1. Introduction
The ability to extract energy from food is essential
for animals [1]. Many obligate herbivores obtain
their energy from angiosperms whose recalcitrant cell wall constituents, cellulose
and lignin, retard digestion and which may additionally contain antifeedants such
as latex, resins, gums, silicates, phenolics and alkaloids. Herbivores include nematodes,
some species of molluscs and crustaceans [2], the
larvae and adults of many insects, the tadpoles of some anurans, certain fish, birds,
reptiles, and odd- and even-toed ungulates and primates among mammals. This variety
obscures similar behavioral adaptations such as avoidance of noxious plants and
parts, and physiological adaptations such as the presence of salivary proteins for
detoxification and digestion [3] and fermentation
chambers with symbionts to assist the digestive process [4].
Plants produce a slew of chemicals as deterrents to
herbivory (Supplementary Table S1). Although
latex functions to plug wounds in the plant body, latex is primarily considered
to be an antifeedant [5]. Latex is an emulsion
with suspended particles including rubber, and may additionally contain other deterrent
metabolites such as cardiac glycosides in milkweed and alkaloids in the opium poppy
[6]. Rubber is a megapolymer of terpene units which
is sequestered in particles with a protein-rich membrane. When exposed to acid,
the membrane is removed and the rubber inside is released like strands of spaghetti
to form insoluble tangles [7]. Latex remains liquid
in neutral and alkaline environments because the membrane is not disrupted. The
exact timeline of the evolution of latex is uncertain, but the major precursor for
latex is the common isoprene molecule whose use spans the spectrum from the synthesis
of fatty acids for essential structural membrane components to secondary metabolites
that provide evolutionary advantage, all the way to terpene compounds with antifungal,
anthelminthic, antibacterial, cytotoxic, and insect-repellent properties [5,8,9].
Studies focused on selection pressures on the evolution
of herbivory have noted that herbivore pressure on plants increases towards the
Equator, and plants respond with a higher density of defenses towards generalist
herbivores [10]. That angiosperms radiated in the
Cretaceous is well accepted [11] and it is also
accepted that “Because some of the oldest and most diverse angiosperm floras
are found in Africa near the Equator, followed by low-latitude, angiosperm-dominated
floras in North America, angiosperms are thought to have radiated from the Equator
and spread to either pole.” [12].
Complementary studies have identified latex and its
component molecules as deterrents for herbivory. Even Monarch butterfly (Danaus
plexippus) caterpillars, which have specialized to feed on milkweed (Ascelpias
spp.), avoid consuming latex, severing latex-carrying tubes and feeding on the latex-depleted
portions of leaves [13]. In a separate experiment,
washing off latex from fig leaves made the leaves more palatable to herbivores [14]. A study of common dandelion (Taxaracum officinale)
showed that latex fouls chewing mouthparts and that a constituent secondary metabolite,
the sesquiterpene lactone taraxinic acid β-D-glucopyranosyl lactone (TA-G), has
a marked effect on the fitness of larvae of the Coleopteran May bug beetle, (Melolontha
melolontha) [15]. Latex may vary in composition,
presumably due to regional selection pressures the plants experience, but the hypothesis
that latex is a herbivory deterrent has gained wide acceptance [5].
Latex-containing plants span 43 families and 20,000 species, and make up a large proportion (>10%) of angiosperms, 1 fern (Regnellidium
diphyllum) and 1 gymnosperm (Gnetum gnemon) [8]. They overlap
with areas with enormous herbivore diversity in the tropics and have a variety of
uses for humans as well. Some are cultivated for food [5], rubber [16],
pharmacologically active molecule production [8], and herbivore-deterrent fencing [17]. Despite clear
evidence of latex’s deterrence of herbivory, and the abundance and diversity of
plants that produce latex, the question of how the two major categories of herbivores
– foregut-fermenters and hindgut-fermenters – tackle the small or large amounts
of latex that must inevitably enter their diet has not been explored, even by the
venerable 454-page volume, The Ecology of Browsing and Grazing II, in which
the word “latex” does not even appear [18]. And
with paper titles like: Grazers and Browsers: How Digestive Morphology Affects
Diet Selection [19], the prevalent assumption
is that gut morphology and, presumably, associated physiology shapes forage selection,
rather than the other way around. Many plants latexes, such as from papaya, Ficus
species, dandelions, mulberry and the rubber tree lack toxicity and the assumption
has been that its sticky nature, which can gum up mouthparts and retard mobility,
is alone responsible for the deterrent effect on herbivory [13]. Konno et al. (2004) even state, “However, the
absence of apparent toxicity from such plants appears to be inconsistent with and
even undermining the widely accepted defense hypothesis.” [14]. I propose, instead, that the deterrent effect of
latex lies in the variable behavior of rubber particles in latex in the alkaline
or acidic conditions encountered in the anterior digestive chambers of foregut-
and hindgut-fermenters, respectively.
Foregut-fermenting herbivores consume latex-containing
shrubs, forbs, and leaves as well as latex-free grasses, and have multiple (polygastric)
chambers with neutral-to-alkaline pH in which fermentative symbionts occur, and
which are located anterior to the acidic stomach (abomasum). Foregut-fermenting
animals include ruminants with a four-chambered digestive system and which regurgitate
and chew cud (deer, goats, cows, sheep,) and nonruminants which do not regurgitate
cud (hippos, kangaroos and sloths) [19]. All ruminating
mammals fall in the order Artiodactyla (even-toed ungulates; also classified as
order Cetartiodactyla). By contrast, hindgut-fermenting herbivores, such as horses,
rabbits, elephants and rhinos, are monogastric animals with an anterior acidic stomach,
and a posterior cecum or colon – the location(s) where symbiont-assisted fermentation
occur(s). Hindgut-fermenting mammals largely belong to the order Perissodactyla
(odd-toed ungulates) or Proboiscidea (elephants) [20].
