Introduction
The burden of nontuberculous mycobacterial pulmonary disease (NTM-PD) is increasing worldwide and the long-term prognosis of NTM-PD is poor [
1,
2,
3]. NTM-PD follows various clinical courses, with some patients improving spontaneously, while others undergo relatively rapid disease progression [
4]. The treatment responses to antibiotic therapy also vary. Therefore, there is a need for an objective quantifiable biomarker that can predict disease progression or reflect severity in a comparable manner. However, despite various research attempts, no clinically useful biomarker has yet been developed.
Serum carbohydrate antigen 19-9 (CA 19-9) is used widely as a tumor marker for many types of cancer, especially pancreatic cancer [
5]. Interestingly, recent studies have reported that CA19-9 can also increase in chronic respiratory diseases, such as bronchiectasis or pulmonary mycobacterial infection [
6]. Moreover, studies observed a decrease in serum CA19-9 levels after antibiotic treatment in NTM-PD patients, which has also been associated with the severity of pulmonary computed tomography (CT) lesions [
7,
8,
9]. Thus, there is increasing interest in how CA19-9 can be used effectively in the management of NTM-PD patients.
Despite these results, the clinical usefulness of CA 19-9 in NTM-PD remains unclear. Therefore, this study compared the clinical characteristics of patients with and without elevated CA19-9 levels, and investigated whether the increase in CA19-9 is related to the microbiological response to antibiotics in NTM-PD patients.
Methods
Study population
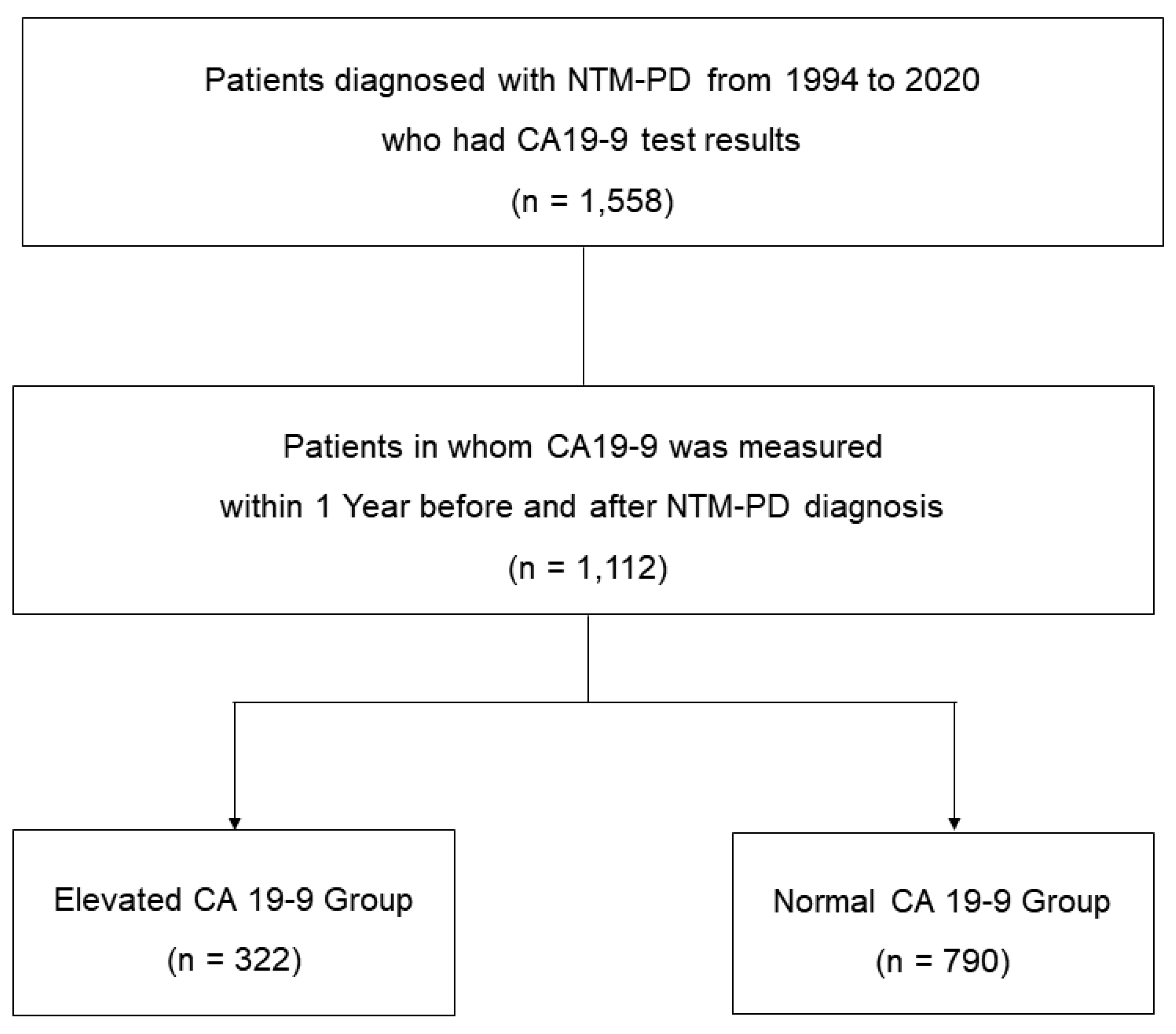
We retrospectively screened patients diagnosed with NTM-PD at Samsung Medical Center in Seoul, South Korea, from January 1994 to December 2020. Most of the patients followed at our institution undergo routine health check-ups at intervals of 1 to 2 years, which include tumor marker blood tests such as CA19-9. During the study period, 1,558 NTM-PD patients who underwent blood test for CA19-9 were identified. Of these, this analysis included 1,112 patients who had serum CA19-9 measured within 1 year before or after the diagnosis of NTM-PD. The patients were categorized into groups with (n = 322) and without (n = 790) elevated CA19-9 levels, and evaluated (
Figure 1). All patients met the NTM-PD diagnostic criteria. From January 1994 to December 2007, data were obtained from a retrospective cohort, and beginning in January 2008, data were obtained from an ongoing Institutional Review Board-approved prospective observational cohort (ClinicalTrials.gov Identifier: NCT00970801, IRB no. 2008-09-016) [
10,
11]. Informed consent was obtained from all participants.
Figure 1.
Study patients.
Figure 1.
Study patients.
Data collection
