1. Introduction
Deciphering the patterns and drivers of biodiversity is one of the fundamental challenges in the fields of biogeography and ecology. Elevational gradients as natural laboratories provide valuable insights into this field. The relationship between elevation and diversity has been a focus of ecological research since Humboldt's early 19th century observations of changes in Andean vegetation [
1]. While early studies largely outlined descriptive patterns, recent advances, supported by sophisticated field tools and mathematical statistical methods, have delved into the mechanistic underpinnings of these patterns [
2,
3].
Traditionally, mountainous areas have commonly shown a pattern of declining species diversity as elevation increases, primarily due to climatic limitations [
4]. However, a number of studies have revealed that many mountain ranges display puzzling peaks in intermediate elevation diversity and have challenged the classical paradigm [
5]. Several hypotheses—ranging from evolutionary dynamics, habitat heterogeneity, to species interactions—have been posited to explain these intricate patterns [
6].
Elevational gradients have long been used as natural laboratories for studying species diversity and distribution [
7]. However, it is becoming increasingly clear that elevation itself is not a direct influence. Rather, it reflects a series of interrelated biotic and abiotic determinants. As an elevation rises or falls, there are certain changes in climate, soil composition, and geographic processes [
8,
9,
10]. The role of climate in shaping biological processes is well known. As elevation increases, decreases in temperature and pressure become pronounced, affecting the physiological and metabolic activities of organisms [
11]. Changes in diurnal temperature differences, frequency of frost events, and changes in precipitation patterns across the elevation gradient determine species' life strategies [
12]. Parallel to climatic influences, elevation significantly alters the soil profile of an area. From depth and texture to nutrient content, soils change with elevation gradients [
13]. Geology and topography resulting from a variety of geographic processes further amplify the biological impacts of elevation [
14]. Processes such as erosion, glaciation, and tectonic activity can both create barriers to species dispersal and open up new habitats [
15]. The orientation of hillsides can determine sunlight exposure, drainage patterns and windbreaks, all of which can regulate local microclimates and influence the life histories of species [
16,
17].
As the most primitive land plants, liverworts are descendants of the first green creatures who settled on land from 470 million years ago [
18]. They are usually non-vascular bundles and lack the special tissue system found in more evolved plants [
19]. On the contrary, they have a simple structure, either thalloid (flat and banded) or leafy [
20]. They mainly fix themselves on wet substrates, from soil, rotten wood to rocks, usually in a cool or humid environment [
21]. Some species have even adapted to more challenging terrain, such as bare rocks or tree trunks. In bryophytes, rhizoids are slender, hair-like appendages that serve to secure the plant to its substrate and aid in the absorption of water and nutrients. However, not only do the rhizoids absorb water, but the entire plant body also has this capability. [
22]. Liverworts show innumerable adaptability to survive in different environments. Many species are resistant to dehydration, can withstand drying, and then rehydrate when water is available [
23]. Others form a symbiotic relationship with fungi to promote nutrient absorption [
24]. They thrive in habitats such as forests, riverbanks and moist meadows [
25]. However, their adaptability also enables them to settle in a range of habitats from tropical rain forests to Arctic tundra [
26,
27].
Tibet is located in the western part of China with an average elevation exceeding 4,000 meters. Its intricate geological and climatic history has shaped a diverse range of habitats, barriers, and crossroads, with the southern regions being more humid and the northern and western parts drier [
28]. Such a unique combination of climatic and topographic features makes Tibet a significant reservoir of liverwort flora [
16]. Studying liverwort plants in this extreme environment offers insights into the resilience and diversity of plants in challenging habitats. Given their role as ecological indicators, liverworts in Tibet can highlight subtle biome shifts and broader climatic changes, enhancing our comprehension of biodiversity dynamics in alpine settings. Specifically, we chose the Sygera Mountain in Tibet, which lies at the transition between semi-arid and humid zones, for our investigation into the altitudinal distribution of bryophytes. Thus, this study aims to address the following questions: (1) How does the diversity of liverwort change along the significant elevational gradient in Tibet? (2) What are the main factors that drive or limit liverwort diversity? (3) Which environmental variables play a crucial role in shaping the liverwort community patterns?
2. Materials and Methods
2.1. Study area
Sygera Mountain is situated in the southeastern region of the Qinghai-Tibet Plateau, characterized by its rugged and varied topography, spanning from 29°30´ to 29°50´N latitude and 94°30´ to 94°54´E longitude. The elevation here varies greatly, from steep cliffs to gentle aspects, from deep valleys to towering ridges, forming a series of habitats. The complex topography has created a large number of microhabitats, each of which has its own unique niche. The vegetation of Sygera Mountain reflects the transitional nature of the southeastern Qinghai-Tibet Plateau. At low elevations, forests are mainly composed of deciduous species, and with the increase of elevation, forests gradually transition to coniferous forests. In alpine areas, there are meadows and bushes. There are two main aspects of Sygera Mountain. The southeastern aspect faces the main direction of the sun in this hemisphere and is warmer with more direct sunlight [
29]. The northwestern aspect is more shaded, receives less direct sunlight, and is cooler and wetter. The climate of Sygera Mountain is affected by its elevation gradient and the position of the southeast edge of the Qinghai-Tibet Plateau. Generally speaking, it belongs to subalpine to alpine climate. Summer is relatively mild, with more precipitation. The winter is cold, and there is a large amount of snow in high elevation areas. The unique climatic conditions of the region, coupled with changes in topography, form different temperature and humidity regions, each with its own special flora and fauna.
