1. Introduction
Mastitis remains one of the most important economic burdens for dairy farmers [
1,
2,
3]. Among all the bacteria involved in mastitis epidemiology, contagious pathogens (
S. aureus and
S. agalactiae) represent a peculiar group because, in addition to the very high direct and indirect costs, they may also have zoonotic and reverse-zoonotic potentials and may contribute to the spread of antimicrobial resistance (AMR) as shown by several studies [
4,
5,
6,
7]. From a One Health perspective, controlling and eradicating these infections have several advantages; such as reducing antimicrobial usage and resistance, improving milk quality and yield, and decreasing the risk of human infections [
6,
8,
9].
One of the most important risk factors for spreading these infections is milking, particularly liner contamination, which may transfer the pathogens from infected to healthy cows [
6,
10,
11,
12]. The segregation of infected cows and their milking last showed to be an effective way to decrease the spread of infections [
6,
13]. However, segregation requires at least three groups of cows (healthy, infected, and to be diagnosed), and an increase in labor for herdsmen. The use of backflush systems that clean and disinfect milking units between cows has been suggested as a way to decrease infection risks without segregation. Unfortunately, the results of this practice are controversial, and these systems alone do not decrease the incidence of infections [
14,
15,
16]. These risks became even higher when automatic milking systems are applied because a single milking unit may milk up to 60-70 cows, with an increase in infection risk and chronic mastitis occurrence [
17,
18,
19].
Recently, the production of elastomers that include antimicrobial components in their formulation has increased the interest in the application of these materials in medical devices and in food production [
20,
21,
22,
23]. Among these new products, an innovative patented technology (Scudo Technologies, Italy) enabled the development of antimicrobial elastomers that can be used to produce both rubber and silicon liners. The availability of these materials allowed us to test them
in vitro against three major mastitis pathogens (
S. aureus,
S. agalactiae, and
E. coli)
, as a preliminary step to identify the elastomers with the highest antibacterial activity. This preliminary study was pivotal for designing field studies to assess their ability to reduce infection risks during milking.
2. Materials and Methods
2.1 Elasatomer characteristics
Two different types of elastomers were considered: rubber and silicon. Two different rubber elastomers were developed, whereas seven different receipts were developed among silicones. In each of them, a diverse patented antimicrobial additive (PAA) was added.
A detailed description of all receipts and relative PAAs were reported in
Table 1 and
Table 2.
2.2 Bacterial strains and culture conditions
The potential antimicrobial activity of elastomers was tested against the following bacterial species, which represent major mastitis pathogens: Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 25922, and Streptococcus agalactiae ATCC 13813. Bacteria were stored at -20°C in 25 % (v/v) glycerol, thawed at room temperature and 10 µL were plated on tryptic Soy Agar (Microbiol, Italy) with aerobic incubation at 37°C overnight. After incubation, bacterial inoculum was prepared by suspending colonies in sterile saline solution (NaCl, 0.9 % w/v) to reach a concentration of 0.5 McFarland units (equivalent to 1.5x108 CFU/mL) determined with a densitometer (BioSan, Medical-Biological Research and Technologies, Riga). Serial dilutions were performed to obtain final concentrations of 104 and 103 CFU/mL. These concentrations were selected to be as close as possible to the values observed under field conditions.
2.3 Preparation of silicone sheets and microplate loading
Squares of 1.21 cm2 from each elastomer sheet were cut with sterile scissors and positioned at the bottom of the wells of 24-well plates (Cellstar, Greiner bio-one, Italy). One mL of each bacterial suspension was dispensed over each square. For each 24-well plate, one sterility control was included. Four different time points were used to assess the antibacterial activity of the silicone sheets: T0 (contact), T1 (1 hour after contact), T2 (6 hours after contact), and T3 (24 hours after contact). At each time point, 50 µL of bacterial suspension was collected from the wells, plated on tryptic soy agar (Microbiol, Italy), and aerobically incubated at 37°C for 24 h. After incubation, the colonies on each plate were counted, and the final results were expressed in CFU/mL. Each time point was tested in triplicate.