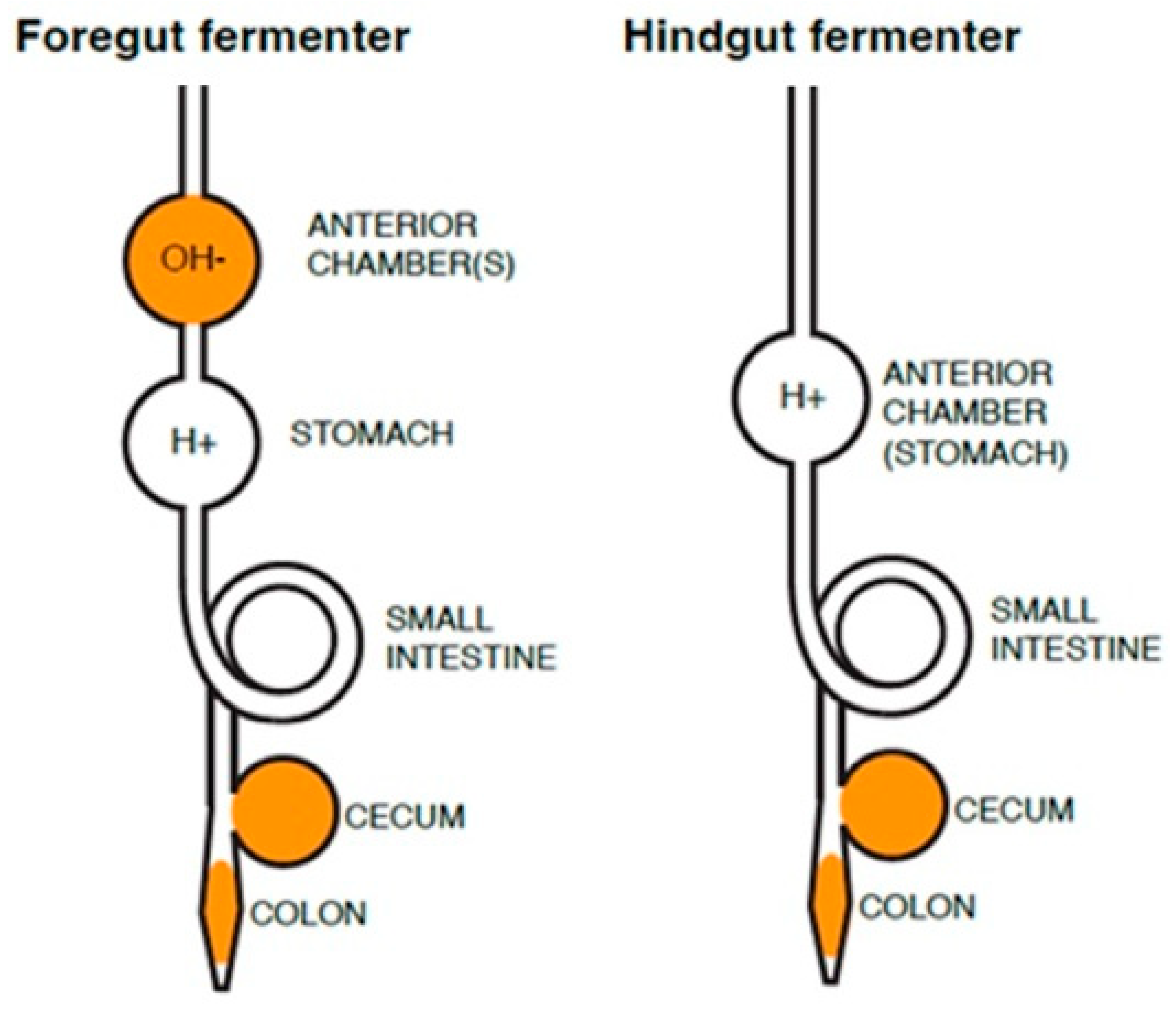
Figure 1 shows a schematic of the location
of anterior and posterior chambers in mammals that have foregut or hindgut (cecal,
colon) fermentation.
Smaller herbivores, such as insect larvae and tadpoles,
lack acidic chambers in the digestive system. Insects have cuticle-lined foreguts
and hindguts, with the midgut providing digestive and absorptive functions and characterized
by neutral to highly alkaline milieus [21]. Captive
tadpoles of the model organism Xenopus laevis are fed pellet food which includes
algae and shrimp flakes [22], and their gut is
reported to be structurally similar to the mammalian gut [23]. However, stomachs of tadpoles do not secrete acid
[24]. Thus, while there may be an anatomical resemblance
to mammalian guts, it does not translate to a functional resemblance.
An alkaline gut, however, renders animals susceptible
to the action of toxins produced by Bacillus thuringiensis and Lysinibacillus
sphaericus (previously Bacillus sphaericus) [25]. These are Gram-positive bacilli which produce sporulation-associated
crystal (Cry) δ-endotoxin, cytolytic (Cyt),
Mtx (mosquitocidal) and Bin (binary) toxins. During vegetative growth they produce
vegetative insecticidal protein (Vip) and secreted insecticidal protein (Sip) [26]. B.thuringiensis Cry δ-endotoxins (Bt) are considered target-specific
biocontrol agents for the larvae of many invertebrate pest species, largely among
Lepidoptera and Diptera, but also Coleoptera and Nematoda. Bt toxins are solubilized
in an alkaline environment as a prerequisite to their proteolytic activation. Activated
toxin inserts into gut membranes to form pores, and cause loss of structural integrity
in environments such as insect guts [27] but potentially
also in foregut-fermenting ruminants and tadpoles. Bt toxins have also been genetically
engineered into a variety of important food and cash crops such as corn, soybean,
eggplant and cotton, which may enter animal feed streams. Additionally, Bt toxins’
reputation as an environmentally-friendly biocontrol agent with few reported off-target
effects has led to its widespread dissemination in the environment, including into
water bodies. The association of susceptibility of foregut-fermenting herbivores
to this type of toxin has not been made before.
In addition to the evolution of alkaline anterior digestive
chambers as an adaptation to latex, I propose that foregut fermenters evolved in
and occupy a wider diversity of habitats from tree canopies to woodland fringe to
pasture, whereas hindgut fermenters occupy grassland or selectively consume latex-free
plants in regionally restricted habitats. Significantly, carnivores, omnivores and
herbivores with hindgut-fermentation and associated acidic stomachs are largely
capable of pivoting to new diets, provided the new diet is latex-free. This was
seen in a population of Italian wall lizards (Podarcis sicula) of which a
few breeding pairs were transported from the island of Pod Kopište where they had
an insectivorous diet to the island of Pod Mrčaru where, in a mere 36 years, they
adapted to a largely herbivorous diet [28]. This habitat pivot, according to the hypothesis
presented in this paper, would not have been possible had the forage consisted of
mostly latex-producing plants.
There are three correlations proposed in this paper
to support the selective pressure of latex as the factor that shaped the evolution
of digestive systems. Although direct evidence will have to be collected to assess
these correlations for latex tolerance, there is circumstantial evidence for the
following that support latex-based selection:
Areas with abundant latex-containing plants correlates with the origins of foregut-fermenting animals. This correlation indicates that latex may have exerted a selective pressure for gut alkalinity.
Foregut-fermenting (often polygastric) animals have higher first chamber pH than hindgut-fermenting (monogastric) animals. This allows the former to tolerate small amounts of latex in the diet, whereas the latter have to avoid latex-containing forage. Awareness of this correlation is important anywhere captive animals are provided forage, such as in farming operations or zoos.
The presence of an alkaline gut pH makes foregut-fermenting vertebrates, metamorphosing tadpoles, and certain orders of insects susceptible to gut damage by Bt δ-endotoxin and related insecticidal toxins. Due to their perceived target-specific nature, these toxins have been widely disseminated and may pose a health threat to foregut-fermenting animals in the wild, as well as to domesticated livestock.
The purpose of this study is to demonstrate that herbivores,
ranging from insect larva and tadpoles to ruminants, could evade the deterrent action
of latex by the presence of alkaline pH in the anterior gut chambers. Alkaline environments
maintain latex in liquid form, thus avoiding gut blockage. However, this physiological
protection against latex may render such herbivores susceptible to the action of
the widely applied Bt toxins. There is little research into examination of the evolution
of the two different types of herbivore gut architecture, and it is hoped that this
paper will stimulate more research into latex as a selective pressure for evolution
of herbivore anatomy and physiology.
