrate e.g., on mudflats (see [
44] for excellent illustrations), and oystercatchers (
Haematopus ostralegus) for instance are experts in their use and will probe by digging with their beak around these cues [
15]. However, locomotion and capture are clearly two distinct facets of foraging, so here we consider that the sensory organ that guides the prey capture is the very last one used by the bird before its beak closes on the prey. The recent work of Ersoy et al. [
51]on a wintering population of red knots (
Calidris canutus) very convincingly demonstrates that there is intraspecific variation in a personality trait, namely a bimodal distribution of individuals’ ability to explore their environment. The exploration abilities of a sample of individuals were scored in an experimental setting and both their food acquisition behavior and their diet were monitored by video recording and stable isotope analyses of droppings, respectively. Scores of slow and fast explorer individuals were shown to remain consistent over time. Results show that slow explorers fed mainly on deeply buried prey that they detect by random probing, whereas fast explorers fed on preys caught near the substrate surface that they detected by probing near visible cues of prey presence. There is thus a difference in the probing behavior, either random or guided by sight. However, the examination of the capture phase on the videos published by Ersoy et al. [
51] indeed confirms that both slow and fast explorers caught their preys by probing.
4.3. Food handling and transport
Prey handling and transport follow prey capture. As previously mentioned, handling is an extremely plastic behavior that depends both on the environmental and social context, and on the condition of each individual (e.g., age, personality, sex). Accordingly, we believe that this behavior is not only very difficult to categorize, but it remains difficult to quantify, which therefore means that it cannot be divided in standardized performances (
sensu Irshick & Higham [
25]). However, we think that it is perhaps possible to find invariants as to the maximum size of piece of prey that constitutes a mouthful for an individual, or as to the maximum time that it will invest in attempts to cut pieces (
e.g., appendices of crabs) or to access soft consumable flesh (
e.g., mollusks). Finding these invariants requires accumulating more precise data than we currently have. It would of course be necessary here to rely on the many very interesting studies which demonstrate the usefulness of Optimal Foraging Theory in understanding the choices made by individuals depending on the environmental context (energy intake and density of prey, presence of potential competitors) and their condition (sex, age, physical condition) (see
e.g., [
44] for a clever introductory review to this topic).
Immediately after prey capture, a successful individual may encounter attempts at kleptoparasitism, both by conspecifics and heterospecifics. We did not measure the frequency of occurrence of these behaviors, which seem rather infrequent. However, it would be interesting to measure their success rates, and to see to what extent they generate an intra- and interspecific hierarchy. During kleptoparasitism attempts, individuals move with their prey in their beaks to escape their competitors. Such movements are also observed when birds search for a particular substrate. It can be a hard substrate for shredding the prey, like oystercatchers looking for “anvils”, i.e., hard rocks to hammer mussels [
15]. It may also be a search for water, perhaps to wash the prey as is often indicated in the literature, but more likely to take advantage of a lubricant that facilitates the transport of the prey from the tip of the beak to the pharynx [
2] by surface tension [
16,
17,
19,
20]. This use of water as lubricant during transport seems however not restricted to small preys enclosed in a drop of water but may also occur with larger preys – this point deserves further investigation. Another black box at this point is the use of the tongue during transport.
Here we demonstrated that shorebirds use three major prey transport: (i) lingual feeding, (ii) surface tension, and (iii) ballistic transport. This is the first time that this last transport mechanism is recorded in shorebirds although a lot of pictures in the literature clearly demonstrate this feeding pattern primarily determined in birds exploiting fruits and large invertebrate and vertebrate prey [
52]. The prey is freely moved between the upper and lower beaks by one or several transport cycles regardless their size, volume and mass. The prey is generally brought to the tongue, and as soon as contacts with the tongue occurs it is moved toward the pharynx by lingual cycles. Despite a large diversity of shapes [
2], the tongue plays a key role in food transport either being the only one involved during lingual transport, or at the final phase of ballistic transport and also surface tension [54]. Surface tension and capillarity have been determined a one specialized mechanism relatively widespread in a lot of shorebirds exploiting small prey at the surface of the water and captured in water and humid sediments [
16,
17,
19,
20]. Alternatively, some species (e.g.
Phalaropus sp.) are able to use ballistic transport for large food items (see supplementary Material in Bels et al. [
21] For those species that use ballistics or surface tension, it should be noted that the length of their tongue is less or even much less than the size of the beak [
2], as if the elongation of the beak had occurred in a decoupled manner, independent of tongue size and shape, forcing these birds to either “invent” a novel transport behaviors or use one of ancestral prey transports involving (i) ballistic transport and (ii) lingual transport. This suggests an adaptive functional response to the properties of the prey. However, two major properties limit the use of surface tension and capillarity: (i) size/volume of the prey, and (ii) the need to evacuate the water used as lubricant, in the form of drops or droplets that are ejected as soon as the lingual prey transport occurs. Water ejection seems based on gravitational mechanism, and either falls on the substrate or is mixed with water as soon as the bird enters again the beak in water for the next pecking/probing event. For those species using surface tension transport, and especially snipes, to what extent the tongue could act as a piston generating a pump effect remains to be determined.
