Submitted:
26 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
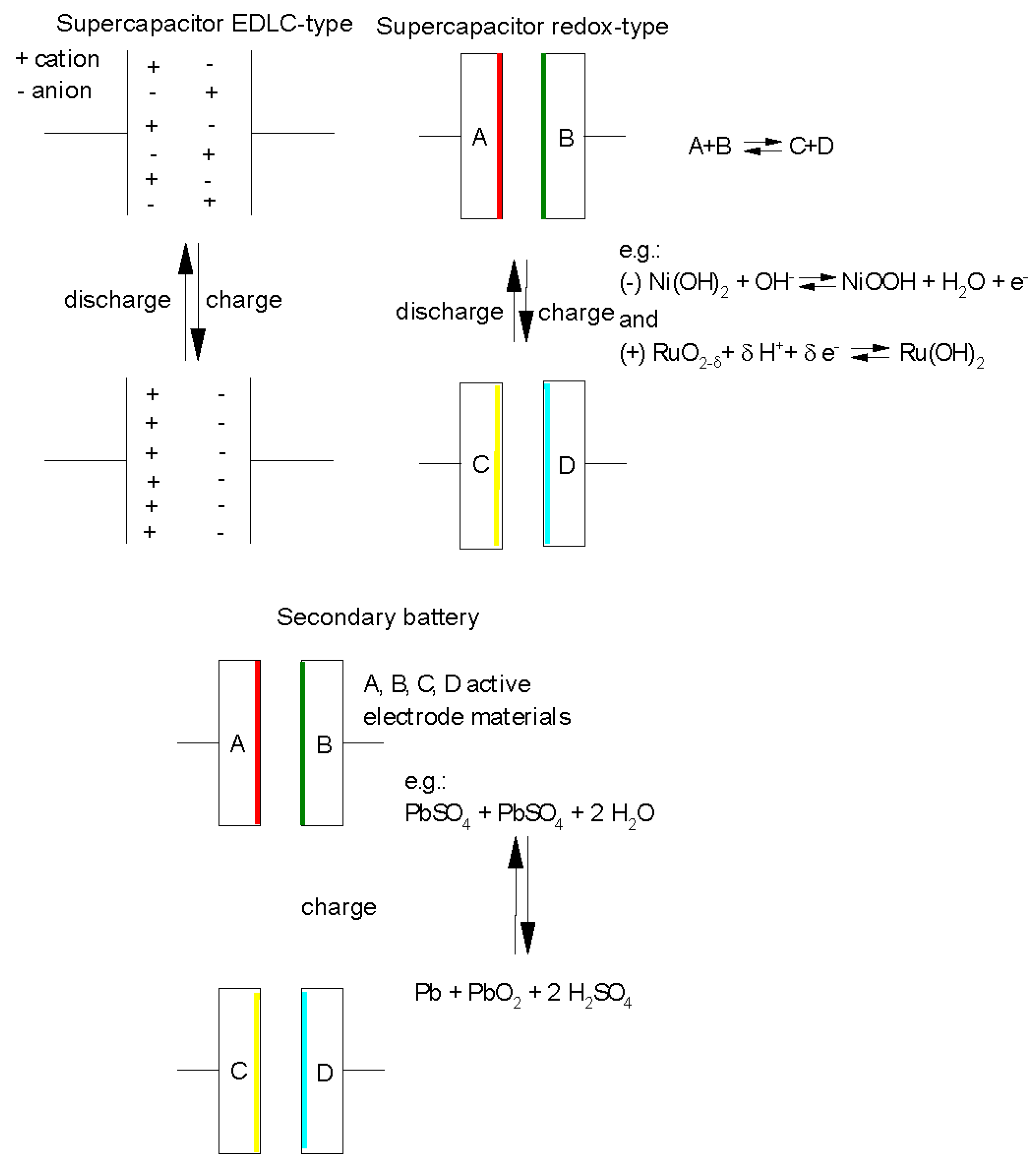
2. The systems
2.1. With carbonaceous supercapacitor electrode materials
2.2. With redox-active supercapacitor electrode materials
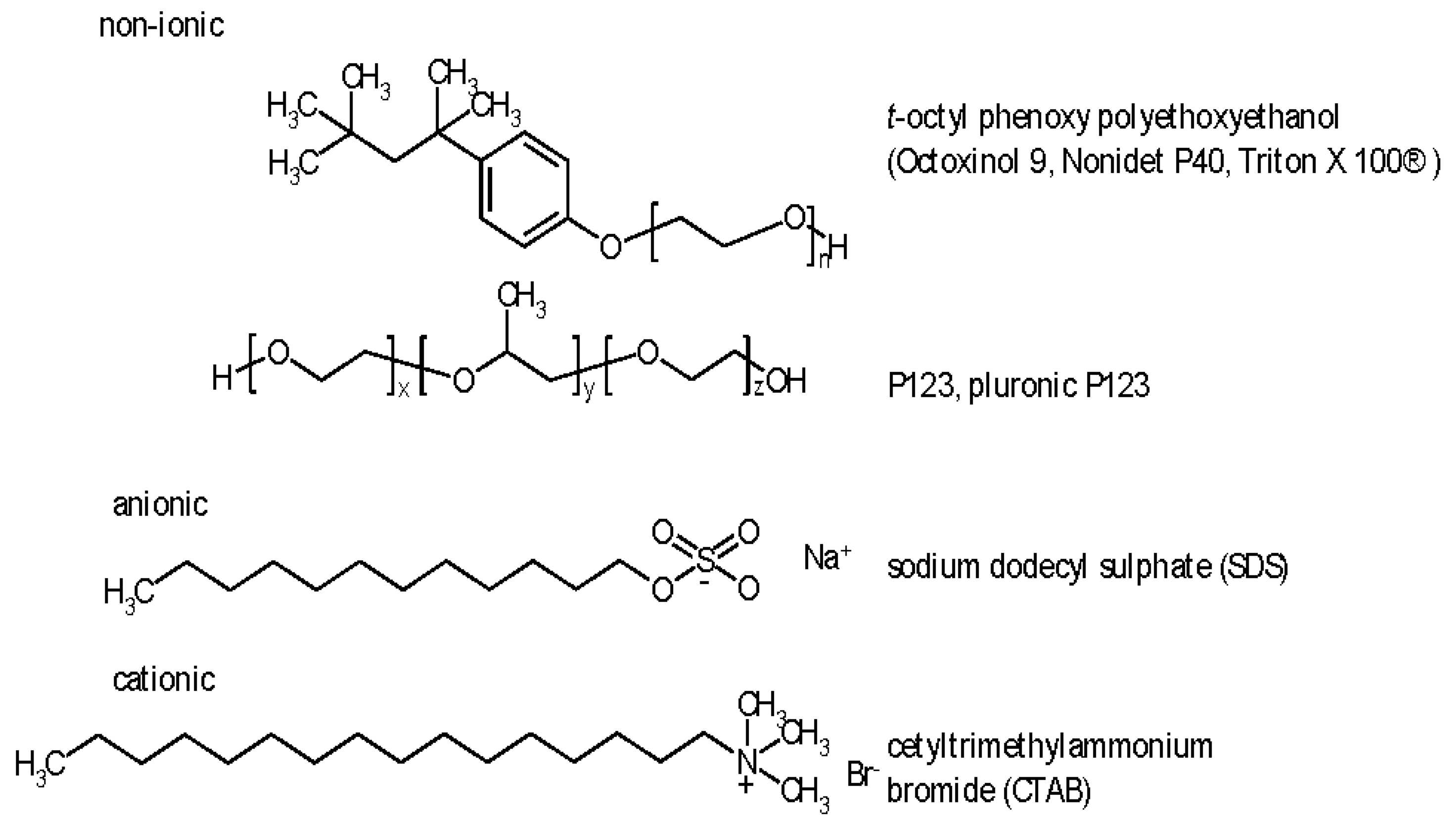
2.3. Other uses of surfactants related to supercapacitors
3. Conclusions
4. Trends and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: from the Leyden jar to electric busses. Chemtexts 2016, 2, 13. [CrossRef]
- Khorate, A.; Kadam, A.V. An overview of patents and recent development in flexible supercapacitors. J. Energy Storage 2022, 52, 104887. [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO-LUMO misconception. Energy Environ. Sci. 2018,11, 2306. [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [CrossRef]
- Xie, X.; Holze, R. Electrode Kinetic Data: Geometric vs. Real Surface Area. Batteries, 2022, 8, 146. [CrossRef]
- Vielstich, W.; Schmickler, W. Elektrochemie II: Kinetik elektrochemischer Systeme, Haase, R., Ed., Steinkopff: Darmstadt, 1976.
- Gileadi, E.; Kirowa-Eisner, E.; Penciner, J. Interfacial Electrochemistry, Addison Wesley: London, 1975.
- Holze, R.; Schneider, J.; Hamann, C.H. Eine neue Methode zur Untersuchung der Elektrosorption reaktiver Verbindungen. Ber. Bunsenges. Phys. Chem. 1988, 92, 1319-1325. [CrossRef]
- Doss, K.S.G.; Kalyanasundaram, A. Effect of surface active substances on the capacity of the electric double layer. Proc. Indian Acad. Sci. 1952, 35A, 27-33. [CrossRef]
- Breyer, B.; Hacobian, S. Tensammetry: A Method of Investigating Surface Phenomena by AC Current Measurements. Aust. J. Sci. Res. Ser. A 1952, 5, 500-520. [CrossRef]
- Plambeck, J.A. Electroanalytical chemistry, Wiley: New York, 1982.
