Submitted:
16 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Chemicals and Zebrafish maintenance
2.2. Experimental design
2.3. Sample collection
2.4. Intestinal histology
2.5. 16S rRNA amplicon sequencing and bioinformatic analysis
2.6. Measurement of biochemical indicators
2.7. Statistical Analysis
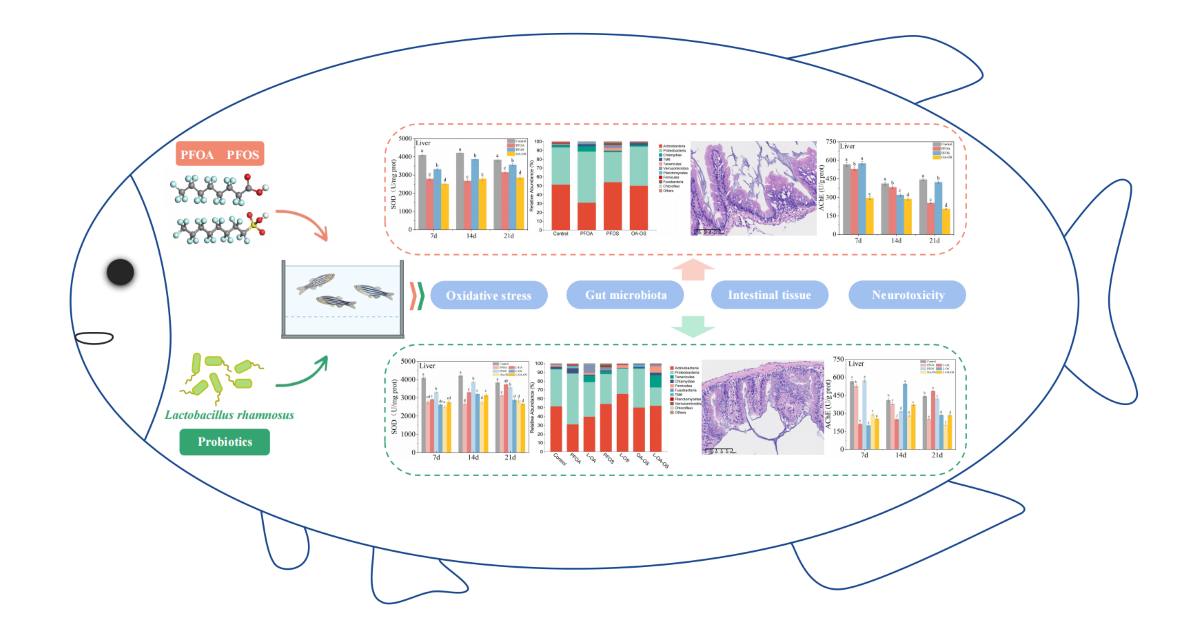
3. Results
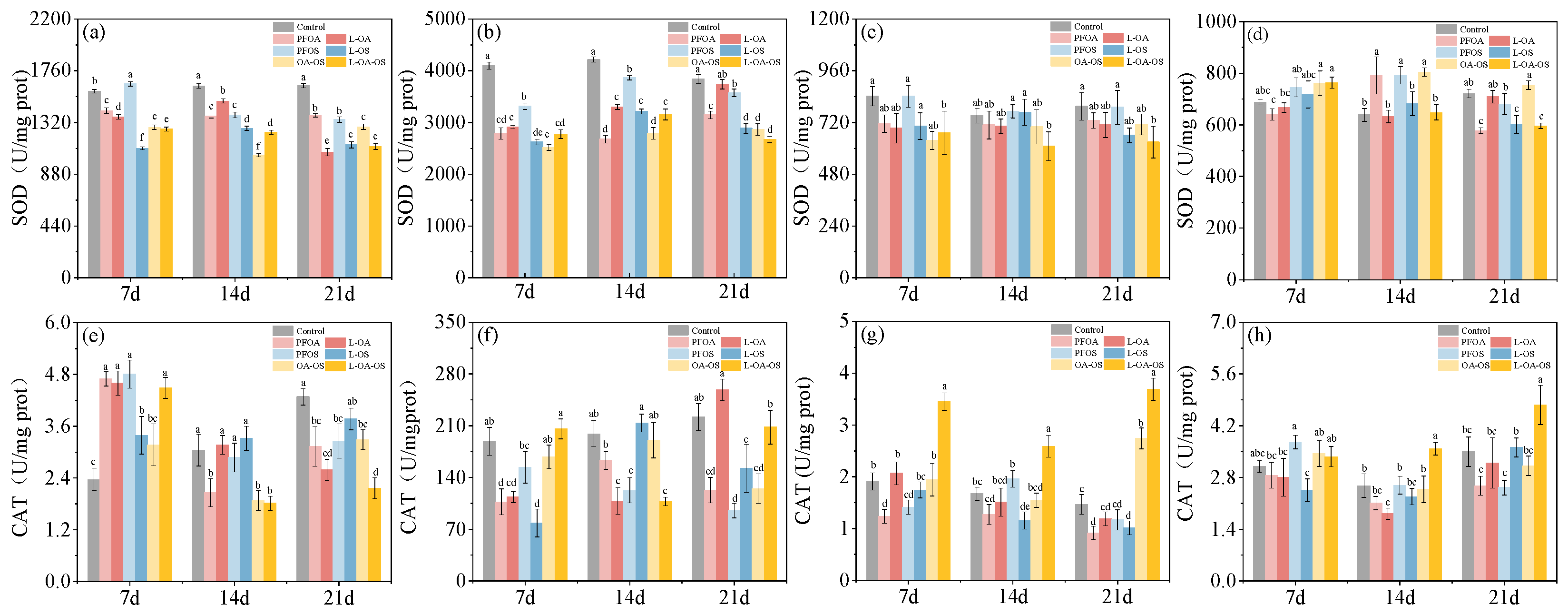
3.1. Effect of different exposure treatments on SOD activity of zebrafish tissues
3.2. Activities of CAT in zebrafish tissues of different treatments
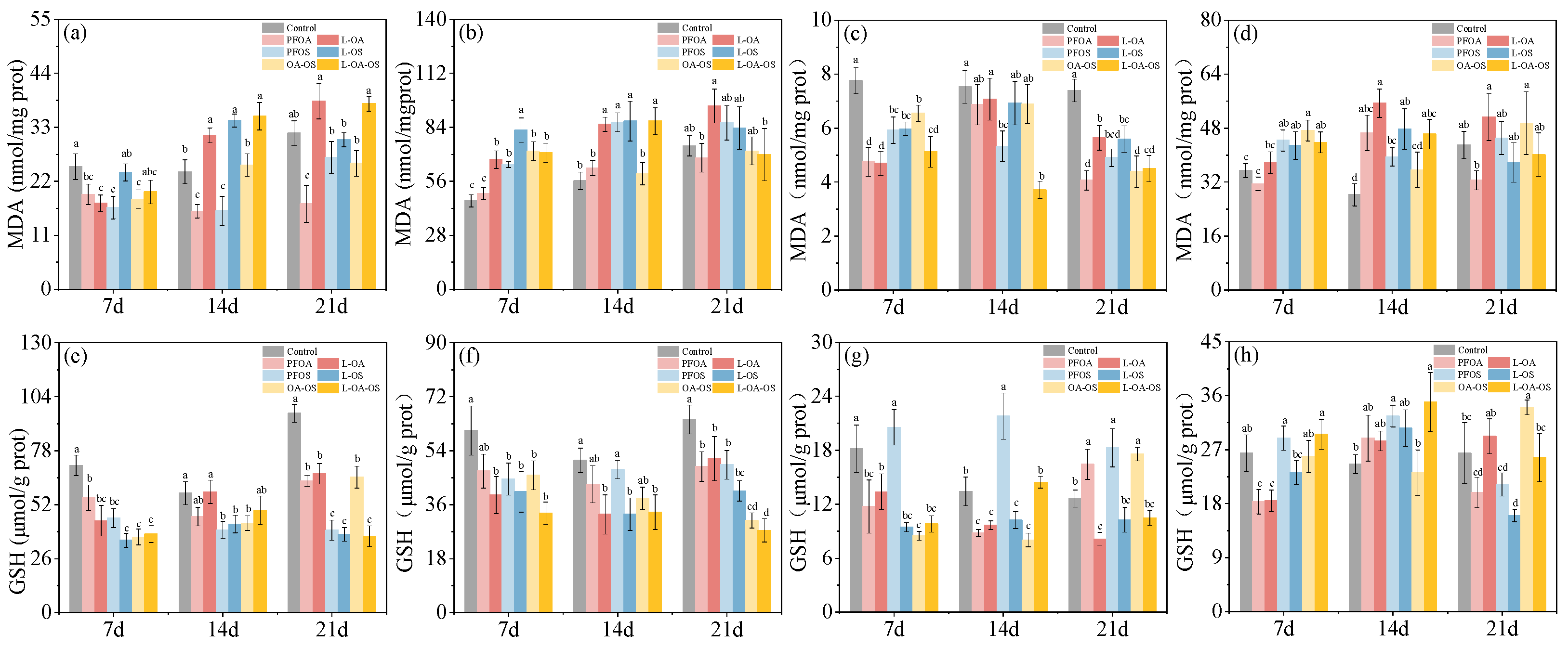
3.3. MDA content in zebrafish tissues of different treatments
3.4. Changes of GSH content in zebrafish tissues of different treatments
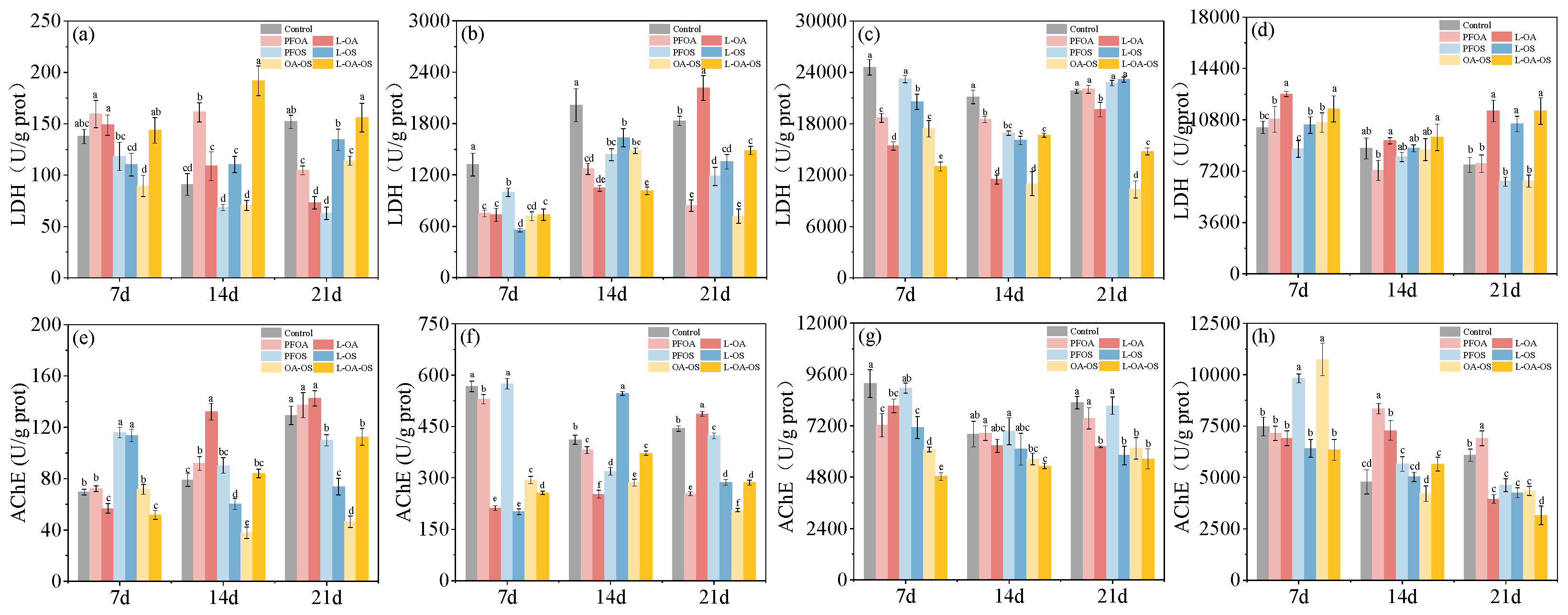
3.5. Influence of various exposure treatments on LDH activity of zebrafish tissues
3.6. Differences in AChE activity of different treatment groups
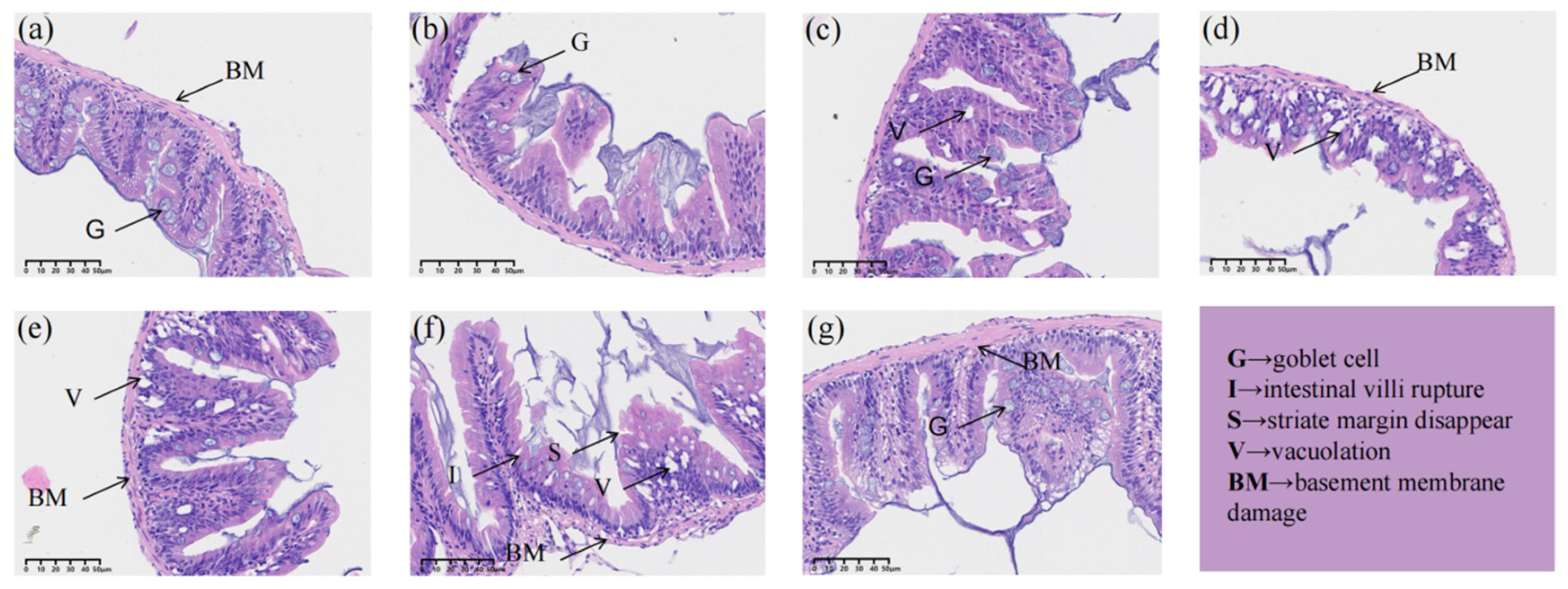
3.7. Changes in zebrafish intestinal tissue morphology
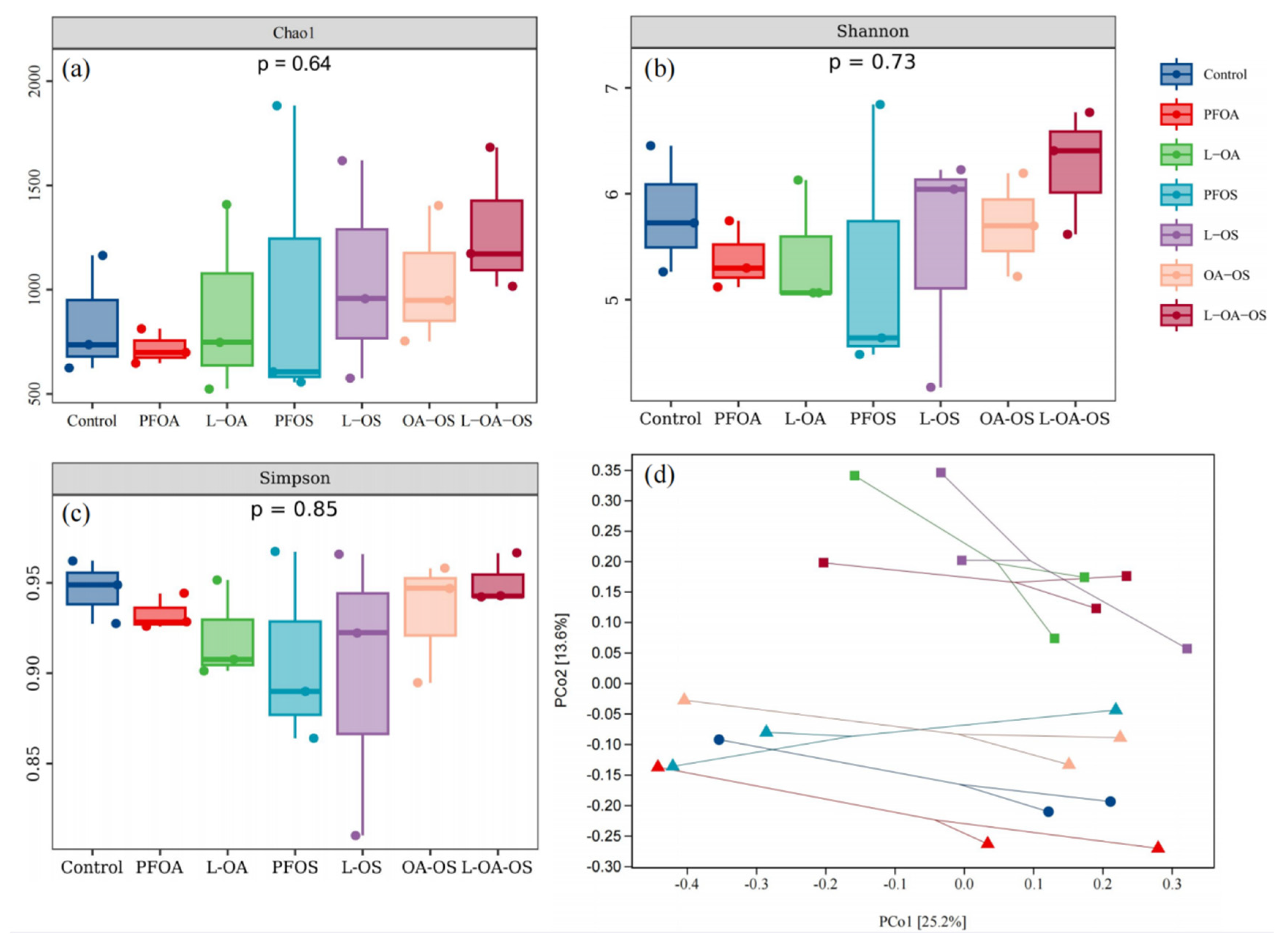
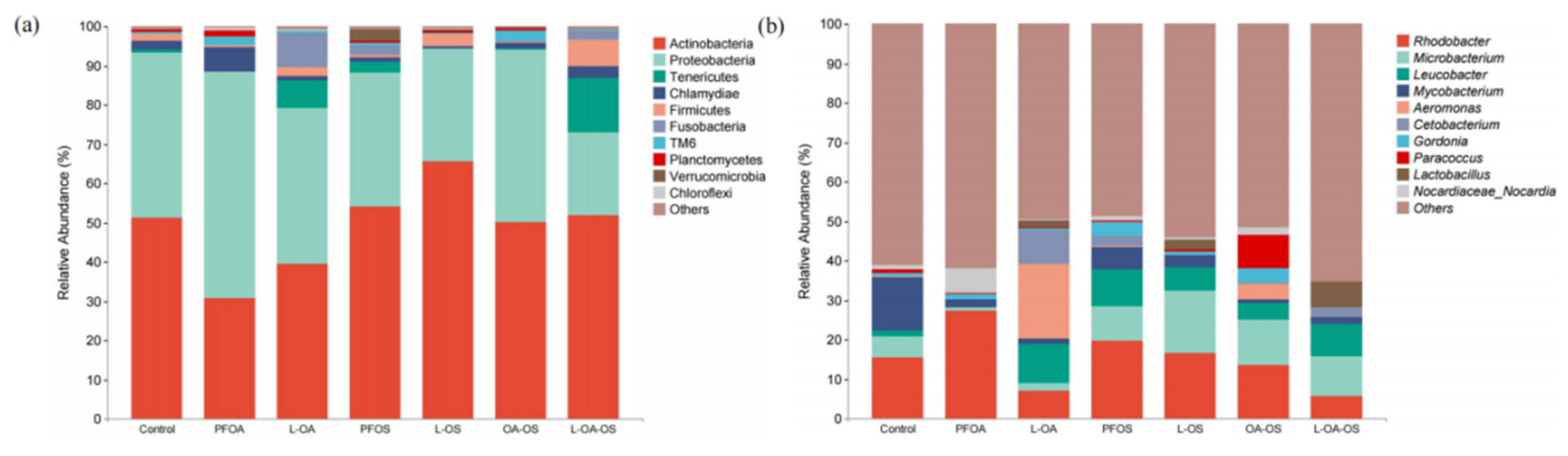
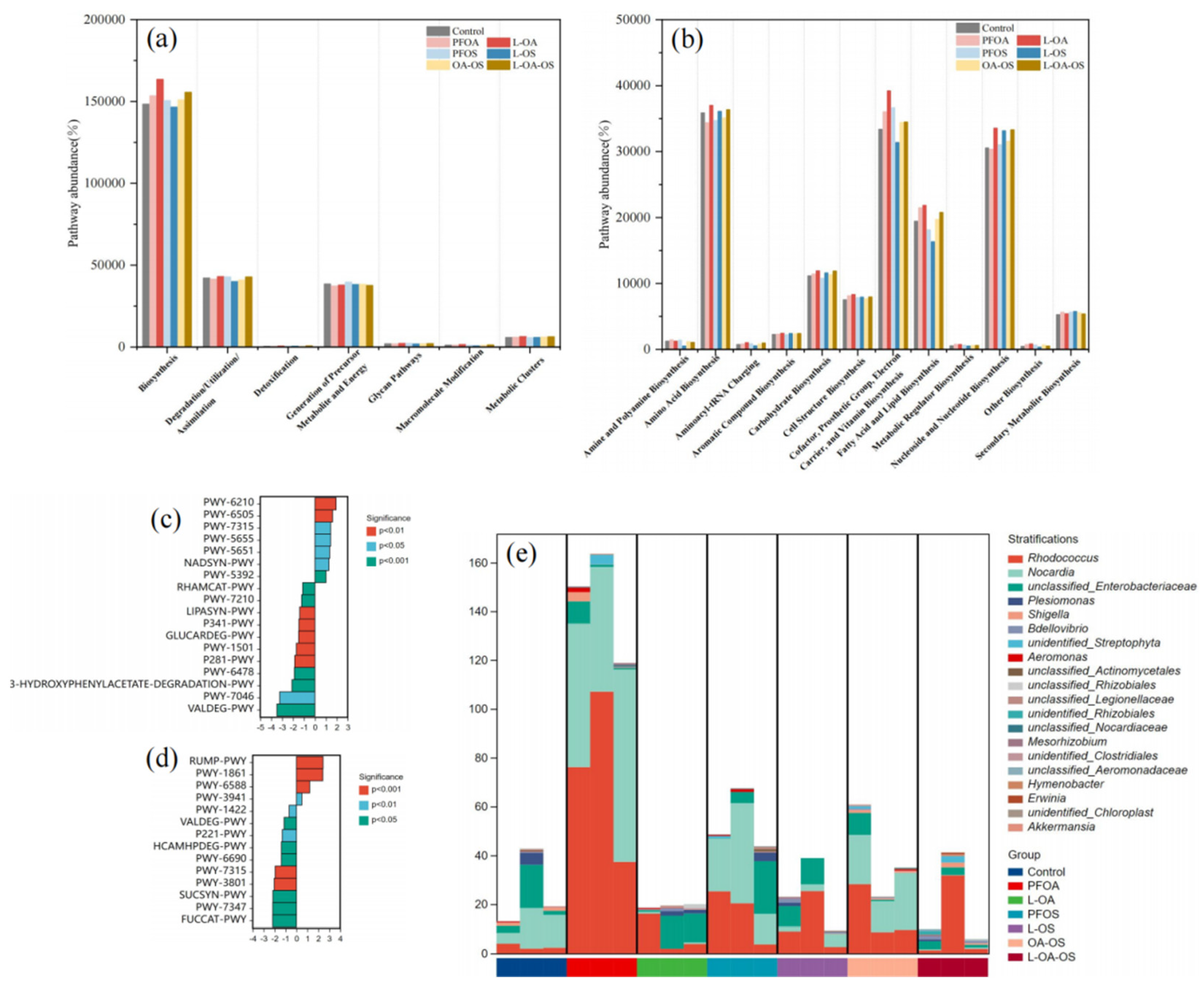
3.8. Community composition and metabolic activities of gut microbiota
4. Discussion
4.1. Probiotic L. rhamnosus relieves zebrafish tissue oxidative stress
4.2. Probiotic L. rhamnosus alleviate neurotoxicity induced by PFOA and PFOS
4.3. Gut histomorphology and microbial community function regulation