5. Conclusions
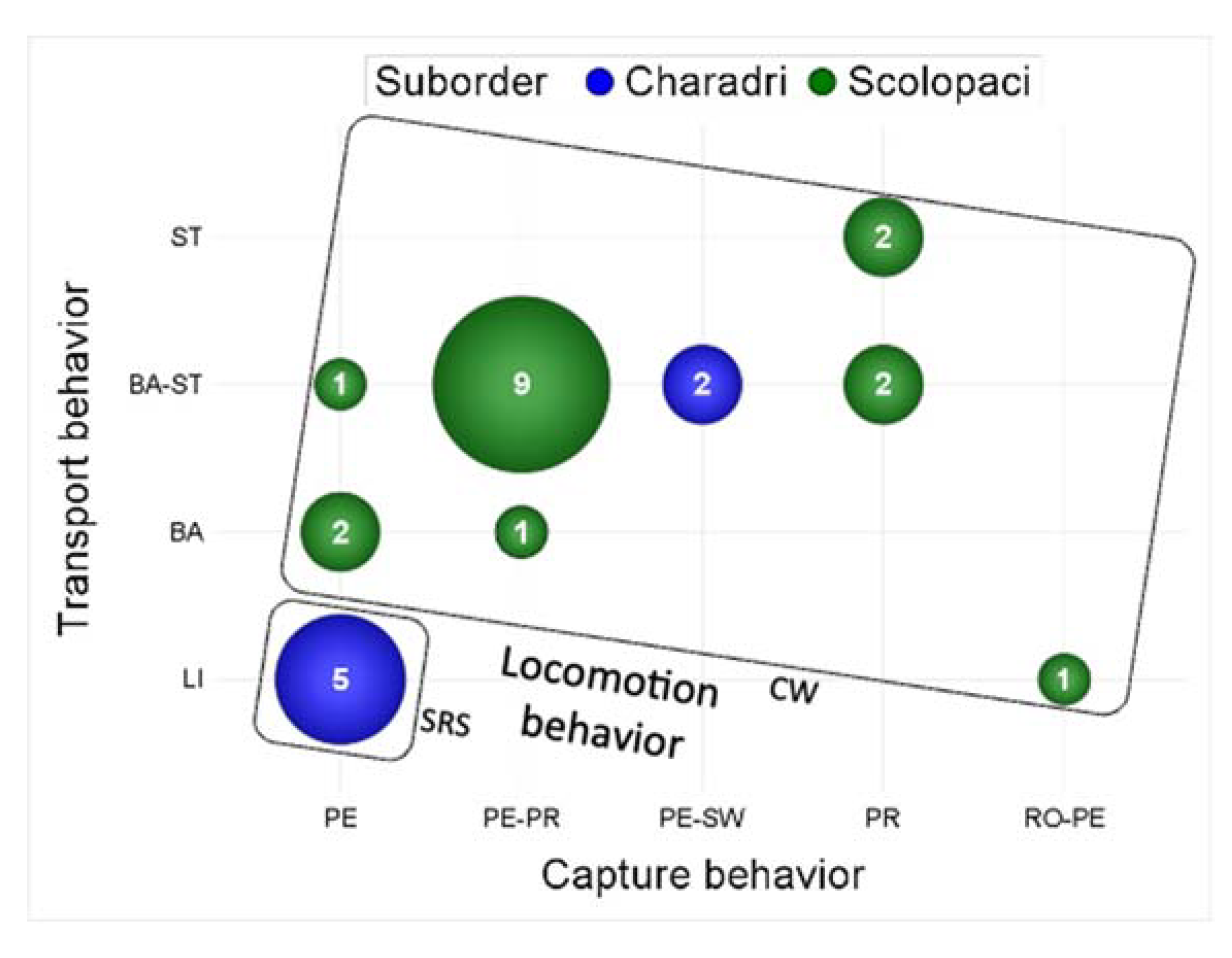
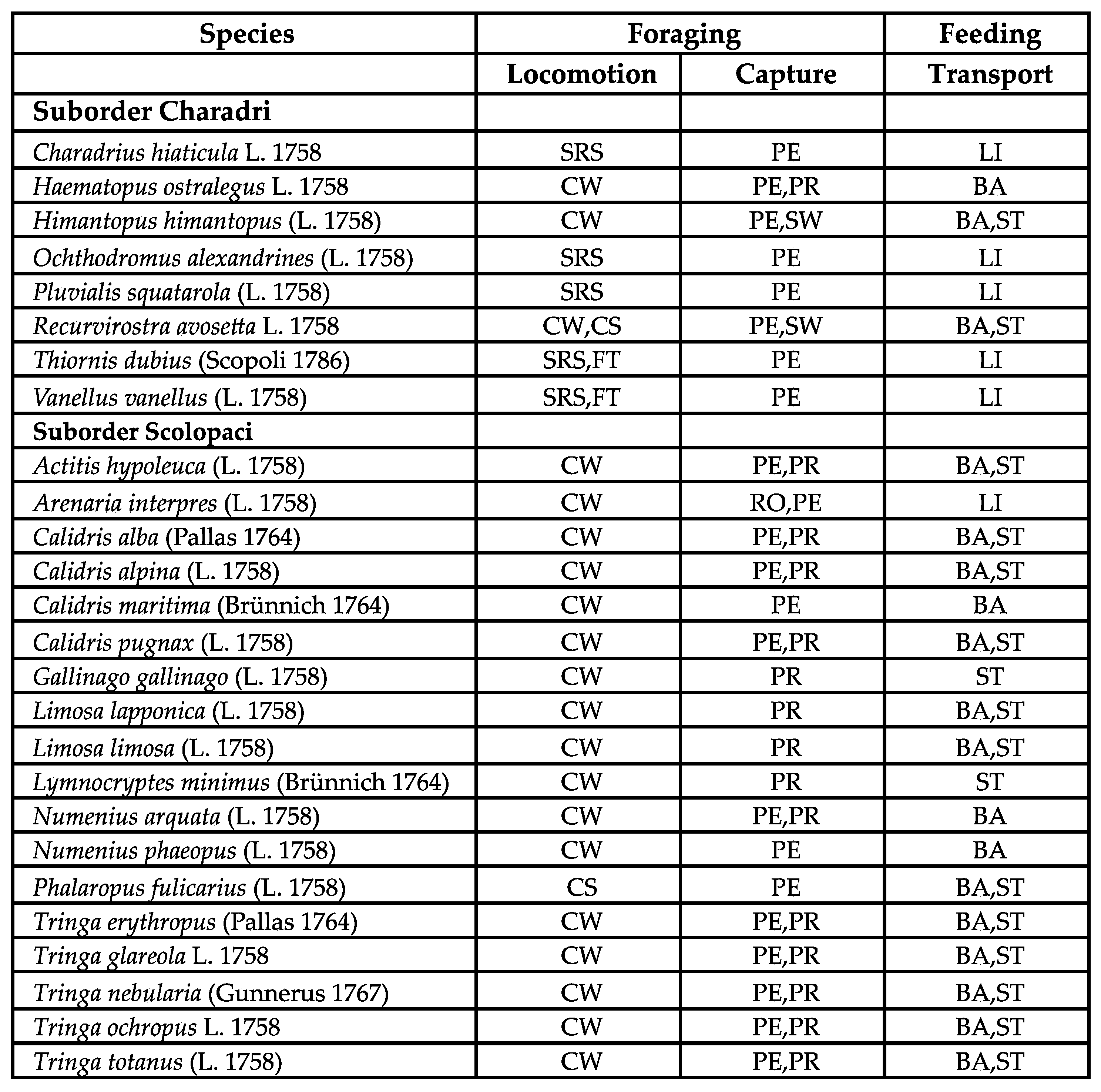
Careful examination of
Table 1 and
Figure 16 indicates the existence of behavioral syndromes of food acquisition in shorebirds. There are indeed covariations between the three behaviors that can be associated with performances
sensu Irshick & Higham [
25],
i.e., locomotion, capture and transport, and there is support for some phylogenetic origin for these syndromes. All the species using a stop-run-stop locomotion behavior (5) use pecking as capture behavior and use lingual transport. These species are all belonging to the Charadri suborder, and to the
Charadriidae family, which seems a basal clade according to the time-dated phylogeny of Cerny and Natale [
22]. The three remaining Charadri species of our data set use continuous walking as locomotion behavior. The two of them that are the only ones in our dataset to use sweeping as capture behavior are the two representatives of the
Recuvirostridae family, which have the most recent origin according to the time-dated phylogeny of Cerny and Natale [
22]. All species members of the Scolopaci suborder using a mixed capture strategy combining pecking and probing (10) move using continuous walking as locomotion behavior, and all but one (
Numenius arquata) use a mixed transport strategy combining ballistic and surface tension transport behaviors. The snipes that are the two species using exclusively probing as capture behavior are also the two species using exclusively surface tension as transport behavior.