- Holze, R. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry, Volume 9: Electrochemistry, Subvolume A: Electrochemical Thermodynamics and Kinetics, Martienssen W, Lechner MD, Eds., Springer: Berlin, 2007.
- Jehring, H. Elektrosorptionsanalyse mit der Wechselstrompolarographie. Akademie-Verlag: Berlin, 1975.
- Randin, J.P.; Yeager, E. Differential Capacitance Study of Stress-Annealed Pyrolytic Graphite Electrodes. J. Electrochem. Soc. 1971, 118, 711-714. [CrossRef]
- Randin, J.P.; Yeager, E. Differential capacitance study on the basal plane of stress-annealed pyrolytic graphite. J. Electroanal. Chem. 1972, 36, 257-276. [CrossRef]
- Gerischer, H. An Interpretation of the Double-Layer Capacity of Graphite-Electrodes in Relation to the Density of States at the Fermi Level. J. Phys. Chem. 1985, 89, 4249-4251. [CrossRef]
- Randin, J.P.; Yeager, E. Effect of boron addition on the differential capacitance of stress-annealed pyrolytic graphite. J. Electroanal. Chem. 1974, 54, 93-100. [CrossRef]
- Randin, J.P.; Yeager, E. Differential capacitance study on the edge orientation of pyrolytic graphite and glassy carbon electrodes, J. Electroanal. Chem. 58 (1975) 313-322. [CrossRef]
- Velicky, M.; Toth, P.S.; Woods, C.R.; Novoselov, K.S.; Dryfe, R.A.W. Electrochemistry of the Basal Plane versus Edge Plane of Graphite Revisited. J. Phys. Chem. C 2019, 123, 11677-11685. [CrossRef]
- Bauer, H.H.; Spritzer, M.S.; Elving, P.J. Double-Layer capacity at a pyrolytic graphite disk electrode. J. Electroanal. Chem. 1968, 17, 299-306. [CrossRef]
- Krüger, A. Neue Kohlenstoffmaterialien, Teubner-Verlag, Wiesbaden 2007.
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties, WILEY, New York 1988.
