I. Introduction
Antibiotics are a classification of medicine that fights off infections by either killing the bacteria or preventing it from growing further. There are two types of antibiotics:bactericidal and bacteriostatic. Bactericidal kills off the bacteria, mainly by either interfering with DNA replication/repair, protein synthesis, or the cell wall (Kohanski et al., 2007). Bacteriostatic antibiotics prevent the bacteria from growing and spreading further but do not kill it (Kohanski et al., 2007).
Moreover, the first antibiotic to be discovered was penicillin in 1928 by Alexander Fleming. This began the golden age of antibiotics (Hutchings et. al. 2019) . In this golden age, a myriad of antibiotics were discovered, revolutionizing modern medicine. Discoveries like penicillin have helped to cure disease and infections that used to be considered lethal. Some examples of these ailments are bacterial endocarditis, meningitis, and pneumococcal pneumonia (Kalvaitis 2008). But over the course of the many years we have been using antibiotics, some bacteria have evolved a resistance to them, causing these diseases to become deadly once more (Davies, J et al., 2010).
These resistant bacteria are commonly referred to as “superbugs”. The first case of resistant bacteria was MRSA, discovered in 1960, and was resistant to all beta-lactam antibiotics, for example:penicillins and cephalosporins (Hutchings et al., 2019). This resistance occurred for a plethora of reasons. For example, the over prescription of antibiotics have caused a lot more bacteria to be exposed to antibiotics and give a chance to develop resistance. Natural selection is also a factor, since the resistant bacteria are more likely to survive and multiply, the bacterial population will become more resistant over time. Patients not taking the full course of the antibiotics prescribed to them is also an issue. Some patients stop taking the antibiotics prescribed to them when their symptoms go away, thinking that the bacteria has all been eliminated. In reality there may be a small amount left that would have been killed off by the remaining antibiotics, but now has been exposed to the antibiotic and has the chance to grow back and evolve resistance. All of these cause changes in selective pressure which promote the increase of prevalence of resistance (Davies, J et al., 2010).
The main two solutions that scientists have been using is to modify existing antibiotics to be effective against the resistant bacteria and discovering new drugs that can eliminate these super bugs.
To further explain, superbugs can evolve to have specialist or general resistance. Special resistance means that the bacteria is resistant to only one antibiotic, but is extremely resistant to it. General resistance means that the bacteria is resistant to multiple different antibiotics, but at a low level. This means that you can eliminate specialist resistant bacteria by using a different antibiotic since it has developed resistance to a different kind of antibiotic. While generalist can be eliminated with a higher dosage than it has developed resistance to (Van Tienderen, 1991).
This experiment will test whether bacteria can evolve resistance to household antibiotics by using two over the counter pharmacy drugs, benzoyl peroxide and bacitracin. They both only contain one antibiotic so the bacteria would evolve specialist resistance. This is because there is only one selective pressure, meaning that there is only one thing causing the bacteria to evolve. Since there is only one antibiotic it needs to develop resistance to, it can become extremely resistant to it, therefore developing specialist resistance. Theorizing that through this experiment, the bacteria will be able to evolve resistance to the antibiotics they are exposed to.
II. Material And Methods
Materials: The materials used in this experiment are petri dishes, agar gel, LB, swabs, plastic wrap, household antibiotics (benzoyl peroxide and bacitracin), sterile water, and a graduated cylinder.
Bacitracin is a polypeptide antibiotic, it is used to treat bacterial skin infections or to prevent infection of minor burns, cuts, or scrapes. It is also bacteriostatic.
Benzoyl peroxide is an over-the-counter topical medication and is also an FDA-approved prescription medication for the treatment of acne vulgaris. It is bactericidal with activity against Cutibacterium acnes on the skin and within the hair follicles.
Bacitracin and benzoyl peroxide will both be used to develop specialist bacteria since they both use only one antibiotic.
Methods: In order to conduct the experiment, the first step was to collect bacteria by running a swab along a household surface. For this experiment, a bathroom sink was used. This bacteria was grown for five days in a petri dish sealed with plastic wrap, so that the bacteria would have time to grow in a controlled environment. One colony was isolated from the dish and grown for 2 weeks. This colony was isolated to ensure that all bacteria used in the experiment were the same species with the same DNA. This was crucial so that any difference in growth could be attributed to any evolved resistance, not from differences in the bacteria itself. This bacteria was referred to as generation 0.
