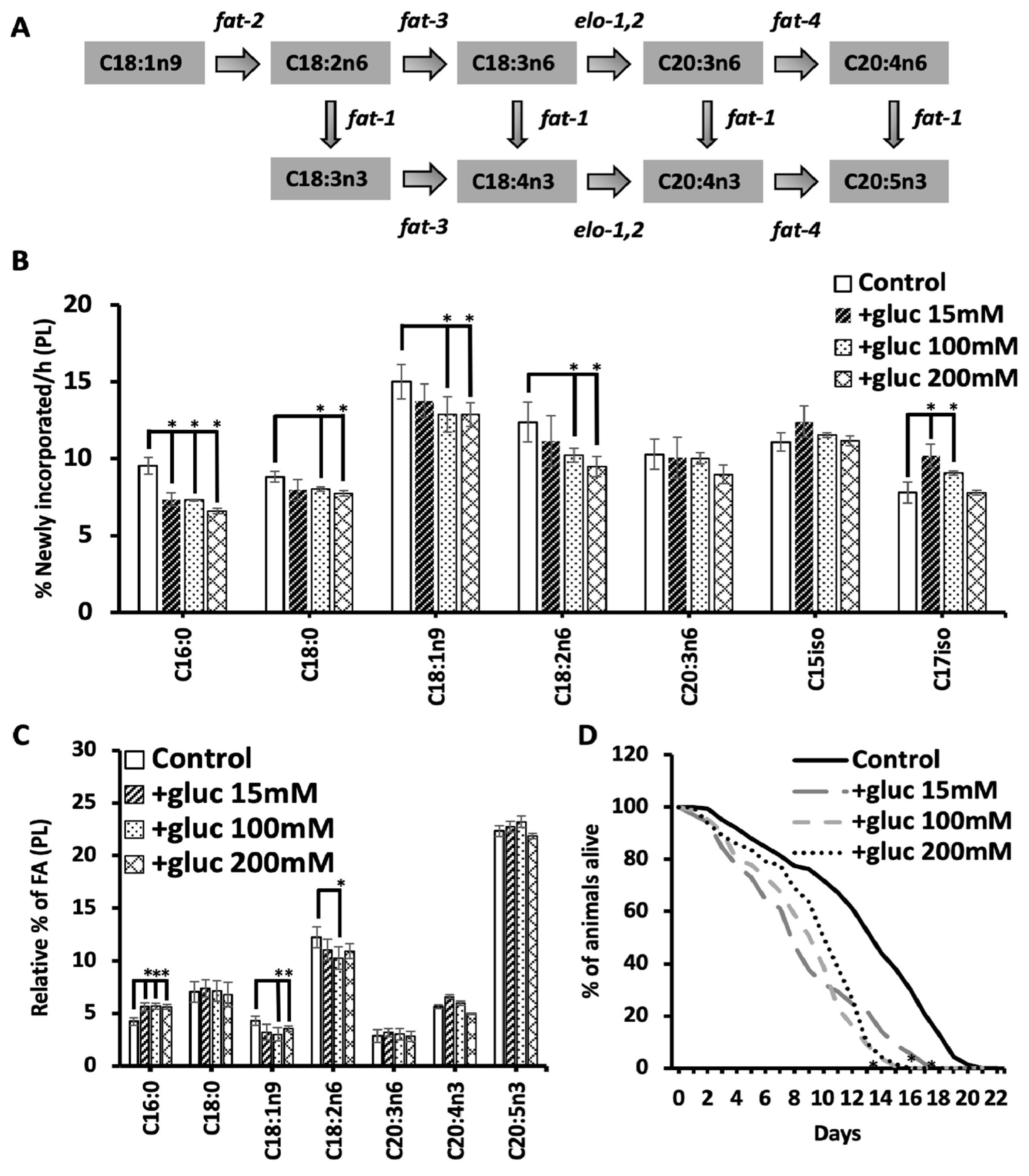
3.1. High dietary glucose alters the allocation of oleic acid to the membrane.
In nematodes supplemented with low levels of glucose (15mM), the dynamics of specific fatty acids are altered as assayed by stable isotope labeling; however, most fatty acid species do not show significant changes [
7]. To further probe the membrane adaptations needed with glucose supplementation, we fed nematodes increasing concentrations of glucose (referred to as +gluc) from 15 mM (mild stress) to 200 mM (high stress) and profiled the dynamics of the fatty acid populations by stable isotope feeding and GC-MS (
Figure 1B). Fatty acids were considered new to the membrane if the molecular weight of intact FA was increased in at least one mass unit (MW+1, MW+2, MW+3, etc.) after correction for natural abundance of stable isotopes in the environment and presence of 12C in the labeled OP50 (Isogro media, Sigma Aldrich, St Louis, MO).