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melo, T.M.; Schauerte, M.; Bluhm, A.; Slany, M.; Paller, M.; Bolan, N.; Bosch, J.; Fritzsche, A.; Rinklebe, J. Ecotoxicological effects of per- and polyfluoroalkyl substances (PFAS) and of a new PFAS adsorbing organoclay to immobilize PFAS in soils on earthworms and plants. J. Hazard. Mater. 2022, 433, 128771. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Balan, S.A.; Scheringer, M.; Trier, X.; Goldenman, G.; Cousins, I.T.; Diamond, M.; Fletcher, T.; Higgins, C.; Lindeman, A.E.; Peaslee, G.; de Voogt, P.; Wang, Z.; Weber, R. The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs). Environ. Health. Persp. 2015, 123, A107–A111. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, J.J.; French, K.; Muir, D.C.; Spencer, C.; Criscitiello, A.; De Silva, A.O.; Young, C.J. Emerging investigator series: a 14-year depositional ice record of perfluoroalkyl substances in the High Arctic. Environ. Sci-Proc. Imp. 2017, 19, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Campo, J.; Lorenzo, M.; Perez, F.; Pico, Y.; Farre, M.; Barcelo, D. Analysis of the presence of perfluoroalkyl substances in water, sediment and biota of the Jucar River (E Spain). Sources, partitioning and relationships with water physical characteristics. Environ. Res. 2016, 147, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, P.; Wang, L.; Li, Q.; Li, X.; Luo, Y. Toxicity of per- and polyfluoroalkyl substances to aquatic vertebrates. Front. Env. Sci-Switz. 2023, 11, 37. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Recent progress in adsorptive removal of per- and poly-fluoroalkyl substances (PFAS) from water/wastewater. Crit. Rev. Env. Sci. Tec. 2022, 52, 90–129. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Abdel-Daim, M.M.; Van Doan, H. Probiotic application for sustainable aquaculture. Rev. Aquacult. 2019, 11, 907–924. [Google Scholar] [CrossRef]