2.2. Sampling and identification
In August 2017 and again in August 2019, systematic field investigations targeting liverworts were conducted on Sygera Mountain. Sampling efforts were strategically dispersed across eight distinct elevational zones, spanning elevations of approximately 3100 m to 4500 m, at intervals of 200 m. Within each of these elevation brackets, multiple substrates were sampled to provide a holistic understanding of liverwort ecology in the region. Specifically, on each aspect, liverworts were sourced from three individual trees, three distinct rock formations, three decaying logs, and two soil sample plots.
For the tree quadrats, we conducted our sampling at various locations along the trunk, precisely at 0m, 0.5m, 1m, and 1.5m. At each of these locations, we collected samples from the four cardinal directions—east, south, west, and north—with each quadrat spanning an area of 0.1 m×0.1 m. Regarding the decaying logs quadrats, we meticulously selected a total of 16 quadrats, maintaining the dimensions of each at 0.1 m×0.1 m. For the soil and rock quadrats, we standardized the size of each quadrat to 0.5 m×0.5 m, ensuring consistency across all samples. At the same time, the coverage of each sample is calculated, and information such as longitude, latitude, and elevation are recorded. This rigorous sampling strategy ensured an encompassing representation of liverwort diversity across these elevational gradients. All procured specimens have been archived and are now accessible at the herbarium of China Agricultural University for future reference and study.
2.3. Environmental variables
Environmental parameters serve as a fundamental framework to understand the intricate interactions and feedback mechanisms within ecosystems. To this end, we sourced high-resolution datasets from globally recognized repositories. Specifically, we extracted metrics for mean diurnal range, temperature, aspect, isothermality, and precipitation from the WorldClim database, which boasts a resolution of approximately 1 km [
30]. Additionally, an index for aridity was procured from the CGIAR-CSI repository via Figshare, presented at an analogous spatial granularity of 30 seconds (circa 1 km) [
31].
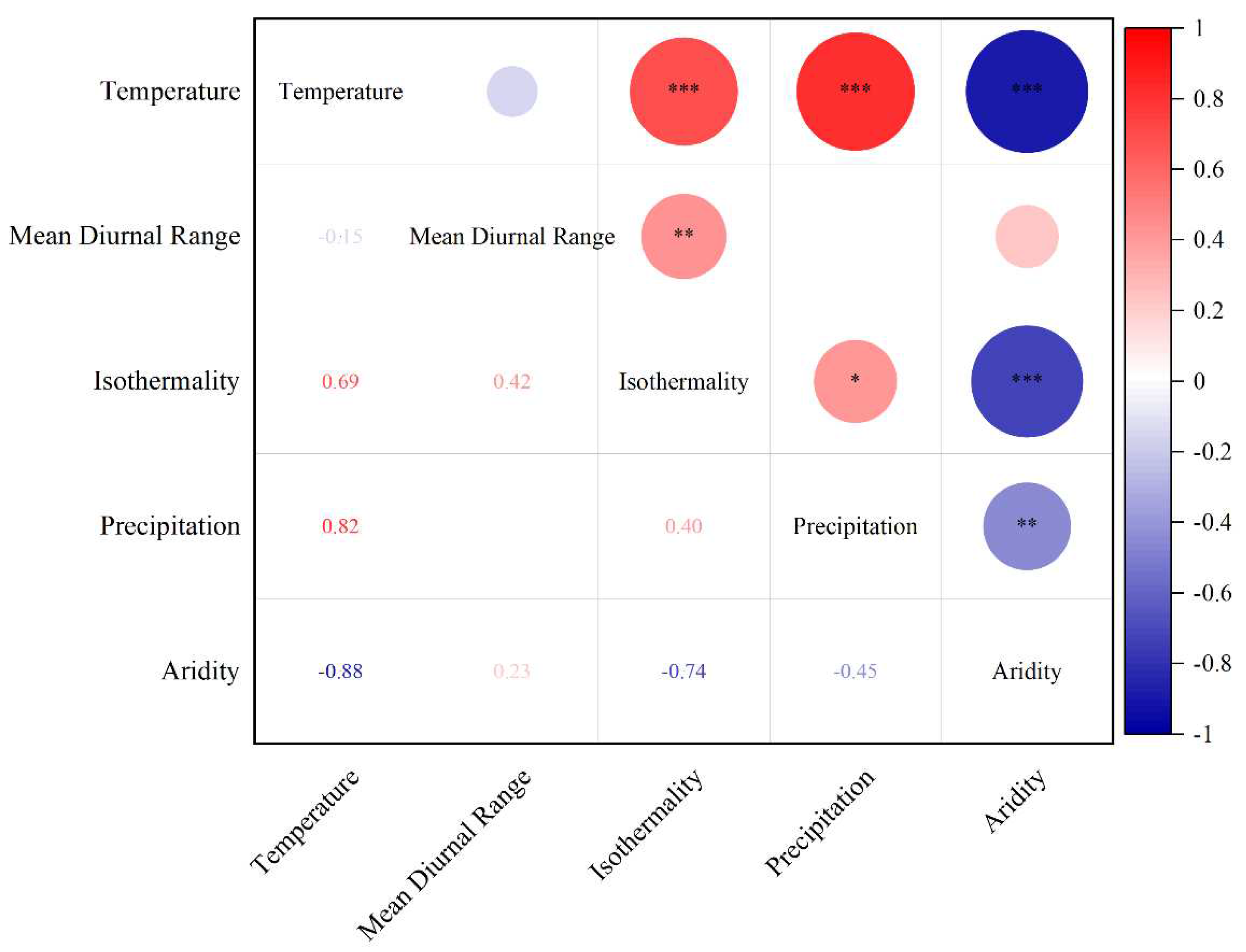
Concurrently, we harnessed the computational capabilities of the R language to extract and synthesize the environmental parameters for 49 distinct plots. Throughout this analytical phase, we upheld stringent criteria against multicollinearity; correlations exceeding a threshold of 0.9 between any pair of selected environmental determinants were meticulously avoided. Such precautionary measures affirmed the independence of our parameters, effectively negating the complexities associated with covariance (
Figure 1).