At the lowest concentration (15 mM), there was a significant reduction in 13C-incorporation into C16:0 and a significant increase in the 13C-incorporation in C17iso consistent with our previous reports [
7]. As the amount of glucose was increased, the levels of newly incorporated C16:0 were reduced from 9.5% ± 0.5 in controls to 7.3% ± 0.1 and 6.6% ± 0.05 in 100 mM and 200 mM, respectively (
Figure 1B). There was no further decrease in C16:0 abundance with increasing glucose concentrations. Interestingly, the increase in C17iso was seen only at the two lower concentrations of 15 mM and 100 mM but not at 200 mM (
Figure 1B) suggesting that the mmBCFA response may only be needed at lower concentrations of glucose. The stable isotope labeling patterns clearly reveal distinct responses to different concentrations of glucose calling for further investigation of other fatty acid species.
The high concentrations of glucose revealed significant decreases in multiple species that are not compromised with lower glucose supplementation including C18:0, C18:1n9, and C18:2n6. Specifically, C18:0 decreases from 8.8 ± 0.3% in control worms to 8.0 ± 0.1% in 100 mM and 7.7 ± 0.1% in 200 mM; C18:1n9 decreases from 15.0 ± 1.2% in control worms to 12.8 ± 0.6% in 100 mM and 12.8 ± 0.4% in 200 mM; and C18:2n6 decreases from 12.3 ± 0.9% in control worms to 10.2 ± 0.6% in 100 mM and 9.4 ± 0.6% in 200 mM (
Figure 1B). The reduction in new C18:1n9 and C18:2n6 suggests that these fatty acid species are not being produced at adequate levels or that any newly synthesized molecules are specifically funneled to maintain highly polyunsaturated species production. Here, the fatty acids are detected using an electron impact (EI) source for the mass spectrometry, and, therefore, the only other PUFA that has sufficient detectable parent ions for analysis is C20:3n6. The amount of newly incorporated C20:3n6 does not change significantly from controls suggesting that C18:1n9 and C18:2n6 are being consumed to funnel resources towards C20 PUFA populations (
Figure 1B).
To further support the funneling hypothesis, the total relative abundance of the fatty acid species involved in C20 PUFA production was quantified using GC-MS. The fatty acid profiles confirmed the increase in palmitate (C16:0) as previously seen at all concentrations with this increase being the only significant change seen in the 15mM treated animals in the species measured (
Figure 1C). There was a similar increase in C16:0 at all concentrations demonstrating that the increase in C16:0 does not continue to rise with higher concentrations of glucose (
Figure 1C). The C16:0 pool is elongated to C18:0 and the levels of C18:0 remain constant at all glucose concentrations showing a specific impact on C16:0 accumulation. C18:0 is converted to C18:1n9 by the FAT-7 desaturase which is implicated in the response to glucose previously [
7,
8]. Here, there is a significant reduction in overall C18:1n9 levels in only the higher +gluc plates (100 mM and 200 mM). Because the precursors to C18:1n9 are elevated or maintained, we suspected that the C18:1n9 was being consumed to produce the C20 PUFAs needed in the nematode. In fact, there is maintenance in the abundance of all measured PUFAs except for C18:2n6 where we find a trend to reduced levels in high +gluc plates but significance was reached only on 100 mM plates (
Figure 1C). Taken together, the stable isotope and GC-MS analysis shows no significant changes in the C20 PUFA population at any glucose concentration suggesting that new dietary carbon is being funneled to maintain these populations.
Elevated glucose in the diet is associated with decreased longevity, and therefore we sought to test whether increasing glucose concentrations would lead to further impacts on lifespan [
17]. For each glucose condition, the mean lifespan of the nematode’s is significantly reduced by approximately 30% (
Figure 1D). There was no further lifespan reduction as the glucose concentration rose to 100 mM and 200 mM glucose. This is consistent with the fatty acid abundance and dynamics data where we see an impact at lower concentrations but not a correlation with glucose concentration for most fatty acid species. We hypothesize that the metabolic rewiring that occurs at 15 mM is largely sufficient to accommodate the increased glucose levels, but this observation dictates more careful probing of fatty acid dynamics.