2.4. Statistical analysis
Data were collected in a database, and the killing rate was calculated using the following equation:
where K(%) is the reduction in the bacterial population (percentage, %), CFU
tn is the bacteria concentration (CFU/mL) at time n>0, and CFU
t0 is the initial bacteria concentration (CFU/mL)
The Kaplan-Meier method was applied to compare the killing activity during the entire follow-up period (24h) (XLstat 2023.1.4 Addinsoft, New York, NY, USA). The Kaplan-Meier analysis allows the comparison of populations through their survival curves, even in the case of irregular time.
3. Results
This study considered 9 different elastomers (2 rubbers and 7 silicones) challenged with three major bovine mastitis pathogens. The results of the antibacterial activities, assessed in triplicate and compared with a control, are reported in the following figures and tables based on the different receipts of the elastomers, as described in
Table 1 and
Table 2.
3.1. Rubber elastomers
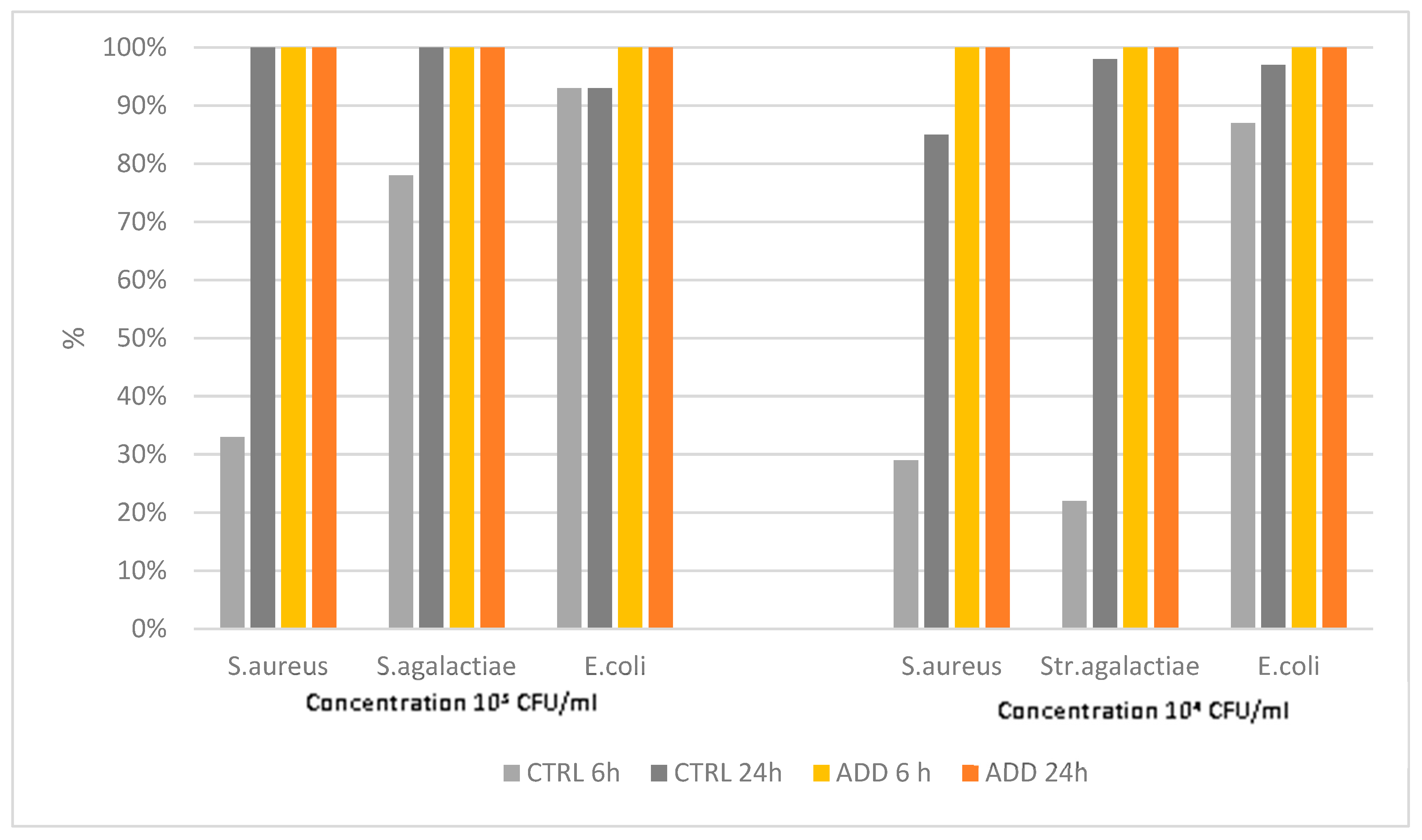
The results of the challenge of rubber elastomer R1 without PAA with the three pathogens considered showed unexpected results. Indeed, antimicrobial activity was observed in the control with a killing rate up to 100% for
S. aureus and
S.agalactiae at 24h post contact (pc), when the initial concentration was 10
3 CFU/ml. A relatively high killing rate was also observed when the 10
4 CFU/ml concentration was used. The addition of antimicrobial components, as expected, raised the killing rate to 100% both at 6h and 24h pc for all three pathogens (
Figure 1).
Table 3 reports the result of Kaplan-Mayer survival curve analysis. The controls confirmed the antibacterial activity against the three pathogens considered, but to a significantly lower level when compared with PAA-additivated rubber, which killed all the bacteria within the 24h pc. To be noticed also that the curve analysis estimated a killing activity of 50% at 6 h/pc for the pathogens considered.