- Nayak, S.K. Probiotics and immunity: a fish perspective. Fish. Shellfish. Immnol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- López Nadal, A.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, Microbiota, and Gut Immunity: Using the Zebrafish Model to Understand Fish Health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.C.W.; Tang, L.; Hu, C.; Liu, M.; Lam, P.K.S.; Zhou, B. Probiotic Modulation of Lipid Metabolism Disorders Caused by Perfluorobutanesulfonate Pollution in Zebrafish. Environ. Sci. Technol. 2020, 54, 7494–7503. [Google Scholar] [CrossRef]
- Sun, B.; Liu, M.; Tang, L.; Hu, C.; Huang, Z.; Chen, L. Probiotics inhibit the stunted growth defect of perfluorobutanesulfonate via stress and thyroid axes in zebrafish larvae. Environ. Pollut. 2021, 290, 118013. [Google Scholar] [CrossRef]
- Sun, B.; Liu, M.; Tang, L.; Hu, C.; Huang, Z.; Zhou, X.; Chen, L. Probiotic supplementation mitigates the developmental toxicity of perfluorobutanesulfonate in zebrafish larvae. Sci. Total. Environ. 2021, 799, 149458. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Ge, X.; Wang, D.; Wang, T.; Zhang, L.; Tanguay, R.L.; Simonich, M.; Huang, C.; Dong, Q. Chronic perfluorooctanesulphonic acid (PFOS) exposure produces estrogenic effects in zebrafish. Environ. Pollut. 