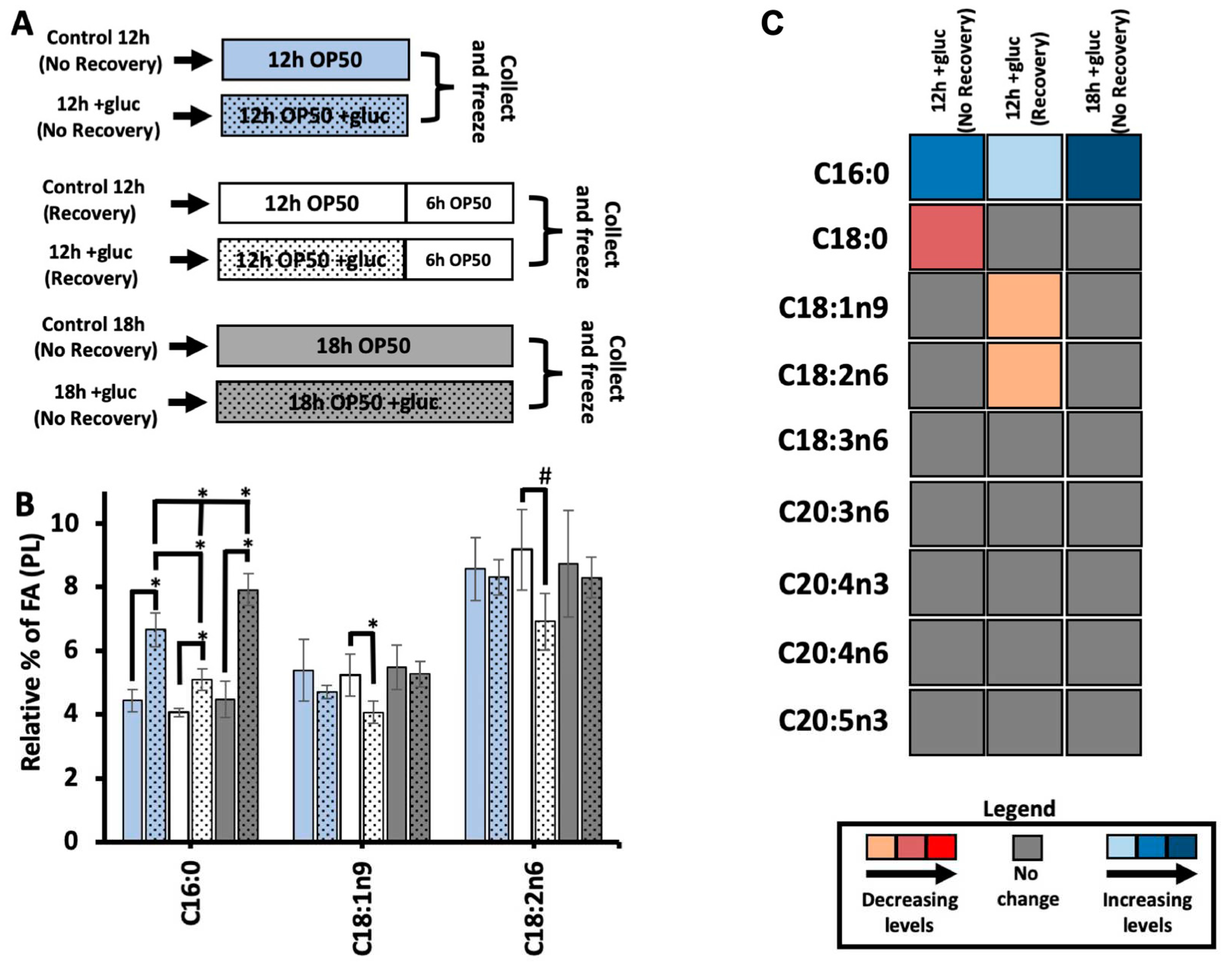
3.2. A recovery period is needed to drive the shift in C18:1n9 abundance.
The higher concentrations of glucose revealed altered oleic acid dynamics consistent with the role of the FAT-7 desaturase in surviving glucose stress. Therefore, we further probed the kinetics of fatty acid metabolism with a focus on C18:1n9. First, the stable isotope labeling technique used here introduces
13C-OP50 on agarose media, which is free of nutrients to prevent the labeled bacteria from incorporating
12C from the unlabeled nutrients in the plate. A consequence of this protocol is that the nematodes are given to a “recovery period” of 6 hours on the labeling plates when there is no glucose stress. Because it was previously shown that removing nematodes from a short glucose stress allows for recovery of development in PAQR-2 mutants, we examined the impact of this recovery period of the fatty acids of the membrane [
9]. To do so, we used GC-MS to quantify the abundance of saturated and unsaturated fatty acids in nematodes stressed for 12 hours and frozen immediately following the stress (+gluc 12h - No Recovery); and nematodes stressed for 12 hours followed by 6 hours of “recovery period” (+gluc 12h - Recovery). We also included nematodes that were stressed for 18 hours and frozen immediately after stress (+gluc 18h – No Recovery) which would allow us to identify changes that were occurring not due to recovery but to the extra hours on the plates (
Figure 2A). To induce the glucose stress, we selected 100 mM of glucose which caused the most significant alterations in membrane dynamics and composition.