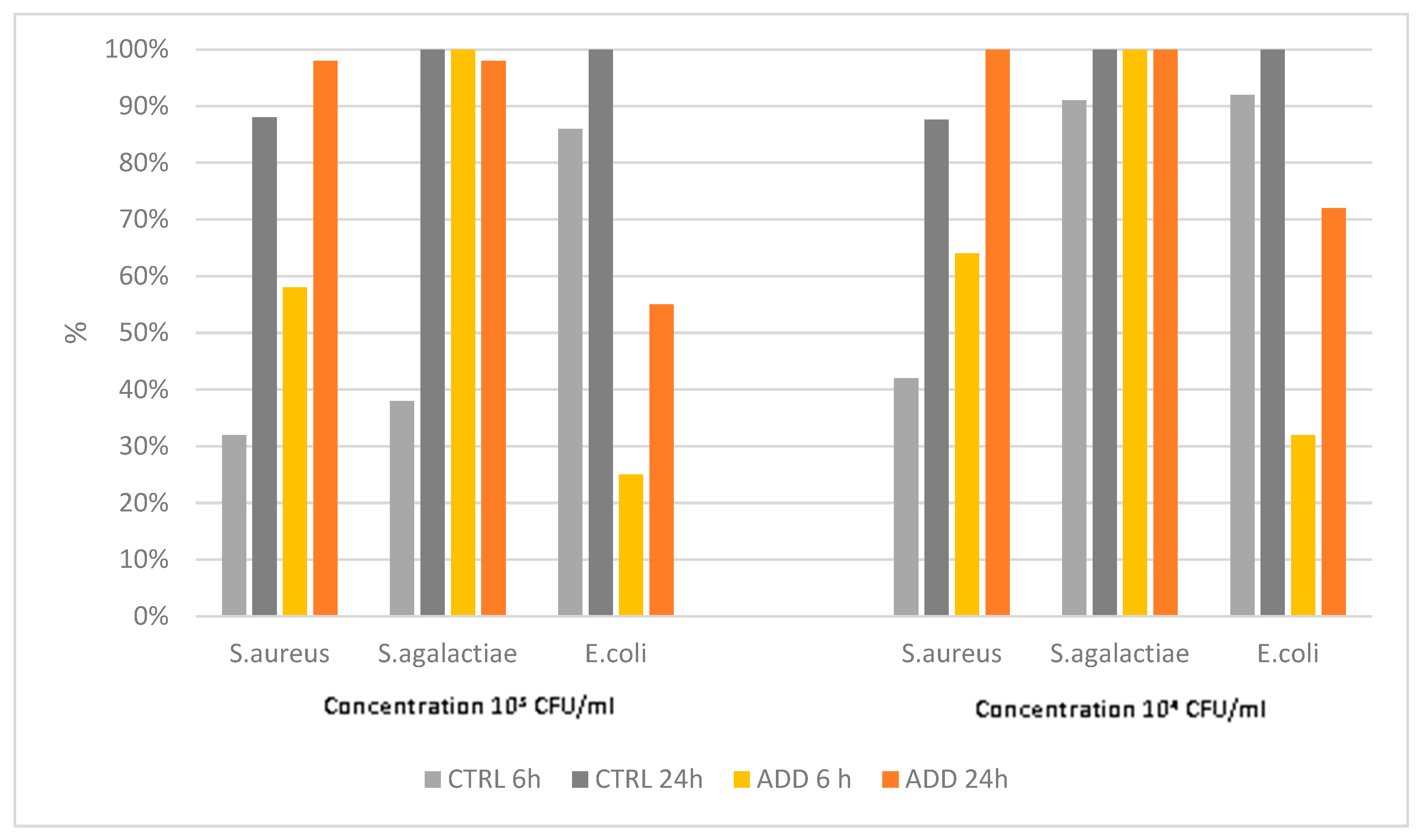
The second rubber elastomer showed a different antimicrobial activity than the R1 elastomer. Indeed, the killing activity of the control was less than 90% at 24 pc for
S. aureus, whereas PAA-added elastomer showed an activity close to or equal to 100% only against
S. agalactiae (
Figure 2), which was significantly higher than of the control (
Table 4). Finally, the activity against
E. coli was significantly lower for PAA-added product vs its control (
Table 4 and
Figure 2).
3.2 Silicon elastomers
The assessment of the antimicrobial activity of silicon elastomers S3 and S4 showed larger differences compared with the rubber ones. Indeed, the control product showed a low antimicrobial activity against the three pathogens considered, and only against
S. aureus an activity > 90% was observed (
Figure 3). PAA-added silicon S3 showed a significantly higher antimicrobial activity vs. all the three pathogens when compared with the control (P<0.0001) at 24h pc (