- Lobato, B.; Suarez, L.; Guardia, L.; Centeno, T.A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434-445. [CrossRef]
- Stoeckli, F.; Centeno, T.A. Optimization of the characterization of porous carbons for supercapacitors, J. Mater. Chem. A 2013, 1, 6865-6873. [CrossRef]
- Gagnon, E.G. Triangular voltage sweep method for determining double-layer capacity of porous-electrodes 4. Porous carbon in potassium hydroxide. J. Electrochem. Soc. 1975, 122, 521-525. [CrossRef]
- Wen, Y.H.; Cao, G.P.; Cheng, J.; Yang, Y.S. Relationship between electrolyte ion and double-layer capacitance of carbon electrode. Acta Phys. Chim. Sin. 2005, 21, 494-498.
- Chmiola, J. Pore-size ion-size correlations for carbon supercapacitors, PhD-thesis Drexel University, 2009.
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730. [CrossRef]
- Feng, G.; Qiao, R.; Huang, J.; Sumpter, B.G.; Meunier, V. Ion distribution in electrified micropores and its role in the anomalous enhancement of capacitance. ACS Nano 2010, 4, 2382. [CrossRef]
- Ania, C.O.; Pernak, J.; Stefaniak, F.; Raymundo-Piñero, E.; Béguin, F. Polarization-induced distortion of ions in the pores of carbon electrodes for electrochemical capacitors. Carbon, 2009, 47, 3158. [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science, 2006, 313, 1760. [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.L.; Simon, P.; Gogotsi, Y. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew. Chem. Int. Ed. 2008, 47, 3392. [CrossRef]
- Xie, X.; Holze, R. Meaning and Determination of Electrode Surface Area. Available online: URL https://encyclopedia.pub/ entry/41569 (accessed on 02.05.2023).
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties. WILEY: New York, 1988.
- Hsieh, C.T.; Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 2002, 40, 667-674. [CrossRef]
- Wu, Y.; Holze, R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem. Energy Technol. 2021, 7, 1-37. [CrossRef]
- X. Chen, Y. Wu, R. HolzeAg(e)ing and Degradation of Supercapacitors: Causes, Mechanisms, Models and Countermeasures. Molecules 2023, 28, 5028. [CrossRef]
- Rosen, M.J. Surfactants and interfacial phenomena. Wiley: New York 1989.
- Rosen, M.J.; Kunjappu, J.T. Surfactants and interfacial phenomena 4th edn., John Wiley and Sons: Hoboken, 2012.
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources, 1997, 66, 1. [CrossRef]
- Conway, B.E.; Gileadi, E.; Kinetic Theory of Pseudo-Capacitance and Electrode Reactions at Appreciable Surface Coverage. Trans. Faraday Soc. 1962, 58, 2493. [CrossRef]
- Conway, B.E. Transition from "Supercapacitor" to "Battery" behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539. [CrossRef]
- Dubal, D.P.; Holze, R. Synthesis, properties, and performance of nanostructured metal oxides for supercapacitors. Pure Appl. Chem. 2014, 86, 611. [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Gomez-Romero, P.; Kim, D.H. Fundamentals of Binary Metal Oxide-Based Supercapacitors in: Metal Oxides in Supercapacitors (D.P. Dubal, P. Gomez-Romero eds.) Elsevier, Amsterdam 2017, p. 79-98. [CrossRef]
- Holze, R. From current peaks to waves and capacitive currents-on the origins of capacitor-like electrode behavior. J. Solid State Electr. 2017, 21, 2601‒2607. [CrossRef]
- Grahame, D.C. Properties of the Electrical Double Layer at a Mercury Surface. I. Methods of Measurement and Interpretation of Results. J. Am. Chem. Soc. 1941, 63, 1207-1215. [CrossRef]
- Szubzda, B.; Szmaja, A.; Halama, A. Influence of structure and wettability of supercapacitor electrodes carbon materials on their electrochemical properties in water and organic solutions. Electrochim. Acta 2012, 86, 255-259. [CrossRef]
- Trasatti, S. Progress in the Understanding of the Structure of the Metal Electrode/Solution Interface. Evolution of the Concept of Hydrophilicity. Croat. Chem. Acta, 1987, 60, 357-370.
- Yoshida, A.; Tanahashi, I.; Nishino, A.; Effect of concentration of surface acidic functional groups on electric double-layer properties of activated carbon fibers. Carbon 1990, 28, 611-615. [CrossRef]
- Gu, W.; Yushin, G.; Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. WIREs Energy Environ. 2014, 3, 424-473. [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81 1075-1078. [CrossRef]
- Le, T.T.Y.; Hussain, S.; Lin, S.Y. A study on the determination of the critical micelle concentration of surfactant solutions using contact angle data. J. Mol. Liq. 2019, 294, 111582. [CrossRef]
- Hunter, R.J. Foundations of colloid science. Oxford University Press: Oxford, 2001.