2. Materials and Methods
Literature search:
A literature search was performed using the Google Scholar
search engine (scholar.google.com) and the checklist from PRISMA-S (Preferred Reporting
Items for Systematic reviews and Meta-Analyses literature search extension) [29]. Searches for articles for the categories were conducted
between August 2023 and October 2023 using the terms listed below. English was the
search language and other language articles were excluded but no other limits were
applied. In Google Scholar, papers were sorted “by relevance”, and “any type” of
articles, except Case Law, were allowed. Each search term pulled up many tens of
thousands, and sometimes even hundreds of thousands of titles, so the search processes
was standardized to identify the most relevant papers as described below.
For the first page of results (10 titles) which were
algorithmically assessed as of highest relevance to the search terms, the abstracts
were perused. If the abstracts were judged to be interesting, the full article was
obtained from Google Scholar, the University of Delaware library, ResearchGate.net
or the Internet Archive (archive.org). For each article that was judged relevant,
“Related articles” were scrutinized by title and abstract as described above. When
available, PDFs of articles were searched using “Find text in document” function
for specific terms of interest, such as alkaline, latex, symbionts, to quickly
establish relevance. The reference list provided in articles of interest were accessed
when in-text citations were of interest. In these cases, the article was obtained
and the abstract was assessed for relevance.
Search categories:
1. Stomach pHs of foregut- and hindgut-fermenting herbivores.
Search terms: Stomach pH levels; stomach acidity levels;
foregut pH; hindgut pH; herbivore stomachs, polygastric monogastric stomachs; foregut
hindgut herbivores
2. Plant deterrents against herbivory, including latex.
Search terms: herbivory deterrent; latex deterrent;
latex plants herbivory; plant antifeedants; secondary metabolite latex deterrents
3. Geography and evolution of foregut- and hindgut-fermenters.
Search terms: Evolution foregut hindgut; evolution of
digestive systems herbivores; where did herbivores evolve; evolution Perissodactyla;
evolution Artiodactyla; evolution Cetartiodactyla
4. The action of Bacillus thuringiensis and related toxins in animals with alkaline gut pH.
Search terms: Bacillus toxin effect animals; Bt alkaline
gut; Bt effect ruminants; Bt mode of action
5. Occasionally, search terms were used to answer specific questions that arose while writing the paper. They were: stomach pH (wild animals by name); hindgut foregut digestion Cretaceous; evolution Artiodactyla Cretaceous; latex levels mature young leaves; Bt effect tadpoles; horses unripe apples; cattle silvopasture
Data analysis:
There is a paucity of information on the phylogeny of
foregut- and hindgut-fermenting herbivores. I sorted the information available with
the purpose of correlating evolutionary origins of herbivores to geographical locations
of origin. This search did not yield unambiguous results, and so was not pursued
further.
After obtaining data on the stomach pHs of herbivores,
I tabulated the existing classifications of the animals as foregut- or hindgut-fermenting
herbivores. Only one animal, the quokka, was mis-classified in one of the references.
Other problems that arose were mean pH being cited in reviews as representative
of whole chamber pH (pointed out by a reviewer). Therefore original work was checked
and the values for anterior chamber (cardiac stomach rather than pyloric stomach)
pH (rather than mean chamber pH) were obtained where available. pH values were rounded
to one decimal place. Averages were taken when ranges were reported.
Then I sorted the data by pH levels and produced a graphic
form of the range of pH the two types of digestive systems for better visualization
of the pH difference in pH between the two groups. The mean of the group’s values
shown in Table 1 (foregut-fermenters) and
Table 2 (hindgut-fermenters) was calculated
using
https://www.socscistatistics.com/tests/studentttest/default2.aspx. The calculator
on the website was used to calculate the independent two-tailed Student’s T-test
values for a significance of p<.05. The standard deviation (SD) was calculated
using
https://www.calculator.net/standard-deviation-calculator.html. The graph was
drawn using Microsoft Excel under a personal license.
The popular biocontrol agent, Bacillus thuringiensis
δ-endotoxin and related insecticidal toxins
are solubilized by alkaline gut pH as a prerequisite to their activation by proteases.
Therefore I further explored the literature for impact of crystal toxins on ruminants
and metamorphosing herbivores such as tadpoles which have alkaline gut pH as larvae
but become carnivores with acidic stomachs as adults.
3. Results
Foregut- and hindgut-herbivores, insects and tadpoles
have dispersed from their areas of origin due to natural and human-mediated actions,
so it is not a straightforward matter to assess the pressures extant during evolution
of the various species, or to delineate the complex interactions influenced by climate,
competition, and biogeography between plants and herbivores. However, since latex-bearing
plants are most abundant in the tropics, which correlates with the greatest herbivore
pressures [10], that latex provided the selection
pressure for the evolution of alkaline guts is a reasonable inference.
It is thought that the mammalian order Perissodactyla
(odd-toed ungulates; monogastric animals; hindgut-fermenters) evolved in North America,
Europe and Asia, all of which were part of the Laurasia supercontinent during the
pre-Cretaceous when flowering plants evolved, diversified, and spread [30]. However, perissodactyl animals would not have been
exposed to latex in their diet. Their presence in Africa and South America is due
to migration from Europe and North America [31].
Most hindgut-fermenting are monogastric grazers which eat grasses and have dentition
to break down silica-containing highly abrasive plant material. Their forage diversity
is limited and poor in nutrition. Therefore they have to eat unremittingly and become
extremely large to accommodate the passage of the large quantity of low-quality
forage they eat (elephants, rhinos, horses) or practice coprophagy (rabbits).
The evolution of artiodactyls is thought to have been
more recent, in what is today’s Pakistan in the Eocene epoch (55-31 Ma) of the Cenozoic
era, and they spread out during the Oligocene (31-23 Ma). By the Pliocene (5.3-2.5
Ma), artiodactyls were established in South America [32].
These animals had/would evolve foregut-fermenting digestive systems. These polygastric
animals have a herbivory-adaptable digestive system due to the presence of foregut-fermentation
as well as cecum fermentation to break down recalcitrant molecules. Old world monkeys
(langur, colobus) and New world monkeys (spider) are foregut-fermenters and they
live in tree canopies and eat leaves which may contain latex. Red howler monkeys
also live in tree canopies but have a diverse diet with not a lot of latex, so their
hindgut-digestive system is adequate to their needs [33].
Although this is a scattershot justification rather
than prediction, the correlations still apply. Interestingly, the giant panda is
an exception to this hueristic. Its forage is extremely protein-rich bamboo shoots
and it has carnivore-like digestion with neither foregut- nor hindgut- fermentation
[34].