For the controls of the experiment, bacteria from generation 0 were grown in three petri dishes under regular conditions, as well as in three petri dishes with sterile water. The water was sterilized by boiling it. The regular control was referred to as control 1, and the control with sterile water was referred to as control 2. The purpose of control 2 is to see if the different growth is caused by the presence of liquid as opposed to the presence of antibiotics. Each control was grown for five days.
For the experimentals, a dilution series was used to build resistance. For the first round, two separate solutions were made of the two antibiotics, benzoyl peroxide in one petri dish and bacitracin in another, diluted to 50% by volume. A scale was used to measure equal parts ointment and sterile water, and they were then mixed together. Two to three drops of benzoyl peroxide, diluted to 50%, were used to cover the surface of a petri dish. Then, bacteria from generation 0 was grown inside it for five days. The same was done with the solution of bacitracin diluted to 50%. These two experimentals were referred to as generation 1.
For the second round, the same process as before was repeated, although the corresponding antibiotic solution was increased to 66.27% dilution. This was referred to as generation. After five days, the process was repeated with full strength ointments and was referred to as generation 3.
Each experiment had two replicas. The experimentals’ growth, as well as the controls’ growth, was observed over the course of the experiment. It was observed how the experimentals grow in comparison to the controls, and if they were able to grow in an environment containing antibiotics and possibly develop any resistance to them. By using methods from the USDA, bacteria growth was measured using the point intercept method to determine the percent covered by bacteria growth. Chi-squared was used to compare the amount of bacteria growth of each generation against the previous generation to see if resistance builds.
III. Results
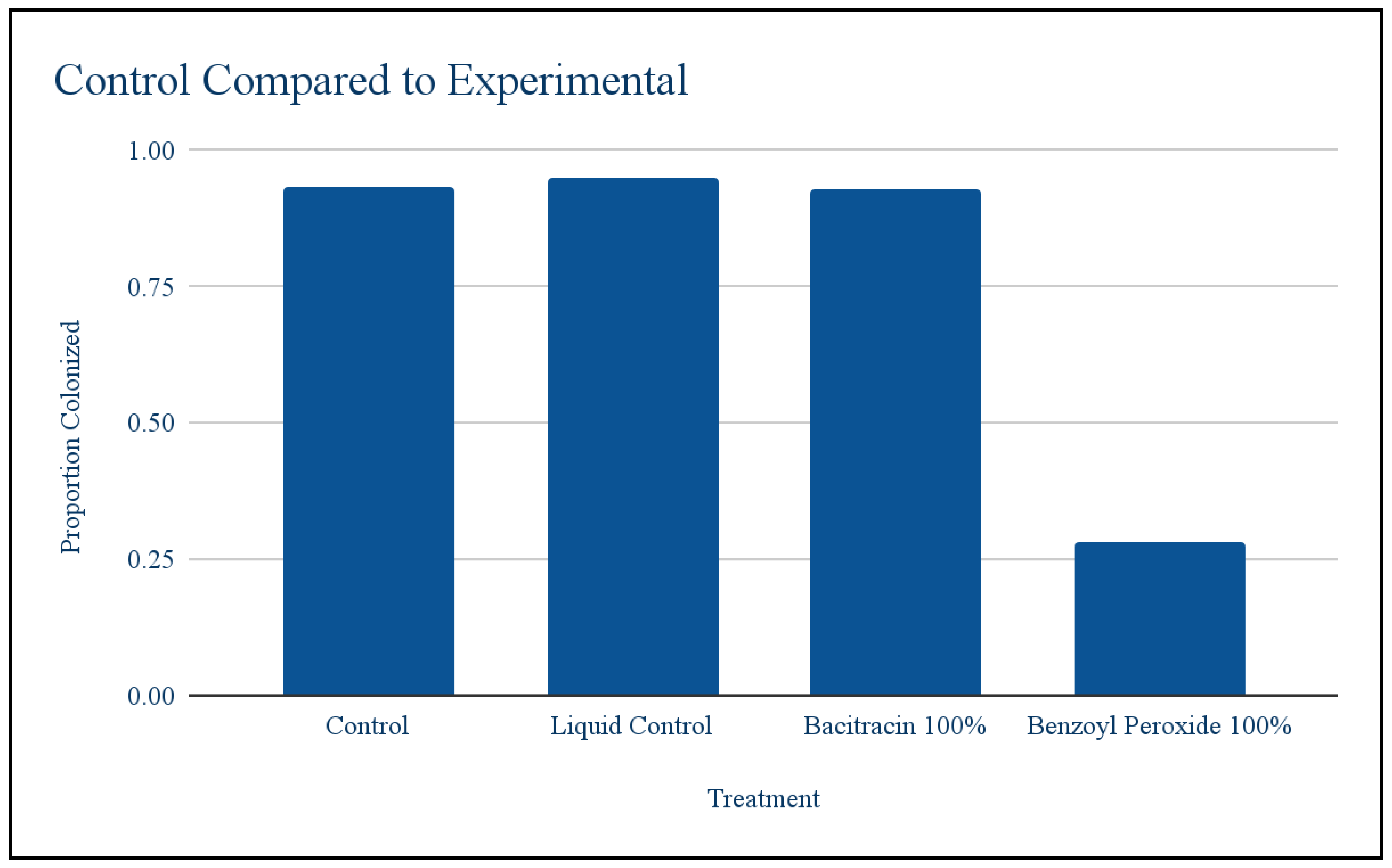
Controls vs expirementals: A T test was conducted to compare the controls to see if having a liquid on the petri dish would have an effect on the bacteria. The colonization proportions were not significantly different.at p < .05 (The t-value is -0.07352. The p-value is .944921). The colonization proportions were not significantly different, meaning that having a liquid present would not affect growth and any changes in growth were due to the antibiotics.
Figure 1.
Control Compared to First Round of Dilution.
Figure 1.
Control Compared to First Round of Dilution.
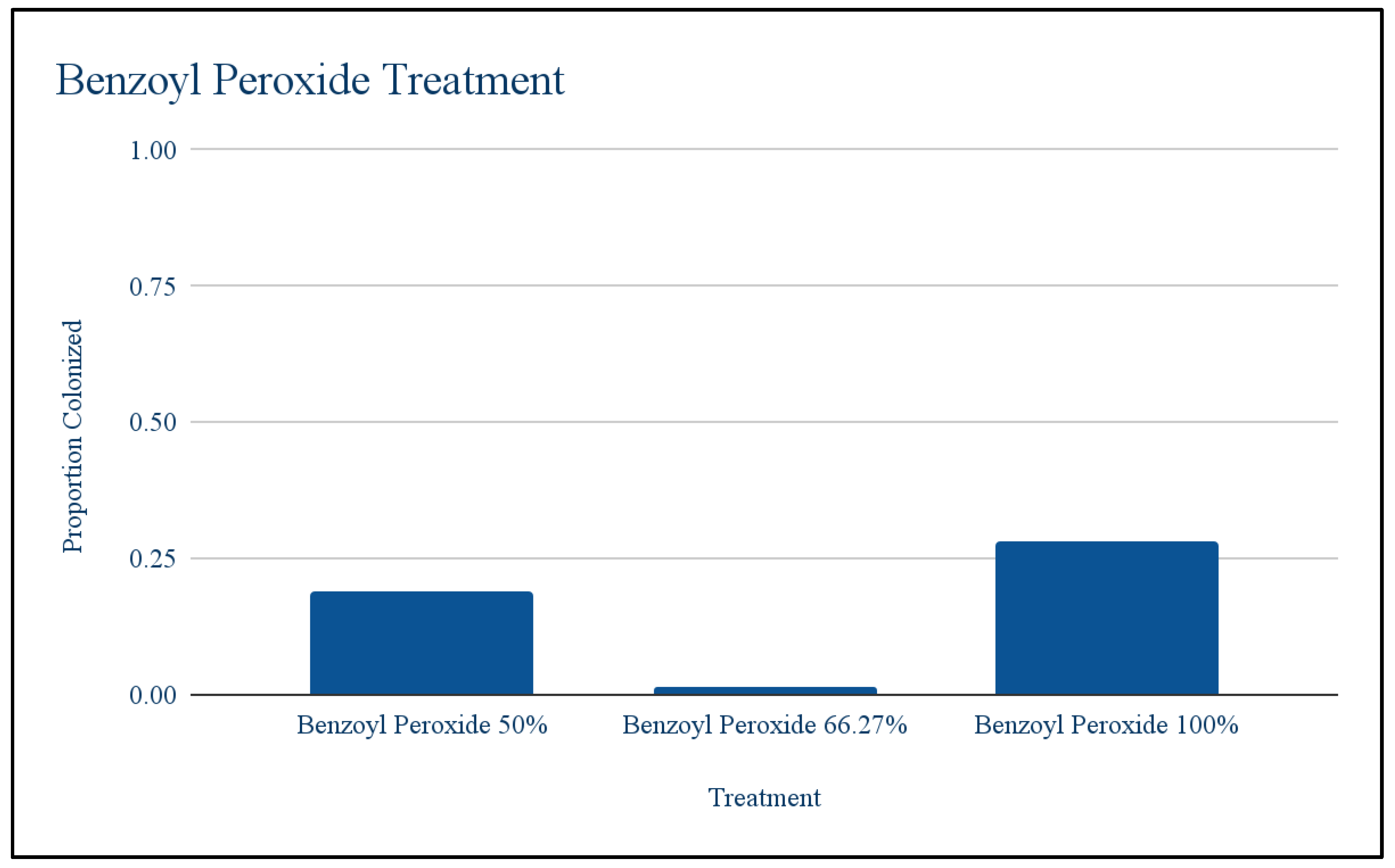
Benzoyl peroxide: Using the T test to compare the first round of benzoyl peroxide against the control, it was found that antibiotic treatment significantly lowered bacterial growth at at p < .05(The t-value is 30.11245. The p-value is .00008). Meaning antibiotics did have an effect on the population but there was some resistance that allowed it to grow little. Chi squared was used to test whether different dilution levels resulted