1. Introduction
The reduced verbal communicability in people with Trisomy 21 (T21, Down syndrome (DS)) is widely recognized (Kumin, 2006), but the nature of their difficulties and effective interventions when co-morbidities are present are relatively unexplored. In a sample of young individuals with T21, Wilson et al. (2019a) showed that 97.8% met the criteria for motor speech disorders of which 37.8% showed dysarthria, 22.2% had both dysarthria and childhood apraxia of speech (CAS) and 11.1% had CAS alone. Thus, approximately 33.3% of their sample demonstrated features of CAS. Among those young individuals with T21 who met the criteria for both dysarthria and CAS, 80% demonstrated reduced intelligibility (Wilson et al., 2019b).
CAS is defined as a neurological disorder with proximal deficits at the level of speech motor planning and/or motor programing of speech movement sequences (ASHA, 2004). CAS is a difficult-to-treat and persistent motor speech disorder (Cassar et al., 2022; Lewis et al., 2004) and in recent years, there has been a push towards the development, refinement, and standardization of intervention approaches for this condition. In particular, treatment approaches based on principles of motor learning have been suggested with varying degrees of effectiveness and evidence levels (see (Morgan et al., 2018)).
A new promising approach capable of promoting neuronal plasticity and producing behavioral improvements in motor practice and learning is the use of non-invasive brain stimulation (NIBS) techniques, such as transcranial Direct Current Stimulation (tDCS) and Transcranial Magnetic Stimulation (TMS). These techniques have gained momentum in the last few years and have been shown to promote successful functional recovery after brain injury (Fregni et al., 2021; Zettin et al., 2021). tDCS has been mostly applied to the post-stroke aphasia population (Marangolo et al., 2013; Marangolo et al., 2011), due to its feasibility and relatively minor side effects (Nitsche et al., 2003; Zettin et al., 2021). Clinical efficacy of tDCS use in this population has been categorized as “possibly effective” (Level C) when applied bilaterally (anode over Broca’s area and cathode over its homologue) (Fregni et al., 2021), but few studies have explored the effects of the tDCS on adult apraxia of speech (AOS). Recently, Themistocleous et al. (2021) measured the duration of vowels and consonants in spoken words and found that segmental duration was significantly shorter after tDCS over the left inferior frontal gyrus (IFG) along with speech therapy in eight adult AOS patients with non-fluent primary progressive aphasia when compared to the sham condition. These gains were generalized to untrained words and present 2-months after treatment. Thus, they suggested that tDCS over the left IFG may facilitate speech production in adult AOS patients (Themistocleous et al., 2021). However, to our knowledge, no studies have reported the use of tDCS in children with CAS.
Furthermore, typical young adult participants receiving tDCS before performing a nonword task showed significantly greater improvement when compared to participants receiving sham or those receiving the tDCS during the speech learning task (Buchwald et al., 2019). Buchwald et al. (2019) suggested that tDCS can improve speech motor learning especially if tDCS is applied immediately before motor practice.
In recent years, there has been a push towards studying the “mode of action” (MoA) by which interventions induce change (e.g., see (Grant et al., 2010)). Understanding the connection(s) between the interventions and the MoA they target would broaden our scientific knowledge on how and why interventions affect change and may result in the development of more effective speech motor interventions (e.g., see (Kadis et al., 2014; A.K.; Namasivayam et al., 2022) and (Yu et al., 2018)).
In the current study, we used a novel way to identify potential MoAs during speech intervention. Specifically, we measured changes in cortical activation induced by tDCS through scalp electroencephalography (EEG) using event-related potentials (ERPs) (Antal et al., 2022; Terranova et al., 2018), and focused in the most studied endogenous ERPs waveform, the P3 (or P300) component (Luck, 2005; Polich, 2007). According to the hypothesis of “context updating”, the P3 ERP component would reflect the updating of working memory content (see (Polich, 2007)). However, its amplitude will decrease (habituate) when stimuli are repetitively presented and when task performance becomes more automatic (Romero & Polich, 1996). These changes mean that fewer resources are then needed.
In this study we explore whether repetitive tDCS would facilitate therapy gains in a young adult individual with CAS and T21, and whether this effect would be related to changes on P3 ERP activity in brain regions associated with speech sound production. Given the exploratory nature of this study, we do not make any directional hypothesis regarding brain regions or activation levels for the ERP data.
2. Methods
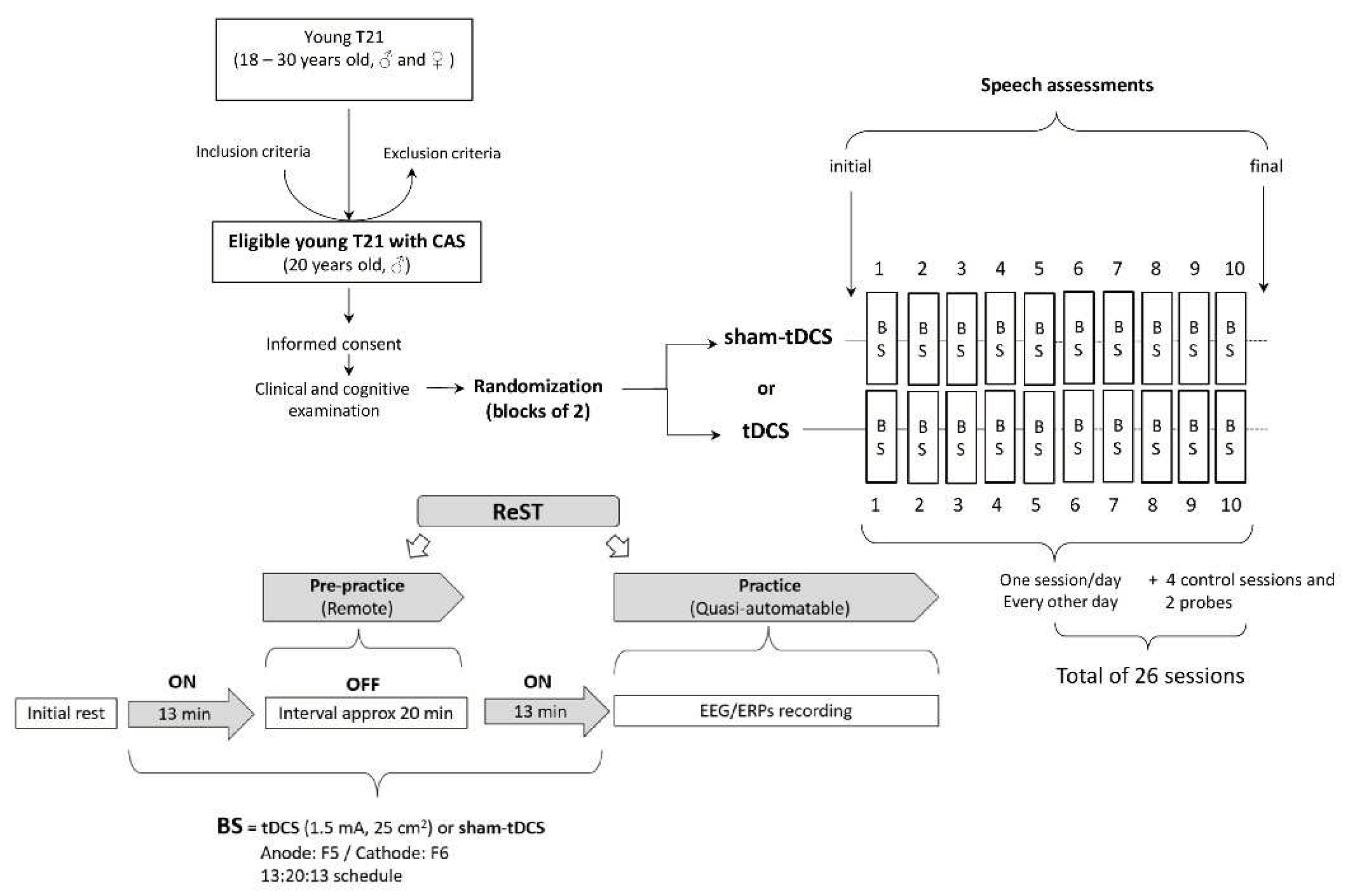
This was a N-of-1 randomized study with a 20-year-old male individual with T21 with moderate-severe apraxia of speech. The research project was approved by the Brazilian Institutional Ethics Review Board of the Federal University of Espírito Santo (CAAE 23866719.8.0000.5060) and conducted in strict adherence to the Declaration of Helsinki. It was registered in the Brazilian Registry of Clinical Trials (ReBEC) under the number RBR-5435x9. Informed consent was obtained from the parent and assent was obtained from the participant prior to start of the study.