- Bard, A.J.; Inzelt, G.; Scholz, F. Electrochemical Dictionary 2edn,. Springer-Verlag: Heidelberg, 2012. [CrossRef]
- Fang, B.; Binder, L. Enhanced surface hydrophobisation for improved performance of carbon aerogel electrochemical capacitor. Electrochim. Acta 2007, 52, 6916-6921. [CrossRef]
- Fang, B.; Binder, L. A modified activated carbon aerogel for high-energy storage in electric double layer capacitors. J. Power Sources 2006, 163, 616-622. [CrossRef]
- Wei, Y.Z.; Fang, B.; Iwasa, S.; Kumagai, M. A novel electrode material for electric double-layer capacitors. J. Power Sources 2005, 141, 386-391. [CrossRef]
- He, T.; Ren, X.; Cai, K.; Wei, Y.; Sun, S. Electrochemical performance of activated carbon treated by vacuum impregnation using fluorinated surfactant. Mater. Technol. 2013, 28, 364-369. [CrossRef]
- Fic, K.; Lota, G.; Frackowiak, E. Effect of surfactants on capacitance properties of carbon electrodes 2011, Mater. Res. Soc. Symp. Proc. 1333. [CrossRef]
- Fic, K.; Lota, G.; Frackowiak, E. Effect of surfactants on capacitance properties of carbon electrodes. Electrochim. Acta 2012, 60, 206-212. [CrossRef]
- Fic, K.; Lota, G.; Frackowiak, E. Electrochemical properties of supercapacitors operating in aqueous electrolyte with surfactants. Electrochim. Acta 2010, 55, 7484-7488. [CrossRef]
- Matile S, Som A, Sord N‚Recent synthetic ion channels and pores. Tetrahedron 2004, 60, 6405-6435. [CrossRef]
- Bo, Z.; Huang, Z.; Zheng, Z.; Chen, Y.; Yan, J.; Cen, K.; Yang, H.; Ostrikov, K. Accelerated ion transport and charging dynamics in more ionophobic sub-nanometer channels. Energy Stor. Mater. 2023, 59, 102797. [CrossRef]
- Kondrat, S.; Wu, P.; Qiao, R.; Kornyshev, A.A. Accelerating charging dynamics in subnanometre pores. Nature Mater. 2014, 13, 387-393. [CrossRef]
- Lota, G.; Centeno, T.A.; Frackowiak, E.; Stoeckli, F. Improvement of the structural and chemical properties of a commercial activated carbon for its application in electrochemical capacitors. Electrochim.Acta 2008, 53, 2210-2216. [CrossRef]
- Holze, R.; Bełtowska-Brzezinska, M. On the adsorption of aliphatic alcohols on gold. Electrochim. Acta 1985, 30, 937-939. [CrossRef]
- Holze, R.; Bełtowska-Brzezinska, M. On the adsorption of aliphatic alcohols on gold Part II. J. Electroanal. Chem. 1986, 201, 387-396. [CrossRef]
- Kurc, B.; Pigłowska, M.; Rymaniak, Ł; Fuć, P. Modern nanocomposites and hybrids as electrode materials used in energy carriers. Nanomaterials 2021, 11, 1-45. [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J.Energy Storage 2019, 21, 801‒825. [CrossRef]
- Chakraborty, S.; Mary, N.L. Review-An Overview on Supercapacitors and Its Applications. J. Electrochem. Soc. 2022, 169, 020552. [CrossRef]
- Dhandapani, E.; Thangarasu, S.; Ramesh, S.; Ramesh, K.; Vasudevan, R.; Duraisamy, N. Recent development and prospective of carbonaceous material, conducting polymer and their composite electrode materials for supercapacitor-A review. J. Energy Storage 2022, 52, 104937. [CrossRef]
- Patel, K.K.; Singhal, T.; Pandey, V.; Sumangala, T.P.; Sreekanth, M.S. Evolution and recent developments of high performance electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103366. [CrossRef]
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Elkodous, M.A.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S. Advanced materials and technologies for supercapacitors used in energy conversion and storage: a review, Environm. Chem. Lett. 2021, 19, 375‒439. [CrossRef]
- Bigdeloo, M.; Kowsari, E.; Ehsani, A.; Chinnappan, A.; Ramakrishna, S.; Ali Akbari, R. Review on innovative sustainable nanomaterials to enhance the performance of supercapacitors. J. Energy Storage 2021, 37, 102474. [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [CrossRef]
- Li, Z.; Xu, K.; Pan, Y. Recent development of Supercapacitor Electrode Based on Carbon Materials. Nanotechnol. Rev. 2019, 8, 35‒49. [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30‒40. [CrossRef]
- Suriyakumar, S.; Bhardwaj, P.; Grace, A.N.; Stephan, A.M. Role of Polymers in Enhancing the Performance of Electrochemical Supercapacitors: A Review. Batteries&Supercaps 2021, 4, 571‒584. [CrossRef]
- Kiamahalleh, M.V.; Zein, S.H.S.; Najafpour, G.; Abd Sata, S.; Buniran, S. Multiwalled carbon nanotubes based nanocomposites for supercapacitors: A review of electrode materials. Nano 2012, 7, 12300022. [CrossRef]
- Dubal, D.P.; Wu, Y.; Holze, R. Supercapacitors as fast storage systems for electric energy. Bunsen-Magazin 2015, 17, 216‒227.
- Shukla, A.K.; Sampath, S.; Vijayamohanan, K. Electrochemical supercapacitors: Energy storage beyond batteries. Curr. Sci. 2000, 79, 1656‒1661.
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: from the Leyden jar to electric busses. ChemTexts 2016, 2, 13. [CrossRef]
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery - Fundamentals and Applications, CRC Press: Boca Raton, 2013.
- Inamuddin, Ahmer, M.F.; Asiri, A.M.; Zaidi, S. Electrochemical Capacitors in: Materials Research Foundations, Materials Research Forum LLC: Millersville, 2018; Volume 26.
- Stevic, Z. Supercapacitor Design and Applications. ExLi4EvA2016 2016.
- Miller, J.M. Ultracapacitor Applications, The Institution of Engineering and Technology: London, 2011.
- Shaikh, N.S.; Ubale, S.B.; Mane, V.J.; Shaikh, J.S.; Lokhande, V.C.; Praserthdam, S.; Lokhande, C.D.; Kanjanaboos, P. Novel electrodes for supercapacitor: Conducting polymers, metal oxides, chalcogenides, carbides, nitrides, MXenes, and their composites with graphene. J. Alloys Compd. 2022, 893, 161998. [CrossRef]
- Beguin, F.; Frackowiak, E. Supercapacitors, Wiley-VCH: Weinheim, 2013.