Fresh latex from plants is liquid and has a pH between
6-7.5 [35]. The neutral-to-alkaline pH of foregut
herbivores is capable of maintaining latex in liquid form. The large gut capacity
of ruminants further provides a dilution factor, so rubber particles are more dispersed
and less likely to occlude the system. Ruminants regurgitate and chew the cud to
break up clumps, and have symbionts to digest recalcitrant molecules such as cellulose,
and possibly rubber as well. Although the presence of rubber-digesting symbionts
from foregut-fermenting herbivores has not been reported in the literature, rubber-degrading
actinomycetes in the genus Gordonia have been isolated from Hevea tree
bark and the environment [36] and are potentially
present in environments where they may be ingested by animals feeding on latex-bearing
plants.
To summarize, foregut-fermenting animals which eat leaves
and forbs as well as grasses appear to be able to handle small amounts of latex
in their mixed forage without adverse effects. Smaller animals like caterpillars
avoid ingesting latex by exhibiting latex-avoidance behaviors like trenching and
vein-cutting [13] but, given their extremely small
size, even tiny amounts can cause gut blockage if it solidifies. Therefore the presence
of an alkaline gut, which keeps latex liquid, is even more critical. Table 1 shows the pH levels of an assortment of
foregut fermenters. Where the pH of cardiac and pyloric regions were measured, the
cardiac portion of the stomach’s pH was selected for the table.
Animals which are likely to encounter substantial amounts of latex on a daily basis such as sloths which feed on the latex-containing
Ficus tree [
49], and colobus monkeys which feed largely on leaves but also fruit when available [
50], have a relatively high pH in the first chamber (
Table 1; sloths=pH 7.4; colobus monkeys=pH 6.8), whereas foregut-fermenting hippos and sheep are largely grass-grazers and have a slightly lower anterior chamber pH [
Table 1; hippos-pH=5.7; sheep=pH 6.4]. This observation indicates a direct relationship between the amount of latex in the diet to levels of alkalinity in the anterior chamber. Another important caveat to these values is the variability of pH during a single day and pre- and post-prandial sampling, and these variations are not part of the record; pH values also varied by sampling technique [
41]. Thus, the values in the table should be treated as indicators of chamber pH, but not as absolute values.
Table 2 shows the anterior digestive chamber pH of hindgut-fermenting animals which primarily eat latex-free forage, including grains, fruits, nuts, and sometimes carrion. The presence of an acidic stomach would hinder hindgut fermenters from tolerating even moderate amounts of latex in the diet. An acidic the first chamber may serve as a barrier against pathogens ingested by the oral route due to the largely grazing habitat increasing the potential for consuming feces deposited by other animals, thereby ingesting pathogens and parasites. Additionally, grains and nuts contain high levels of proteins which are broken down by proteases in the presence of stomach acid.
Latex feeding studies in hindgut-fermenters have not been reported. Remarkably, however, there is documentation of a woman (humans are hindgut-fermenters) who ingested liquid latex. The latex solidified into a solid mass, which the surgeons attributed to its exposure to stomach acid, took on the shape of the stomach and completely blocked it. It reportedly bounced like a rubber ball upon surgical removal [
56].
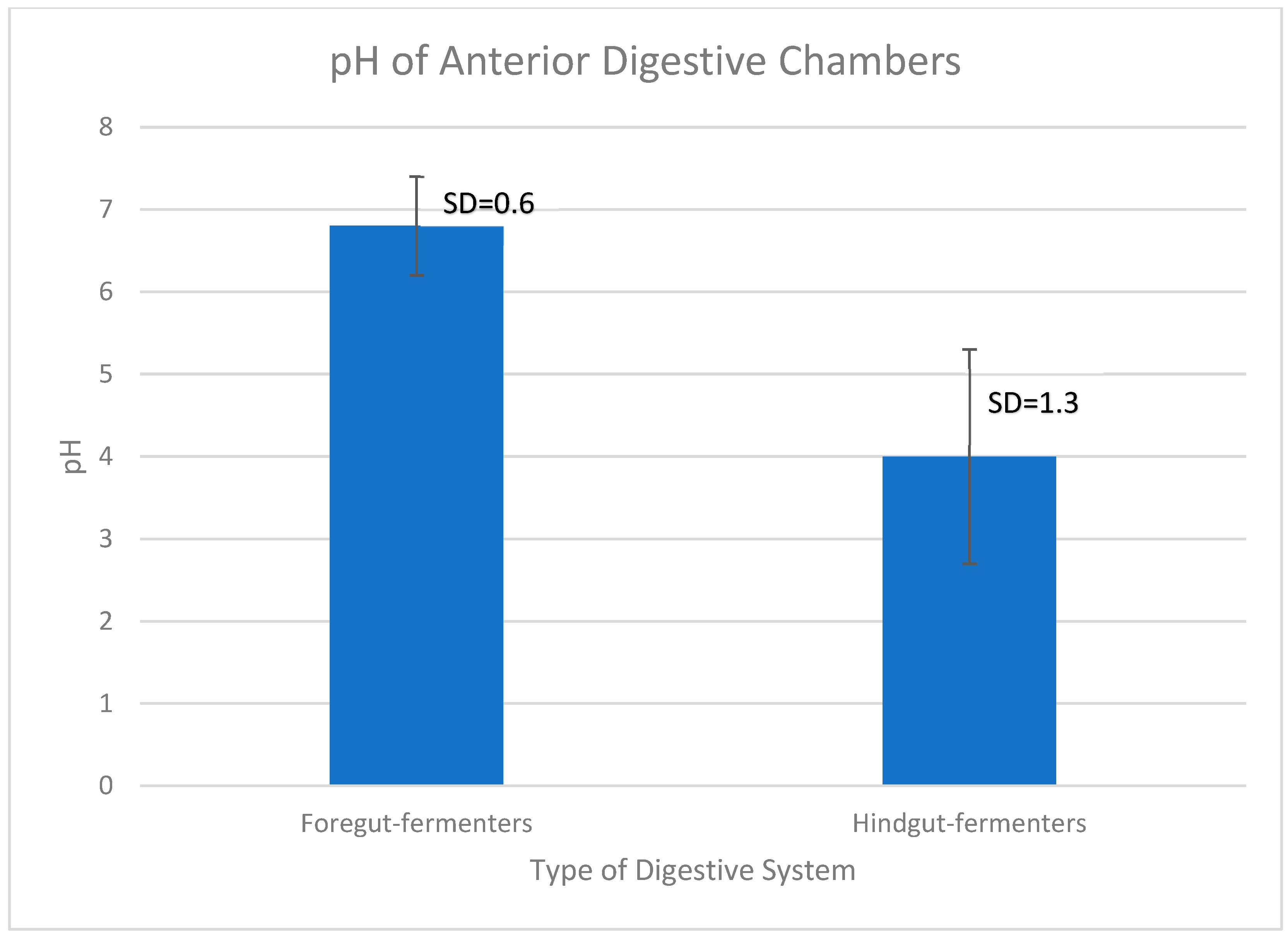
In order to assess if the pH values between foregut- and hindgut-fermenters’ first chambers were significantly different to support the conjectures above, an independent two-tailed Student’s T-test was done. The graph in
Figure 2 shows that there is a significant difference in the average chamber pH between the groups of animals whose anterior chamber pH is listed in
Table 1 and
Table 2.