We first quantified the abundance of C16:0 in the three conditions and found a significant increase in all glucose treatment groups regardless of the timing and recovery period. The increase in C16:0 with the recovery period was significantly less than in either of the treatment groups without a recovery period (
Figure 2A). This trend suggests that the input of C16:0 to the membrane is higher in glucose conditions and that higher input is reduced once glucose is removed from the plate. The amount of C16:0 does continue to significantly rise between 12 hours and 18 hours of glucose feeding supporting the hypothesis that the input of C16:0 is linked to glucose supplementation (
Figure 2A). Next, we analyze the relative levels of C18:1n9 in the PL membrane with and without a recovery. If the nematodes are not given to a recovery period, oleic acid levels were not significantly different in 12-hour glucose-stressed animals versus controls (
Figure 2B). When given a recovery period, +gluc 12h (Recovery) showed a significant decrease when compared to controls where the level of C18:1n9 decreased from 5.2% ± 0.6 to 4.0% ± 0.3 (
Figure 2B). C18:1n9 is converted to C18:2n6 via FAT-2, and we quantified similar trends in this species with a change from 9.1% ± 1.2 to 6.9% ± 0.8 after the recovery period (
Figure 2B). This reduction was not a result of the longer time period as these fatty acid species after 18 hours of glucose stress had no change in overall levels (
Figure 2B).
The reduction in both C18:1n9 and C18:2n6 abundance after a recovery may show an overall reduction in PUFA production or a specific focus on the production of C20 PUFAs. Therefore, we quantified the relative fatty acid abundances for all major species in the nematode in the three glucose treatment groups versus their respective controls (
Figure 2C,
Supplementary Figure 1A). We found that there are no significant modifications in the relative abundance of any C20 PUFAs in any stress conditions (
Figure 2C). The fatty acids that had significant changes in our previous results remained consistent: increased levels of C16:0 in all treatment groups, decreased C18:1n9 abundance in the 12h plus recovery and decreased C18:2n6 abundance in the 12h plus recovery treatment group (
Figure 2C). In addition, there was a small but significant decrease in C18:0 in the 12 h group without recovery. Taken together, our data suggests that the reduction in C18:1n9 and C18:2n6 occurs as these fatty acids are converted to C20 PUFAs in the period following glucose exposure.
Figure 2.
Recovery from glucose is required to observe the shift in C18:1n9. (A) For each comparison, the addition of glucose is represented by the inclusion of black dots vs control with no dots. The blue bars represent nematodes collected immediately after 12 hours of stress (12h; No Recovery), the white bars are nematodes allowed to recover for 6 hours in agarose plates seeded 0.15mg/mL of OP50 (12h: Recovery), and the gray bars are nematodes collected immediately after 18 hours of stress (18h; No Recovery). (B) C16:0 SFA showed significant increase in all glucose stressed animals relative to its controls, and nematodes stressed for a longer period (18h) showed significantly larger accumulation of C16:0 compared to all other two stress conditions. Both C18:1n9 and C18:2n6 maintained stable levels in “No Recovery” animals but showed significant decrease in +gluc 12h (Recovery) compared to its respective control. C18:1n9 decreased from 5.2% ± 0.6 in control 12h (Recovery) to 4% ± 0.3 in +gluc 12h (Recovery), and C18:2n6 decreased from 9.1% ± 1.2 in control 12h (Recovery) to 6.9% ± 0.8 in +gluc 12h (Recovery). (C) A heat map shows the alteration in FA levels comparing stressed animals to controls (+gluc/controls). Significant decreases are shown in light orange (little), dark orange (intermediate), and red (substantial). Significant increases are shown in light blue (little), median blue (intermediate), and dark blue (substantial). Gray bars indicate FAs that did not have significant alteration. For all GC-MS analysis, values represent means ± SEM of at least 9 replicates. Statistical significance, P<0.05 indicated by * and P<0.1 is indicated by #, was calculated using unpaired T tests and F tests to compare variances. .
Figure 2.
Recovery from glucose is required to observe the shift in C18:1n9. (A) For each comparison, the addition of glucose is represented by the inclusion of black dots vs control with no dots. The blue bars represent nematodes collected immediately after 12 hours of stress (12h; No Recovery), the white bars are nematodes allowed to recover for 6 hours in agarose plates seeded 0.15mg/mL of OP50 (12h: Recovery), and the gray bars are nematodes collected immediately after 18 hours of stress (18h; No Recovery). (B) C16:0 SFA showed significant increase in all glucose stressed animals relative to its controls, and nematodes stressed for a longer period (18h) showed significantly larger accumulation of C16:0 compared to all other two stress conditions. Both C18:1n9 and C18:2n6 maintained stable levels in “No Recovery” animals but showed significant decrease in +gluc 12h (Recovery) compared to its respective control. C18:1n9 decreased from 5.2% ± 0.6 in control 12h (Recovery) to 4% ± 0.3 in +gluc 12h (Recovery), and C18:2n6 decreased from 9.1% ± 1.2 in control 12h (Recovery) to 6.9% ± 0.8 in +gluc 12h (Recovery). (C) A heat map shows the alteration in FA levels comparing stressed animals to controls (+gluc/controls). Significant decreases are shown in light orange (little), dark orange (intermediate), and red (substantial). Significant increases are shown in light blue (little), median blue (intermediate), and dark blue (substantial). Gray bars indicate FAs that did not have significant alteration. For all GC-MS analysis, values represent means ± SEM of at least 9 replicates. Statistical significance, P<0.05 indicated by * and P<0.1 is indicated by #, was calculated using unpaired T tests and F tests to compare variances. .