- Holze, R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes-An Introduction. Polymers 2020, 12, 1835. [CrossRef]
- Ul Hoque, Md.I.; Holze, R. Intrinsically Conducting Polymer Composites as Active Masses in Supercapacitors. Polymers 2023, 15, 730. [CrossRef]
- Wang, B.; Guo, P.; Bi, H.; Li, Q.; Zhang, G.; Wang, R.; Liu, J.; Zhao, X.S. Electrocapacitive properties of MnFe2O4 electrodes in aqueous LiNO3 electrolyte with surfactants. Int. J. Electrochem. Sci. 2013, 8, 8966-8977. [CrossRef]
- Goddard, E.D.; Ananthapadmanabhan, K.P. Interactions of Surfactants with Polymers and Protein.s CRC Press: Boca Raton, 1993.
- Kuo S L, Wu N L. Electrochemical capacitor of MnFe2O4 with NaCl electrolyte. Electrochem. Solid-State Lett. 2005, 8, A495-A499. [CrossRef]
- Aydin, A.; Patat, S.; Ulgen, A.; Sahan, H.; Fatma; Dokan, K.; Veziroglu, S. Synthesis, characterization and improvement of α-Co(OH)2 for supercapacitor applications 2014 International Conference on Optimization of Electrical and Electronic Equipment, OPTIM 2014, 1087-1091. [CrossRef]
- Ge, Y.; Roscher, J.; Holze, R.; Increased capacitance of metal oxide-based supercapacitor electrodes caused by surfactant addition to the electrolyte solution. J. Nanosci. Nanotechnol. 2020, 20, 7544-7552. [CrossRef]
- Ghasemi, S.; Ahmadi, F. Effect of surfactant on the electrochemical performance of graphene/iron oxide electrode for supercapacitor. J.Power Sources 2015, 289, 129-137. [CrossRef]
- Malik, R.; Lata, S.; Malik, R.S. Electrochemical behavior of composite electrode based on sulphonated polymeric surfactant (SPEEK/PSS) incorporated polypyrrole for supercapacitor. J. Electroanal. Chem. 2019, 835, 48-59. [CrossRef]
- Sumboja, A.; Foo, C.Y.; Yan, J.; Yan, C.; Gupta, R.K.; Lee, P.S. Significant electrochemical stability of manganese dioxide/polyaniline coaxial nanowires by self-terminated double surfactant polymerization for pseudocapacitor electrode. J. Mater. Chem. 2012, 22, 23921-23928. [CrossRef]
- Zhang, K.; Mao, L.; Zhang, L.L.; On Chan, H.S.; Zhao, X.S.; Wu, J. Surfactant-intercalated, chemically reduced graphene oxide for high performance supercapacitor electrodes. J. Mater. Chem. 2011, 21, 7302-7307. [CrossRef]
- Chen, X.; Kierzek, K.; Jiang, Z.; Chen, H.; Tang, T.; Wojtoniszak, M.; Kalenczuk, R.J.; Chu, P.K.; Borowiak-Palen, E. Synthesis, growth mechanism, and electrochemical properties of hollow mesoporous carbon spheres with controlled diameter J. Phys. Chem. C 2011, 115, 17717-17724. [CrossRef]
- Chen, X.; Cendrowski, K.; Srenscek-Nazzal, J.; Rümmeli, M.; Kalenczuk, R.J.; Chen, H.; Chu, P.K.; Borowiak-Palen, E.; Fabrication method of parallel mesoporous carbon nanotubes. Coll. Surf. A 2011, 377, 150-155. [CrossRef]
- Chen, X.; Kierzek, K.; Kalenczuk, R.J.; Mijowska, E.; Jiang, Z.; Tang, T. New synthesis method of sword-sheath structured carbon nanotubes. 2012 2012 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, 3M-NANO 2012 - Conference Proceedings, 297-300. [CrossRef]
- Chen, X.; Kierzek, K.; Wenelska, K.; Cendrowski, K.; Gong, J.; Wen, X.; Tang, T.; Chu, P.K.; Mijowska, E. Electrochemical characteristics of discrete, uniform, and monodispersed hollow mesoporous carbon spheres in double-layered supercapacitors. Chem. Asian J. 2013, 8, 2627-2633. [CrossRef]
- Wilgosz, K.; Chen, X.; Kierzek, K.; Machnikowski, J.; Kalenczuk, R.J.; Mijowska, E. Template method synthesis of mesoporous carbon spheres and its applications as supercapacitors. Nanoscale Res. Lett. 2012, 7, 269. [CrossRef]
- Kong, L.B.; Lu, C.; Liu, M.C.; Luo, Y.C.; Kang, L. Effect of surfactant on the morphology and capacitive performance of porous NiCo2O4. J. Solid State Electr. 2013, 17, 1463-1471. [CrossRef]
- Fang, B.; Wei, Y.Z.; Suzuki, K.; Kumagai, M.; Surface modification of carbonaceous materials for EDLCs application. Electrochim. Acta 2005, 50, 3616-3621. [CrossRef]
- Fang, B.; Wei, Y.Z.; Kumagai, M. Modified carbon materials for high-rate EDLCs application. J. Power Sources 2006, 155 487-491. [CrossRef]
- Dettlaff, A.; Wilamowska, M. Electrochemical synthesis and characterization of nanocomposites based on poly(3,4-ethylenedioxythiophene) and functionalized carbon nanotubes. Synth. Met. 2016, 212, 31-43. [CrossRef]
- Liu, P.; Wang, X.; Li, H.; Facile preparation of string-like composite of hollow PPy nanospheres decorated on the carbon nanotubes. Synth. Met. 2014, 189 173-176. [CrossRef]
- Alshahrie, A.; Ansari, M.O. High Performance Supercapacitor Applications and DC Electrical Conductivity Retention on Surfactant Immobilized Macroporous Ternary Polypyrrole/Graphitic-C3N4@Graphene Nanocomposite. Electron. Mater. Lett. 2019, 15, 238-246. [CrossRef]