2016, 218, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; He, M.Y.; Ling, Y.; Wang, X.J.; Zhang, F.; Feng, X.S.; Zhang, Y.; Xing, S.G.; Li, J.; Qiu, X.; Li, Y.R. Tissue distribution study of perfluorooctanoic acid in exposed zebrafish using MALDI mass spectrometry imaging. Environ. Pollut. 2022, 293, 118505. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2 ' s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Liu, H.; Shi, L.; Giesy, J.P.; Yu, H. Polychlorinated diphenyl sulfides can induce ROS and genotoxicity via the AhR-CYP1A1 pathway. Chemosphere 2019, 223, 165–170. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, Q.; Wu, C.; Zhu, A.; Shen, B. Zinc acclimation mitigated high zinc induced oxidative stress by enhancing antioxidant defenses in large yellow croaker Pseudosciaena crocea. Aquat. Toxicol. 2016, 172, 21–29. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Liu, S.; Lai, H.; Tu, W. Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish. J. Hazard. Mater. 2022, 425, 127950. [Google Scholar] [CrossRef]
- Beauchamp, C.O.; Fridovich, I. Isozymes of superoxide dismutase from wheat germ. Biochim. Biophys. Acta. 1973, 317, 50–64. [Google Scholar]
- Son, V.M.; Chang, C.C.; Wu, M.C.; Guu, Y.K.; Chiu, C.H.; Cheng, W.T. Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish. Shellfish. Immun. 2009, 26, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Miao, L.; Pan, W.; Huang, X.; Dengu, J.M.; Zhang, W.; Ge, X.; Liu, B.; Ren, M.; Zhou, Q.; Xie, J.; Pan, L.; Xi, B. Effect of nitrite exposure on the antioxidant enzymes and glutathione system in the liver of bighead carp, Aristichthys nobilis. Fish. Shellfish. Immun. 2018, 76, 126–132. [Google Scholar] [CrossRef]