2.1. N-of-1 study design
The N-of-1 study design is often used to investigate the effects of treatments for subjects presenting with unique conditions. It has been used to investigate the effects of neurorehabilitation (Edgington, 1987; Perdices & Tate, 2009) in communication disorders (Rvachew, 1988; Rvachew & Matthews, 2017). In N-of-1 designs, a randomization of treatment allocation at multiple points in time is introduced, allowing the application of parametric statistical analyzes like those used in randomized clinical trials employing groups of multiple subjects (Edgington, 1987; Ferron & Ware, 1994; Rvachew, 1988; Rvachew & Matthews, 2017).
2.2. Experimental procedures
As this study took place during the 2020-2021 global pandemic, strict COVID-19 health protocols were followed (adequate masks, sanitization, physical distancing – only the experimenter and the participant was allowed in the experimental set) for the duration of this study.
The participant underwent a non-invasive brain stimulation paradigm using the tDCS or sham-tDCS in conjunction with a motor speech intervention known as the Rapid Syllable Transition Training (ReST) (
Figure 1).
2.2.1. Non-Invasive Brain Stimulation
A portable tDCS device (1x1 mini-CT, Model 1601-LTE, Soterix Medical Inc., New York, USA) was used to deliver tCDS. Ten tDCS sessions (current intensity of 1.5 mA, electrode size of 25 cm
2, anode over F5 [Broca’s area - BA44/45] and cathode over F6 [right contralateral region]) and ten sham-tDCS sessions were randomly (
www.randomizer.org) distributed in blocks of 2 to be applied one session per day every other day (three times a week). To maximize cortical effects, they were administered in two 13-min applications with a 20-min interval (13:20:13 protocol; Klauss et al., 2014; Monte-Silva et al., 2013). During both
13-minute tDCS/sham-tDCS
applications, the participant was kept seated at rest (i.e., free to listen to music or watch short movies of his choice on his mobile phone). During the 20-min interval, the training (i.e., pre-practice) phase of ReST was applied remotely (see the description below), and the ReST practice phase was conducted immediately after the second 13-min of tDCS or sham-tDCS application (Fig. 1).
2.2.2. Speech intervention
The ReST (Murray et al., 2015) is a speech intervention based on principles of motor learning and has been recommended for CAS. ReST aims to improve speech production and prosody by training nonsense words (NSWs) with varied stress patterns. Due to limitations for face-to-face delivery during the COVID-19 pandemic, this intervention was chosen in part for its suitability for remote tele-health administration. ReST has sufficient data to warrant its use for children with CAS in both in-person and tele-health formats (Bahar et al., 2021; Murray et al., 2015; Thomas et al., 2016).
In the current study, trisyllabic NSWs with two stress patterns (strong-weak-weak or weak-strong-weak) were used (e.g., gótabe, faduque). All NSWs used in ReST treatment were balanced according to the patient’s inventory of sounds, level of motor complexity (based on mandible-lip-tongue movement transitions (e.g., Namasivayam et al., 2021) and met phonotactic constraints of Brazilian Portuguese words. These NSWs were checked and validated by two licensed Brazilian linguists. A licensed speech-language pathologist (ACEV), blind to the treatment conditions remotely provided the ReST intervention and presented the NSWs to the participant during training (pre-practice) phase of the ReST. The speech-language pathologist was formally trained to administer ReST with fidelity in Brazilian Portuguese.
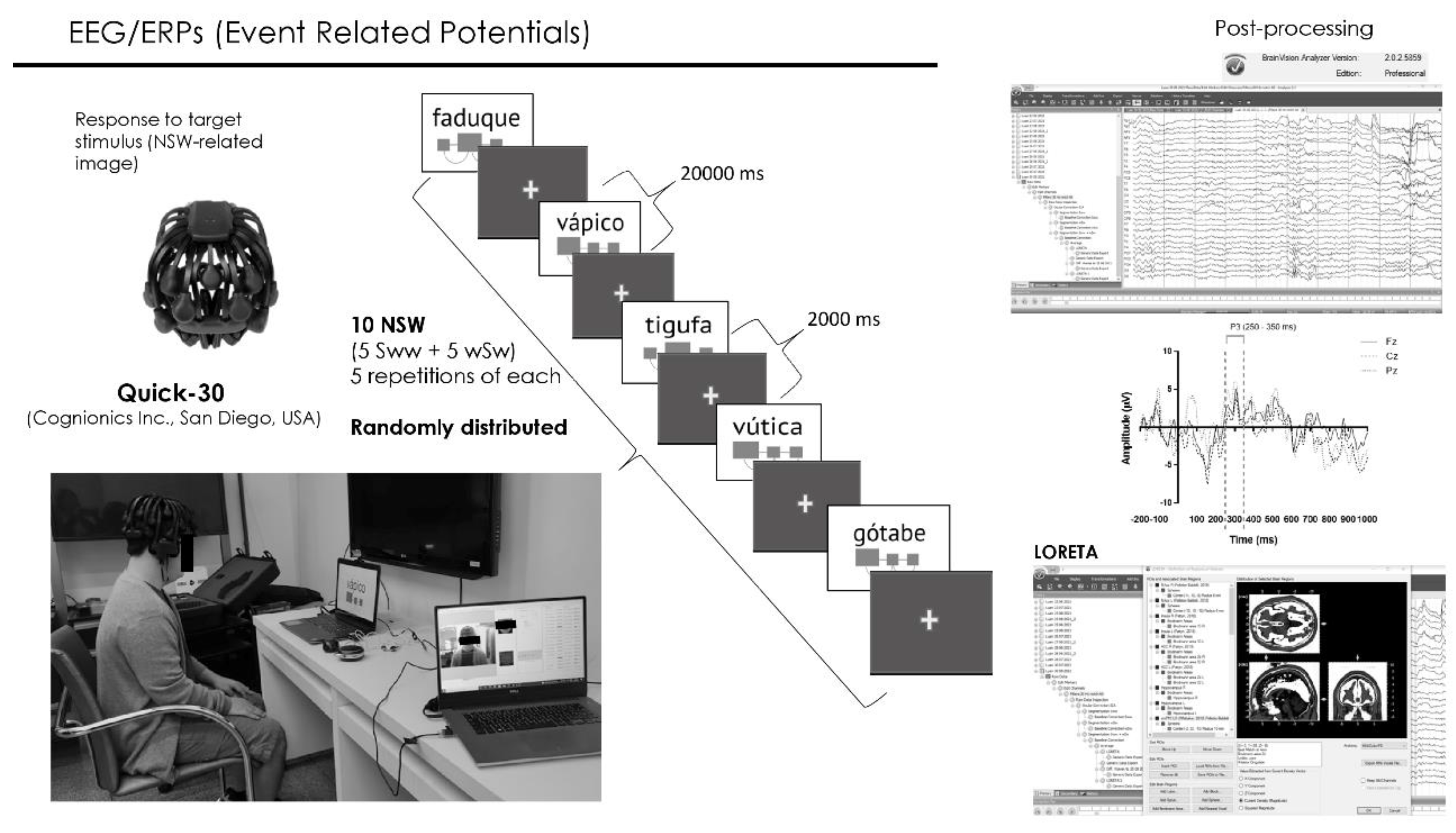
For the practice phase of the ReST, the target NSWs utterances employed in the pre-practice phase were pre-recorded and randomly presented via a computer using Presentation® software (Version 18.0, Neurobehavioral Systems, Inc., Berkeley, CA,
www.neurobs.com) in a quasi-automatable way. Written NSWs were shown as pictures with diagrams cueing the strong syllable (
Figure 2), simultaneously, with a pre-recorded audio (all trisyllabic NSWs were pre-recorded by the speech-language pathologist) with approximately 1000 milliseconds duration. Each NSW presentation lasted for 20000 milliseconds. An interval of 2000 milliseconds was interposed between them with a default screen consisted of a black background with a small yellow cross mark in the center to keep the subjects’ attention to the screen. The practice phase lasted approximately 20 minutes where the participant repeated each of the 10 NSW 5 times for a total of 50 productions per session. The speech production of each practice phase of ReST was recorded using OBS studio software and transcribed offline.
Ten NSWs were randomly chosen for tDCS, and other set of ten different NSWs was chosen for sham-tDCS condition. These 10-NSWs sets were kept constant over 10 sessions of each condition to facilitate speech motor practice and learning. Within each session the 10 NSWs were randomly presented. A third set of ten other NSWs constituted the control condition which was tested on 4 days randomly distributed across NIBS sessions when no sham-tDCS or tDCS applications were conducted. These control sessions were free of brain stimulation procedures, allowing to verify the potential occurrence of any placebo effect when comparing to sham-tDCS condition. Finally, a fourth set of ten different NSWs was used as a probe and applied at the beginning (initial) and at the end (final) of the study protocol. This allowed the verification of any effects related to repeated presentation of a NSWs set. Each session was about 90 minutes in duration and was carried out every other day (~3 sessions per week, over the 10 weeks), for a total of 26 sessions. Although it is possible to run two tDCS training sessions in a day, we only ran 1 session per day due to logistics.