Documenting such patterns of covariation among behaviors belonging to the different stages of food acquisition offers a key framework to understand the evolution of shorebirds. The huge variation in morphological structures associated with food acquisition among shorebird species, especially beak length and shape does not translate into a huge variety of behaviors. Besides, this variation in morphological structures (beak and legs) occur similarly in recent Charadri and in Scolopaci that share locomotion, capture and transport behaviors. The existence of behavioral syndromes involving a small number of behaviors we highlight here provides evidence of differences between the evolution speed of morphological structures and behaviors involved in food acquisition. It is as if rapid evolution could occur on some morphological structures like the beak or the legs that are obviously the key innovations of shorebird adaptive radiation, while the evolution of neuro-sensory, hormonal, and muscular circuits involved in behaviors, and of other morphological structures like the tongue, was much more constrained, and therefore slower. This point might be elucidated by (1) collecting morphological and behavioral data on a larger sample of species and (2) performing in-depth quantitative comparisons of morphological structures in a phylogenetic context.
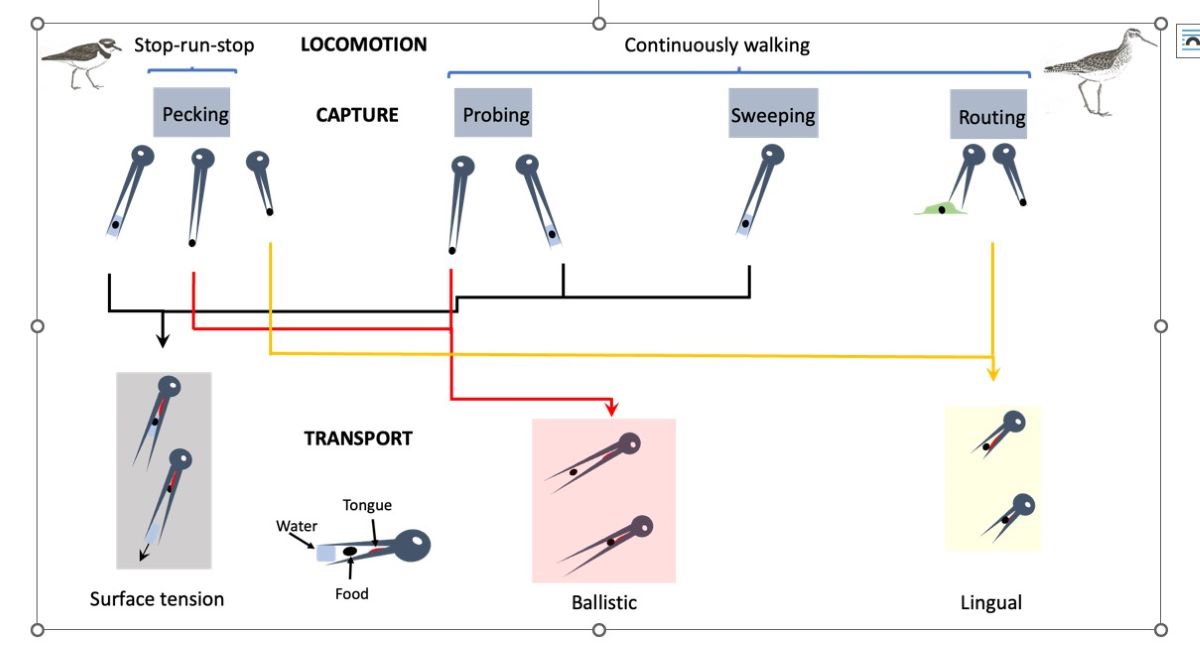
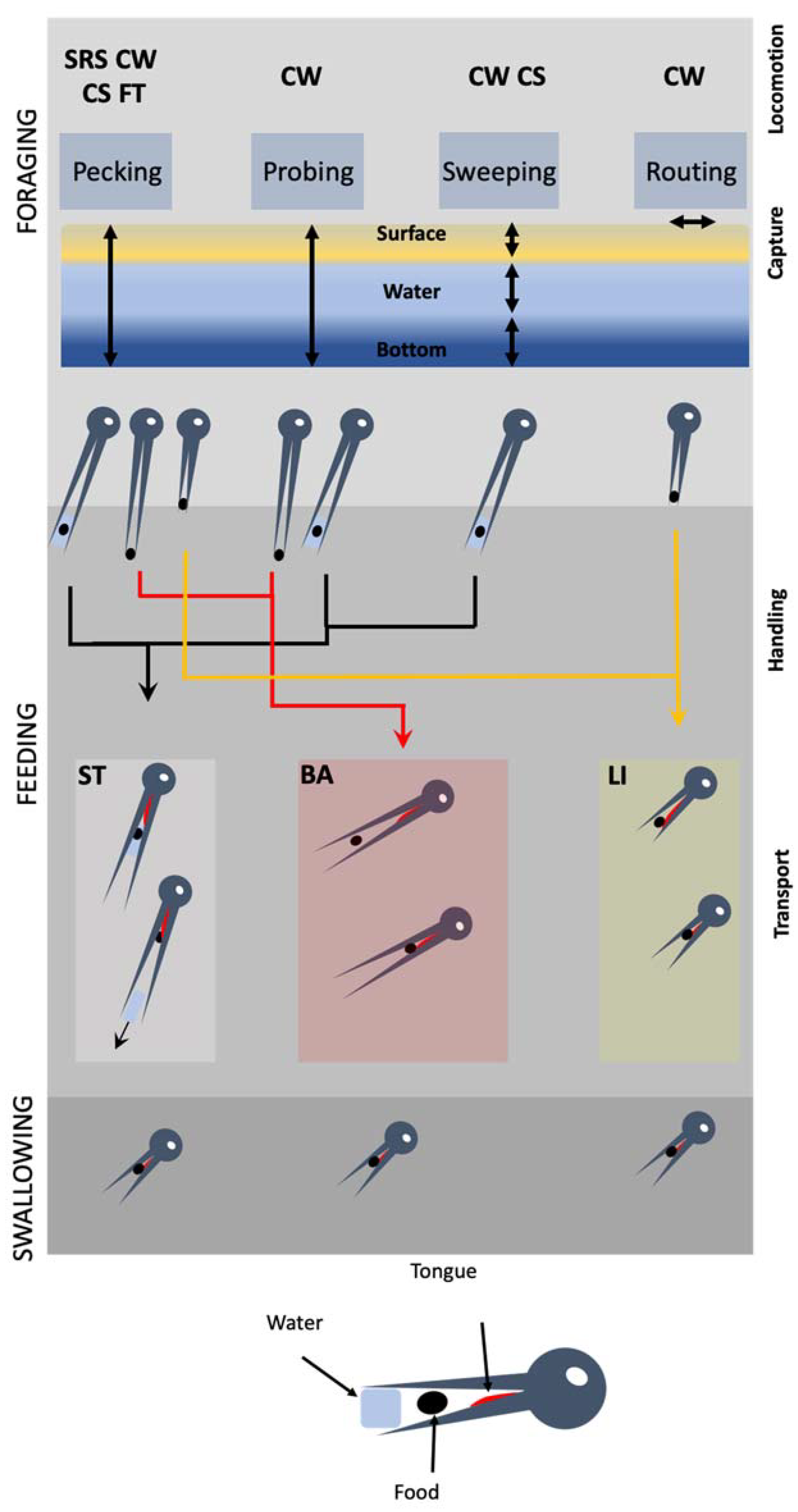
Figure 1.