2.4. Data analyses
Variations in liverwort diversity across different elevational gradients were rigorously examined using Analysis of Variance (ANOVA). Post hoc evaluations were subsequently conducted using Fisher's exact test to discern the significance of observed disparities.
In order to delineate the role of environmental determinants on liverwort diversity, the mixed effect model was used [
32]. Specifically, elevation, mean diurnal range, temperature, aspect, isothermality, aridity, and precipitation were incorporated as fixed effects. Crucially, to account for inherent variability and site-specific deviations, location was integrated as a random effect in our model [
33,
34,
35,
36].
In our effort to understand the differences in liverwort community composition at various elevations, we ued non-metric multidimensional scaling (NMDS). Subsequent to this, a Hellinger transformation was applied to the species-location matrix, enhancing the analytical robustness. By calculating a 95% confidence ellipse around the centroid, we were able to define a representation for a 'typical' elevation sample [
37]. Moreover, to rigorously test distinctions across elevation, orientation, and liverwort species communities, an analysis of similarity (ANOSIM) was conducted using 999 permutations based on Bray-Curtis distances [
38].
To thoroughly examine the relationship between liverwort community patterns and environmental factors, we conducted canonical correspondence analysis (CCA) [
38]. Pairwise distances for each environmental variable were meticulously computed. Leveraging partial Mantel tests with 9,999 permutations, we delineated the intricate associations between liverwort community composition and the myriad environmental parameters under investigation.
3. Results
3.1. Diversity of liverworts on elevation and aspect
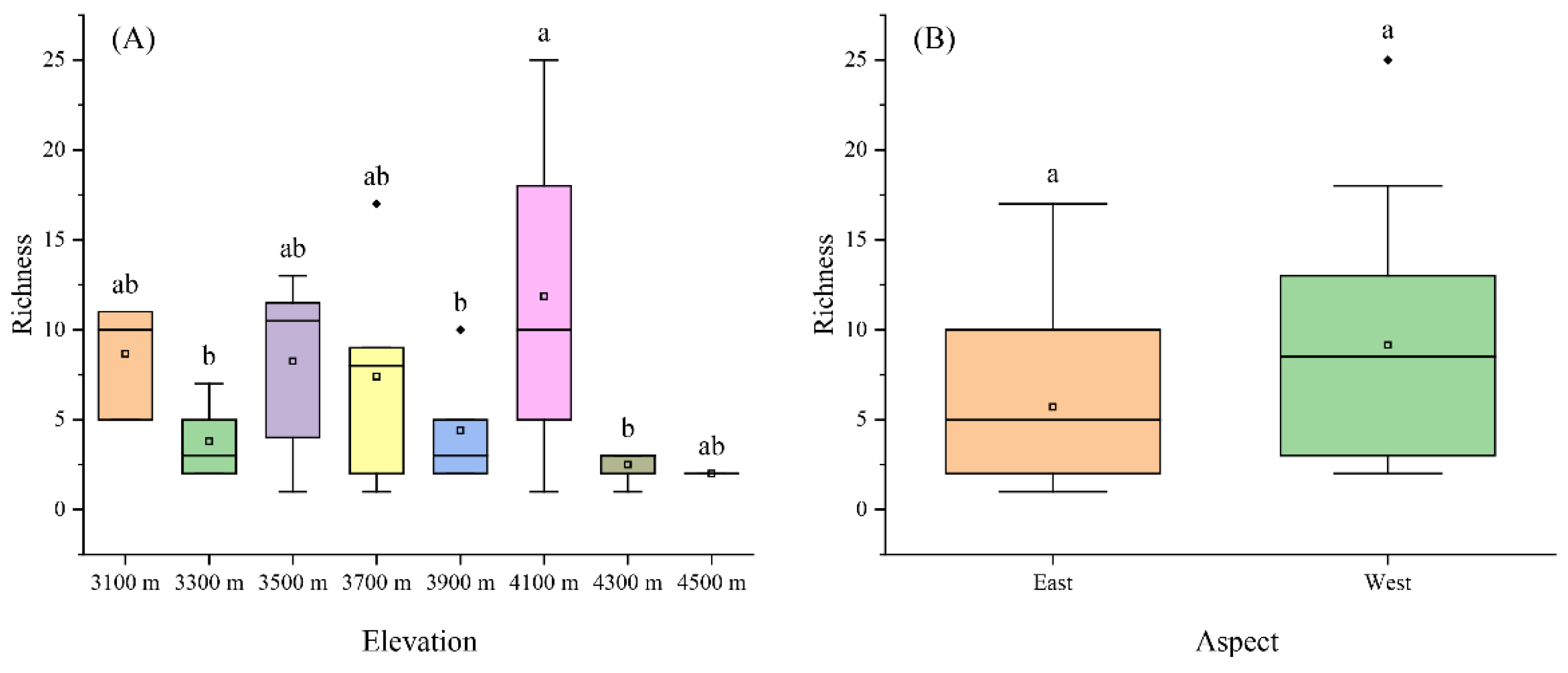
The richness of liverworts was bimodal with elevation, reaching the highest value at an elevation of 4100 m (
Figure 2). The eastern aspect had lower richness compared to the western aspect, although the difference in aspect direction was not statistically significant (
Figure 2B).