Among different parameters analyzed in the ReST treatment program (sounds, beats, and smoothness), speech sound accuracy during NSWs production and the speech sound production, was chosen as the main outcome for this study as it seemed to be the most representative of the participant’s efforts in motor programming and planning to pronounce the NSWs. Besides, this parameter could be objectively extracted as all the three syllables in a trisyllabic NSW must be produced correctly for the utterance to be scored as correct and computed as 1, and 0 (zero) was computed when one, two or all syllables were incorrectly pronounced, as recommended by the ReST therapy data sheet (
https://rest.sydney.edu.au/). The mean percentage (%) of correct responses (± standard error of the mean - SEM) was calculated for each ReST practice session considering 50 trisyllabic NSWs utterances in each practice session (10 NSWs repeated 5 times each).
2.2.3. Speech assessments
The following tests were remotely administered: (1) The ABFW Child Language Test (ABFW) is used to test areas of phonology, vocabulary, fluency, and pragmatics. It was created and validated for the Brazilian child population (Andrade et al., 2004). The vocabulary evaluation consists of nine different semantic fields (clothing, animals, food, transportation, furniture and fixtures, professions, sites, shapes and colors, toys, and musical instruments), providing percentage scores. The phonological test consists of 34 pictures of objects for naming and 39 words for imitation. From this test the correct consonants can be counted, and the PCC index can be calculated (Shriberg & Kwiatkowski, 1982); (2) Montreal-Toulouse Language battery (Brazilian version; MTL-BR for the evaluation of language comprehension (Pagliarin et al., 2015; Pagliarin et al., 2014). This test assesses spoken and written language, praxis and arithmetical skill (Pagliarin et al., 2014); (3) FOCUS-34 parent and clinician (Oddson et al., 2019) (Brazilian Portuguese version) designed to measure functional outcomes in everyday life. Additionally, within session consistency of production was assessed by examining the number of correct repetitions of NSWs. All speech outcome measures were double checked for reliability (no errors or disagreements were present).
2.2.4. Event-Related Potentials
Electrophysiological event-related potentials (EEG/ERPs) were recorded during ReST practice phase of each session (Fig. 1) through a 30-channel wireless system operated by lithium battery and with dry electrodes (Quick-30, Cognionics Inc., San Diego, USA) (Fig. 2). Electrodes were placed over the scalp according to the international 10/20 EEG system. Data were recorded with a sampling rate of 500 Hz filtering between 0.5 Hz and 100 Hz with auricular electrode (A1) as reference and having NSWs presented during the ReST practice as stimuli.
EEG data was post-processed using BrainVision Analyzer 2.1.2 Professional software (BrainProducts GmbH, Munich, Germany) (Fig. 2). Data was filtered from 0.5305164 (order 2, time constant 0.3) to 30 Hz (order 2) with the notch enabled at 60 Hz. Ocular correction was done by independent component analysis having the Fp1 channel as blink marker. Next, artifact removal was inspected semi-automatically. Finally, all datasets were segmented into epochs from −200 to 1000 ms relative to picture and audio onset and averaged. All epochs were retained. Control correction was performed using the pre-stimulus interval (i.e. −200 to 0 ms). Low-resolution brain electromagnetic tomography analysis (LORETA) was applied to estimate the three-dimensional intracerebral current density distribution (μA/mm2) (Anderer et al., 2000; Pascual-Marqui et al., 2002; Pascual-Marqui et al., 1999; Worrell et al., 2000).
Current source densities (CSDs) of P3 segment were measured within the interval between 250 and 350 milliseconds (Fig. 2) from regions of interest (ROIs). We specifically extracted measurements from regions related to speech and to regions surrounding tDCS electrodes position: Broca’s area (left BA 44/45), right contralateral region (right BA 44/55), Wernicke’s area (left BA 22), Sylvian temporal parietal junction (left BA 22/39), left and right supramarginal gyrus, left and right inferior parietal lobule, left and right dorsolateral prefrontal cortex (left and right BA 9/46), left and right frontal eye field (left and right BA 8), left and right ventrolateral prefrontal cortex (coordinates left: -32, 56, 6; right: 34, 54, -4; radius: 10 mm (Androulakis et al., 2018)), ventromedial prefrontal cortex (coordinates: -2, 32, -10, radius: 10 mm (Pelletier-Baldelli et al., 2020; Whittaker et al., 2018)). The brain activity in these different regions was then compared between treatment conditions (tDCS vs sham-tDCS) and within therapy sessions and correlated to sound production during ReST practice performance.
2.2.5. Statistical Analysis
SPSS Statistics Base 24.0 (SPSS Inc., USA) and GraphPad Prism 7.0 (GraphPad Software Inc, USA) were employed for statistical analysis and graphic presentations.
A two-way analysis of variance (ANOVA) with repeated measures was performed to localize the significant differences. We matched the data by both factors (2 conditions: tDCS vs sham-tDCS) vs 10 ReST practice sessions for all comparisons. These results were then followed by Bonferroni’s multiple comparisons test.
To estimate whether brain stimulation procedure could predict speech performance, a linear regression analysis was applied on the percentage (%) of correct responses of speech sound production of trisyllabic NSWs of ReST practice performance across 10-sessions under tDCS or sham-tDCS conditions. Slopes of linear curves were further compared between conditions. A paired t-test was also applied to compare the number of correct utterances of the trisyllabic NSWs of ReST practice performance from initial and final probe sessions.
P3-CSDs from selected ROIs obtained during ReST practice performance were analyzed by two-way ANOVAs with repeated measures matched by both factors [2 conditions (tDCS vs sham-tDCS) vs 10 sessions] followed by Bonferroni’s multiple comparisons tests. Cross-correlations between P3-CSD from these selected ROIs were also done.
Linear regressions were applied on the percentage (%) of correct productions of trisyllabic NSWs of ReST practice performance over the mean of P3-CSDs obtained across 10 sessions under both tDCS and sham-tDCS conditions, and between P3-CSDs from main ROIs.
3. Results
3.1. Participant
The participant of this study was a 20-years old male individual with clinical and genetic diagnosis of Trisomy of chromosome 21, fulfilling criteria for intellectual disability (Diagnostic and Statistical Manual of Mental Disorders - fifth edition, DSM-5) or intellectual development disorder (International Classification of Diseases 11th Revision, ICD 11), and for communication disorders, more specifically for speech sound disorder.
In the absence of a gold standard test for CAS diagnosis, the clinical identification of CAS was based on the checklist published by Namasivayam et al. (2015). This checklist states that the presence of at least seven of 12 behavioral features suggests a diagnosis of CAS. The participant of this study presented ten of 12 features on this CAS checklist and was remotely diagnosed by one of the co-authors (ACEV), a qualified speech-language pathologist, as having CAS.
Additionally, to stablish the severity of the speech disorder the Percentage of Correct Consonants (PCC) was calculated (Shriberg & Kwiatkowski, 1982). This index is obtained by dividing the Number of Correct Consonants (NCC) by the total number of consonants [NCC added to the Number of Incorrect Consonants (NIC)], multiplied by one hundred. Based on the PCC result, the speech disorder is classified into four categories: severe (PCC < 50%), moderate-severe (50% < PCC < 65%), mild-moderate (65% < PCC < 85%) and mild (85% < PCC < 100%) (Shriberg & Kwiatkowski, 1982). In the PCC index, omissions, substitutions and distortions are considered as errors (Andrade et al., 2004; Shriberg & Kwiatkowski, 1982; Wertzner et al., 2006). In this study, the PCC index was calculated from picture naming and word imitation scored in the ABFW test (Barrozo et al., 2017). As per PCC scores, the participant demonstrated moderate-severe speech disorder (PCC = 61.6%; 52.2% on figure naming and 71% for word naming).
Language comprehension was evaluated by the vocabulary section for ABFW and MTL-BR at the beginning of the study. In the vocabulary section of ABFW, the participant showed percentages of usual verbal designation of pictures representing nine different conceptual fields expected for 7-year-old children which is the maximum age that this test was formulated for. In the MTL-BR test, the participant showed 100% (5 out of 5) of word comprehension, and 57% of phrases comprehension (8 out of 14), giving a total score of 68.4%. Both tests showed that participant had adequate language comprehension.
Thus, despite intellectual limitation and the severity of the CAS, the participant showed adequate language comprehension, was able to understand Brazilian Portuguese and to carry out the experimental instructions. Besides, he was in good general health condition, with no other diagnosis of mental disorders and any restrictions for brain stimulation procedures and did not have past or current illnesses or abnormalities in laboratory tests that could be aggravated during the treatment.