Hierarchical organization of the involvement of the cranial system during the three stages of food acquisition in shorebirds (foraging, feeding, swallowing). Foraging is divided into locomotion and capture. During locomotion, birds move their legs looking for foods using four stereotyped behaviors: SRS, stop-run-stop, CW, continuous walking, CS, continuous swimming, FT, foot trembling. The four capture behaviors (pecking, probing, sweeping, routing) are used at various water heights. Feeding begins by food handling in which the food particle is neutralized and fragmented if necessary, and oriented to be transported into the pharynx. Three modes of transport exist, each associated with more than one capture behavior: ST, surface tension, BA, ballistic and LI, lingual. Swallowing is the end point of the food acquisition process that is characterized by the entrance of the food particle within the pharynx. This stage does not show interspecific differences.
Figure 1.
Hierarchical organization of the involvement of the cranial system during the three stages of food acquisition in shorebirds (foraging, feeding, swallowing). Foraging is divided into locomotion and capture. During locomotion, birds move their legs looking for foods using four stereotyped behaviors: SRS, stop-run-stop, CW, continuous walking, CS, continuous swimming, FT, foot trembling. The four capture behaviors (pecking, probing, sweeping, routing) are used at various water heights. Feeding begins by food handling in which the food particle is neutralized and fragmented if necessary, and oriented to be transported into the pharynx. Three modes of transport exist, each associated with more than one capture behavior: ST, surface tension, BA, ballistic and LI, lingual. Swallowing is the end point of the food acquisition process that is characterized by the entrance of the food particle within the pharynx. This stage does not show interspecific differences.
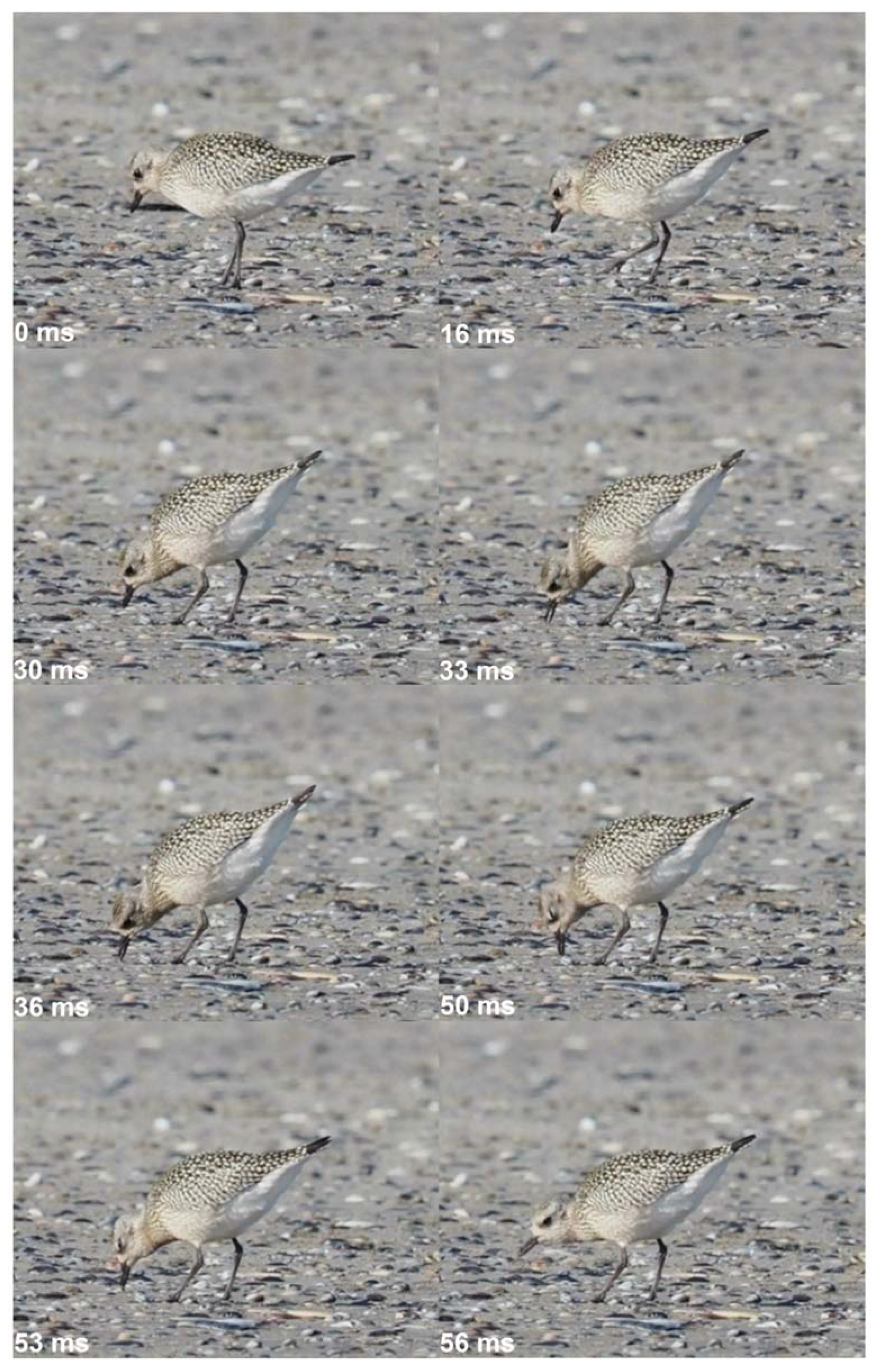
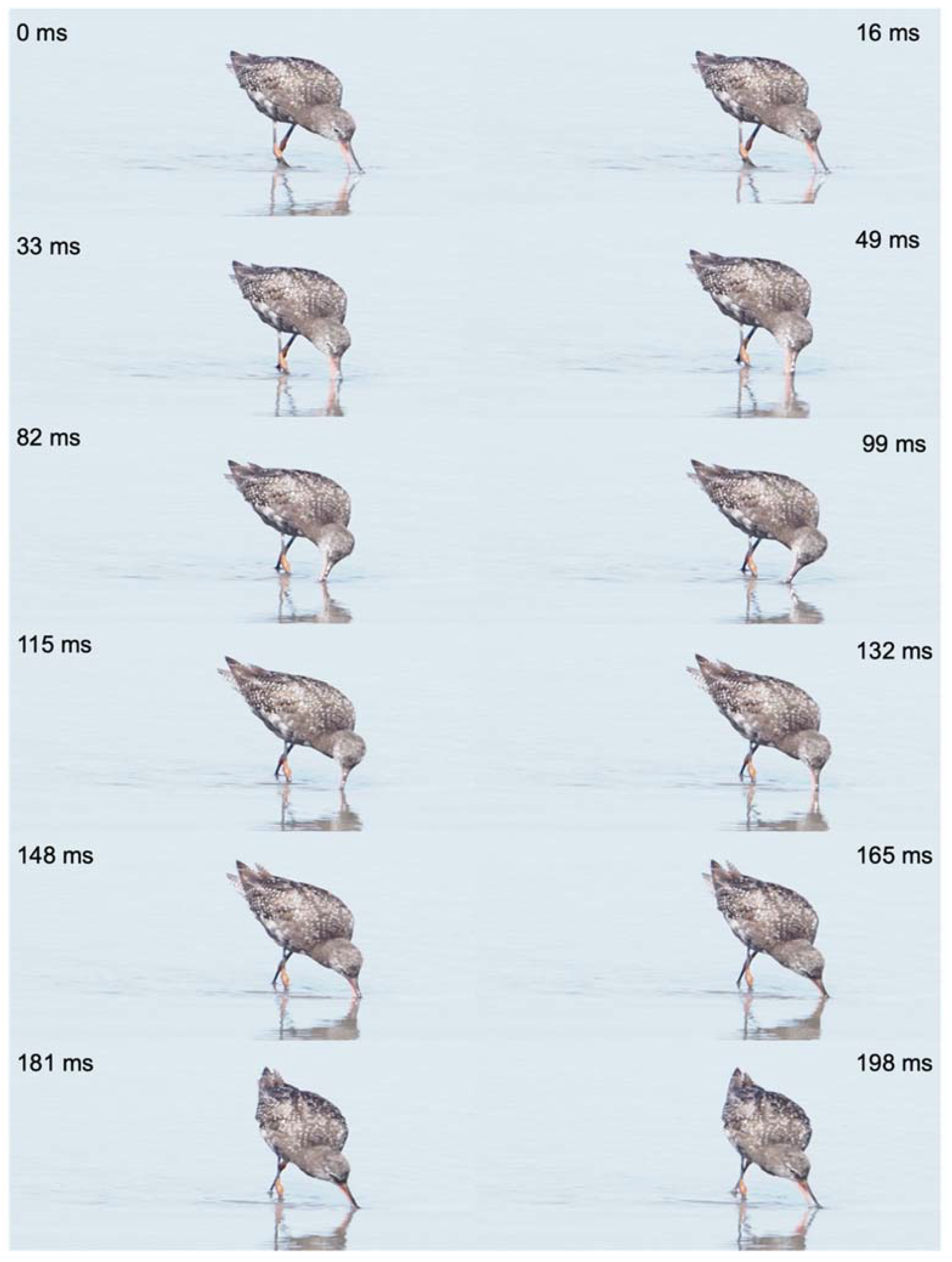
Figure 2.
Foraging and pecking behavior of a grey plover (Pluvialis squatarola), beach of Nieuwpoort (B), September 22nd, 2022. At time 0 ms, the bird locates the prey, and then heads to it (16 ms). The capture behavior begins at time 30 ms, with a rapid spring of the head and the neck, directed towards the prey. The prey is caught on the surface of the ground by opening and closing movements of the beak (33-50 ms), and the capture ends when the prey is at the tip of the beak and the bird raises its head (53-56 ms). Pictures © Michel Baguette, all rights reserved.
Figure 2.
Foraging and pecking behavior of a grey plover (Pluvialis squatarola), beach of Nieuwpoort (B), September 22nd, 2022. At time 0 ms, the bird locates the prey, and then heads to it (16 ms). The capture behavior begins at time 30 ms, with a rapid spring of the head and the neck, directed towards the prey. The prey is caught on the surface of the ground by opening and closing movements of the beak (33-50 ms), and the capture ends when the prey is at the tip of the beak and the bird raises its head (53-56 ms). Pictures © Michel Baguette, all rights reserved.
Figure 3.