Fixed variables explained a larger proportion of variation in richness of liverworts (
R2m = 0.415). Also, the random variable effect explained a small proportion of variation (
R2c -
R2m = 0.342) (
Table 1). Elevations mainly explained the variation in the richness of liverworts (0.285). Secondly, mean Diurnal Range can also significantly affect the richness of liverworts. Liverwort was negatively correlated with mean diurnal range.
3.2. Relationship between liverwort communites and environmental factors
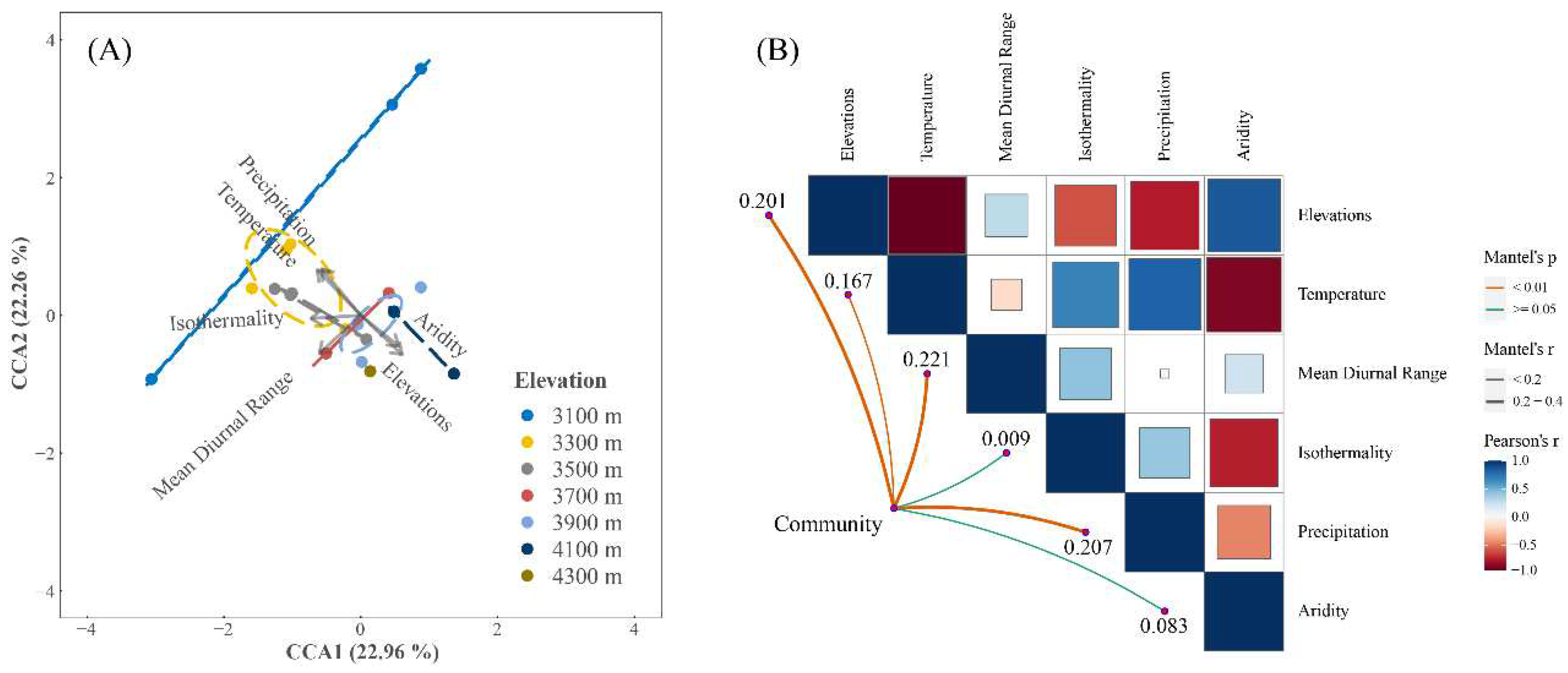
Distinct variations were observed across elevation gradients, with pronounced disparities manifesting especially between communities at 4100 m and their counterparts (
Figure 3). The canonical axes derived from the CCA elucidated 45.22% of the overarching variance (
Figure 4A). Liverwort communities at elevations of 4100 m and 4300 m predominantly align with environments characterized by heightened aridity. In contrast, those at 3100 m and 3300 m are more commonly found in regions with increased mean annual temperatures and augmented annual precipitation. While multiple factors including mean diurnal range, precipitation, elevation and temperature exerted significant influences on liverwort communities, the variance in mean diurnal range emerged as the most influential determinant, isothermality and aridity are not significant for liverwort communities (
Figure 4B).