3.2. ReST performance
3.2.1. Speech sound production
The percentage of correct responses for sound production at 1
st, 2
nd and 3
rd sessions was larger under sham-tDCS condition 8.0, 3.7 and 1.25 times (between 10 to 22%), respectively, over the tDCS condition (between 2 to 8%). However, the performance under tDCS surpassed the sham-tDCS performance after the 4
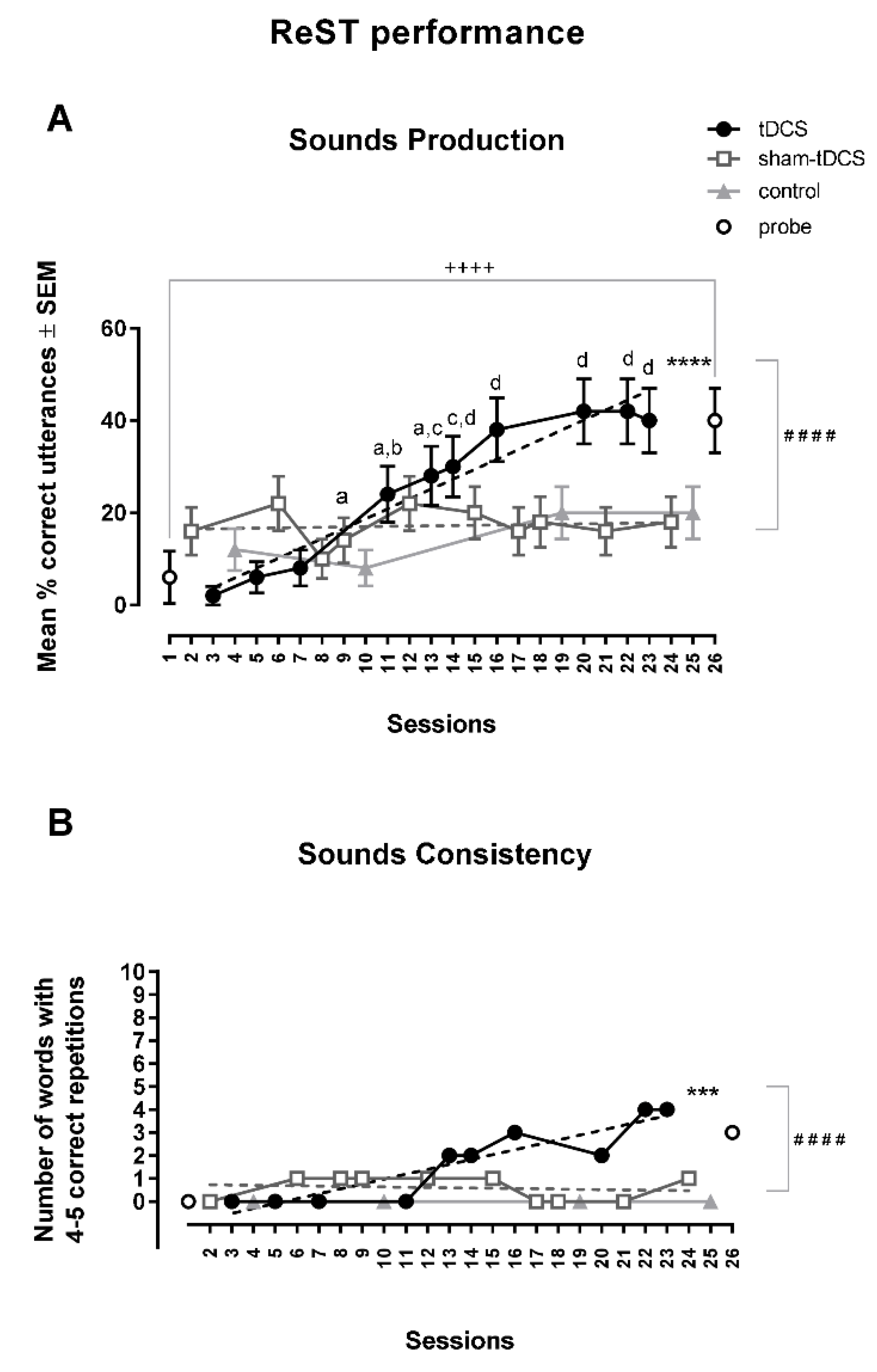
th session reaching a plateau in the last three sessions in which the percentage of correct responses (around 40%) were shown to be 2.3, 3.0, 2.2 times over sham-tDCS condition (around 20%) (
Figure 3). The percentage of correct responses for sound production was between 8 to 20% in the four control sessions, while it increased 10 times in probe test sessions, from 6% at the initial to 40% at the end of the study protocol (
Figure 3A).
The two-way ANOVA with repeated measures was not different in the between-condition analysis [F(1,49) = 2.72, MSE = 0.71], but it showed a statistically significant difference of correct utterance of trisyllabic NSWs in ReST practice performance in the within-condition analysis [F(9,441) = 5.92, MSE = 0.68, p < 0.0001, ωp2 = 0.0363] and also a significant interaction between factors [F(9,441) = 5.71, p < 0.0001, MSE = 0.10, ωp2 = 0.0315]. Bonferroni’s multiple comparisons test showed statistically significant differences when comparing data from 4th to 10th sessions from those obtained in the 1st to 3rd sessions under tDCS condition. No within-condition differences were found across sessions under sham-tDCS condition.
Linear regression analysis showed a statistically significant increase in the % of correct responses under tDCS condition [Y = -2.714 + 2.143X, r2 = 0.93; F(1,8) = 112.4, p < 0.0001]. The slope under sham-tDCS condition was not statistically significant [Y = 16.38 + 0.0625X, r2 = 0.014; F(1,8) = 0.12, p = 0.74]. There was a significant difference when comparing slopes under tDCS and sham-tDCS conditions [F(1,16) = 57.87, p < 0.0001] (Fig. 3A).
3.2.2. Probe: pre- and post-analysis
A statistically significant increase of correct utterance of trisyllabic NSWs in ReST performance was observed between initial and final probes (t = 4.63, df = 49; p < 0.0001, paired t-test) (Fig. 3A).
3.2.3. Consistency of NSWs utterances
Differences in the consistency of trisyllabic NSWs utterances were observed between the tDCS and the sham-tDCS condition. Under the tDCS condition, 4 of 10 NSWs were correctly repeated 4 or 5 times in the last two sessions. In comparison, under the sham-tDCS condition, 0 to 1 NSW was consistently repeated in the last two sessions. No NSWs was consistently repeated in control sessions and 3 NSWs were correctly repeated 4-5 times in the final probe test. A statistically significant upwards slope of sounds consistency under tDCS condition was clearly shown by the linear regression analysis [Y = -1.15 + 0.213X, r2 = 0.83; F(1,8) = 39.7, p = 0.0002]. This pattern was not observed with the slope under the sham-tDCS condition [Y = 0.75 – 0.012X, r2 = 0.03, p = 0.66]. The slope in the sham-tCDS condition was significantly different compared to the slope under tDCS condition [F(1,16) = 27.98, p < 0.0001] (Fig. 3B).
3.3. Speech assessments
An analysis of the phonological ABFW showed a significant clinical improvement in figure naming (about 28.9%); the PCC index increased from moderate-severe (PCC = 47 out of 90, i.e., 52.22%) to mild-moderate (PCC = 73 out of 90, i.e., 81.11%). There was no change in severity for word imitation (i.e., stayed at mild-moderate severity). There was a slight increase (7.5%) in the PCC index from 71.0% (76 out of 107) to 78.5% (84 out of 107).
The FOCUS scores did not significantly change in the study (<9 difference meaning not likely a meaningful clinical change, according to guidelines from Preschool Speech and Language Outcome Measurement Guide, 2015). The scores of FOCUS-34 clinician form were of 70 and 75 at the initial and final evaluations, respectively, and of FOCUS-34 parental form were of 56 to 60 at the initial and final evaluations, respectively. FOCUS assesses participation of the child in a broader social-communication context. We potentially attribute this lack of change in functional communication to the strict social restrictions placed during the COVID-19 pandemic.