Pecking of a black-winged stilt (Himantopus himantopus), Domaine des Oiseaux, Mazère (F-09), April 28th, 2023. The bird keeps its eyes open when submersing its head. Picture © Michel Baguette, all rights reserved.
Figure 3.
Pecking of a black-winged stilt (Himantopus himantopus), Domaine des Oiseaux, Mazère (F-09), April 28th, 2023. The bird keeps its eyes open when submersing its head. Picture © Michel Baguette, all rights reserved.
Figure 4.
Foraging, almost vertical probing behavior and prey handling by a black tailed godwit (Limosa limosa), Pont de Gau, Saintes Maries de la Mer (F-13), July 7th 2023. The bird explores the sediment by using various number of head vertical movements that are organized in cycles according to their vertical back and forth. For instance, cycle 1 ends when the bird lifted its head and began to plunge it back. Cycle 1-4: successive probing beak cycles 1 to 4. Probing can occur either when the bird stops during beak movements, or when the bird moves continuously. Eyes are closed during probing under water. Handling: worm handling after capture, using rynchokinesis (opening of the distal part of the beak while the proximal part remains closed) at 1092 ms. Pictures © Michel Baguette, all rights reserved.
Figure 4.
Foraging, almost vertical probing behavior and prey handling by a black tailed godwit (Limosa limosa), Pont de Gau, Saintes Maries de la Mer (F-13), July 7th 2023. The bird explores the sediment by using various number of head vertical movements that are organized in cycles according to their vertical back and forth. For instance, cycle 1 ends when the bird lifted its head and began to plunge it back. Cycle 1-4: successive probing beak cycles 1 to 4. Probing can occur either when the bird stops during beak movements, or when the bird moves continuously. Eyes are closed during probing under water. Handling: worm handling after capture, using rynchokinesis (opening of the distal part of the beak while the proximal part remains closed) at 1092 ms. Pictures © Michel Baguette, all rights reserved.
Figure 5.
Oblique probing behavior of a greenshank (Tringa nebularia), Ijzermondig, Nieuwpoort (B), September 20th, 2022. The bird advances pushing its ajar beak in front of it, which scrapes away the sediment and excavates food particles. Picture © Michel Baguette, all rights reserved.
Figure 5.
Oblique probing behavior of a greenshank (Tringa nebularia), Ijzermondig, Nieuwpoort (B), September 20th, 2022. The bird advances pushing its ajar beak in front of it, which scrapes away the sediment and excavates food particles. Picture © Michel Baguette, all rights reserved.
Figure 6.
Lateral probing behavior of a spotted redshank (Tringa erythropus), Pont de Gau, Sainte Marie de la Mer (F-13), July 7th 2023. The bird advances forwards, balancing its ajar beak from side to side, which scrapes away the sediment and excavates food particles. Pictures © Michel Baguette, all rights reserved.
Figure 6.
Lateral probing behavior of a spotted redshank (Tringa erythropus), Pont de Gau, Sainte Marie de la Mer (F-13), July 7th 2023. The bird advances forwards, balancing its ajar beak from side to side, which scrapes away the sediment and excavates food particles. Pictures © Michel Baguette, all rights reserved.