While direct measurement may be difficult in very small animals, there is an indirect way to test the pH of the first chamber of the gut by measuring the organism’s susceptibility to
Bacillus thuringiensis crystal δ-endotoxin (Bt). Bt toxins are proteins which are solubilized and proteolytically activated in an alkaline pH [
57]. When activated, they punch holes in the gut lining, causing leakage and death [
58]. If the proteins encounter acid in the first chamber, they remain in inactive form and are degraded by proteases, and thus made innocuous.
Larvae of Lepidoptera (moths and butterflies), Coleoptera (beetles), Diptera (flies and mosquitoes), Hemiptera (true bugs) and Hymenoptera (bees, wasps and ants) typically have neutral to alkaline midguts and many include plant material in their diets. The larvae are susceptible to Bt toxins, which are used as biocontrol agents. Coleoptera larvae, which have a slightly more acidic to neutral midgut (pH 5–7), are less susceptible than Lepidoptera and Diptera larvae which have a more alkaline midgut (pH > 9) [
59]. Interestingly, a data point that Huber et al. (2016) could not explain in their study of the Coleopteran larvae of May bugs feeding on dandelion roots was that the larvae gained mass when they fed on latex, even as the concentration of the secondary metabolite TA-G in the latex negatively correlated with
M. melolontha growth [
15]. This observation can be explained if the latex was solidifying inside the larva due to Coleopterans’ more acidic gut pH, and therefore failing to be digested and eliminated, resulting in the observed increase in mass.
The presence of alkaline guts in foregut-fermenters has significant implications for the use of insect larvicidal toxins for biological control. In sporadic reports, Bt was claimed to be toxic to cows, goats, buffaloes and sheep [
60,
61,
62], which would support the hypothesis of the alkaline pH of their guts activating the toxin. The presence of alkaline gut chambers has not been addressed before in the scientific literature as a causative agent for Bt-engineered plants and their products being detrimental to foregut-herbivores. Instead, other reports have focused on allergenicity and leakage of toxin into bloodstream or milk of cows as pathways by which Bt might cause damage, and little has been done to examine long-term effect of Bt in ruminant feed [
63]. Silaging appears to remove the toxic effect, consistent with the process breaking down Bt toxin [
64,
65].
In an experiment conducted by Lajmanovich et al. (2015), Bt proved toxic to the tadpoles of the South American common frog,
Leptodactylus latrans [
66]. Herbivorous tadpoles have a stomach with a neutral pH but, as these tadpoles metamorphose to adulthood and obligate carnivory, their stomach pH changes to acidic [
67]. Although not all gut pHs have been measured, an indirect measurement can be applied by assessing susceptibility to Bt toxins which, as mentioned before, requires alkaline pH for solubilization followed by proteolytic activation. Lajmanovich et al. [
66] did not extend their findings to foregut-fermenting herbivores. A master’s dissertation by Zaayman (2012) also documented the possibility of Bt in maize leaves as being a stressor during
Xenopus laevis tadpole development, but handling and other complications diluted the clarity of the effect [
68].
Thus, animals with an alkaline first chamber will both activate Bt toxin and not cause latex to coagulate, whereas animals with an acidic gut will break down crystal toxins, but also cause latex to coagulate. In the presence of both latex and Bt, gut blockage or gut leakage are the options on offer.
4. Discussion
The primary hypothesis presented in this paper is that the neutral-to-alkaline pH of the anterior digestive chambers of foregut-fermenting herbivores has a straightforward evolutionary purpose: to enable tolerance of the widespread plant metabolite, latex. Obviously, this does not preclude the adaptation of the chamber to symbionts, since the moderate pH is amenable to supporting a variety of organisms. This hypothesis is further supported by the presence of neutral-to-alkaline anterior digestive chambers in folivores, which eat leaves with high levels of latex, and frugivores, where latex is a gatekeeper for fruit ripeness (see
Table 1). Mature leaves and ripe fruit typically have lower latex levels, and are more often browsed than tender shoots [
69]. Some plant latexes, like papaya latex, do not contain toxic chemicals but show high levels of proteases which may provide an additional deterrent to herbivory [
14]. Thus, a secondary hypothesis emerges that toxic compounds, where they exist in latex, are directed towards foregut-fermenting herbivores which can tolerate latex ingestion; but latex-producing plants would prefer to not be eaten at all, and so produce still other deterrents to herbivory (see Supplemental
Table S1).
The choice of food for animals that are confined, as in zoos or domesticated or not able range on their own, is critical for their health. In zoos, care is taken to monitor nutrition, and usually zoos follow the precedents of other zoos which have successfully nurtured a picky herbivore. But it is as well to know why, so that the diet can be modified to resemble the wild diet [
70], but within the bounds of the capacity of the animal’s digestive system. Domesticated herbivores are also at risk if food is offered without foresight. Apples and persimmons, among many other fruits, contain latex as a ripeness gatekeeper, and may be commonly planted in pastures. It is known that horses should not eat a lot of unripe apples. The National Equine site has the following comment:
Yes, horses can eat unripe apples. However, horses prefer ripe apples because they are sweeter and easier to eat.
Unripe apples can be quite tart and might not be enjoyable for horses.” [
71]. The bolding in the preceding sentences represents my emphasis; the “preference” may be due to other things besides “tartness”, which may not be appreciated without an understanding of the role of latex. Since horses are hindgut-fermenters with acidic first chambers, latex in feed will coagulate rather than break down, potentially causing obstruction and pain. Regarding foregut-fermenting cattle, a recent and highly successful move to silvopasture where animals are released into lightly forested woodland can be attributed to their being returned to the environment in which they evolved to eat grasses, forbs and leaves [
72]. Foregut-fermenting bison and deer are notorious for eating young saplings and preserving and producing grasslands, but they can cope with the latex produced by those very saplings. Horses do not forage on trees. Such observations can now be put into a physiological context.
Another interesting consequence of alkaline guts as protection against latex relates to the widespread use of Bt and similar protein biopesticides which are thought of as highly target-specific. Although Bt’s safety as an ecologially responsible insecticide has devoted champions [
73], it is not clear that the distinction between digestive modes of different animals was taken into consideration. Rather, safety tests were carried out on non-target insects and laboratory animals such as hindgut-fermenting rats and mice [
74], making Bt’s off-target impact and consequent ecological safety questionable. Specifically, the effect of Bt on alkaline-gut vertebrates, such as ruminants and tadpoles was not considered in assessing the safety profile. By using the metric that these proteins are activated in an alkaline gut, the target spectrum widens to include any animal which has foregut-fermentation as well as metamorphosing insects and amphibians. Indeed, Lajmanovich et al. [
66] show that Bt can target herbivorous tadpoles. This is concerning, given the already stressful environmental conditions that amphibians are encountering. Even those who may be working to diminish the presence of chemical pesticides in the environment by moving to more “natural” control may be causing unintended damage. For livestock fed on the remnants of Bt-containing crops, the impact on their digestion may cause morbidity and even mortality [
60,
61,
62]. It seems that silaging may break down the Bt toxin [
64,
65], which is a simple fix for a consequential problem.