- Zhang, L.L.; Zhao, S.; Tian, X.N.; Zhao, X.S. Layered graphene oxide nanostructures with sandwiched conducting polymers as supercapacitor electrodes. Langmuir 2010, 26, 17624-17628. [CrossRef]
- Meng, Q.; Wang, K.; Guo, W.; Fang, J.; Wei, Z.; She, X. Thread-like supercapacitors based on one-step spun nanocomposite yarns. Small 2014, 10, 3187-3193. [CrossRef]
- Athira, A.R.; Deepthi, S.; Xavier, T.S. Impact of an anionic surfactant on the enhancement of the capacitance characteristics of polyaniline-wrapped graphene oxide hybrid composite. Bull. Mater. Sci. 2021, 44, 178. [CrossRef]
- Ma, Z.; Zhao, J.; Fan, Y.; Qin, X.; Shao, G.; High surface area of crystalline/amorphous ultrathin MnO2 nanosheets electrode for high-performance flexible micro-supercapacitors. J. Alloys Compd. 2022, 920, 166012. [CrossRef]
- Maheswari, N.; Muralidharan, G. Supercapacitor Behavior of Cerium Oxide Nanoparticles in Neutral Aqueous Electrolytes. Energy Fuels 2015, 29, 8246-8253. [CrossRef]
- Yuan, R.; Li, H.; Zhang, X.; Zhu, H.; Zhao, J.; Chen, R. Facile one-pot solvothermal synthesis of bifunctional chrysanthemum-like cobalt-manganese oxides for supercapacitor and degradation of pollutants. J. Energy Storage 2020, 29, 101300. [CrossRef]
- Yoon, S.; Oh, S.M.; Lee, C. Direct template synthesis of mesoporous carbon and its application to supercapacitor electrodes. Mater. Res. Bull. 2009, 44, 1663-1669. [CrossRef]
- Idisi, D.O.; Oke, J.A.; Bello, I.T. Graphene oxide/Au nanoparticles: Synthesis, properties, and application: A mini-review., Int. J. Energy Res. 2021, 45, 19772-19788. [CrossRef]
- Díez, N.; Sevill,a M.; Fuertes, A.B. Dense (non-hollow) carbon nanospheres: synthesis and electrochemical energy applications. Mater. Today Nano 2021, 16, 100147. [CrossRef]
- Castro-Gutiérrez, J.; Díez, N.; Sevill,a M.; Izquierdo, M.T.; Celzard, A.; Fierro, V. Model carbon materials derived from tannin to assess the importance of pore connectivity in supercapacitors. Renew. Sustain. Energy Rev. 2021, 151, 111600. [CrossRef]
- Jia, S.; Guo, Q.; Shen, M.; Gao, Q.; Wang, K. Controlled synthesis of carbon spheres via the modulation of the hydrophobic length of fatty aldehyde for supercapacitors. Coll. Surf. A 2022, 636, 128064. [CrossRef]
- Kim, Y.S.; Sohn, J.S.; Ju, H.R.; Inamdar, A.I.; Im, H.; Kim, H. Effect of surfactants on PANI morphologies and supercapacitive properties. J. Kor. Phys. Soc. 2012, 60, 1767-1771. [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [CrossRef]
- Zhang, X.; Wang, X.;, Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Sources 2012, 216, 290-296. [CrossRef]
- Zhang, X.; Wang, X.; Su, J.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; The effects of surfactant template concentration on the supercapacitive behaviors of hierarchically porous carbons. J. Power Sources 2012, 199, 402-408. [CrossRef]
- Reiman, K.H.; Brace, K.M.; Gordon-Smith, T.J.; Nandhakumar, I.; Attard, G.S.; Owen, J.R. Lithium insertion into TiO2 from aqueous solution - Facilitated by nanostructure. Electrochem. Commun. 2006, 8, 517-522. [CrossRef]
- Yang, L.; Wu, W.; Ohki, Y.; Feng, Y.; Li, S.; Enhanced conductivity of polyaniline in the presence of nonionic amphiphilic polymers and their diverse morphologies. J. Appl. Polym. Sci. 2017, 134, 45547. [CrossRef]
- Zhang, L.; Zheng, W.; Jiu, H.; Ni, C.; Chang, J.; Qi, G. The synthesis ofNiO and NiCo2O4 nanosheets by a new method and their excellent capacitive performance for asymmetric supercapacitor. Electrochim. Acta 2016, 215, 212-222. [CrossRef]
- Liu, A.; Lv, Y.; Mu, J.; Guo, Z.; Pei, Z.; Zhang, X.; Bai, Y.; Xie, H.; Che, H. Facile synthesis of hollow Ni0.2Mn0.8O1.5 twin microspheres for electrochemical energy storage. J. Appl. Electrochem. 2018, 48, 15-26. [CrossRef]
- Krishnan, S.G.; Harilal, M.; Yar, A.; Vijayan, B.L.; Dennis, J.O.; Yusoff, M.M.; Jose, R. Critical influence of reduced graphene oxide mediated binding of M (M = Mg, Mn) with Co ions, chemical stability and charge storability enhancements of spinal-type hierarchical MCo2O4 nanostructures Electrochim. Acta 2017, 243, 119-128. [CrossRef]
- Patra, S.; Munichandraiah, N. Supercapacitor studies of electrochemically deposited PEDOT on stainless steel substrate. J. Appl. Polym. Sci. 2007, 106, 1160-1171. [CrossRef]
- Zhang, H.; Wang, Y.; Wang, C. Influence of surfactant on the capacitive performance of manganese dioxide prepared at different temperatures. Energy Convers.Management 2013, 74, 286-292. [CrossRef]
- Kim, Y.S.; Sohn, J.S.; Ju, H.R.; Inamdar, A.I.; Im, H.; Kim, H. Effect of surfactants on PANI morphologies and supercapacitive properties. J. Kor. Phys. Soc. 2012, 60 ,1767-1771. [CrossRef]
- Kathuroju, P.K.; Nagaraju, J. Supercapacitor studies on globular polypyrrole microstructures developed by a facile electrochemical route. Micro Nano Lett. 2011, 6, 1002-1006. [CrossRef]