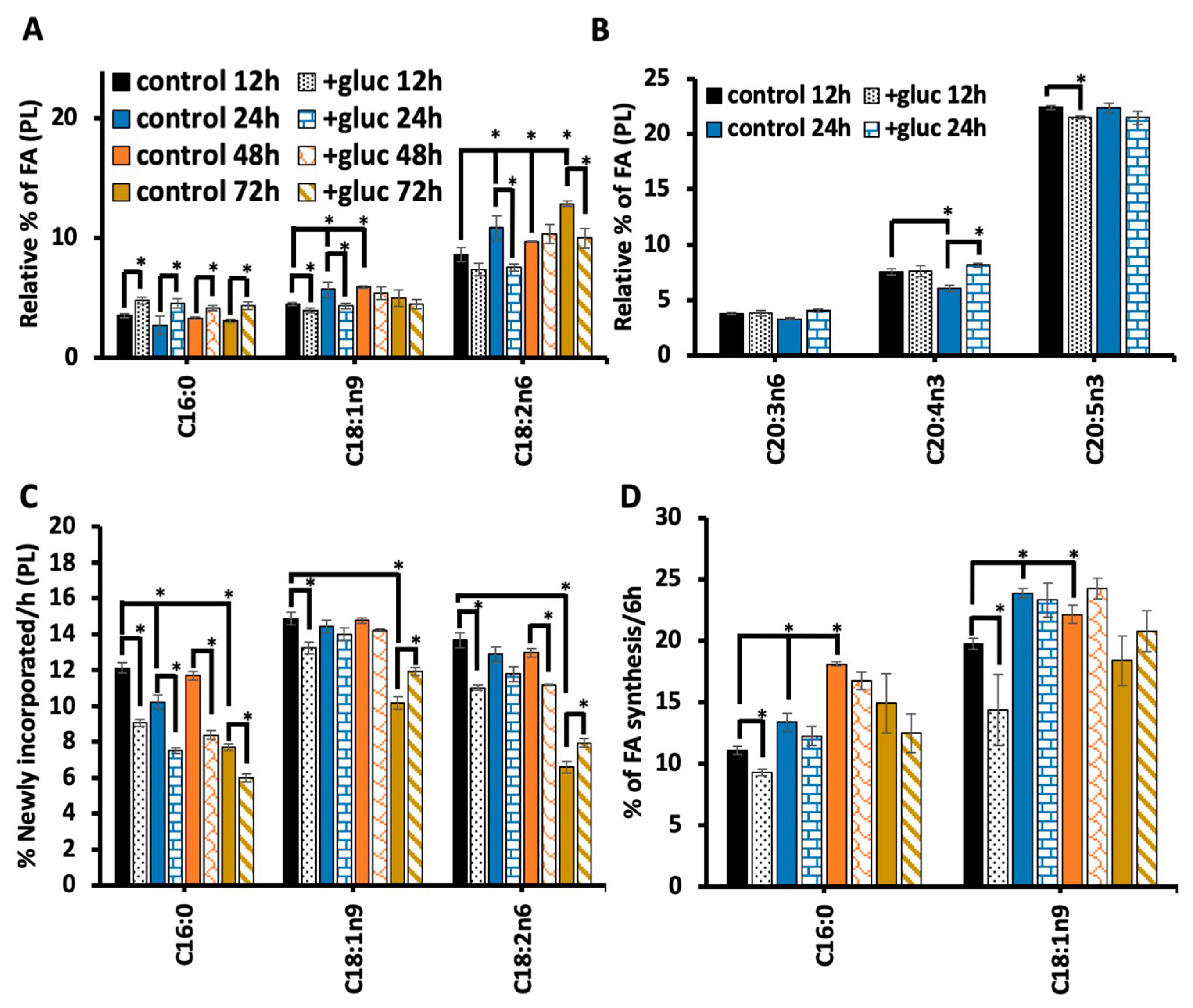
3.3. The abundance of oleate and linoleate stabilizes with longer glucose exposure.
Once we found that the recovery period was essential to see the alterations in C18:1n9 metabolism, we next tested the impact of longer durations of glucose stress. Nematodes were subjected to 100 mM glucose for 12 hours, 24 hours, 48 hours and 72 hours, and all treatment groups had a 6-hour recovery period to elicit the reduction in C18:1n9 and in C18:2n6 as well as to allow for stable isotope labeling. First, C16:0 abundance was considered, and, with all durations of glucose stress, there was a significant increase in C16:0 (
Figure 3A). Interestingly, the longer periods of glucose stress did not lead to further increases in C16:0 abundance. Because we saw an increase in C16:0 with 18 hours compared to 12 hours, we believe that the recovery period along with metabolic rewiring can compensate for the longer exposure to glucose.