in different levels of growth. Chi-square test: 31.97; Degree of freedom: 2; P-value: <0.001. This means the different dilutions did result in different levels of growth. Growth at 100% was higher than expected and growth at 66.27% was lower than expected. Throughout the levels of dilution bacterial growth was low at 50%, decreased at 66.27% and then increased at 100%.
Figure 2.
Different Dilutions of Benzoyl Peroxide.
Figure 2.
Different Dilutions of Benzoyl Peroxide.
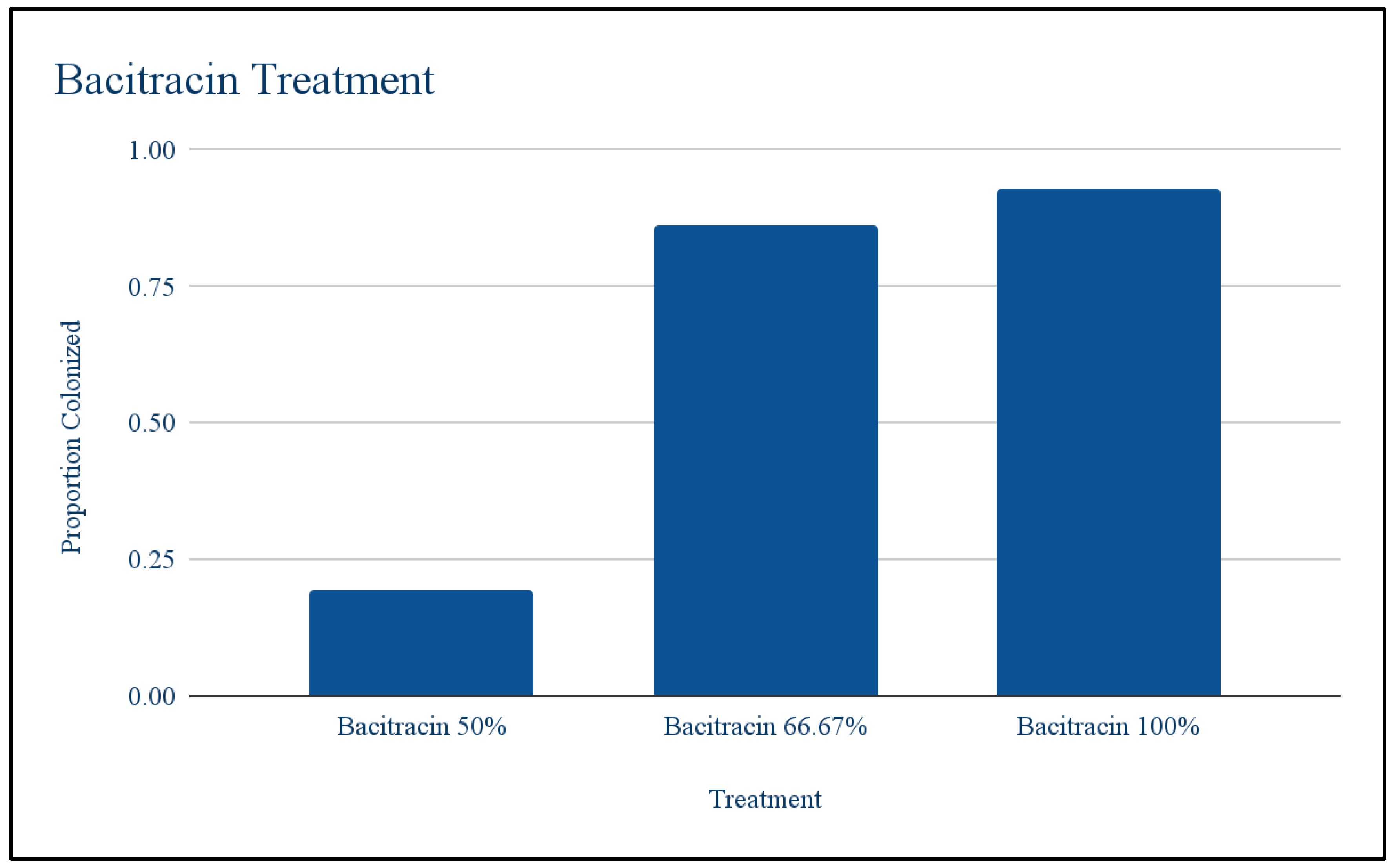
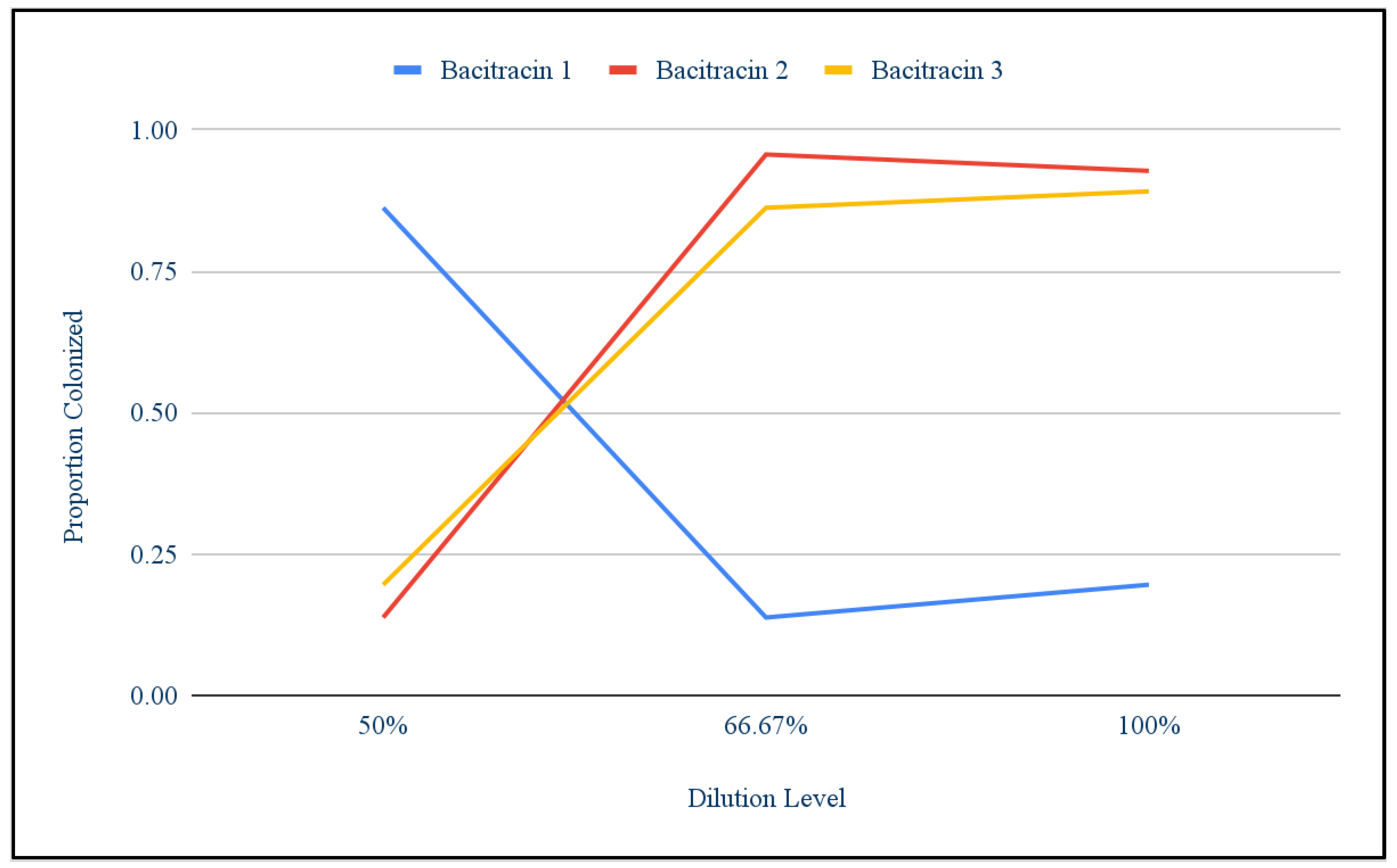
Bacitracin: Using the T test to compare the first round of bacitracin against the control, it was found that antibiotic treatment did not significantly lower bacteria growth. The t-value is 2.28859. The p-value is .083988. The result is not significant at p < .05. Chi squared was used to show that there were differences among the different replicants of dilutions. These differences are what caused the growth of the first round of bacitracin to not appear significantly different from the control growth. The chi-square statistic is 96.7728. The p-value is < 0.00001. The result is significant at p < .05. Figure 5 shows that population bacitracin replicant 1 responded very differently to the concentrations than bacitracin replicant 2 and 3. Where bacitracin replicant 2 and 3 started off with low growth at 50% and then increased, bacitracin had high growth at 50% and then decreased.
Figure 3.
Different Dilutions of Bacitracin.
Figure 3.
Different Dilutions of Bacitracin.
Figure 4.
Growth Across Bactrincin Replicants.