- Dale, K.; Yadetie, F.; Muller, M.B.; Pampanin, D.M.; Gilabert, A.; Zhang, X.; Tairova, Z.; Haarr, A.; Lille-Langoy, R.; Lyche, J.L.; Porte, C.; Karlsen, O.A.; Goksoyr, A. Proteomics and lipidomics analyses reveal modulation of lipid metabolism by perfluoroalkyl substances in liver of Atlantic cod (Gadus morhua). Aquat. Toxicol. 2020, 227, 105590. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Shi, X.; Wang, J.; Lam, P.K.; Wu, R.S.; Zhou, B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat. Toxicol. 2007, 82, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Zomkowski, A.D.; Engel, D.; Santos, D.B.; dos Santos, A.A.; Moretti, M.; Valvassori, S.S.; Ornell, F.; Quevedo, J.; Farina, M.; Rodrigues, A.L. Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Exp. Neurol. 2013, 240, 112–121. [Google Scholar] [CrossRef]
- Guedes, R.P.; Dal Bosco, L.; Araújo, A.S.; Belló-Klein, A.; Ribeiro, M.F.; Partata, W.A. Sciatic nerve transection increases gluthatione antioxidant system activity and neuronal nitric oxide synthase expression in the spinal cord. Brain. Res. Bull. 2009, 80, 422–427. [Google Scholar] [CrossRef]
- Kodali, V.P.; Sen, R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J. 2008, 3, 245–251. [Google Scholar] [CrossRef]
- Serrano-Lorenzo, P.; Coya, O.N.; López-Jimenez, A.; Blázquez, A.; Delmiro, A.; Lucia, A.; Arenas, J.; Martín, M.A. Plasma LDH: A specific biomarker for lung affectation in COVID-19? Pract. Lab. Med. 2021, 25, e00226. [Google Scholar] [CrossRef]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006, 10, 172–172. [Google Scholar] [CrossRef]