We retrospectively collected baseline demographics, comorbidities, medical history, and laboratory and radiological findings of the study population at the time of NTM-PD diagnosis. Radiologically, the NTM-PD was categorized into nodular bronchiectatic (NB), fibrocavitary (FC), and non-classifiable forms according to chest CT images. The NB form was defined by the presence of multifocal bronchiectasis and clusters of small nodules on chest CT, regardless of the presence of small cavities in the lungs. The FC form was defined by the presence of cavitary opacities and pleural thickening. When the disease did not belong to either the FC or NB form, it was deemed non-classifiable [
11].
MAC-PD severity was determined using the BACES [body mass index < 18.5 kg/m
2, age ≥ 65 years, presence of cavity, elevated erythrocyte sedimentation rate (ESR) in men > 15 mm/h and in women > 20 mm/h, and male sex; each one point] score. One point was given for each item, and the total score is considered an indicator of mild (0–1 point), moderate (2–3 points), or severe (4–5 points) disease [
12,
13,
14].
Measurement of CA 19-9 levels
Serum CA 19-9 levels were measured using a COBAS e 801 analyzer (Roche Diagnostics, Mannheim, Germany) in an electrochemiluminescence immunoassay or using a Dream Gamma-10 gamma counter (Shinjin Medics, Korea) with an immunoradiometric assay (reference < 37 U/mL). For the study analysis, we defined the reference value for CA 19-9 elevation as ≥ 37 U/mL.
Microbiological response
‘Negative culture conversion’ was defined as at least three consecutive negative sputum cultures, collected at least 4 weeks apart based on the NTM-NET consensus statement [
15]. The time to culture conversion was defined as the time to the date of the first negative culture. ‘Microbiological cure’ was defined as multiple consecutive negative cultures, but no positive cultures with the causative species after culture conversion until the end of anti-mycobacterial treatment.
Statistical analysis
Statistical analyses utilized SPSS software (IBM SPSS statistics version 27, Chicago, IL, USA) and GraphPad Prism 9 (GraphPad, San Diego, CA, USA). Data were presented as number (%) or interquartile range (IQR). Categorical variables were compared using Pearson’s chi-square or Fisher’s exact test, while continuous variables were compared using the Mann-Whitney U test. Correlation analysis explored the relationship between laboratory tests and CA 19-9 levels. Kaplan-Meier method visualized cumulative culture conversion rates based on CA 19-9 levels, compared with the log-rank test. Multivariable logistic regression identified factors associated with microbiological cure in our study population. All tests were two-sided, with statistical significance set at p < 0.05
Results
Comparison of the characteristics of patients with and without an elevated CA19-9
Table 1 summarizes the clinical characteristics of the study patients at the time of NTM-PD diagnosis. Of the total, 61% were female, with a median age of 60 years. The majority (72%) were never-smokers. Bronchiectasis was observed in most patients (83%) and 37% had past pulmonary tuberculosis treatment.