- Lin, Y.; Zhang, H.; Liao, H.; Zhao, Y.; Li, K. A physically crosslinked, self-healing hydrogel electrolyte for nano-wire PANI flexible supercapacitors. Chem. Eng. J. 2019, 367, 139-148. [CrossRef]
- Cui, L.; Huang, L.; Ji, M.; Wang, Y.; Shi, H.; Zuo, Y.; Kang, S. High-performance MgCo2O4 nanocone arrays grown on three-dimensional nickel foams: Preparation and application as binder-free electrode for pseudo-supercapacitor. J. Power Sources 2016, 333, 118-124. [CrossRef]
- Sharma, A.K.; Chaudhary, G.; Bhardwaj, P.; Kaushal, I.; Duhan, S. Studies on Metal Doped Polyaniline-Carbon Nanotubes Composites for High Performance Supercapacitor. Curr. Anal. Chem. 2017, 13, 277-284. [CrossRef]
- Singu, B.S.; Palaniappan, S.; Yoon, K.R. Polyaniline-nickel oxide nanocomposites for supercapacitor. J. Appl. Electrochem. 2016, 46, 1039-1047. [CrossRef]
- Saranya, S.; Selvan, R.K.; Priyadharsini, N. Synthesis and characterization of polyaniline/MnWO4 nanocomposites as electrodes for pseudocapacitors. Appl. Surf. Sci. 2012, 258, 4881-4887. [CrossRef]
- Zou, B.; Gong, S.; Wang, Y.; Liu, X. Tungsten Oxide and Polyaniline Composite Fabricated by Surfactant-Templated Electrodeposition and Its Use in Supercapacitors. J. Nanomater. 2014, 2014, 813120. [CrossRef]
- Sidhu, N.K.; Rastogi, A.C. Vertically aligned ZnO nanorod core-polypyrrole conducting polymer sheath and nanotube arrays for electrochemical supercapacitor energy storage. Nanoscale Res.Lett. 2014, 9, 453. [CrossRef]
- Shanmugavadivel, M.; Violet Dhayabaran, V.; Subramanian, M. Fabrication of a Novel Polymer Nanohybrid Electrode Material PANI-BaMnO3 for High Power Supercapacitor Application. Port. Electrochim. Acta 2017, 35, 225-232. [CrossRef]
- Sen, P.; De, A. Electrochemical performances of poly(3,4-ethylenedioxythiophene)-NiFe2O4 nanocomposite as electrode for supercapacitor. Electrochim. Acta 2010, 55, 4677-4684. [CrossRef]
- Krishnan, S.G.; Harilal, M.; Misnon, I.I.; Reddy, M.V.; Adams, S.; Jose, R. Effect of processing parameters on the charge storage properties of MgCo2O4 electrodes. Ceram. Int. 2017, 43, 12270-12279. [CrossRef]
- Singu, B.S.; Male, U.; Srinivasan, P:; Pabba, S. Use of surfactant in aniline polymerization with TiO2 to PANI-TiO2 for supercapacitor performance. J. Solid State Electr. 2014, 18, 1995-2003. [CrossRef]
- Qian, A.; Zhuo, K.; Shin, M.S.; Chun, W.W.; Choi, B.N.; Chung, C.H. Surfactant Effects on the Morphology and Pseudocapacitive Behavior of V2O5·H2O. ChemSusChem 2015, 8, 2399-2406. [CrossRef]
- Zhao, W.; Wang, W.; Peng, J.; Chen, T.; Jin, B.; Liu, S.; Huang, W.; Zhao, Q. Wrinkled two-dimensional ultrathin Cu(ii)-porphyrin framework nanosheets hybridized with polypyrrole for flexible all-solid-state supercapacitors. Dalton Trans. 2019, 48, 9631-9638. [CrossRef]
- Jeong, G.H.; Lee, I.; Kang, J.G.; Lee, H.; Yoon, S.; Kim, S.W. Mesoporous hollow carbons on graphene and their electrochemical properties. RSC Adv. 2015, 5, 73119-73125. [CrossRef]
- Patra, S.; Munichandraiah, N. Supercapacitor studies of electrochemically deposited PEDOT on stainless steel substrate. J. Appl. Polym. Sci. 2007, 106, 1160-1171. [CrossRef]
- Zhang, H.; Wang, Y.; Wang, C. Influence of surfactant on the capacitive performance of manganese dioxide prepared at different temperatures. Energy Convers. Managem. 2013, 74, 286-292. [CrossRef]
- Devaraj, S.; Munichandraiah, N.; The effect of nonionic surfactant Triton X-100 during electrochemical deposition of MnO2 on its capacitance properties. J. Electrochem. Soc. 2007, 154, A901-A909. [CrossRef]
- Kathuroju, P.K.; Nagaraju, J,.;Supercapacitor studies on globular polypyrrole microstructures developed by a facile electrochemical route. Micro Nano Lett. 2011, 6, 1002-1006. [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S.; Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56-77. [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Wang, H.; Lei, Z. High-performance supercapacitor based on multi-structural CuS@polypyrrole composites prepared by in situ oxidative polymerization. J. Mater. Chem. A 2014, 2, 3303-3307. [CrossRef]
- Nagaraju, P.; Vasudevan, R.; Alsalme, A.; Alghamdi, A.; Arivanandhan, M.; Jayavel, R. Surfactant-Free Synthesis of Nb2O5 Nanoparticles Anchored Graphene Nanocomposites with Enhanced Electrochemical Performance for Supercapacitor Electrodes. Nanomaterials 2020, 10, 160. [CrossRef]
- Guo, N.; Lin, Y.; Cui, Y.; Su, S.; Dai, H.; Yang, J.; Zhu, X. Effect of MWCNTs additive on preservation stability of rGO powder. J. Mater. Sci. Mater. Electron. 2022, 33, 6766-6779. [CrossRef]