- Venkateswara, R.J. Sublethal effects of an organophosphorus insecticide (RPR-II) on biochemical parameters of tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2006, 143, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Xia, X.; Zhou, D.; Wang, H.; Zhai, Y.; Lin, H.; Chen, J.; Hu, D. Bioconcentration and tissue distribution of shorter and longer chain perfluoroalkyl acids (PFAAs) in zebrafish (Danio rerio): Effects of perfluorinated carbon chain length and zebrafish protein content. Environ. Pollut. 2019, 249, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Kang, M.; Shen, Y.; Yun, L.; Yang, G.; Zhu, L.; Meng, X.; Zhang, J.; Su, X. Bacillus coagulans SCC-19 maintains intestinal health in cadmium-exposed common carp (Cyprinus carpio L.) by strengthening the gut barriers, relieving oxidative stress and modulating the intestinal microflora. Ecotox. Environ. Safe. 2021, 228, 112977. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.; Fingerman, M. Inhibition of acetylcholinesterase activity in the central nervous system of the red swamp crayfish, Procambarus clarkii, by mercury, cadmium, and lead. Bull. Environ. Contam. Toxicol. 1995, 55, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, Z.; Yang, Y.; Jiao, Y.; Qu, J.; Wang, Y.; Zhang, Y. Effects of common environmental endocrine-disrupting chemicals on zebrafish behavior. Water. Res. 2022, 208, 117826. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Dasriya, V.L.; Nataraj, B.H.; Nagpal, R.; Behare, P.V. Lacticaseibacillus rhamnosus-Derived Exopolysaccharide Attenuates D-Galactose-Induced Oxidative Stress and Inflammatory Brain Injury and Modulates Gut Microbiota in a Mouse Model. Microorganisms 2022, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim. Nutr. 2020, 6, 69–79. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Tang, J.; Cai, J.; Yu, H.; Wang, Z.; Abarike, E.D.; Lu, Y.; Li, Y.; Afriyie, G. In vivo assessment of the probiotic potentials of three host -associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 2020, 527, 735440. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, E92–E97. [Google Scholar] [CrossRef] [PubMed]
- Roshchina, V.V. New trends and perspectives in the evolution of neurotransmitters in microbial, plant, and animal cells. Adv. Exp. Med. Biol. 2016, 874, 25–77. [Google Scholar] [PubMed]
- Tian, X.; Yu, Z.; Feng, P.; Ye, Z.; Li, R.; Liu, J.; Hu, J.; Kakade, A.; Liu, P.; Li, X. Lactobacillus plantarum TW1-1 Alleviates Diethylhexylphthalate-induced testicular damage in mice by modulating gut microbiota and decreasing inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 221. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Baumler, A.J. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ran, C.; Wang, Y.; Zhang, Z.; Ding, Q.; Yang, Y.; Olsen, R.E.; Ringo, E.; Bindelle, J.; Zhou, Z. Use of probiotics in aquaculture of China-a review of the past decade. Fish. Shellfish. Immun. 2019, 86, 734–755. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Prasannan Geetha, P.; Thavarool Puthiyedathu, S.; Vattringal Jayadradhan, R.K. Applications of Actinobacteria in aquaculture: prospects and challenges. 