Table 4). Silicon S4 had a slightly lower killing activity when compared to S3, showing to be effective against
S. agalactiae at 6h pc, but very few colonies may be present at 24h pc when challenged with
E. coli. The differences between additivated and control silicones were statistically different in Kaplan-Meyer analysis also in this case (P<0.0001).
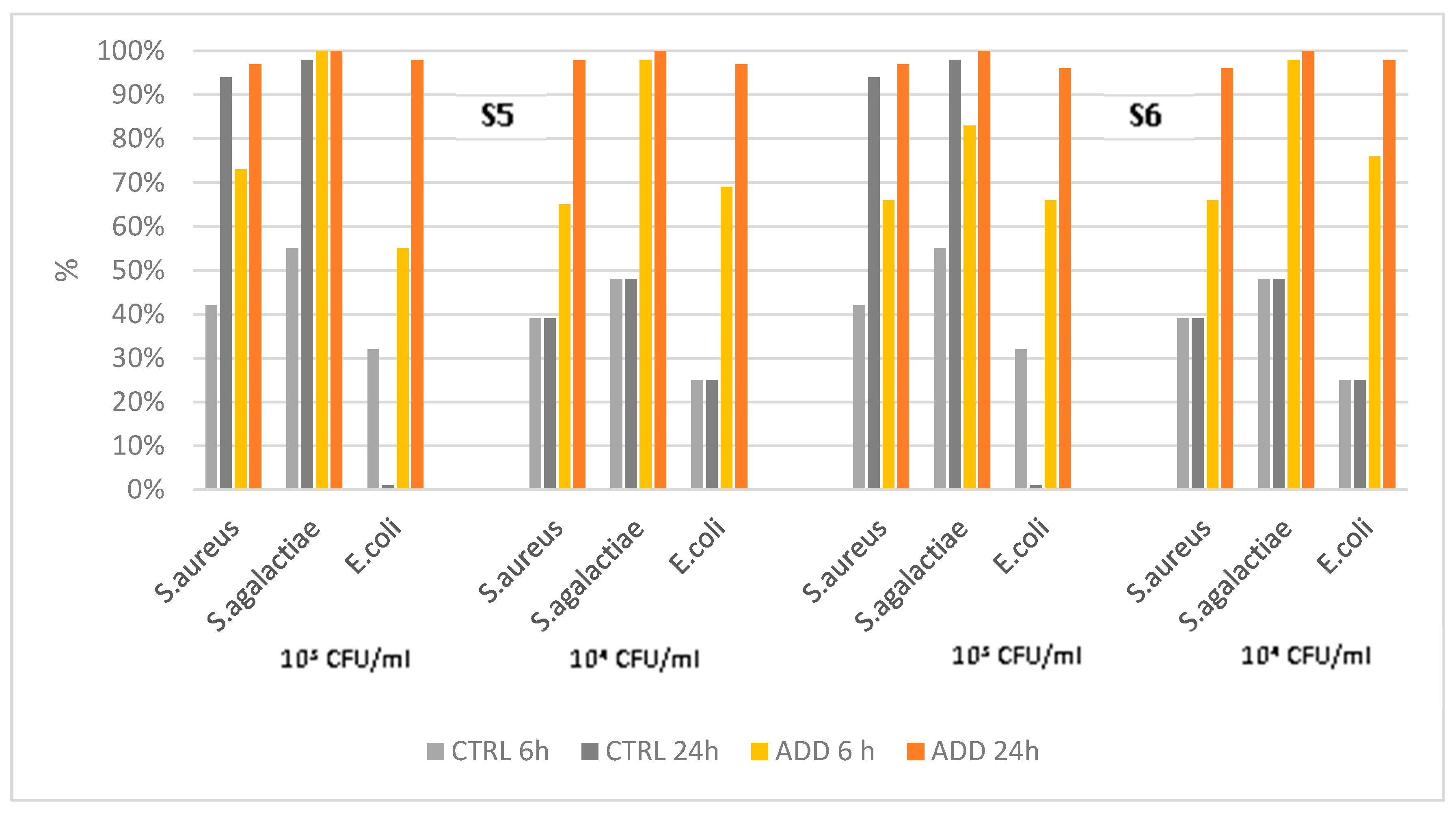
The antimicrobial activity of PAA-added silicones S5 and S6 was slightly lower than that observed for S3-S4, and it achieved a level of 100% only against
S. agalactiae at 24h pc (
Figure 4). Despite the formulation of the two silicones being different, the antimicrobial activity was similar (
Table 5), even though S6 had a quicker action with lower survival rates at 6h pc when compared with S5. All the additivated silicones survival curves significantly differed from the control by Kaplan-Mayer analysis (P<0.0001).