4. Discussion
4.1. The Influence of Elevation on Liverwort Diversity and Community Composition.
Liverwort richness thrives in the Sygera Mountain, and our study underscores the nuanced relationship between elevation and biodiversity. The bimodal pattern of liverwort richness observed across the sampled elevations unravels the intricate ecological nuances of this mountainous region. Specifically, two prominent peaks in liverwort richness at elevations of 3500 m and 4100 m indicate two distinct zones favoring liverwort diversity.
The peak at 3500 m may coincide with a vegetative transition zone, characterized by an amalgamation of coniferous and broad-leaved forests with mild climatic conditions, which creates a unique interplay between low and high elevation conditions and promotes a range of microhabitats, thereby enriching liverwort diversity. Furthermore, the presence of a richness peak at mid-elevations can be attributed to several biologically plausible processes that result in hump-shaped elevational patterns. This pattern is primarily driven by the accumulation of species at mid-elevations, which migrate from both lower and higher elevations. This phenomenon, as explained by previous research [
39,
40], can be attributed to the overlap zone created by either non-self-sustaining sink populations or a more permanent overlap of different floras [
39,
40]. Considering the significant ecological variations that occur within short geographical distances in mountainous regions, liverwort community composition undergoes substantial changes along the elevation gradient [
41,
42]. Therefore, the observed peak in liverwort richness at mid-elevation is likely the result of an overlap in species ranges, where species from lower elevations mix with those from higher elevations in Sygera Mountain. This finding aligns with previous research on liverworts in Colombia [
43].
The 4100 m peak appears to be a bastion of high ecological diversity for liverworts in the Sygera Mountain [
16]. Our winter field surveys revealed a significant presence of snow, which, although not perennial, tends to melt later in the season. This delayed melting of snow provides a consistent moisture source for the liverworts, and the insulating properties of the snow could offer some thermal protection [
44,
45]. However, richness starts to decline past 4100 meters, plunging substantially at 4500 meters. This suggests the constraining factors of high elevation: diminished atmospheric pressure, colder temperatures, limited liquid water due to freezing conditions, and potentially hostile soil conditions that can restrict the diversity and abundance of liverworts.
The observed bimodal species richness pattern isn't unique to Sygera and has been noted in other mountain systems worldwide, such as the oceanic island of La Réunion [
46]. Often, such patterns are credited to mid-elevation zones serving as ecological sanctuaries or unique environmental conditions at specific heights fostering heightened species diversity.
4.2. The Impact of Climatic Factors on the Community Structure of Liverworts.
In the realm of liverwort communities, aridity and isothermality have typically stood out as pivotal variables [
47]. They're the yardstick against which we often gauge ecosystems. However, our research in the Sygera Mountain presents an intriguing deviation. Here, the most salient determinant for liverwort communities appears to be the variance in the mean diurnal range, temperature, mean diurnal range, precipitation emerging as major determinants suggests a unique microclimate or set of ecological conditions. Mountainous regions often have complex topographies, which can create a multitude of microclimates within a relatively small area. As elevation changes, so does the temperature and precipitation patterns. This can lead to areas with sharp temperature drops at night (a high mean diurnal range) and varied annual average temperatures and precipitation depending on the elevation [
48].
In the Sygera Mountain, the complex interplay between topographical and vegetative features crafts a unique thermal landscape. The orientation of mountain slopes, distinguishing between northwestern and southeastern aspects, dictates sunlight exposure, subsequently affecting temperature. This leads to pronounced variations in the mean diurnal range and annual average temperatures, often observed even between adjacent areas. Compounding this, the area's inherent elevational gradients foster a plethora of microclimates. Unlike typical ecosystems where isothermality ensures consistent temperatures between day and night, the Sygera Mountain exhibit pronounced daily temperature swings. Such diurnal variability, potentially more impactful on liverwort ecology than previously estimated and influencing processes like evaporation and condensation on liverwort surfaces, is modulated by the region's vegetation [
49,
50]. Dense canopies act as buffers, mitigating temperature extremes and providing a protective umbrella for liverworts beneath [
51]. Conversely, in areas with sparser vegetation of high elevation, liverworts face the full brunt of these temperature fluctuations, possibly amplifying their adaptive strategies to navigate these daily thermal challenges.
Mountains, due to their imposing topography, play a pivotal role in influencing local precipitation patterns. The windward side (southeastern aspects) of a mountain range typically receives more precipitation than the leeward side (northwestern) influenced by the Indian Ocean monsoon, resulting in the rain shadow effect [
52]. This leads to variations in annual average precipitation within the same range. However, in such terrains, the rainfall can be intermittent and often unpredictable, leading to sporadic hydration episodes for liverworts [
25,
53]. Consequently, these liverworts might develop adaptations that favor rapid response to these short-lived hydration periods rather than enduring extended dry or wet conditions. In this context, even if an area registers as arid based on broader metrics, it's precipitation that predominantly shapes liverwort community pattern.
The evolutionary trajectory of organisms is often intimately intertwined with their environmental milieu [
54]. Over prolonged evolutionary timescales, it's conceivable that these communities have undergone selective pressures shaped by the unique climatic determinants of the region. Instead of the broader factors of isothermality or aridity, which conventionally influence liverwort distributions in other terrains, the Sygera liverworts appear to show a heightened sensitivity to changes in the mean diurnal range, annual average temperature, and precipitation patterns. Such evolutionary adaptations could be the outcome of consistent exposure to these specific environmental variables, driving them to develop traits or strategies that optimize their survival and propagation in the face of these localized challenges [
55,
56].