M. avium complex (70%) was the most common causative organism. Overall patients, 322 (29%) had elevated serum CA19-9 levels, while the remaining 790 (71%) had normal levels.
Only the ESR, C-reactive protein (CRP) level, and rate of initiation of antibiotic treatment differed significantly between the two patient groups. ESR and CRP were higher in the elevated CA19-9 group (p < 0.001 and p = 0.029, respectively), as was the antibiotic initiation rate (74% vs. 57%, p < 0.001). For the 811 patients for which ESR data were available, serum CA19-9 levels were weakly positively correlated with the ESR (Spearman’s rank correlation coefficient, ρ = 0.139, p < 0.001,
Supplementary Figure S1).
Microbiological response in patients who received antibiotic therapy
The microbiological response in patients who received antibiotic treatment is shown in
Table 2. Among the 688 patients who received antibiotic treatment, 239 (35%) had an elevation of serum CA19-9 levels, while the remaining 449 (65%) had normal levels. The median duration of antibiotic therapy for both groups was 19.0 months and 19.6 months, respectively, with no statistical difference. The rate of culture conversion within one year after starting antibiotics showed no statistical difference between the CA19-9 elevation group and the normal group (80% versus 72%, p = 0.055)
Of the 551 patients who completed antibiotics and were eligible for microbiological cure evaluation, 466 (85%) achieved microbiological cure; the serum CA19-9 levels did not differ between those who achieved microbiological cure and those who did not (
Supplementary Figure S2).
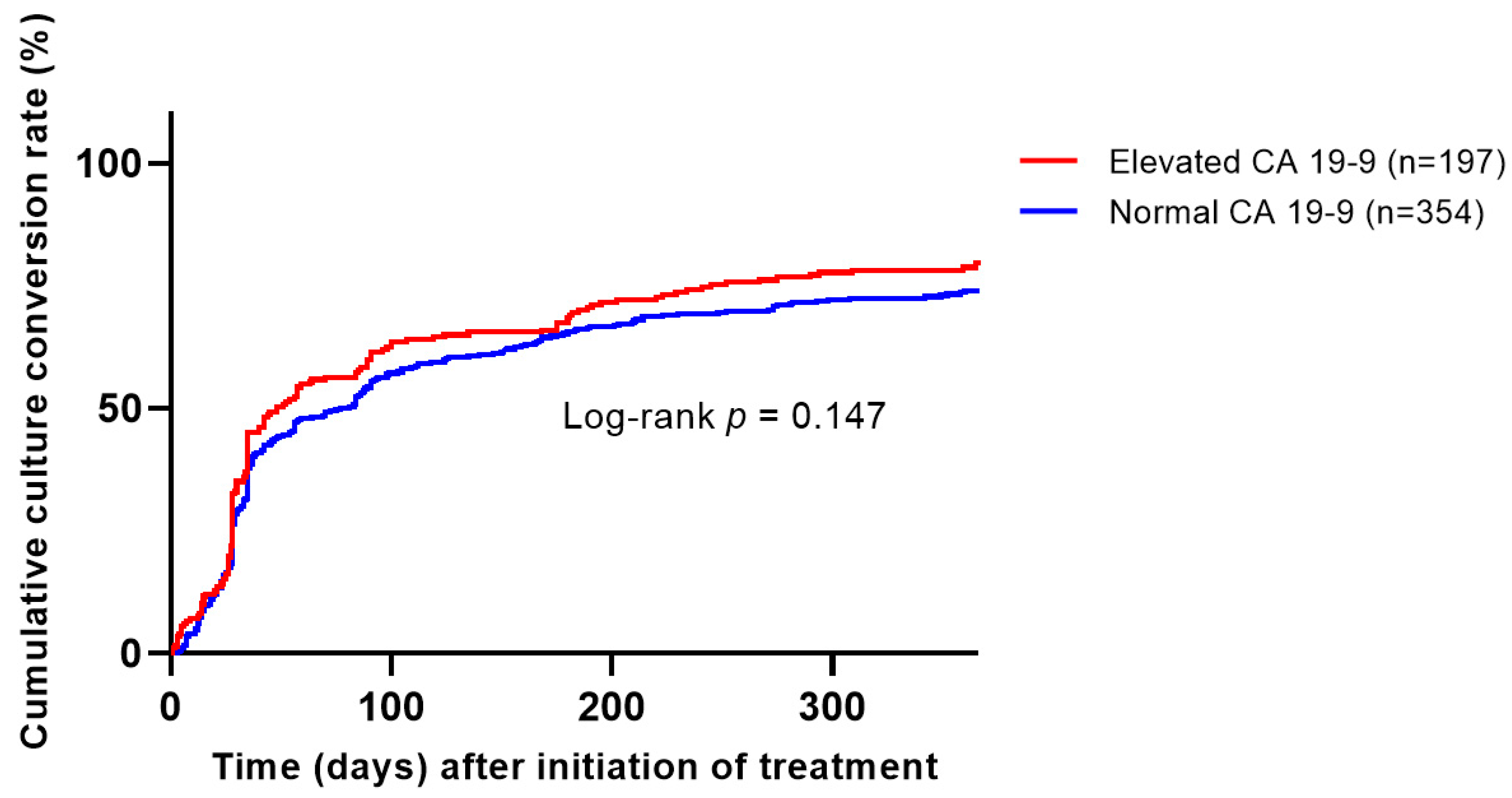
Of the patients who underwent microbiological cure evaluation, 89% of the CA19-9 elevation group and 82% of the normal group achieved microbiological cure, with a slightly higher tendency in the CA19-9 group (p = 0.039). However, when assessing the cumulative culture conversion rate in patients eligible for microbiological cure evaluation, no significant difference was observed between the two groups based on CA19-9 elevation status (Kaplan–Meier, log-rank test, p = 0.147,
Figure 2).
Factors associated with microbiological cure including CA 19-9 levels
To assess the impact of serum CA19-9 on microbiological cure, multivariate analysis was conducted. Analysis was performed on the subset of 434 individuals for whom adjusted variable analysis was possible, including other factors along with CA19-9 (
Table 3). Current smoking, bronchiectasis, AFB smear positivity, and the