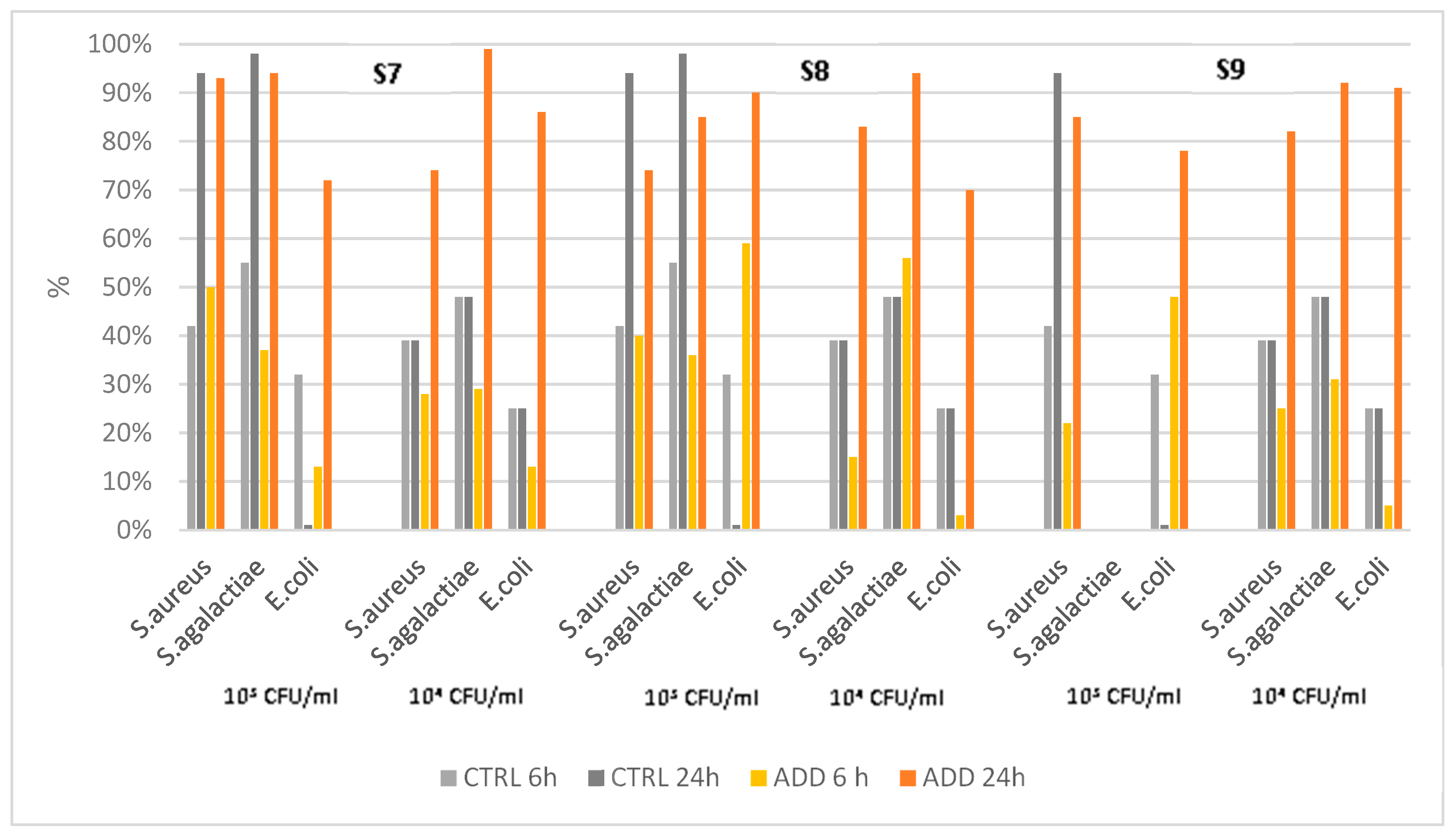
The fourth set of analysis concerns a silicon elastomer additivated with three different concentrations of the same PAA. The assessment results showed different patterns of killing activity (
Figure 5), and in very few cases a 100% of killing activity was observed, despite a significant difference vs control was always observed with Kaplan-Mayer analysis (
Table 6). The PAA-added silicones were more active than the control against
E. coli, independent of the concentration of additives, but there was no correlation between concentration and killing rate. Only S7 showed a high killing rate vs
S. agalactiae, and the control showed better activity than additivated silicones compared with
S. aureus.
4. Discussion
The severe problems related to the spread of AMR worldwide require the development of new protocols and tools to prevent and/or control microorganisms, thus reducing the use of antimicrobials. Among the potential solutions, increasing interest is devoted to developing elastomers with antimicrobial activity [
23,
24,
25].
The potential applications of these materials are wide; among them are the food industry and primary milk production. Indeed, contagious pathogens still have a relatively high prevalence in many herds [
6,
8,
26,
27], and the two major contagious pathogens (
S. aureus and
S. agalactiae) may have a zoonotic potential both directly and as vectors of AMR [
4,
5,
7,
28].