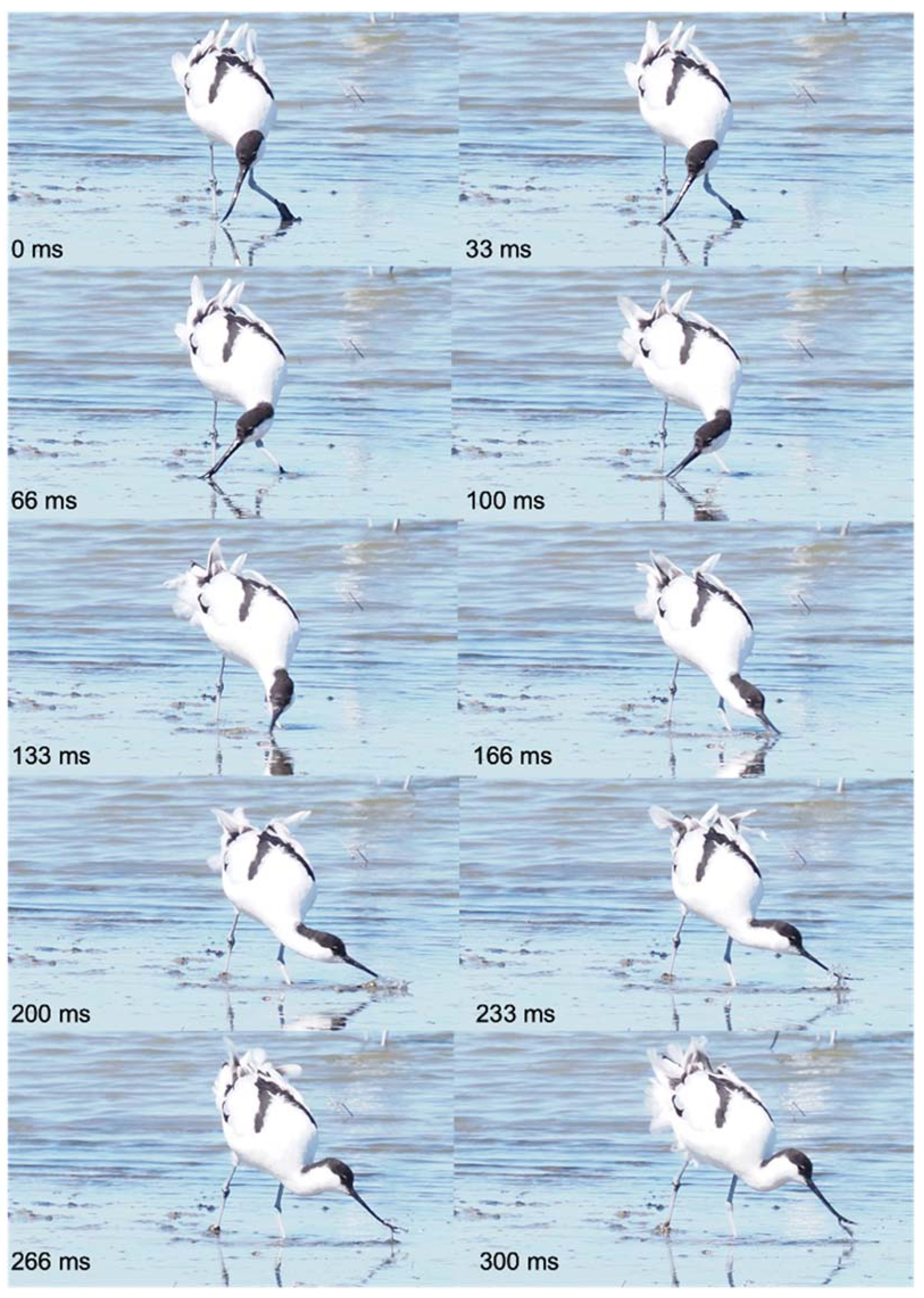
Figure 7.
Foraging and sweeping behavior of an avocet Recuvirostra avosetta, Réserve onithologique du Teich, bassin d’Arcachon (F-33), April 9th, 2022. The bird walking continuously moves its head from side to side at various water depths and stops for food transport. Small preys are captured at the distal tip of the slightly ajar beak during the wide lateral movements of the head, which give this capture behavior its name sweeping. Pictures © Michel Baguette, all rights reserved.
Figure 7.
Foraging and sweeping behavior of an avocet Recuvirostra avosetta, Réserve onithologique du Teich, bassin d’Arcachon (F-33), April 9th, 2022. The bird walking continuously moves its head from side to side at various water depths and stops for food transport. Small preys are captured at the distal tip of the slightly ajar beak during the wide lateral movements of the head, which give this capture behavior its name sweeping. Pictures © Michel Baguette, all rights reserved.
Figure 8.
Sweeping by an avocet (Recuvirostra avosetta), Salin de Giraud, Arles (F-13), September 20th, 2021. The bird closes its eyes when submersing its head. Picture © Michel Baguette, all rights reserved.
Figure 8.
Sweeping by an avocet (Recuvirostra avosetta), Salin de Giraud, Arles (F-13), September 20th, 2021. The bird closes its eyes when submersing its head. Picture © Michel Baguette, all rights reserved.
Figure 9.
Routing behavior of a ruddy turnstone (Arenaria interpres), pier of Oostende (B), September 20th, 2022. The bird lifts kelp by using various powerful and rapid head and beak movements. Eyes are closed and beak is open when the bird is in close contact with kelp. The prey will finally be caught by pecking. Pictures © Michel Baguette, all rights reserved.
Figure 9.
Routing behavior of a ruddy turnstone (Arenaria interpres), pier of Oostende (B), September 20th, 2022. The bird lifts kelp by using various powerful and rapid head and beak movements. Eyes are closed and beak is open when the bird is in close contact with kelp. The prey will finally be caught by pecking. Pictures © Michel Baguette, all rights reserved.
Figure 10.
Stabbing by an oystercatcher (Haematopus ostralegus), beach of Oostende (B), September 20th, 2022. The bird inserts its beak between the valves of a slightly open razor clam, to dissect the shell retractor muscles. Picture © Michel Baguette, all rights reserved.
Figure 10.
Stabbing by an oystercatcher (Haematopus ostralegus), beach of Oostende (B), September 20th, 2022. The bird inserts its beak between the valves of a slightly open razor clam, to dissect the shell retractor muscles. Picture © Michel Baguette, all rights reserved.