We next quantified the abundance of C18:1n9 with different lengths of 100 mM exposure. Here, we found a decrease of C18:1n9 from 4.4% ± 0.1 in control 12h to 3.9 ± 0.1 in +gluc 12h, and a greater decrease from 5.6% ± 0.6 in control 24h to 4.3 ± 0.2 in +gluc 24h. However, the 48h and the 72h treatment did not lead to significant changes in C18:1n9 (
Figure 3A). To interpret this data, we considered the oleic acid populations in the control populations which revealed that the baseline C18:1n9 levels increased in the 24h and the 48h controls (
Figure 3A). The longer glucose exposure dictates that the lipid populations are being examined in older animals, and, although 24 h is a brief period, the first three days of adulthood are the peak reproductive period within the nematodes and associated with glucose-independent metabolic changes [
22,
23]. Despite the impact of aging, this data is consistent with an upregulation of FAT-7 that requires time to stabilize the higher levels of the enzyme.
To further understand the impact of the altered C18:1n9 levels on fatty acid elongation and desaturation pathway, we next examined C18:2n6, the immediate product of C18:1n9 desaturation (
Figure 1A). The trends in C18:2n6 were similar to C18:1n9 with significantly decreased abundance from 10.8 ± 1.0% in control 24h to 7.5 ± 0.3 in +gluc 24h (
Figure 3A). Like C18:1n9, there is no significant change with 48 hours of glucose feeding. However, the 72-hour analysis revealed significantly higher levels of C18:2n6 in control animals compared to the young 12 h stressed controls and a significant decrease in C18:2n6 with 72h glucose exposure (
Figure 3A). Next, we examined if the reduction in C18:2n6 and C18:1n9 affected the abundance of the C20 PUFAs downstream. To do so, we examined the levels of the major C20 PUFAs with 24 hours glucose stress as that treatment had the greatest impact on the precursor populations (see
Supplementary Figure 1B for all treatment durations). For C20:3n6, C20:4n3 and C20:5n3, there was some small but significant change in these pools; however, the extent of these changes was relatively minor. In fact, the C20:4n3 levels increased following 24 hours of glucose (
Figure 3B), supporting the hypothesis that the C18:1n9 produced by FAT-7 upregulation is funneled to preserve C20 PUFAs (
Figure 3B).
In addition to monitoring the overall abundance of these fatty acid pools, we implemented a stable isotope labeling strategy to determine the flux in these populations. Consistent, with the 12-hour data, the amount of newly incorporated or isotopically labeled C16:0 was reduced with all durations of 100 mM glucose exposure (
Figure 3C). In both C18:1n9 and C18:2n6, there was a significant reduction in the number of 13C-fatty acids at 12 hours as seen previously. There are no significant changes in the labeling of C18:1n9 at 24 or 48 hours despite reducing C18:1n9 abundance at 24 hours. This data suggests that FAT-7 levels reach their peak within 24 hours and the turnover of the fatty acids requires 48 hours for the fatty acid pool to stabilize. A similar trend is seen with C18:2n6 but there are significant differences at 48 hours after labeling. We hypothesize that the upregulation of FAT-3, the enzyme that drives the conversion between C18:2n6 and C18:3n6 has slower kinetics.
Finally, each of the fatty acid pools examined had a dramatic reduction of labeling at 72 hours in the control nematodes. This reduction likely indicates an alteration in phospholipid metabolism as the animals leave the reproductive period, but it also uncovers the relative production of these fatty acids. New C16:0 production continues to be reduced (
Figure 3C), consistent with the fact that the overall levels of C16:0 are still elevated at 72 hours (
Figure 3A). Both C18:1n9 and C18:2n6 have significant increases in new fatty acids compared to the unstressed controls (
Figure 3C). The increases in these populations likely reflect the stabilization or activation of FAT-7 and FAT-3.