3.4. EEG/ERPs
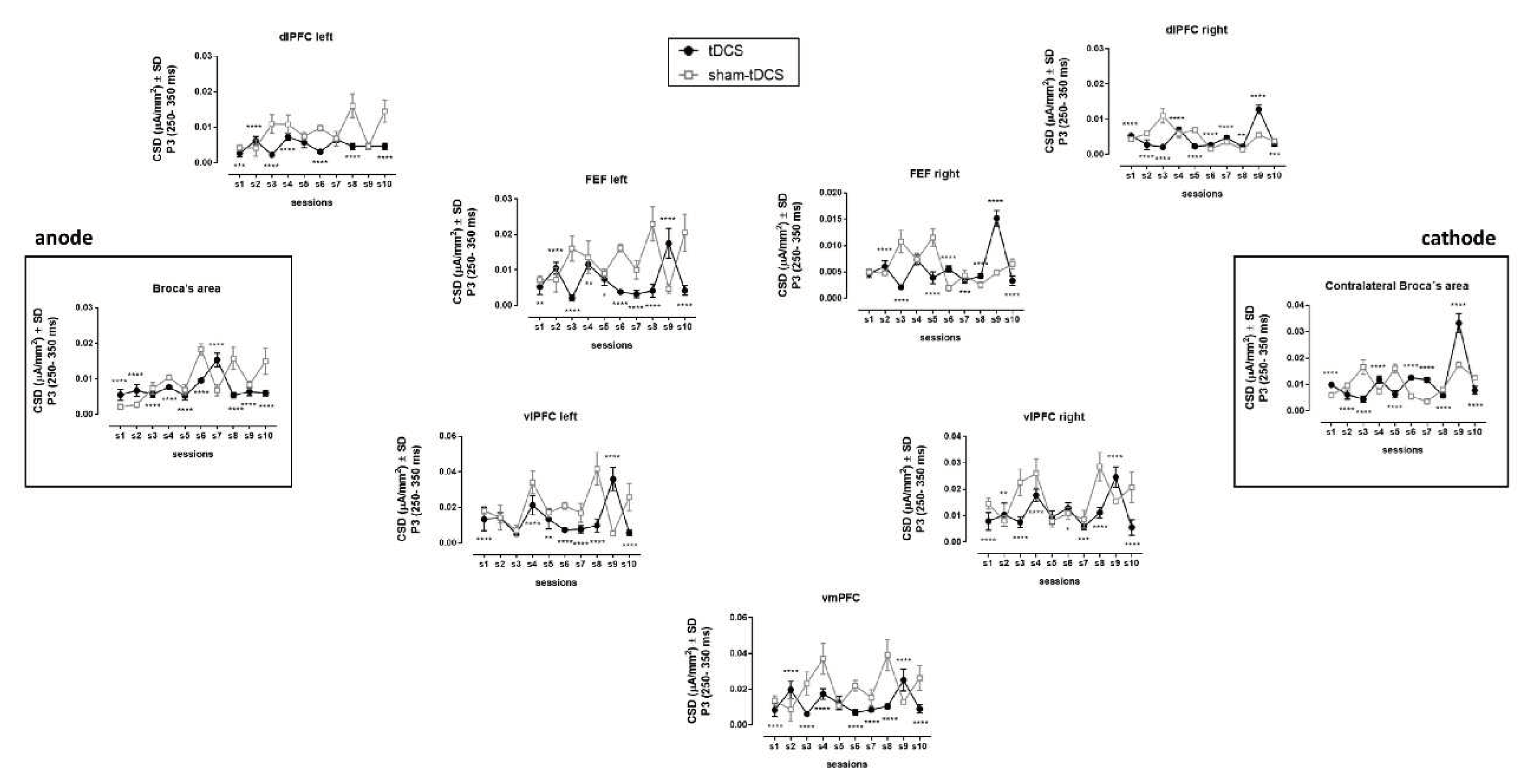
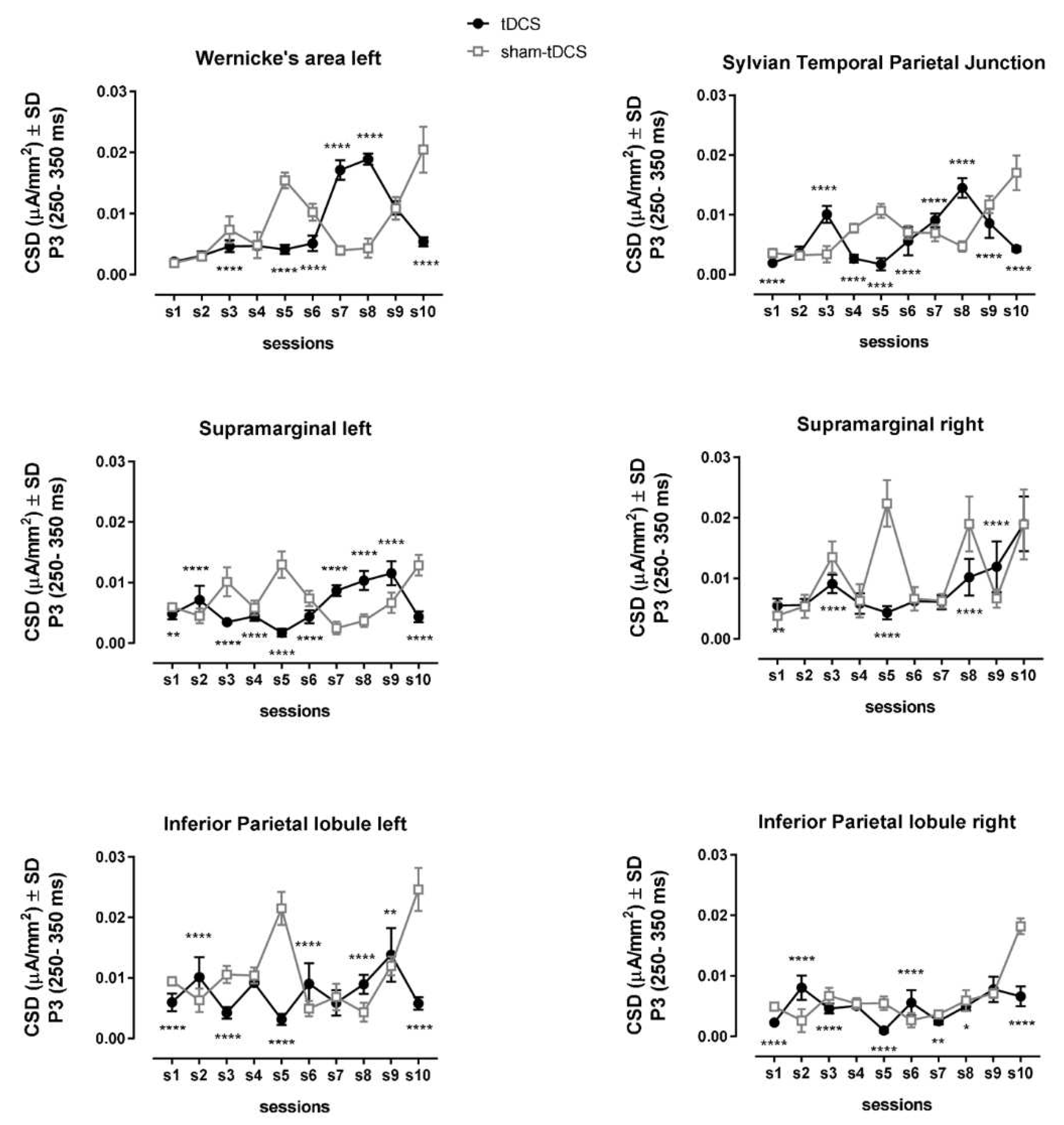
Differences in brain activation were observed during P3 interval (250 – 350 ms). The current source densities (CSDs) of prefrontal region were, in general, reduced under tDCS condition over the sessions when compared to sham-tDCS condition (
Figure 4,
Table 1). This reduction was especially from the left side of the brain, including the Broca’s area and the ventromedial prefrontal cortex (vmPFC).
An opposite pattern was observed for Wernicke’s area, left supramarginal gyrus, and Sylvian Temporal Parietal Junction, regions related to speech motor function (
Figure 5,
Table 1). These brain regions followed inverted U-shaped curves from sessions 6 to 10 under tDCS condition, meanwhile there was a reverse pattern, U-shaped curves, over these sessions under sham-tDCS condition (
Figure 5).