- Huo, W.C.; Liu, X.L.; Yuan, Y.S.; Li, N.; Lan, T.; Liu, X.Y.; Zhang, Y.X. Facile synthesis of manganese cobalt oxide/nickel cobalt oxide composites for high-performance supercapacitors. Front. Chem. 2019, 7, 661. [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Lee, S.H.; Ryu, K.S. Porous thin layered nanosheets assembled ZnCo2O4 grown on Ni-foam as an efficient electrode material for hybrid supercapacitor applications. Int. J. Hydrogen Energy 2017, 42, 3122-3129. [CrossRef]
- Du, H.; Li, Y.; Ding, F.; Zhao, J.; Zhang, X.; Li, Y.; Zhao, R.; Cao, M.; Yu, T.; Xu, X. Boosting the capacitance of NiCo2O4 hierarchical structures on nickel foam in supercapacitors. Int. J. Hydrogen Energy 2018, 43, 15348-15357. [CrossRef]
- Aghazadeh, M.; Golikand, A.N.; Ghaemi, M. Synthesis, characterization, and electrochemical properties of ultrafine beta- Ni(OH)2 nanoparticles. Int. J. Hydrogen Energy 2011, 36, 8674-8679. [CrossRef]
- Li, J.; Wei, M.; Chu, W.; Wang, N. High-stable alpha-phase NiCo double hydroxide microspheres via microwave synthesis for supercapacitor electrode materials. Chem. Eng. J. 2017, 316, 277-287. [CrossRef]
- Naveen, A.N.; Selladurai, S. Fabrication and performance evaluation of symmetrical supercapacitor based on manganese oxide nanorods-PANI composite. Mater. Sci. Semicon. Proc. 2015, 40, 468-478. [CrossRef]
- Yao, W.; Zhou, H.; Lu, Y. Synthesis and property of novel MnO2@polypyrrole coaxial nanotubes as electrode material for supercapacitors. J. Power Sources 2013, 241, 359-366. [CrossRef]
- Jiang, F.; Li, W.; Zou, R.; Liu, Q.; Xu, K.; An, L.; Hu, J. MoO3/PANI coaxial heterostructure nanobelts by in situ polymerization for high performance supercapacitors. Nano Energy 2014, 7, 72-79. [CrossRef]
- Nie, G.; Lu, X.; Zhu, Y.; Chi, M.; Gao, M.; Chen, S.; Wang, C. Reactive Template Synthesis of Inorganic/Organic VO2@ Polyaniline Coaxial Nanobelts for High-Performance Supercapacitors. ChemElectroChem 2017, 4, 1095-1100. [CrossRef]
- Wang, S.; Wang, Z.; Wang, Y.; Chen, J.; Chen, Z.; Chen, Y.; Fu, J. Environmentally friendly room temperature synthesis of hierarchical porous alpha-Ni(OH)2 nanosheets for supercapacitor and catalysis applications. Green Chem. 2019, 21, 5960-5968. [CrossRef]
- Nagaraju, P.; Arivanandhan, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced electrochemical performance of α-MoO3/graphene nanocomposites prepared by an in situ microwave irradiation technique for energy storage applications. RSC Adv. 2020, 10, 22836-22847. [CrossRef]
- Zheng, X.; Han, Z.; Yang, W.; Qu, F.; Liu, B.; Wu, X. 3D Co3O4@MnO2 heterostructures grown on a flexible substrate and their applications in supercapacitor electrodes and photocatalysts. Dalton Trans. 2016, 45, 16850-16858. [CrossRef]
- Liu, Y.; Teng, X.; Mi, Y.; Chen, Z. A new architecture design of Ni-Co LDH-based pseudocapacitors. J. Mater. Chem. A 2017, 5, 24407-24415. [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel scalable synthesis of highly conducting and robust PEDOT paper for a high performance flexible solid supercapacitor. Energy Environ.Sci. 2015, 8, 1339-1347. [CrossRef]
- Pech, D.; Brunet, M.; Taberna, P.L.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conedera, V.; Durou, H. Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J. Power Sources 2010, 195, 1266-1269. [CrossRef]
- Yang, X.H.; Wang, Y.G.; Xiong, H.M.; Xia, Y.Y. Interfacial synthesis of porous MnO2 and its application in electrochemical capacitor. Electrochim. Acta 2007, 53, 752-757. [CrossRef]
- Hsu, C.T.; Hu, C.C. Synthesis and characterization of mesoporous spinel NiCo2O4 using surfactant-assembled dispersion for asymmetric supercapacitors. J. Power Sources 2013, 242, 662-671. [CrossRef]
- Devaraj, S.; Munichandraiah, N. The effect of nonionic surfactant triton X-100 during electrochemical deposition of MnO2 on its capacitance properties. J. Electrochem. Soc. 2007, 154, A901-A909. [CrossRef]
- Tan, G.; Cheng, Z.; Qiu, Y.; Huang, W.; Fan, H.; Ren, B. Supercapacitors based on polyelectrolyte/ferrocenyl-surfactant complexes with high rate capability. RSC Adv. 2016, 6, 31632-31638. [CrossRef]
- Xu, N.; Klein, J.M.; Huang, P.; Alwusaydi, H.A.; Mann, E.K.; Gurkan, B.E. Improved accessibility of porous carbon electrodes with surfactant ionic liquids for supercapacitors. J. Appl. Electrochem. 2019, 49, 151-162. [CrossRef]
- Lin, Y.; Xie, X.; Wang, X.; Zhang, B.; Li, C.; Wang, H.; Wang, L. Understanding the enhancement of electrochemical properties of NiCo layered double hydroxides via functional pillared effect: an insight into dual charge storage mechanisms. Electrochimica Acta, 2017, 246, 406-414. [CrossRef]
- Lee, J.; Weingarth, D.; Grobelsek, I.; Presser, V. Use of surfactants for continuous operation of aqueous electrochemical flow capacitors. Energy Technol. 2016, 4, 75-84. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