There is still a lot of work to be done to clarify the impact of latex on digestive systems. In 1989, C.C. Webster and W. J. Baulkwill concluded that “…
the function of latex and rubber in the plant remains unknown.” [
75]. Three decades later, Abarca et al. (2019) stated that
“There is no scientific evidence of the metabolic role of latex in plants.”[
9]. In 1999, Shipley bemoaned “
In addition, virtually all studies comparing anatomy and physiology of browsers and grazers focus on ruminants, and thus fail to consider similar adaptations by other types of herbivores, such as hindgut-fermenters (e.g., rodents, rabbits, horses) and non-ruminant foregut-fermenters (e.g., kangaroos, sloths)” [
19]. But there is progress. Besides plugging wounds, there may be no functional role for latex in the plant itself, except to prevent browsing by herbivores.
5. Conclusion
In summary, in this paper, I explain how differences in the pH of the anterior chambers of foregut- and hindgut-fermenting herbivores may be an adaptation to latex in forage. Since latex is liquid in neutral to alkaline pH, but solidifies to rubber in acid pH, I concur that latex is primarily an antifeedant as has been suggested by Agrawal and Konno [
13], but that it is latex
itself, not the additional chemicals it contains, that is the primary deterrent. Additional work to examine the latex-adapted foregut herbivore vs. latex-maladapted hindgut herbivore hypothesis will have impact on feed and forage provided to livestock and captive animals.
I further propose that foregut-fermenting animals’ alkaline chambers evolved as an adaptation to cope with the presence of latex in the environment. This protection from latex also makes these animals susceptible to the action of Bt toxin which is widely used as an insect biocontrol agent. Providing guidelines for Bt use to minimize off-target impact will benefit foregut-fermenting animals already stressed by environmental or captive conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org Author Contributions
VR conceived the hypothesis, did the research, and wrote the paper.
Funding
This research received no external funding.
Acknowledgements
VR thanks Professor I. Barry Holland and Drs. Stephen Streatfield, Anthony Herrel, Lewis Kinter and Krishna Doraiswamy for helpful comments, and Penelope Malish for drawing
Figure 1.
Conflicts of Interest
The author declares no conflict of interest.
References
- Schoener, T.W. (1971) Theory of feeding strategies. Annu Rev Ecol and Syst, 2, 369-404. URL: https://www.annualreviews.org/doi/abs/10.1146%2Fannurev.es.02.110171.002101.
- Allan, E. and Crawley, M.J. (2011) Contrasting effects of insect and molluscan herbivores on plant diversity in a long-term field experiment. Ecol Lett, 14(12), 1246-1253. [CrossRef]
- Rivera-Vega, L. J., Acevedo, F.E., and Felton, G.W. (2017) Genomics of Lepidoptera Saliva Reveals Function in Herbivory. Curr Opin Insect Sci, 19, 61–69. [CrossRef]
- Furness, J. B., Cottrell, J. J., and Bravo, D. M. (2015) Comparative Gut Physiology Symposium: Comparative physiology of digestion. J Anim Sci, 93(2), 485–491. [CrossRef]
- Agrawal, A.A., and Hastings, A.P. (2019) Plant Defense by Latex: Ecological Genetics of Inducibility in the Milkweeds and a General Review of Mechanisms, Evolution, and Implications for Agriculture. J Chem Ecol, 45 (11-12), 1004–1018. [CrossRef]
- Warowicka, A., Nawrot, R. and Goździcka-Józefiak, A., (2020) Pharmacologically active compounds from latex-bearing plants. In Adv Bot Res. Vol. 93, pp. 119-151. Academic Press. [CrossRef]
- Kerche-Silva, L.E., Cavalcante, D.G.S.M. and Job, A.E. (2018) Natural rubber latex biomaterials in bone regenerative medicine. Biomater Regen Med, 1, p.13. [CrossRef]
- Gracz-Bernaciak J, Mazur O, Nawrot R. (2021) Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int J Mol Sci, 22(22), p. 12427. [CrossRef]
- Abarca L.F.S., Kinkhamer, P.G.L. and Choi, Y.H. (2019) Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med, 85(11/12), 856–868. [CrossRef]
- Salazar, D., and Marquis, R.J. (2012) Herbivore Pressure Increases toward the Equator. Proc Natl Acad Sci U.S.A., 109 (31), 12616–12620. [CrossRef]
- Friis, E.M., Pedersen, K.R. and Crane, P.R., (2005) When Earth started blooming: insights from the fossil record. Curr Opin Plant Biol, 8(1), pp.5-12. [CrossRef]
- Angiosperm-Classification. (n.d.) Encyclopedia Britannica. https://www.britannica.com/plant/angiosperm/Classification (accessed on 24 August 2023).
- Agrawal, A.A., and Konno, K. (2009) Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu Rev Ecol Evol Syst, 40, 311-331. [CrossRef]
- Konno, K., Hirayama, C., Nakamura, M., Tateishi, K., Tamura, Y., et al. (2004) Papain protects papaya trees from herbivorous insects: role of cysteine protease in latex. Plant J, 37, 370–378. [CrossRef]
- Huber, M., Epping, J, Gronover, C.S., Fricke, J., Aziz, Z., Brillatz, T., Swyers, M. et al. (2016) A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol, 14(1), e1002332. [CrossRef]
- Yasuyuki, H. (2009) Production of natural rubber from Para rubber tree. Plant Biotechnol, 26(1), 67-70. [CrossRef]
- Yohanna C.T., Onaji A.I., Nyam M.A., and Azila J.J. (2023) Suitability of Latex-Producing Plant Species as Bio-security for some Landed Properties in Jos South, Jos, Plateau State. Int J Biol Sci, 6(05), 01-15. http://ijojournals.com/index.php/bs/article/view/650.
- Gordon, I.J., & Prins, H.H., eds (2019) The ecology of browsing and grazing II. Vol 239. Springer International Publishing. PDF available at: https://www.researchgate.net/profile/Rene-Van-Der-Wal/publication/337202219_The_Ecology_of_Browsing_and_Grazing_in_Other_Vertebrate_Taxa/links/5edde7df4585152945445f41/The-Ecology-of-Browsing-and-Grazing-in-Other-Vertebrate-Taxa.pdf#page=409.
- Shipley, L.A. (1999) Grazers and browsers: how digestive morphology affects diet selection. Grazing behavior of livestock and wildlife, Idaho Forest, Wildlife & Range Exp. Sta. Bull. #70m (Editors: K.L. Launchbaugh, K.D. Sanders, J.C. Mosley.), pp. 20-27. PDF available at: https://www.webpages.uidaho.edu/range456/readings/shipley.pdf.