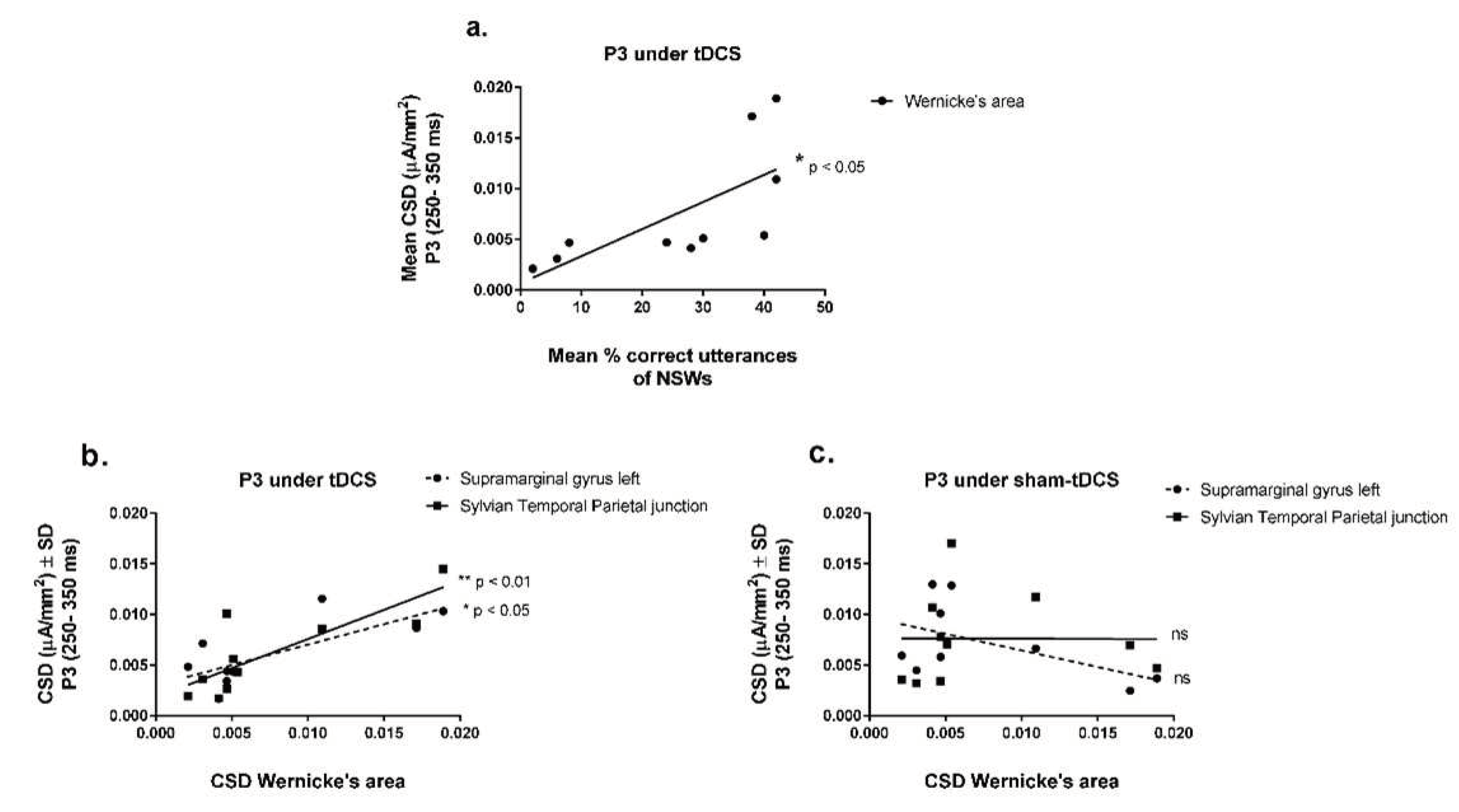
Interestingly, CSDs from Wernicke’s area under tDCS condition progressively increased with the improvement of speech sound productions over the learning sessions (
Figure 6a) [Y = 0.000667 + 0.0002672X, r
2 = 0.49, F(1,8) = 7.54, p = 0.0252, Linear regression analysis]. No other brain regions of interest were linearly related to speech utterances during ReST practice performance.
Additionally, the CSDs from Wernicke’s area progressively increased with increasing CSDs from supramarginal gyrus from the left hemisphere [Y = 0.003 + 0.407X, r2 = 0.56, F(1,8) = 10.28, p = 0.0125] and from Sylvian Temporal Parietal junction [Y = 0.00183 + 0.575X, r2 = 0.67, F(1,8) = 15.96, p = 0.004] under tDCS condition (Fig. 6b). Surprisingly, no other brain region depicted in this study was related to Wernicke’s area activation, not even the Broca’s area. There was also no relation found between Wernicke’s area and other brain regions under the sham-tDCS condition (Fig. 6c).
4. Discussion
The present study investigated the effects of tDCS combined with a motor learning task in developmental apraxia of speech co-existing with T21. The bilateral brain stimulation using tDCS (anodal stimulation of Broca’s area (left IFG) and cathodal stimulation of its homologue contralateral region (right IFG)) progressively increased the accuracy of speech sound production (best performance reached ~ 40%), indicating a significant clinical gain. In contrast, the performance under sham-tDCS condition did not change and was around 20% from the beginning to the end of the study protocol. Improvements were also noted for speech consistency and phonological ABFW (figure naming) test.
Marangolo et al. (2011) showed that anodal tDCS over the left IFG (with cathode over the right supraorbital region) produced long-term speech improvements in three patients with chronic aphasia and apraxia. They observed an increase in the mean percentage of response accuracy from 7.1% to about 34% after five tDCS sessions (1 mA, 35 mm2, for 20 min) compared to change of 18.3% after five sham sessions. Between pre- and post-training, there was a mean difference of percentage on response accuracy of 26.7% for anodal tDCS and 11.7% for sham condition.
In a follow-up tDCS study on eight chronic patients with aphasia and apraxia, Marangolo et al. (2013) demonstrated that active bihemispheric stimulation (2 mA, 35 cm2, for 20 min) over left and right IFG over 10 sessions increased the accuracy of correct words by 22% relative to sham-tDCS.
In the current study, similar gains in speech accuracy were observed. The mean difference between the first and fifth tDCS sessions, was of 26%, while between the first and fifth sham-tDCS sessions, was only 6%. The overall accuracy increased by 38% after 10 sessions of tDCS and only by 2% after 10 sessions of sham in the current study. Thus, gains produced by the tDCS on speech accuracy in apraxia of speech in a single individual with T21 resemble those reported by Marangolo et al. (Marangolo et al., 2013; Marangolo et al., 2011) in apraxia of speech in adult patients with aphasia.
Marangolo et al. (2013) observed that tDCS-induced changes generalized to other tasks administered before and after the treatment. A generalization effect was also observed by Themistiocleous et al. (2021) as they found that sounds of untrained words were 47% shorter in tDCS condition compared to sham immediately after treatment. In our study, some transference could be inferred by comparing the sound production of untrained 10-NSWs set (probe) applied at the beginning and at the end of the study protocol. The gain of speech sound accuracy was 34% (from initial 6% to the final 40%). Also, some transference could be inferred from the 28.9% increase in the PCC index scores obtained from the ABFW test.
The pattern of Wernicke’s area activity during ReST training seemed to predict the pattern of gain of speech sound accuracy over the 10 sessions under the tDCS condition. Changes in Wernicke’s area following speech motor intervention has been reported earlier by Kadis et al. (2014). They investigated cortical thickness changes in response to 8-weeks of PROMPT intervention (a type of speech motor intervention) in children (ages 3-6 years) with CAS. Following therapy, 8 of 9 children with apraxia demonstrated a significant thinning of the left posterior superior temporal gyrus (canonical Wernicke’s area). They argued that these findings demonstrated experience-dependent structural plasticity in children with CAS. However, in their study, the degree of cortical thinning was not significantly correlated to the change in standardized speech assessments (Kadis et al., 2014).
Much beyond of what has been classically conceived as related to language comprehension, the left posterior Superior Temporal Gyrus (pSTG) together with adjacent supramarginal gyrus, named as Wernicke’s area, has been recently considered to be critical for speech production (Binder, 2015, 2017; Tremblay & Dick, 2016). According to Binder (2017), neuroimaging studies have provided evidence that Wernicke’s area is not critical for speech perception or word comprehension. Instead, Wernicke’s area supports the retrieval of phonological forms (mental representations of phoneme sequences), which are essential for speech output. Binder (2017) even suggested that Wernicke’s area should no longer be referred as critical for speech comprehension because it does not actually support language comprehension.
In the present study, the anodal tDCS, but not sham-tDCS, over Broca’s area (having the cathode placed over the contralateral region) may have triggered the recruitment of the Wernicke’s area when the subject was trained to speak trisyllabic NSWs (i.e., with no associated semantic meaning), successively presented in written and audio formats. Furthermore, the activation of Wernicke’s area triggered by the Broca’s anodal tDCS was positively correlated to the activation of supramarginal gyrus from the left hemisphere and of Sylvian Temporal Parietal Junction.
In contemporary view, the left pSTG and adjacent cortex in the superior temporal sulcus and supramarginal gyrus regions are thought to store and mentally activate phonological (speech sounds) forms, a process termed as phonological representation (or phonological encoding, phonological access, phonological retrieval, etc.) (see (Binder, 2017). This author specifies that “phonological” refers to the spoken form of the word, not the written form or the meaning. According to Binder (2017), the phonological representation is a necessary stage prior to all speech output tasks and is also needed to maintain speech sounds in short-term memory. Patients with lesions in the left pSTG and supramarginal gyrus are specifically unable to retrieve an internal mental image of the phonemes represented by the written words.
In a series of left hemisphere stroke patients, Pillay et al. (2014) identified the pre-articulatory phonological representation (phonological access, or phonological retrieval) as correlated with damage to a focal region of the cortex and white matter caudal to the posterior sylvian fissure, including the posterior supramarginal gyrus and adjacent anterior angular gyrus, planum temporale, and pSTG, and no correlation was observed with Broca’s area, insula, or sensorimotor cortex. Additionally, they found no correlation between damage in this posterior perisylvian region and spoken word comprehension.