M. abscessus strain demonstrated a statistically significant negative impact on microbiological cure. On the other hand, the
M. massiliense strain was found to have a significantly positive association with microbiological cure (adjusted odds ratio 3.108, p = 0.040). On the other hand, the serum CA 19-9 level did not show a statistically significant association with microbiological cure in both univariate and multivariate analyses.
To assess the impact of serum CA19-9 on microbiological cure, multivariate analysis was conducted on the subset of 434 individuals for whom adjusted variable analysis was possible, including other factors along with CA19-9 (
Table 3). Current smoking, bronchiectasis, acid-fast bacilli smear positivity, and the
M. abscessus strain had significant negative impacts on microbiological cure, while the
M. massiliense strain had a significant positive association with microbiological cure (adjusted odds ratio 3.108, p = 0.040). The serum CA 19-9 level did not show a significant association with microbiological cure in either the univariate or multivariate analysis.
Discussion
This study compared the clinical characteristics between NTM-PD patients with elevated and normal serum CA19-9 levels to identify clinical factors associated with the elevated CA19-9 levels in NTM-PD patients. Although the serum CA19-9 level had positive associations with the inflammatory markers ESR and CRP, it was not associated with the microbiological response to antibiotic therapy in NTM-PD patients. Thus, while serum CA19-9 may reflect the extent of the inflammatory response in NTM-PD patients with elevated CA19-9 levels, it has a limited role as a predictor of antibiotic treatment outcomes.
When we initially planned this study, we anticipated that higher serum CA19-9 levels would be associated with poorer treatment responses in NTM-PD patients based on a study that found a positive association between serum CA19-9 levels and the extent of lung lesions on chest CT [
8]. Contrary to our expectations, our analysis revealed that elevated serum CA19-9 levels did not lead to worse microbiological responses to antibiotic treatment. Additionally, there were no distinct clinical characteristics that differentiated those with elevated and normal CA19-9 levels. These findings suggest that CA19-9 is unlikely to be a specific biomarker associated with the pathogenesis of NTM-PD. Furthermore, the presence of an elevated serum CA19-9 at the time of NTM-PD diagnosis does not necessarily indicate a poor treatment outcome or the need for more aggressive antibiotic therapy.