- Ahrestani, F.S., Heitkönig, I.M., Matsubayashi, H., Prins, H.H. (2016). Grazing and Browsing by Large Herbivores in South and Southeast Asia. In: Ahrestani, F., Sankaran, M. (eds) The Ecology of Large Herbivores in South and Southeast Asia. Ecol Stud, Vol 225. Springer, Dordrecht. [CrossRef]
- Caccia, S., Casartelli, M. and Tettamanti, G. (2019) The amazing complexity of insect midgut cells: types, peculiarities, and functions. Cell Tissue Res, 377, 505-525. [CrossRef]
- McNamara, S., Wlizla, M. and Horb, M.E. (2018) Husbandry, general care, and transportation of Xenopus laevis and Xenopus tropicalis. Xenopus: methods and protocols, pp.1-17. [CrossRef]
- Chalmers, A.D. and Slack, J.M., (2000) The Xenopus tadpole gut: fate maps and morphogenetic movements. Development, 127(2), 381-392. PDF available at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=baf7c5d4eab41df3d3b1f1c6435b5a90dc9f6aa9.
- Kohl, K.D., Cary, T.L., Karasov, W.H. and Dearing, M.D. (2013) Restructuring of the amphibian gut microbiota through metamorphosis. Env Microbiol Rep, 5(6), 899-903. [CrossRef]
- Berry, C. (2012) The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J Invertebr Pathol, 109(1), 1-10. [CrossRef]
- Baranek, J., Pogodziński, B., Szipluk, N. et al. (2020) TOXiTAXi: a web resource for toxicity of Bacillus thuringiensis protein compositions towards species of various taxonomic groups. Sci Rep, 10, 19767. [CrossRef]
- Palma, L., Muñoz, D., Berry, C., Murillo, J. and Caballero, P. (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins, 6(12), 3296-3325. [CrossRef]
- Herrel, A., Huyghe, K., Vanhooydonck, B., Backeljau, T., Breugelmans, K., Grbac, I., Van Damme, R. and Irschick, D.J. (2008) Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc Natl Acad Sci U.S.A., 105(12), 4792-4795. [CrossRef]
- Rethlefsen, M.L., Kirtley, S., Waffenschmidt, S., Ayala, A.P., Moher, D., Page, M.J. and Koffel, J.B. (2021) PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Systematic reviews, 10(1), 1-19. [CrossRef]
- Silvestro, D., Bacon, C.D., Ding, W., Zhang, Q., Donoghue, P.C., Antonelli, A. and Xing, Y. (2021) Fossil data support a pre-Cretaceous origin of flowering plants. Nat Ecol Evol, 5(4), 449-457. [CrossRef]
- Amnh.org. (2014). Evolution | Perissodactyl. [online] Available at: https://research.amnh.org/paleontology/perissodactyl/evolution/intro. [Accessed 14 Oct. 2023].
- Cassini, G.H., Muñoz, N.A., Merino, M.L., Agnolin, F.L., Lio, G.L., Brissón-Egli, F., Chimento, N.R. and Novas, F.E. (2016) Evolutionary history of South American Artiodactyla. Historia evolutiva y paleobiogeográfica de los vertebrados de américa del sur. Contribuciones del MACN, 6, 673-689. PDF available at: https://www.researchgate.net/profile/Guillermo-Cassini/publication/331071363_Evolutionary_History_of_South_American_Artiodactyla/links/5c641ed845851582c3e5aeb0/Evolutionary-History-of-South-American-Artiodactyla.pdf.
- Julliot, C. and Sabatier, D. (1993) Diet of the red howler monkey (Alouatta seniculus) in French Guiana. Int J Primatol, 14, 527-550. [CrossRef]
- Yong, E. (2019). The Giant Panda Is a Closet Carnivore. [online] The Atlantic. Available at: https://www.theatlantic.com/science/archive/2019/05/giant-panda-closet-carnivore/588553 [Accessed 15 Oct. 2023].
- Ng, J. W., Othman, N. and Yusof, N. H. (2022). Various Coagulation Techniques and Their Impacts towards the Properties of Natural Rubber Latex from Hevea Brasiliensis — a Comprehensive Review Related to Tyre Application. Ind Crops Prod, 181, 114835. [CrossRef]
- Sarkar, B. and Mandal, S. (2021) Gordonia sp. BSTG01 isolated from Hevea brasiliensis plantation efficiently degrades polyisoprene (rubber). 3 Biotech. 11(12), p.508. [CrossRef]
- Smith H.W. (1965) Observations on the flora of the alimentary tract of animals and factors affecting its composition. J Pathol, 89, 95–122.
- Clemens, E.T. and Maloiy, G.M.O. (1982) The digestive physiology of three East African herbivores: the elephant, rhinoceros and hippopotamus. J Zool, 198(2), 141-156. PDF available at: http://www.rhinoresourcecenter.com/pdf_files/151/1519596941.pdf.
- Bodmer, R.E. (1989) Frugivory in Amazonian Artiodactyla: evidence for the evolution of the ruminant stomach. J Zool, 219(3), 457-467.
- Bauchop T, Martucci RW. (1968) Ruminant-like digestion of the langur monkey. Science, 161, 698–700. PMID: 4969750. [CrossRef]
- Duffield, T., Plaizier, J.C., Fairfield, A., Bagg, R., Vessie, G., Dick, P., Wilson, J., Aramini, J. and McBride, B. (2004) Comparison of techniques for measurement of rumen pH in lactating dairy cows. J Dairy Sci, 87(1), 59-66. [CrossRef]
- Kay, R.N.B., Hoppe, P. and Maloiy, G.M.O. (1976) Fermentative digestion of food in the colobus monkey, Colobus polykomos. Experientia, 32(4), 485-487. [CrossRef]
- Williams VJ (1963) Rumen function in the camel. Nature. 4873, 1221. PDF available at: https://www.nature.com/articles/1971221a0.pdf.
- Grajal, A. (1995) Structure and Function of the Digestive Tract of the Hoatzin (Opisthocomus hoazin): A Folivorous Bird with Foregut Fermentation. Auk. 112(1), 20–28. [CrossRef]
- Dehority, B.A. (1997) Foregut fermentation. In Gastrointestinal Microbiology: Volume 1 Gastrointestinal Ecosystems and Fermentations (pp. 39-83). Boston, MA: Springer US. PDF available at: https://link.springer.com/content/pdf/10.1007/978-1-4615-4111-0_3.pdf?pdf=inline%20link.
- Heller, R., Gregory, P.C. and v Engelhardt, W. (1984) Pattern of motility and flow of digesta in the forestomach of the llama (Lama guanacoe f. glama). J Comp Physiol B, 154, 529-533. [CrossRef]
- Moir, R.J., Somers, M. and Waring, H. (1956) Studies on marsupial nutrition I. Ruminant-like digestion in a herbivorous marsupial (Setonix brachyurus Quoy & Gaimard). Aus J Biol Sci, 9(2), 293-304. PDF available from: https://www.publish.csiro.au/bi/pdf/bi9560293.