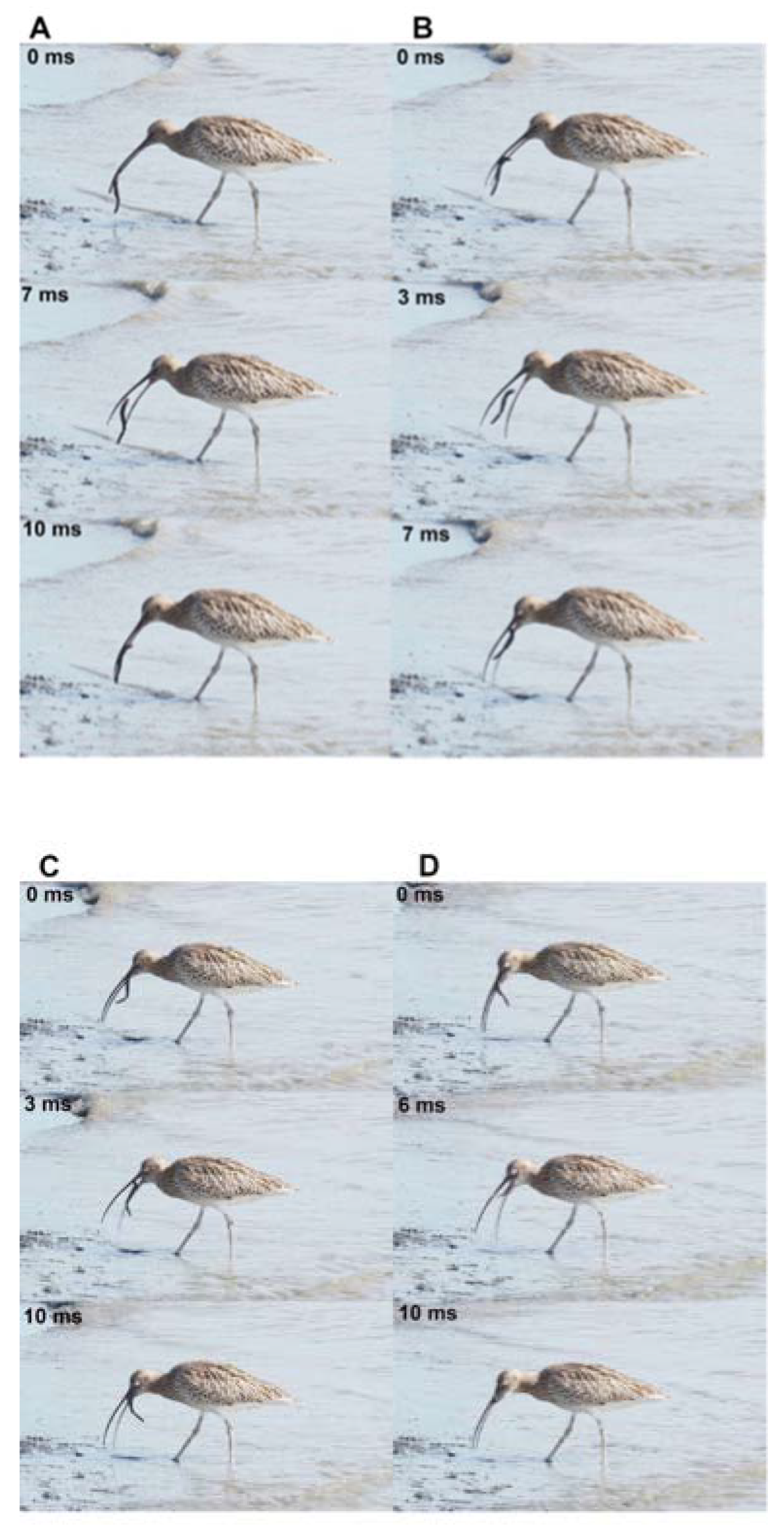
Figure 11.
Four cycles of ballistic transport of a large worm by a Eurasian curlew (Numenius arquata), Ijzermondig, Nieuwpoort (B), September 20th, 2022. A and B, two successive ballistic transports showing the prey moving from the tip of the beak to the tongue. The prey is freed from the beak, which then suddenly moves forwards and recaught the prey so fast that the prey "levitates" during this brief interval. C and D, two lingual cycles allowing the bird to move the prey toward the pharynx and finally swallow this large prey. These successive cycles show how this bird with a long thin beak transports any prey without using water (surface tension). Lingual transport only occurs as soon as the tongue contacts the prey and can act on its posterior displacement toward the pharynx. Pictures © Michel Baguette, all rights reserved.
Figure 11.
Four cycles of ballistic transport of a large worm by a Eurasian curlew (Numenius arquata), Ijzermondig, Nieuwpoort (B), September 20th, 2022. A and B, two successive ballistic transports showing the prey moving from the tip of the beak to the tongue. The prey is freed from the beak, which then suddenly moves forwards and recaught the prey so fast that the prey "levitates" during this brief interval. C and D, two lingual cycles allowing the bird to move the prey toward the pharynx and finally swallow this large prey. These successive cycles show how this bird with a long thin beak transports any prey without using water (surface tension). Lingual transport only occurs as soon as the tongue contacts the prey and can act on its posterior displacement toward the pharynx. Pictures © Michel Baguette, all rights reserved.
Figure 12.
Surface tension transport by a redshank (Tringa totanus), Domaine des Oiseaux, Mazère (F-09), March 28th 2023. The food particle caught by the beak tips is moved along the beak by integrated head movements and surface tension mechanism due to the food inclusion in a water droplet. The black arrow shows the food position along the head cycles. From time 0 ms to 5 ms, bird suddenly moves the head backwards to give energy to the food included in the droplet and opens the beak. The food glides by surface tension along the beak to reach a posterior position at time 10 ms. The bird moves the head forward from time 10 ms to time 15 ms for helping movement of the food in the droplet. Between time 15 ms and 30 ms, the bird does not move the head, and it is the tongue that transports the food to the pharynx. At the same time, the water droplet glides to the beak tip due to the gravity and is expulsed from the beak cavity when the beak closes at time 40 ms. Black arrows: food particle included in water droplet. Red arrow: water droplet expulsed from the beak of the bird. Pictures © Michel Baguette, all rights reserved.
Figure 12.
Surface tension transport by a redshank (Tringa totanus), Domaine des Oiseaux, Mazère (F-09), March 28th 2023. The food particle caught by the beak tips is moved along the beak by integrated head movements and surface tension mechanism due to the food inclusion in a water droplet. The black arrow shows the food position along the head cycles. From time 0 ms to 5 ms, bird suddenly moves the head backwards to give energy to the food included in the droplet and opens the beak. The food glides by surface tension along the beak to reach a posterior position at time 10 ms. The bird moves the head forward from time 10 ms to time 15 ms for helping movement of the food in the droplet. Between time 15 ms and 30 ms, the bird does not move the head, and it is the tongue that transports the food to the pharynx. At the same time, the water droplet glides to the beak tip due to the gravity and is expulsed from the beak cavity when the beak closes at time 40 ms. Black arrows: food particle included in water droplet. Red arrow: water droplet expulsed from the beak of the bird. Pictures © Michel Baguette, all rights reserved.

Figure 13.
Surface transport by a jack snipe (Lymnocryptes minimus), Domaine des Oiseaux, Mazère (F-09), March 28th, 2023. Snipes use surface tension as their only transport behavior. The capture of a prey is followed by the adoption of this very recognizable posture during which the bird raises its head and partially or entirely takes its beak out of the water, maintaining it at approximately 45° to the surface, probably to facilitate the transport compared to a beak held vertically as during probing cycles. Picture © Michel Baguette, all rights reserved.