4.3. Future Research Directions.
Given the intricate relationships between liverwort diversity, community composition, and environmental variables observed in the Sygera Mountain, future research should focus on understanding species-specific responses to these ecological conditions in greater detail. One promising direction is longitudinal studies that look at the dynamic responses of liverwort species over time, particularly in relation to climate change and anthropogenic impacts. This would provide valuable insights into the resilience and adaptation of liverwort communities, contributing to broader ecological understanding and biodiversity conservation strategies. In addition, these studies can employ advanced ecological modeling techniques to predict how future climate change will affect the distribution and diversity of liverworts in montane ecosystems [
57]. Such predictive models, combined with empirical data, will help to develop conservation plans aimed at predicting and mitigating the effects of climate change.
In addition, there is an urgent need for comparative studies of different mountain ecosystems around the world. By extending the study to other mountain ranges, a more thorough understanding of the similarities and contrasts in liverwort diversity patterns and ecological determinants can be achieved. Recent studies have begun to explore the impact of geographical heterogeneity on plants [
58,
59]. Similarly, we can investigate whether geographic diversity and climatic variability can drive the current distribution patterns of mosses, combining perspectives on traits and species diversity. Such cross-system analyses, potentially combined with molecular and phylogenetic tools, could reveal the evolutionary trajectories of these species and their ecological niches [
60]. The use of interdisciplinary approaches such as remote sensing and geographic information system (GIS) techniques could enhance the spatial analysis of liverwort communities, leading to a more comprehensive understanding of their ecological dynamics. These approaches will greatly assist the field of mountain ecology by providing a deeper understanding of species-environment interactions and informing global biodiversity conservation efforts.
5. Conclusions
Our study in the Sygera Mountain provides a detailed understanding of the complex interactions between environmental factors and liverworts diversity and community composition. The main findings of this study emphasize the unique bimodal pattern of liverworts richness along the altitudinal gradient, with two significant peaks observed at 3500 m and 4100 m. The peaks in geophyte richness at these elevations are characterized by their unique microhabitat and climatic conditions. These elevations, characterized by their unique microhabitats and climatic conditions, appear to provide optimal conditions for the diversity of liverwort species. The critical role of mean diurnal range, precipitation, and annual temperature in shaping the liverworts communities in the Sygera Mountain. The diversity and distribution of liverworts in the Sygera Mountain is evidence of the complex and layered interactions between organisms and their environment. Future research and conservation efforts in this and similar mountain ecosystems should take these complex interrelationships into account to ensure the protection and understanding of this unique and valuable ecosystem.
Supplementary Materials
Appendix S1. Data on Liverwort diversity and environmental factors in Sygera Mountain.
Author Contributions
Conceptualization: Xiaoming Shao and Xiaotong Song; methodology: Xiaotong Song, Jiqi GU and Xiaoming Shao; software: Jiqi GU, Yanhui Ye and Yujia Liao; validation: Heping Ma, Xiaoming Shao; formal analysis: Xiaotong Song, Jiqi GU, and Xiaoming Shao; investigation: Xiaoming Shao, Xiaotong Song, Yanhui Ye, Ruihong Wang and Heping Ma; resources: Xiaoming Shao; data curation: Xiaotong Song, Jiqi GU, and Xiaoming Shao; writing—original draft preparation: Xiaotong Song, Jiqi GU and Xiaoming Shao; writing—review and editing: Xiaotong Song, Jiqi GU, and Xiaoming Shao; visualization: Xiaotong Song, Jiqi GU, Yanhui Ye and Xiaoming Shao; supervision: Ruihong Wang and Heping Ma; project administration: Xiaoming Shao; funding acquisition: Xiaoming Shao. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Natural Science Foundation of China (Grant No. 41771054), the Flexible talent support project of Tibet Agricultural and Animal Husbandry University (Grant No. 604419044), and the Joint Scientific Research Fund Project of China Agricultural University-Tibet Agriculture and Animal Husbandry University, Chinese Universities Scientific Fund (Grant No. 2022TC126).
Data Availability Statement
The data that support the findings of this study are openly available in Supporting Information.
Acknowledgments
Sincerest thanks are given to Youfang Wang (School of life science, East China Normal University), Min Li (College of Life Sciences, Hebei Normal University), Xiaorui Wang (College of Resources and Environmental, Shijiazhuang University), Yingjie Fan, Xinyuan Jiang, Mengzhen Wang, Ling Liu from College of Resources and Environmental Sciences, China Agricultural University, for their contributions to the collection of bryophytes specimens in Tibet.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Humboldt, A.V.; Bonpland, A. Essai sur la géographie des plantes accompagné d’un tableau physique des régions équinoxiales. Levrault, Schoell et Cie, Paris 1805. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical ecology with R. Springer: 2011; Vol. 2, p.
- Ovaskainen, O.; Abrego, N. Joint species distribution modelling: With applications in R. Cambridge University Press: 2020; p.