In terms of the clinical utility of CA19-9 in NTM-PD, studies have shown a decrease in CA19-9 levels following antibiotic treatment [
7,
9]. However, no studies focused on the association between serum CA19-9 levels and antibiotic responses in NTM-PD patients. In this context, we believe that our study has unique clinically significant implications. Considering both previous studies and our data, we believe that the utility of CA19-9 lies mainly in monitoring the antibiotic treatment response in NTM-PD patients. No research has examined whether a rise in CA19-9 levels after completing treatment for NTM-PD is associated with relapse and further studies are required to determine whether CA19-9 can be used as a biomarker for monitoring the treatment response or predicting relapse.
Our study has several limitations. First, our participants were primarily patients who had CA19-9 measurements as part of routine health check-ups, which could introduce selection bias as CA19-9 was not measured consecutively in a prospective manner. Second, using CA19-9 measurements taken approximately 1 year before and after the diagnosis of NTM-PD as a reference point may have introduced bias. Finally, some patients did not undergo aggressive testing for underlying conditions that could cause an increase in CA19-9, such as gastrointestinal malignancy. Despite these limitations, however, we believe our data hold clinical relevance, as no study has evaluated the association between CA19-9 and outcomes in NTM-PD patients.
In conclusion, our data revealed a positive association between serum CA19-9 levels and the inflammatory markers ESR and CRP in NTM-PD patients. However, the CA19-9 level was not associated with the microbiological response to treatment. Therefore, CA19-9 has a limited role as a predictor of antibiotic treatment outcomes.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1. Analysis of the correlation between serum CA19-9 levels and ESR levels (n = 811). Figure S2. Comparison of CA 19-9 level between microbiological cure (n = 466) and failure (n = 85) groups (total n = 551).
References
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Pasipanodya, J.G.; Ogbonna, D.; Deshpande, D.; Srivastava, S.; Gumbo, T. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother 2017, 72, i3–i19. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Myung, W.; Koh, W.J.; Moon, S.M.; Jhun, B.W. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007-2016. Emerg Infect Dis 2019, 25, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.A.; Kim, S.; Jo, K.W.; Shim, T.S. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J 2017, 49, 1600537. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Uchida, E.; Takasaki, H.; Burnett, D.A.; Steplewski, Z.; Pour, P.M. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 1987, 47, 5501–5503. [Google Scholar] [PubMed]
- Mukae, H.; Hirota, M.; Kohno, S.; Komori, K.; Fukushima, K.; Hiratani, K.; Kadota, J.; Hara, K. Elevation of tumor-associated carbohydrate antigens in patients with diffuse panbronchiolitis. Am Rev Respir Dis 1993, 148, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Han, S.G.; Kim, W.; Ko, Y.; Song, J.; Hong, G.; Eom, J.S.; Lee, J.H.; Jhun, B.W.; Koh, W.J. Normalization of elevated CA 19-9 level after treatment in a patient with the nodular bronchiectatic form of Mycobacterium abscessus lung disease. Tuberc Respir Dis (Seoul) 2013, 75, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yong, S.H.; Lee, S.H.; Lee, S.H.; Leem, A.Y.; Kim, S.Y.; Chung, K.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; et al. Correlation between serum carbohydrate antigen 19-9 levels and computed tomography severity score in patients with nontuberculous mycobacterial pulmonary disease. Sci Rep 2021, 11, 2777. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Jang, S.H.; Kim, S.Y.; Chung, K.S.; Song, J.H.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Kang, Y.A. Elevated serum CA 19-9 levels in patients with pulmonary nontuberculous mycobacterial disease. Braz J Infect Dis 2016, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.W.; Kim, S.Y.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Development of macrolide resistance and reinfection in refractory Mycobacterium avium complex lung disease. Am J Respir Crit Care Med 2018, 198, 1322–1330. [Google Scholar] [CrossRef]
- Jhun, B.W.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Yoo, H.; Carriere, K.C.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: a 15-year follow-up study. Eur Respir J 2020, 55, 1900798. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kwak, N.; Hong, H.; Kang, N.; Im, Y.; Jhun, B.W.; Yim, J.J. BACES score for predicting mortality in nontuberculous mycobacterial pulmonary disease. Am J Respir Crit Care Med 2021, 203, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Jhun, B.W.; Kim, H.; Kwon, O.J. Treatment outcomes of Mycobacterium avium complex pulmonary disease according to disease severity. Sci Rep 2022, 12, 1970. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, M.J.; Kwon, B.S.; Kim, Y.W.; Lim, S.Y.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Lee, C.T.; Lee, J.H. Usefulness of the BACES score in nontuberculous mycobacterial pulmonary disease for various clinical outcomes. Sci Rep 2023, 13, 7495. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Aksamit, T.; Andrejak, C.; Böttger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J 2018, 51, 1800170. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).