Figure 13.
Surface transport by a jack snipe (Lymnocryptes minimus), Domaine des Oiseaux, Mazère (F-09), March 28th, 2023. Snipes use surface tension as their only transport behavior. The capture of a prey is followed by the adoption of this very recognizable posture during which the bird raises its head and partially or entirely takes its beak out of the water, maintaining it at approximately 45° to the surface, probably to facilitate the transport compared to a beak held vertically as during probing cycles. Picture © Michel Baguette, all rights reserved.
Figure 14.
Lingual transport in a grey plover (Pluvialis squatarola), beach of Nieuwpoort (B), September 22nd, 2022. The bird uses one jaw-tongue coordinated cycle to move to prey from the beak tips toward the pharynx. The amplitude of the gape cycle is small, and the tongue elongation corresponds to the lower beak length. In plovers, food transport occurs during locomotion, showing an independent motor organization for the cranial and post cranial systems. Black arrow: prey. Red arrow: tongue. Pictures © Michel Baguette, all rights reserved.
Figure 14.
Lingual transport in a grey plover (Pluvialis squatarola), beach of Nieuwpoort (B), September 22nd, 2022. The bird uses one jaw-tongue coordinated cycle to move to prey from the beak tips toward the pharynx. The amplitude of the gape cycle is small, and the tongue elongation corresponds to the lower beak length. In plovers, food transport occurs during locomotion, showing an independent motor organization for the cranial and post cranial systems. Black arrow: prey. Red arrow: tongue. Pictures © Michel Baguette, all rights reserved.
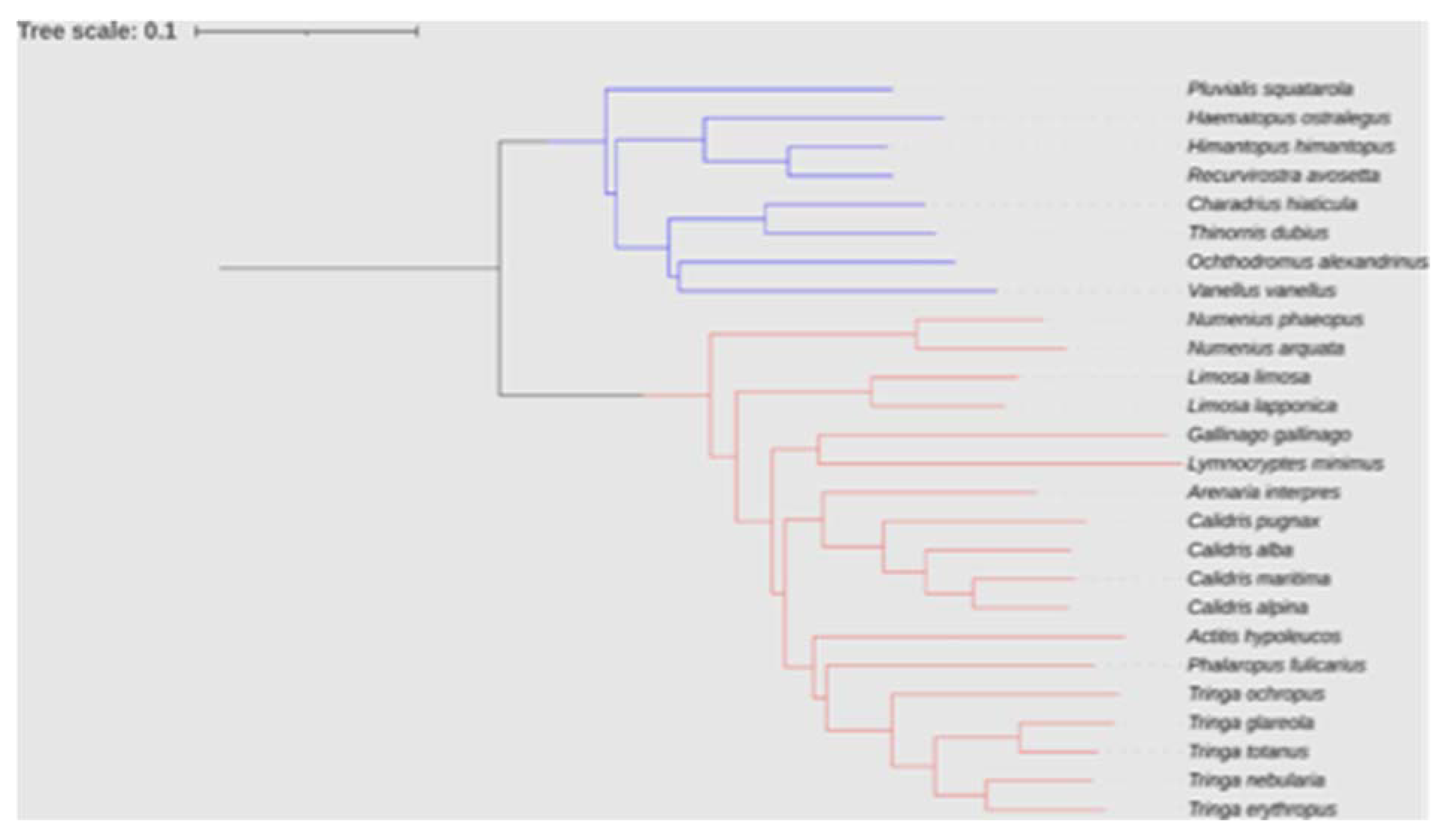
Figure 15.
Phylogenetic relationships between the 26 shorebird species of our data set drawn from the consensus tree of Cerny and Natale [
22], pruned to our 26 study species using iTOL [
23]. Blue and red bars correspond to species belonging to Charadri and Scolopaci, respectively.
Figure 15.
Phylogenetic relationships between the 26 shorebird species of our data set drawn from the consensus tree of Cerny and Natale [
22], pruned to our 26 study species using iTOL [
23]. Blue and red bars correspond to species belonging to Charadri and Scolopaci, respectively.
Table 1.
Locomotion, capture, and transport behaviors observed in our subset of 26 Western Palearctic shorebird species. The order of behaviors is alphabetical, and does not reflect any preference. Abbreviation for locomotion: CW: continuously walking. SRS: stop-run-stop. FT: foot trembling. CS: continuously swimming. Abbreviation for capture: PE: pecking. RO: routing. PR: probing. SW: sweeping. Abbreviation for transport: BA: ballistic. ST: superficial tension. LI: lingual.
Table 1.
Locomotion, capture, and transport behaviors observed in our subset of 26 Western Palearctic shorebird species. The order of behaviors is alphabetical, and does not reflect any preference. Abbreviation for locomotion: CW: continuously walking. SRS: stop-run-stop. FT: foot trembling. CS: continuously swimming. Abbreviation for capture: PE: pecking. RO: routing. PR: probing. SW: sweeping. Abbreviation for transport: BA: ballistic. ST: superficial tension. LI: lingual.