- McCain, C.M.; Grytnes, J. Elevational gradients in species richness. eLS 2010. [Google Scholar]
- Zhang, W.; Huang, D.; Wang, R.; Liu, J.; Du, N. Altitudinal patterns of species diversity and phylogenetic diversity across temperate mountain forests of northern China. Plos One 2016, 11, e159995. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Anderson, M.J.; Arita, H.T.; Chao, A.; Colwell, R.K.; Connolly, S.R.; Currie, D.J.; Dunn, R.R.; Graves, G.R.; Green, J.L. Patterns and causes of species richness: a general simulation model for macroecology. Ecol Lett 2009, 12, 873–886. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kluge, J.; Worm, S.; Lange, S.; Long, D.; Böhner, J.; Yangzom, R.; Miehe, G. Elevational seed plants richness patterns in Bhutan, Eastern Himalaya. J Biogeogr 2017, 44, 1711–1722. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Y.; Tang, Y.; Du, W.; Shao, C.; Wu, J.; Zhao, L.; Zhang, L.; Liu, J.; Xu, X. The importance of including soil properties when disentangling the drivers of species richness: the case of the alpine genus Saxifraga L. in China. Front Ecol Evol 2020, 8, 244. [Google Scholar] [CrossRef]
- Descombes, P.; Leprieur, F.; Albouy, C.; Heine, C.; Pellissier, L. Spatial imprints of plate tectonics on extant richness of terrestrial vertebrates. J Biogeogr 2017, 44, 1185–1197. [Google Scholar] [CrossRef]
- Körner, C. Significance of temperature in plant life. Plant growth and climate change 2006, 48–69. [Google Scholar]
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M.R. Global patterns in plant height. J Ecol 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Kewlani, P.; Negi, V.S.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Soil nutrients concentration along altitudinal gradients in Indian Western Himalaya. Scand J Forest Res 2021, 36, 98–104. [Google Scholar] [CrossRef]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J. Geological and climatic influences on mountain biodiversity. Nat Geosci 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Perrigo, A.; Hoorn, C.; Antonelli, A. Why mountains matter for biodiversity. J Biogeogr 2020, 47, 315–325. [Google Scholar] [CrossRef]
- Gu, J.; Song, X.; Liao, Y.; Ye, Y.; Wang, R.; Ma, H.; Shao, X. Tree Species Drive the Diversity of Epiphytic Bryophytes in the Alpine Forest Ecosystem: A Case Study in Tibet. Forests 2022, 13, 2154. [Google Scholar] [CrossRef]
- Chen, Z.; Hsieh, C.; Jiang, F.; Hsieh, T.; Sun, I. Relations of soil properties to topography and vegetation in a subtropical rain forest in southern Taiwan. Plant Ecol 1997, 132, 229–241. [Google Scholar] [CrossRef]
- Graham, L.; Lewis, L.A.; Taylor, W.; Wellman, C.; Cook, M. Early terrestrialization: transition from algal to bryophyte grade. Photosynthesis in bryophytes and early land plants 2014, 9–28. [Google Scholar]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. Conducting tissues and phyletic relationships of bryophytes. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 2000, 355, 795–813. [Google Scholar] [CrossRef]
- Mishler, B.D.; Churchill, S.P. Transition to a land flora: phylogenetic relationships of the green algae and bryophytes. Cladistics 1985, 1, 305–328. [Google Scholar] [CrossRef]
- Huttunen, S.; Bell, N.; Hedenäs, L. The evolutionary diversity of mosses–taxonomic heterogeneity and its ecological drivers. Crit Rev Plant Sci 2018, 37, 128–174. [Google Scholar] [CrossRef]
- Valarezo, E.; Meneses, M.A.; Jaramillo-Fierro, X.; Radice, M.; Benítez, Á. Volatile Compounds and Oils from Mosses and Liverworts. In Bioactive Compounds in Bryophytes and Pteridophytes, Springer: 2022; pp 1-53.
- Marks, R.A.; Burton, J.F.; McLetchie, D.N. Sex differences and plasticity in dehydration tolerance: insight from a tropical liverwort. Ann Bot-London 2016, 118, 347–356. [Google Scholar] [CrossRef]
- Humphreys, C.P.; Franks, P.J.; Rees, M.; Bidartondo, M.I.; Leake, J.R.; Beerling, D.J. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat Commun 2010, 1, 103. [Google Scholar] [CrossRef] [PubMed]
- Hallingbäck, T.; Hodgetts, N.G. Mosses, liverworts, and hornworts: status survey and conservation action plan for bryophytes. IUCN in collaboration with the Swedish Threatened Species Unit Gland …: 2000; p.
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspectives in Plant Ecology, Evolution and Systematics 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Dos Santos, N.D.; Da Costa, D.P. Altitudinal zonation of liverworts in the Atlantic Forest, Southeastern Brazil. The bryologist 2010, 113, 631–645. [Google Scholar] [CrossRef]
- Corlett, R. The ecology of tropical East Asia. Oxford University Press, USA: 2014; p.
- Shen, Z.; Lu, J.; Hua, M.; Tang, X.; Qu, X.; Xue, J.; Fang, J. Population structure and spatial pattern analysis of Quercus aquifolioides on Sejila Mountain, Tibet, China. J Forestry Res 2018, 29, 405–414. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global aridity index and potential Evapo-Transpiration (ET0) climate database v2. CGIAR Consortium for Spatial Information (CGIAR-CSI). Published online, available from the CGIAR-CSI GeoPortal 2018. [Google Scholar]
- Schielzeth, H.; Nakagawa, S. Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol Evol 2013, 4, 14–24. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv preprint 2014, arXiv:1406.5823 2014. [Google Scholar]
- Dray, S.; Dufour, A. The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Package ‘lmertest’. R package version 2015, 2, 734. [Google Scholar]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm. hp: an R package for computing individual effect of predictors in generalized linear mixed models. J Plant Ecol 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr:‘ggplot2’based publication ready plots. R package version 0.4. 0. In 2020.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.; Wagner, H. Package ‘vegan’: Community ecology package. R package version 2012, 2. [Google Scholar]
- Grytnes, J.A. Ecological interpretations of the mid-domain effect. Ecol Lett 2003, 6, 883–888. [Google Scholar] [CrossRef]
- Kessler, M.; Hofmann, S.; Krömer, T.; Cicuzza, D.; Kluge, J. The impact of sterile populations on the perception of elevational richness patterns in ferns. Ecography 2011, 34, 123–131. [Google Scholar] [CrossRef]
- Frahm, J.; Gradstein, S.R. An altitudinal zonation of tropical rain forests using byrophytes. J Biogeogr 1991, 669–678. [Google Scholar] [CrossRef]
- Gradstein, S.R.; Churchill, S.P.; Salazar-Allen, N. Guide to the bryophytes of tropical America. Memoirs-New York Botanical Garden 2001.