The concept of phonological representation of speech sound production seems to fit well with the core concepts of the ReST approach. The principles of (speech) motor learning procedure employed in ReST requires the subject to build a mental image of the spoken forms of NSWs and retrieve them from a verbal short-term memory to produce them correctly. Thus, it may be possible that the ReST task requires the function of the Wernicke’s area, triggered by the repetitive anodal tDCS over the Broca’s area in the present study. The involvement of Wernicke’s area with adjacent supramarginal gyrus and superior temporal sulcus, including the Sylvian Temporal Parietal Junction may be necessary to the processing of the phonological representation of NSWs.
Studies on phonological short-term memory have shown that the posterior end of the Sylvian Temporal Parietal area, a region in the posterior portion of the planum temporale, is activated during stimulus encoding (perception) and covert rehearsal (Buchsbaum et al., 2011). This area seems to be maximally activated during phonological rehearsal tasks, and it has been thought that it would function as an interface site for the integration of sensory and vocal tract-related motor representations of complex sound sequences, including speech and music (see (Buchsbaum et al., 2011)). The Sylvian Temporal Parietal region may be critical for the transformation of an auditory input code to an articulatory (or output) code occurring during tests of simple repetition as well as phonological working memory (Buschsbaum et al. 2011).
Ferpozzi et al. (2018) observed that Broca’s area does not have a direct control on the phono-articulatory apparatus, not being a proper motor area by itself. Instead, it could be involved in more cognitive pre-articulatory function (i.e., operating as a functional gate), authorizing the phonetic translation preceding speech articulation executed by the motor areas. In the present study, the activation of the Broca’s area was not strong under anodal tDCS over this region during the motor speech training. Considering the “functional gate” hypothesis, Broca’s area may have not been the one with the greater change induced by the anodal tDCS. However, its stimulation allowed the recruitment and activation of the other regions required for the phonological representation and translation, cognitively orchestrating the phonological working memory.
Under tDCS condition, the activity in brain regions that were correlated to the speech accuracy mentioned above followed an “inverted U” shape. It mostly reached the maximum activation in sessions 7 and 8 and decreasing afterwards, in sessions 9 and 10. This pattern of the activation curvature may suggest that these brain regions were increasingly recruited up to the maximum speech accuracy. After reaching a plateau of speech performance, these regions were possibly no longer in demand, leading to a reduction in the resources needed for phonological working memory processing (Temistocleous et al. (2021)). Ficek et al. (2018) observed a lower functional connectivity of stimulated areas (between frontal and temporal areas in the language network) after repeated anodal tDCS over the left IFG in patients with primary progressive aphasia, which was correlated with the improved performance in language therapy.
Limitations: In this study, we used a N-of-1 randomized study because of the rare co-occurrence of CAS and T21. Although this N-of-1 randomized study was carefully designed and conducted, it is still limited to one single participant. To strengthen the evidence, further replication (potentially in a multi-center clinical trial) with more participants is needed. Generalizability of study findings is also limited because longitudinal data could not be collected due to restrictions put in place during the height of the first wave of COVID-19 pandemic lockdown in Brazil (in early 2020).
5. Conclusion
Multiple sessions of anodal tDCS over Broca’s area (with cathodal over the contralateral region) improved the speech sound accuracy during training of NSWs in the ReST protocol for a young individual with T21 with CAS.
The activation of Wernicke’s area seems to predict the progressive gain in speech performance seen under repetitive anodal bihemispheric tDCS condition. This activation appeared to predict the activation of the supramarginal gyrus and Sylvian Temporal Parietal Junction from the left hemisphere. These brain regions are essential for phonological working memory processes to provide accurate speech sound production.
Bearing in mind the need of replication in other young adult individuals with T21 and their individual characteristics, we may suggest that NIBS, such as tDCS, over speech sound network could be useful to help with the treatment of apraxia of speech in this population.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability Statement (DAS)
The authors confirm that the data supporting the findings of this study are available within the article. However, supplementary data/statistical analysis of this study are available on request from the corresponding author, EMN-P.
Acknowledgments
The authors would like to thank the participant for participating in this research. We also want to thank Soterix Medical Inc. (New York, USA) for providing a unit of their portable tDCS device that has been used in this study. .
Conflicts of Interest Statement
The authors report no conflict of interest.
References
- Anderer, P.; Saletu, B.; Pascual-Marqui, R.D. Effect of the 5-HT(1A) partial agonist buspirone on regional brain electrical activity in man: a functional neuroimaging study using low-resolution electromagnetic tomography (LORETA). Psychiatry Res 2000, 100, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Andrade CR, F.; Béfi-Lopes, D.M.; Fernandes FD, M.; Wertzner, W.H. ABFW: Teste de linguagem infantil nas áreas de Fonologia, Vocabulário, Fluência e Pragmática. In Pró-Fono (Ed.); 2004.
- Androulakis, X.M.; Krebs, K.A.; Jenkins, C.; Maleki, N.; Finkel, A.G.; Rorden, C.; Newman, R. Central Executive and Default Mode Network Intranet work Functional Connectivity Patterns in Chronic Migraine. J Neurol Disord 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Luber, B.; Brem, A.K.; Bikson, M.; Brunoni, A.R.; Cohen Kadosh, R.; Dubljevic, V.; Fecteau, S.; Ferreri, F.; Floel, A.; Hallett, M.; Hamilton, R.H.; Herrmann, C.S.; Lavidor, M.; Loo, C.; Lustenberger, C.; Machado, S.; Miniussi, C.; Moliadze, V.; Nitsche, M.A.; Rossi, S.; Rossini, P.M.; Santarnecchi, E.; Seeck, M.; Thut, G.; Turi, Z.; Ugawa, Y.; Venkatasubramanian, G.; Wenderoth, N.; Wexler, A.; Ziemann, U.; Paulus, W. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract 2022, 7, 146–165. [Google Scholar] [CrossRef] [PubMed]
- ASHA. Evidence-Based Practice in Communication Disorders: An Introduction [Technical Report]. 2004. Available online: https://www.asha.org/policy/tr2004-00001/.
- Bahar, N.; Namasivayam, A.K.; van Lieshout, P. Telehealth intervention and childhood apraxia of speech: a scoping review. Speech, Language and Hearing 2021, 1–13. [Google Scholar] [CrossRef]
- Barrozo, T.F.; Pagan-Neves, L.O.; Pinheiro da Silva, J.; Wertzner, H.F. Sensitivity and specificity of the Percentage of Consonants Correct-Revised in the identification of speech sound disorder. Codas 2017, 29, e20160038. [Google Scholar] [CrossRef]
- Binder, J.R. The Wernicke area: Modern evidence and a reinterpretation. Neurology 2015, 85, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R. Current Controversies on Wernicke's Area and its Role in Language. Curr Neurol Neurosci Rep 2017, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, B.R.; Baldo, J.; Okada, K.; Berman, K.F.; Dronkers, N.; D'Esposito, M.; Hickok, G. Conduction aphasia, sensory-motor integration, and phonological short-term memory - an aggregate analysis of lesion and fMRI data. Brain Lang 2011, 119, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, A.; Calhoun, H.; Rimikis, S.; Lowe, M.S.; Wellner, R.; Edwards, D.J. Using tDCS to facilitate motor learning in speech production: The role of timing. Cortex 2019, 111, 274–285. [Google Scholar] [CrossRef]
- Cassar, C.; McCabe, P.; Cumming, S. "I still have issues with pronunciation of words": A mixed methods investigation of the psychosocial and speech effects of Childhood Apraxia of Speech in adults. Int J Speech Lang Pathol 2022, 1–13. [Google Scholar] [CrossRef]
- Edgington, E.S. Randomized single-subject experiments and statistical tests. Journal of Counseling Psychology 1987, 34, 437–442. [Google Scholar] [CrossRef]
- Ferpozzi, V.; Fornia, L.; Montagna, M.; Siodambro, C.; Castellano, A.; Borroni, P.; Riva, M.; Rossi, M.; Pessina, F.; Bello, L.; Cerri, G. Broca's Area as a Pre-articulatory Phonetic Encoder: Gating the Motor Program. Front Hum Neurosci 2018, 12, 64. [Google Scholar] [CrossRef] [PubMed]