- Payne, A.I. (1978) Gut ph and digestive strategies in estuarine grey mullet (Mugilidae) and tilapia (Cichlidae). J Fish Biol, 13 (5), 627-629. [CrossRef]
- Mureb, L.S., Rocha-Santos, L., Cassano, C.R., da Silva Lopes, G., Rosa, B., Miranda, F.R., Miranda, C.R.R. and Giné, G.A.F. (2023) Tree diversity mediates individual diet specialization of the maned sloth (Bradypus torquatus). Mamm Biol, 103(2), 145-159. [CrossRef]
- Harris, T.R. and Chapman, C.A. (2007) Variation in diet and ranging of black and white colobus monkeys in Kibale National Park, Uganda. Primates, 48, 208-221. [CrossRef]
- Kitts, W.D., Bose, R.J., Wood, A.J. and Cowan, I.M. (1957) Preliminary observations on the digestive enzyme system of the beaver (Castor canadensis). Can J Zoolog, 35(3), 449-452. [CrossRef]
- Hume I.D. (1982) Herbivorous marsupials—the non-macropodids. In: Hume ID, editor. Digestive physiology and nutrition of marsupials, Cambridge: Press Syndicate of the University of Cambridge. Pp. 69 – 110.
- Milton K., McBee R.H. (1983) Rates of fermentative digestion in the howler monkey, Alouatta palliata (Primates: Ceboidea). Comp Biochem Physiol A: Comp Physiol, 74: 29–31. [CrossRef]
- Beasley, D.E., Koltz, A.M., Lambert, J.E., Fierer, N. and Dunn, R.R. (2015) The evolution of stomach acidity and its relevance to the human microbiome. PloS one, 10(7), p.e0134116. [CrossRef]
- Williams, P.J. and Taylor, T.G. (1985) A comparative study of phytate hydrolysis in the gastrointestinal tract of the golden hamster (Mesocricetus auratus) and the laboratory rat. Brit J Nutr, 54(2), 429-435. PDF available at: https://www.cambridge.org/core/services/aop-cambridge-core/content/view/A8C80DC8946FDA1D4FF515011C871C86/S0007114585000472a.pdf/div-class-title-a-comparative-study-of-phytate-hydrolysis-in-the-gastrointestinal-tract-of-the-golden-hamster-span-class-italic-mesocricetus-auratus-span-and-the-laboratory-rat-div.pdf.
- Thirunavukkarasu K, Yoheswaran K. (1967) Coagulation of rubber latex in the stomach. Br Med J, 4(5577), 484. [CrossRef]
- Cohen, Z.P. (2015). Bacillus thuringiensis, bio-pesticide. Cornell.edu. URL: https://biocontrol.entomology.cornell.edu/pathogens/bacillus.php. (Accessed on 24 August 2023).
- Palma, L., Muñoz, D., Berry, C., Murillo, J. and Caballero, P. (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins. 6(12), 3296-3325. [CrossRef]
- De Maagd, R.A., Bravo, A. and Crickmore, N. (2001) How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet, 17(4), 193-199. [CrossRef]
- Glöckner, G. and Séralini, G.É. (2016) Pathology reports on the first cows fed with Bt176 maize (1997–2002). Sch J Agric Sci, 6, 1-8.PDF available at: https://jeffreydachmd.com/wp-content/uploads/2016/07/Pathology-reports-cows-fed-Bt176-maize-Gl%C3%B6ckner-and-S%C3%A9ralini-2016.pdf.
- Ramdas, S.R. (2010) Bt cotton and livestock: Health impacts, bio-safety concerns and the legitimacy of public scientific research institutions.. In National workshop on Genetically Modified Crops/Foods and Heath Impacts. PDF available at: http://indiaenvironmentportal.org.in/files/bt-cotton-and-livestock-health-impacts-dr-sagari-r-ramdas.pdf.
- Hashim, M.A., ElObied, G.H. and Adawi, I.A. (2017) Respondents Evolution of the Effect of Grazing on Bt-cotton Crop Residues by Ruminants on Health and Milk Characteristics in Gezira State, Sudan. Int J Res Agric Sci (IJRAS), 4 (6), 304-309. PDF available at: http://www.ijras.org/administrator/components/com_jresearch/files/publications/IJRAS_610_FINAL.pdf.
- Rubio-Infante, N. and Moreno-Fierros, L. (2016) An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. J Appl Toxicol, 36(5), 630-648. [CrossRef]
- Folmer JD, Grant RJ, Milton CT, Beck J. (2002) Utilization of Bt corn residues by grazing beef steers and Bt corn silage and grain by growing beef cattle and lactating dairy cows. J Anim Sci, 80(5):1352-1361. [CrossRef]
- Faust, M., Smith, B., Rice, D., Owens, F., Hinds, M., Dana, G. and Hunst, P. (2007) Performance of lactating dairy cows fed silage and grain from a maize hybrid with the cry1F trait versus its nonbiotech counterpart. J Dairy Sci, 90(12), 5706-5713. [CrossRef]
- Lajmanovich, R.C., Junges, C.M., Cabagna-Zenklusen, M.C., Attademo, A.M., Peltzer, P.M., Maglianese, M., Márquez, V.E. and Beccaria, A.J. (2015) Toxicity of Bacillus thuringiensis var. israelensis in aqueous suspension on the South American common frog Leptodactylus latrans (Anura: Leptodactylidae) tadpoles. Environ Res, 136, 205-212. [CrossRef]
- Bjorndal, K.A. (1997) Fermentation in Reptiles and Amphibians. In: Mackie, R.I., White, B.A. (eds) Gastrointestinal Microbiology. Chapman & Hall Microbiology Series. Springer, Boston, MA. pp.199-230. [CrossRef]
- Zaayman, J.L. (2012) Bt maize and frogs: An investigation into possible adverse effects of Bt toxin exposure to amphibian larvae (master’s dissertation, North-West University South Africa.) PDF available at: https://repository.nwu.ac.za/bitstream/handle/10394/9869/Zaayman_JL.pdf?sequence=1.
- Spilatro, S.R. and Mahlberg, P.G. (1986) Latex and laticifer starch content of developing leaves of Euphorbia pulcherrima. Am J Bot, 73(9), 1312-1318. [CrossRef]
- Crissey, S. (2005) The complexity of formulating diets for zoo animals: a matrix. Int Zoo Yearbook, 39(1), 36-43. [CrossRef]
- Anon (2022). Can Horses Eat Apples? - National Equine. [online] Available at: https://www.nationalequine.org/feeding/horses-eat-apples/ [Accessed 16 Oct. 2023].
- Jose, S. and Dollinger, J. (2019) Silvopasture: a sustainable livestock production system. Agroforest Syst, 93, 1-9. [CrossRef]
- Raymond, B. and Federici, B.A. (2017) In defence of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—a response to EFSA. FEMS Microbiol Ecol, 93(7), p.fix084. [CrossRef]
- Bishop, A., Johnson, C. & Perani, M. (1999) The safety of Bacillus thuringiensis to mammals investigated by oral and subcutaneous dosage. World J Microbiol Biot, 15, 375–380. [CrossRef]
- Webster C.C. and Paardekooper, E.C. (1989) The botany of the rubber tree. In: Rubber (CC Webster and WJ Baulkwill, eds.) Tropical Agriculture Series. Longman Scientific & Technical/John Wiley & Sons, Inc., New York.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).