- WOLF, J. DIVERSITY PATTERNS AND BIOMASS OF EPIPHYTIC BRYOPHYTES AND LICHENS ALONG AN ALTITUDINAL GRADIENT IN THE NORTHERN ANDES. Ann Mo Bot Gard 1993, 80, 928–960. [Google Scholar] [CrossRef]
- Glime, J.M. Volume 1, Chapter 10-2: Temperature: Cold. 2013. [Google Scholar]
- Górski, P.; Gądek, B.; Gąbka, M. Snow as a parameter of bryophyte niche partitioning in snow-beds of the Tatra Mountains (Western Carpathians). Ecol Indic 2020, 113, 106258. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Flores, O.; Wilding, N.; Bardat, J.; Marline, L.; Hedderson, T.A.; Strasberg, D. Functional diversity of subalpine bryophyte communities in an oceanic island (La Réunion). Arctic, Antarctic, and Alpine Research 2014, 46, 841–851. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, T.; Wu, Y.; Hu, R.; Huang, K.; Shao, X. Past distribution of epiphyllous liverworts in China: The usability of historical data. Ecol Evol 2018, 8, 7436–7450. [Google Scholar] [CrossRef] [PubMed]
- Horwath, A.B.; Royles, J.; Tito, R.; Gudiño, J.A.; Salazar Allen, N.; Farfan-Rios, W.; Rapp, J.M.; Silman, M.R.; Malhi, Y.; Swamy, V. Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent. Proceedings of the Royal Society B 2019, 286, 20182284. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Poeschl, Y.; Blatt-Janmaat, K.L.; Uthe, H. Ecometabolomics Studies of Bryophytes. In Bioactive Compounds in Bryophytes and Pteridophytes, Springer: 2023; pp 637-679.
- Yadav, S.; Srivastava, A.; Biswas, S.; Basu, S.; Singh, S.K.; Mishra, Y. Seasonal changes in the antioxidative defence system of a liverwort Dumortiera hirsuta. J Plant Growth Regul 2022, 41, 1265–1275. [Google Scholar] [CrossRef]
- Fenton, N.J.; Frego, K.A.; Sims, M.R. Changes in forest floor bryophyte (moss and liverwort) communities 4 years after forest harvest. CANADIAN JOURNAL OF BOTANY-REVUE CANADIENNE DE BOTANIQUE 2003, 81, 714–731. [Google Scholar] [CrossRef]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Gerlitz, L.; Heyken, H.; Lange, J.; Müller, M.; Scholten, T. Do Himalayan treelines respond to recent climate change? An evaluation of sensitivity indicators. Earth Syst Dynam 2015, 6, 245–265. [Google Scholar] [CrossRef]
- Bates, J.W. Effects of intermittent desiccation on nutrient economy and growth of two ecologically contrasted mosses. Ann Bot-London 1997, 79, 299–309. [Google Scholar] [CrossRef]
- Song, X.; Fang, W.; Chi, X.; Shao, X.; Wang, Q. Geographic pattern of bryophyte species richness in China: The influence of environment and evolutionary history. Front Ecol Evol 2021, 9, 680318. [Google Scholar] [CrossRef]
- Newsham, K.K. The biology and ecology of the liverwort Cephaloziella varians in Antarctica. Antarct Sci 2010, 22, 131–143. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspectives in Plant Ecology, Evolution and Systematics 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Cerrejon, C.; Valeria, O.; Mansuy, N.; Barbe, M.; Fenton, N.J. Predictive mapping of bryophyte richness patterns in boreal forests using species distribution models and remote sensing data. Ecol Indic 2020, 119, 106826. [Google Scholar] [CrossRef]
- Chang, Y.; Gelwick, K.; Willett, S.D.; Shen, X.; Albouy, C.; Luo, A.; Wang, Z.; Zimmermann, N.E.; Pellissier, L. Phytodiversity is associated with habitat heterogeneity from Eurasia to the Hengduan Mountains. New Phytol 2023. [Google Scholar] [CrossRef] [PubMed]
- Vernham, G.; Bailey, J.J.; Chase, J.M.; Hjort, J.; Field, R.; Schrodt, F. Understanding trait diversity: the role of geodiversity. Trends Ecol Evol 2023. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Eklund, D.M.; Cronberg, N.; Bowman, J.L.; Lagercrantz, U. Rates and patterns of molecular evolution in bryophyte genomes, with focus on complex thalloid liverworts, Marchantiopsida. Mol Phylogenet Evol 2021, 165, 107295. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).