- Ferron, J.; Ware, W. Using randomization tests with responsive single-case designs. Behav Res Ther 1994, 32, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Ficek, B.N.; Wang, Z.; Zhao, Y.; Webster, K.T.; Desmond, J.E.; Hillis, A.E.; Frangakis, C.; Vasconcellos Faria, A.; Caffo, B.; Tsapkini, K. The effect of tDCS on functional connectivity in primary progressive aphasia. Neuroimage Clin 2018, 19, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; El-Hagrassy, M.M.; Pacheco-Barrios, K.; Carvalho, S.; Leite, J.; Simis, M.; Brunelin, J.; Nakamura-Palacios, E.M.; Marangolo, P.; Venkatasubramanian, G.; San-Juan, D.; Caumo, W.; Bikson, M.; Brunoni, A.R.; Neuromodulation Center Working, G. Evidence-Based Guidelines and Secondary Meta-Analysis for the Use of Transcranial Direct Current Stimulation in Neurological and Psychiatric Disorders. Int J Neuropsychopharmacol 2021, 24, 256–313. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.L.; Combs, A.B.; Acosta, D. Experimental models for the investigation of toxicological mechanisms. In Comprehensive Toxicology, 2nd ed.; M. C., A., Ed.; Elsevier Ltd., 2010; pp. 203–224. [Google Scholar]
- Kadis, D.S.; Goshulak, D.; Namasivayam, A.; Pukonen, M.; Kroll, R.; De Nil, L.F.; Pang, E.W.; Lerch, J.P. Cortical thickness in children receiving intensive therapy for idiopathic apraxia of speech. Brain Topogr 2014, 27, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Klauss, J.; Penido Pinheiro, L.C.; Silva Merlo, B.L.; de Almeida Correia Santos, G.; Fregni, F.; Nitsche, M.A.; Miyuki Nakamura-Palacios, E. A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 2014, 17, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Kumin, L. Speech intelligibility and childhood verbal apraxia in children with Down syndrome. Downs Syndr Res Pract 2006, 10, 10–22. [Google Scholar] [CrossRef]
- Lewis, B.A.; Freebairn, L.A.; Hansen, A.J.; Iyengar, S.K.; Taylor, H.G. School-age follow-up of children with childhood apraxia of speech. Lang Speech Hear Serv Sch 2004, 35, 122–140. [Google Scholar] [CrossRef]
- Luck, S.J. An introduction to the event-related potential technique; MIT Press, 2005. [Google Scholar]
- Marangolo, P.; Fiori, V.; Cipollari, S.; Campana, S.; Razzano, C.; Di Paola, M.; Koch, G.; Caltagirone, C. Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. Eur J Neurosci 2013, 38, 3370–3377. [Google Scholar] [CrossRef]
- Marangolo, P.; Marinelli, C.V.; Bonifazi, S.; Fiori, V.; Ceravolo, M.G.; Provinciali, L.; Tomaiuolo, F. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res 2011, 225, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 2013, 6, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.T.; Murray, E.; Liegeois, F.J. Interventions for childhood apraxia of speech. Cochrane Database Syst Rev 2018, 5, CD006278. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; McCabe, P.; Ballard, K.J. A Randomized Controlled Trial for Children With Childhood Apraxia of Speech Comparing Rapid Syllable Transition Treatment and the Nuffield Dyspraxia Programme-Third Edition. J Speech Lang Hear Res 2015, 58, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.K.; Pukonen, M.; Goshulak, D.; Hard, J.; Rudzicz, F.; Rietveld, T.; Maassen, B.; Kroll, R.; van Lieshout, P. Treatment intensity and childhood apraxia of speech. Int J Lang Commun Disord 2015, 50, 529–546. [Google Scholar] [CrossRef]
- Namasivayam, A.K.; Huynh, A.; Bali, R.; Granata, F.; Law, V.; Rampersaud, D.; Hard, J.; Ward, R.; Helms-Park, R.; Van Lieshout, P.H.H.M.; Hayden, D. Development and Validation of a Probe Word list to Assess Speech Motor Skills in Children. Am J Speech Lang Pathol 2021, 30, 622–648. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, A.K.; Yan, T.; Bali, R.; Hayden, D.; van Lieshout, P. Cross-Modal Somatosensory Repetition Priming and Speech Processing. J. Integr. Neurosci. 2022, in press. [CrossRef] [PubMed]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 2003, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Oddson, B.; Thomas-Stonell, N.; Robertson, B.; Rosenbaum, P. Validity of a streamlined version of the Focus on the Outcomes of Communication Under Six: Process and outcome. Child Care Health Dev 2019, 45, 600–605. [Google Scholar] [CrossRef]
- Pagliarin, K.C.; Ortiz, K.Z.; Barreto Sdos, S.; Pimenta Parente, M.A.; Nespoulous, J.L.; Joanette, Y.; Fonseca, R.P. Montreal-Toulouse Language Assessment Battery: evidence of criterion validity from patients with aphasia. J Neurol Sci 2015, 357, 246–251. [Google Scholar] [CrossRef]
- Pagliarin, K.C.; Ortiz, K.Z.; Parente, M.A.; Arteche, A.; Joanette, Y.; Nespoulous, J.L.; Fonseca, R.P. Montreal-Toulouse language assessment battery for aphasia: validity and reliability evidence. NeuroRehabilitation 2014, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol 2002, 24 Suppl C, 91–95. [Google Scholar] [PubMed]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.; Hell, D.; Koukkou, M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res 1999, 90, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Pelletier-Baldelli, A.; Orr, J.M.; Bernard, J.A.; Mittal, V. A. Social reward processing: A biomarker for predicting psychosis risk? Schizophr Res 2020, 226, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Perdices, M.; Tate, R.L. Single-subject designs as a tool for evidence-based clinical practice: Are they unrecognised and undervalued? Neuropsychol Rehabil 2009, 19, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.B.; Stengel, B.C.; Humphries, C.; Book, D.S.; Binder, J.R. Cerebral localization of impaired phonological retrieval during rhyme judgment. Ann Neurol 2014, 76, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Romero, R.; Polich, J. P3(00) habituation from auditory and visual stimuli. Physiol Behav 1996, 59, 517–522. [Google Scholar] [CrossRef]
- Rvachew, S. Application of single subject randomization designs to communicative disorders research. Human Communication Canada 1988, 12, 7–13. [Google Scholar]
- Rvachew, S.; Matthews, T. Demonstrating treatment efficacy using the single subject randomization design: A tutorial and demonstration. J Commun Disord 2017, 67, 1–13. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Kwiatkowski, J. Phonological disorders III: a procedure for assessing severity of involvement. J Speech Hear Disord 1982, 47, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Terranova, C.; Rizzo, V.; Cacciola, A.; Chillemi, G.; Calamuneri, A.; Milardi, D.; Quartarone, A. Is There a Future for Non-invasive Brain Stimulation as a Therapeutic Tool? Front Neurol 2018, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Themistocleous, C.; Webster, K.; Tsapkini, K. Effects of tDCS on Sound Duration in Patients with Apraxia of Speech in Primary Progressive Aphasia. Brain Sci 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C.; McCabe, P.; Ballard, K.J.; Lincoln, M. Telehealth delivery of Rapid Syllable Transitions (ReST) treatment for childhood apraxia of speech. Int J Lang Commun Disord 2016, 51, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, P.; Dick, A. S. Broca and Wernicke are dead, or moving past the classic model of language neurobiology. Brain Lang 2016, 162, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wertzner, H.F.; Papp, A.C.; Galea, D.E. [Picture naming and imitation tests as tools for the diagnosis of phonological disorder]. Pro Fono 2006, 18, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.R.; Foley, S.F.; Ackling, E.; Murphy, K.; Caseras, X. The Functional Connectivity Between the Nucleus Accumbens and the Ventromedial Prefrontal Cortex as an Endophenotype for Bipolar Disorder. Biol Psychiatry 2018, 84, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.; Abbeduto, L.; Camarata, S.M.; Shriberg, L.D. Estimates of the prevalence of speech and motor speech disorders in adolescents with Down syndrome. Clin Linguist Phon 2019, 33, 772–789. [Google Scholar] [CrossRef]
- Wilson, E.M.; Abbeduto, L.; Camarata, S.M.; Shriberg, L.D. Speech and motor speech disorders and intelligibility in adolescents with Down syndrome. Clin Linguist Phon 2019, 33, 790–814. [Google Scholar] [CrossRef]
- Worrell, G.A.; Lagerlund, T.D.; Sharbrough, F.W.; Brinkmann, B.H.; Busacker, N.E.; Cicora, K.M.; O'Brien, T.J. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr 2000, 12, 273–282. [Google Scholar] [CrossRef]
- Yu, V.Y.; Kadis, D.S.; Goshulak, D.; Namasivayam, A.K.; Pukonen, M.; Kroll, R.M.; De Nil, L.F.; Pang, E.W. Impact of Motor Speech Intervention on Neural Activity in Children with Speech Sound Disorders: Use of Magnetoencephalography. Journal of Behavioral and Brain Science 2018, 8, 415–429. [Google Scholar] [CrossRef]
- Zettin, M.; Bondesan, C.; Nada, G.; Varini, M.; Dimitri, D. Transcranial Direct-Current Stimulation and Behavioral Training, a Promising Tool for a Tailor-Made Post-stroke Aphasia Rehabilitation: A Review. Front Hum Neurosci 2021, 15, 742136. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).