The changes in fatty acid abundance can ultimately reflect increased production, increased dietary absorption or decreased consumption. The stable isotope strategy we used here lets us define the origin of the oleate pool as altered with glucose supplementation. Under basal conditions, 11 ± 0.3% C18:1n9 is derived from
de novo fatty acid synthesis consistent with past reports11. The contribution of synthesis to C18:1n9 production falls to 9.2 ± 0.2% in +gluc 12h (
Figure 3D). The amount of synthesis is not statistically different in any of the longer treatment periods; however, it is interesting to note that the amount of synthesized fatty acids increases in 24 hours and 48 hours compared to 12 hours in the controls (
Figure 3D). The trends in synthesized C18:1n9 closely mirror the overall amount of stable isotope incorporation suggesting that the processing of synthesized fatty acids is the driver of those dynamics. This is consistent with the composition of the bacterial diet which has few overall changes in fatty acids [
7].
Because C16:0 is the main product of
de novo fatty acid synthesis, we quantified the amount of synthesized palmitate in the time course. Similar to C18:1n9, there is a decrease in synthesized C16:0 at 12 hours and no significant changes at other timepoints (
Figure 3D). We believe that this quantification shows that synthesis is initially reduced to prevent further production of C16:0. This also shows that the elevated levels of C16:0 are not derived from using the excess glucose to drive fatty acid synthesis in the nematode. Furthermore, there is an increase in synthesized C16:0 in 24 hour and 48 hours populations reinforcing the altered fatty acid metabolism over the first few days of adulthood. Unlike the C18:1n9, the pattern of synthesized C16:0 does not match the overall labeling implicating fatty acid absorption as a key contributor to palmitate metabolism.