3 Biotech. 2023, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.A.; Petty, B.D. Mixed Metazoan and Bacterial Infection of the Gas Bladder of the Lined Seahorse-A Case Report. J. Aquat. Anim. Health. 2013, 25, 42–52. [Google Scholar] [CrossRef]
- Zhan, M.; Huang, Z.; Cheng, G.; Yu, Y.; Su, J.; Xu, Z. Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2022, 23, 14037. [Google Scholar] [CrossRef]
- Tinh, N.T.; Dierckens, K.; Sorgeloos, P.; Bossier, P. A review of the functionality of probiotics in the larviculture food chain. Mar. Biotechnol. 2008, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, P.; Kunze, W.A.; Bienenstock, J. On communication between gut microbes and the brain. Curr. Opin. Gastroen. 2012, 28, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Picchietti, S.; Rodiles, A.; Cossignani, L.; Merrifield, D.L.; Taddei, A.R.; Maradonna, F.; Olivotto, I.; Gioacchini, G.; Carnevali, O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci. Rep. 2015, 5, 1–11. [Google Scholar]
- Sun, H.; Zhang, A.; Song, Q.; Fang, H.; Liu, X.; Su, J.; Yang, L.; Yu, M.; Wang, X. Functional metabolomics discover pentose and glucuronate interconversion pathways as promising targets for Yang Huang syndrome treatment with Yinchenhao Tang. Rsc. Adv. 2018, 8, 36831–36839. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Ren, Z.; Cui, Z. The toxic effects of deltamethrin on Danio rerio: the correlation among behavior response, physiological damage and AChE. Rsc. Adv. 2016, 6, 109826–109833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