Figure 4.
Growth Across Bactrincin Replicants.
IV. Discussion
So based on this study antibiotic resistance did show up as a trait but the level evolved across the populations was inconsistent. For the benzoyl peroxide the results were surprising because there was so much bacterial growth in the 100% compared to 66.27%, because the dilution is less you would not expect more growth. The replicants of bacteria responded differently to the dilutions that were unexpected because this was an uncontrolled lab environment and contamination was possible. For instance, fungal growth was observed in a dish demonstrating that it was not completely sterile, however any visibly contaminated dishes were not included in the study. The results of T test between the control and bacitracin didn’t seem significant because there seems to be an outlier with a much percent than the other replicants, across the other replicants of antibiotic treatment bacterial growth decreased as expected however one plate had similar amounts of a control plate and this could be due to contamination or accidentally inoculating a higher starting population. Antibiotic resistance is a persistent ongoing issue and if households use antibiotics no longer work it would pose a huge issue for increasing infections among people. Further studies would involve developing possible solutions to bacteria that are resistant to household antibiotics.
V. Conclusion
In conclusion, if common bacteria evolved resistance to over the counter antibiotics, then people would not be able to prevent infections as easily as we are able to now. In order to test whether bacteria could develop resistance to over the counter antibiotics, bacteria were grown in antibiotic ointments in a dilution series to see if resistance would develop. It was found that antibiotic resistance did appear as a trait, but the level of it was inconsistent across the different bacteria populations. Further research necessary on this topic would be to test if resistance evolves to other over the counter antibiotics and potential ways to eliminate this resistant bacteria.
References
- Kohanski, M. A., Dwyer, D. F., Hayete, B., Lawrence, C. J., & Collins, J. J. (2007). A common mechanism of cellular death induced by bactericidal antibiotics. Cell, 130(5), 797–810. 5). [CrossRef]
- Hutchings, M. I., Truman, A. W., & Wilkinson, B. (2019). Antibiotics: past, present and future. Current Opinion in Microbiology, 51, 72–80. [CrossRef]
- Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews, 74(3), 417–433. 3). [CrossRef]
- Kalvaitis, K. (2008, August 10). Penicillin: An accidental discovery changed the course of medicine. Healio. Retrieved October 6, 2023, from https://www.healio.com/news/endocrinology/20120325/penicillin-an-accidental-discovery-changed-the-course-of-medicine#:~:text=Since%20then%2C%20the%20discovery%20of,pneumococcal%20pneumonia%2C%20gonorrhea%20and%20syphilis. 10 August.
- Van Tienderen, P. H. (1991). EVOLUTION OF GENERALISTS AND SPECIALISTS IN SPATIALLY HETEROGENEOUS ENVIRONMENTS. Evolution, 45(6), 1317–1331. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).