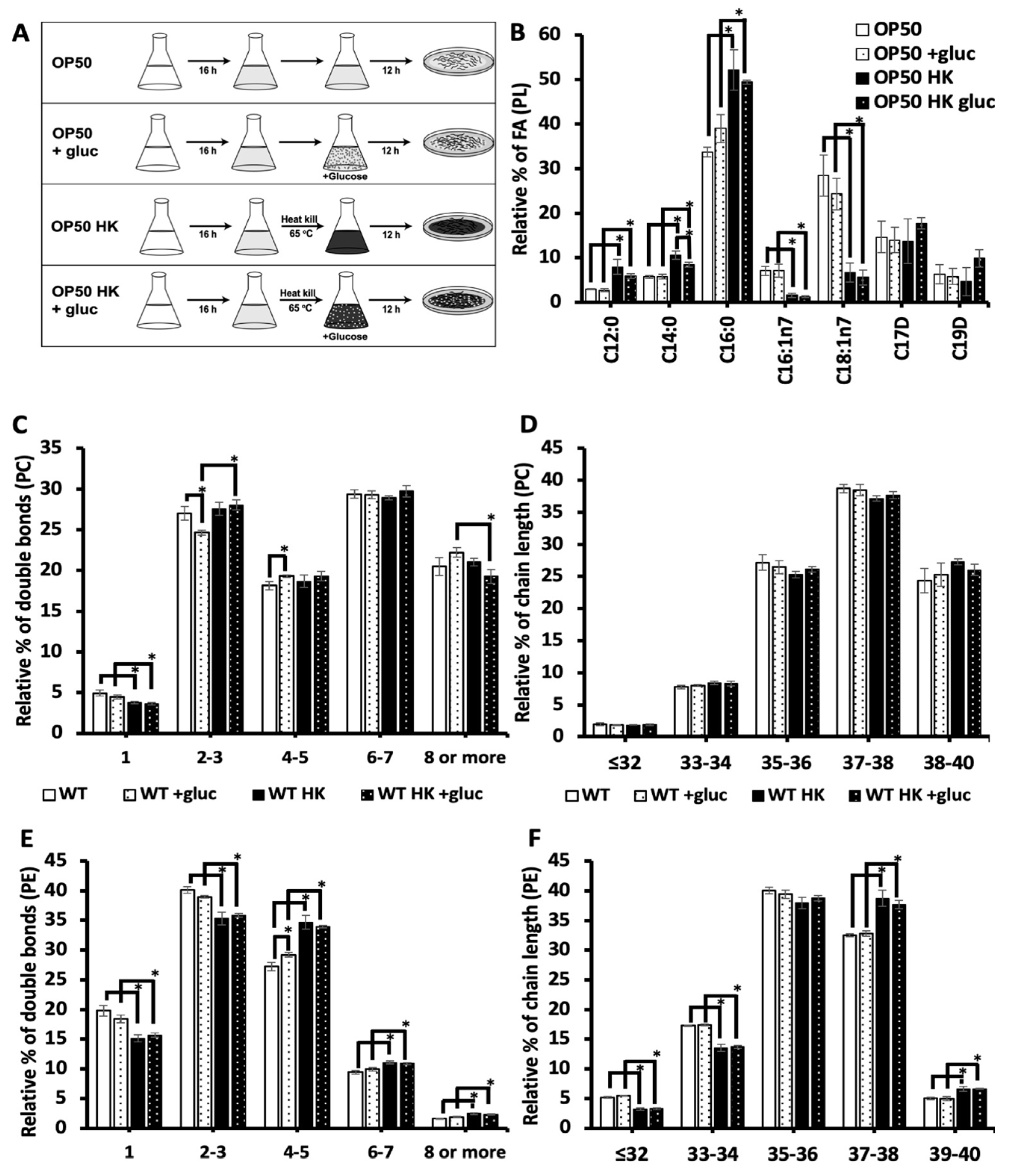
3.4. Living bacteria is needed for the impact of glucose stress on the membrane.
The addition to glucose to the diet may induce excess saturated fatty acid production or alter membrane metabolism through another mechanism. For instance, it has been seen that glucose can cause oxidative stress through increased production of advanced glycation agents[
20]. Because the bacterial food source (OP50) is present during glucose exposure, the bacteria could theoretically metabolize glucose and contribute to the effects of glucose stress. To test the impact of bacteria processing glucose on the membrane composition, the OP50 bacteria was grown in LB broth media, and then bacteria were separated into two groups: living and heat killed bacteria (scheme shown in
Figure 4A). Living bacteria was resuspended into fresh LB (OP50) for the control treatment and LB media containing 100 mM glucose (OP50 +gluc) for the stress. In the other treatment group, bacteria were heat killed at 65 °C for 20 mins and resuspended into fresh LB media (OP50 HK (Heat Killed)) and LB media containing 100 mM glucose (OP50 HK + gluc).
To determine the impact of heat killing on the bacteria food source, we analyzed the fatty acid composition of the bacteria using GC-MS. Notably, we found that killing the bacteria significantly increased the level of saturated fatty acids, C12:0, C14:0 and C16:0, even in the absence of glucose (
Figure 4B). Specifically, the level of C16:0, the most abundant saturated fatty acid in OP50, increased from 33.7 ± 1.0% of the dietary fatty acids to 52 ± 4.5% in heat killed OP50. Additionally, there was a corresponding decrease in the level of unsaturated fatty acids particularly C18:1n7 which decreased from 28.4 ± 4.5% in OP50 to 6.7 ± 2.1% in killed OP50. We next examined the impact of glucose in both populations and found that glucose did not affect the composition of living bacteria. There was a small but not significant increase in C16:0 which suggests that the accumulation of C16:0 in the nematodes is not a result of increased dietary C16:0. There was a small but significant decrease in C14:0 glucose in the killed OP50 with glucose, but the GC-MS analysis confirmed that the glucose did not have a major impact on the fatty acid composition of the killed bacterial populations (
Figure 4B).
To assess the impact of bacteria processing glucose on the lipid composition of the nematodes, the phospholipid composition was analyzed using HPLC/MS-MS in animals fed living or killed bacteria. Here, we focused on the most abundant phospholipids of the membrane, phosphatidylcholine (PC) and phosphatidylethanolamine (PE) (see
supplementary table 1 for full list). First, we considered the total number of double bonds present in the two fatty acid tails (i.e., 0-1, 2-3, 4-5, 6-7 and 8 or more) (
Figure 4C) and the chain length (
Figure 4D) of the PC populations. Feeding the animals with live OP50 +gluc led to a significant decrease in the level of species with 2-3 double bonds and an increase in species with 4-5 double bonds (
Figure 4C). The decrease in lipid species with 2-3 double bonds is consistent with GC-MS data as the fatty acids C18:1n9 and C18:2n6 are mostly present in this population. Animals that were fed a heat killed OP50 +gluc did not have any significant change in their degree of unsaturation, suggesting that the living bacteria is needed for processing glucose. Except for a reduction in the populations with 1 double bond, there was no significant difference between the PC profiles in living versus killed bacteria without glucose treatment (
Figure 4C). The phospholipid was also binned according to their chain length (total number of carbon present) as the length of phospholipids can influence the biophysical property of the membrane such as thickness. However, there were no significant changes in the chain length of PC lipids when the worms were fed either living bacteria or heat killed bacteria (
Figure 4D).
In analyzing the double bond distribution of the PE population, the animals were fed OP50 + gluc diet only had a significant increase in phospholipids with 4-5 double bonds (
Figure 4E) and no changes in the distribution of chain lengths (
Figure 4F). Again, there were no significant alterations in the level of unsaturation or chain length when the nematodes were fed a HK OP50 +gluc diet versus the killed diet without glucose supplementation (
Figure 4E-F). Notably, there were significant changes in the chain length distribution of OP50 compared with OP50 HK which makes it difficult to disentangle the impact of the different dietary source from the requirement for living bacteria to process the glucose. However, these results indicate that living bacteria may have to process glucose to drive the changes in the membrane lipid composition.