The milking phase is critical for spreading contagious pathogens within the dairy herd. The segregation of cows during milking is the only effective way to significantly decrease this risk [
6,
13], but it is often considered troublesome in many herds and requires additional labor. Other methods, such as cluster disinfection, have yielded controversial results [
14,
15,
16]. The risk of transmission is even higher when automatic milking systems are used because segregation is practically impossible to apply due to the need to keep a proper and constant number of cows per automatic milking unit, and t hygiene procedures are not always effective [
29,
30,
31].
The availability of elastomers with antimicrobial activities may represent a step forward in reducing the risk of infection spread during milking, thus decreasing the need to implement other control measures such as segregation or cluster disinfection. The opportunity to have several elastomers with different compositions both as material (rubber or silicon) and with different antibacterial components (additives), allowed us to compare the killing capacity of these materials with some unexpected results.
Indeed, to the best of our knowledge, for the first time, we observed that basic rubber materials have an intrinsic antimicrobial activity that could be up to 100% in a few cases. In contrast, unadditivated silicon elastomers showed less bactericidal properties. The “intrinsic" antimicrobial activity may explain the high level of activity observed for both rubber elastomers when antimicrobial components were added. The addition of PAA significantly increased the killing rate of both elastomers, and R1 demonstrated greater killing activity than R2, which has the same PAA addition, but a different basal composition. Therefore, the differences in killing activity between these two rubber elastomers should be related to the rubber formulations rather than PAA addition. Bacteria, as expected, also play a role. Indeed, while R1 led to similar survival curves for all three pathogens, R2 showed the highest killing activity for S. agalactiae and the lowest for E. coli.
Silicones have a much lower basal antibacterial activity, and the additives play a major role in modifying it. Indeed, silicones without additives could not kill all the bacteria at 24h pc, as observed for rubber ones. It is noticed that the basal formulation is ineffective vs E. coli, but some activity was observed for the other two pathogens. Different results were obtained by adding PAA to the base silicon receipt. The addition of silver phosphate glass, silver chloride, and zinc pyrithione significantly decreased survival rates (S3). However, when magnesium oxide and zinc oxide were added to the formulation (S4) very little difference was observed. The same formulation as S4, but without zinc oxide (S5), gave similar results as S4, as well as the same formulation with zinc oxide, instead of zinc pyrithione (S6).
Finally, when different concentrations of silver phosphate glass were added together with zinc pyrithione (S7-9), but without the other antimicrobial components, the proportional decrease in survival rates expected, was not observed, and the elastomer with lower concentration showed a better activity than the higher concentrations. Moreover, the addition of only these two components (silver phosphate glass and zinc pyrithione) resulted in a lower reduction in the killing activity compared with the other formulations.
The different results may also be due to the diverse chemical reactions that occur during the manufacturing process, which could affect the antibacterial activity of the single and combined components, as previously observed [
21,
32,
33].
These findings suggest that the development of new antibacterial elastomers should necessarily include a fine-tuned evaluation of the mix of different components, and the effects of the manufacturing process on their antimicrobial capability. Indeed, the antimicrobial activity was shown to be related to a proper combination of components, while it is not only the results of additive effects either of components or their concentration. This preliminary in vitro assessment is pivotal before proceeding to field trials to evaluate their antimicrobial effects on the various bacterial species.
5. Conclusions
From a One Health perspective, controlling infectious disease in dairy herds with zoonotic and AMR potential is critical. Moreover, the need to apply prudent antimicrobial protocols is required to improve the health management of herds. The availability of new tools such as liners produced from elastomers with antimicrobial activity, thus reducing the risks for the spread of infection and generally decreasing the bacteria load at milking, would be a step forward in achieving a higher global sustainability of dairy production.
6. Patents
International application PCT/IB2023/050905 “A composition suitable for the production of a thermosetting elastomer with antimicrobial capabilities by means of vulcanization by molding”.
International patent classification C08K3/04 C08K3/32 C08K5/00 C08L7/00 C08L23/16 C08L27/16 C08L43/04
Author Contributions
Conceptualization, A.Z; methodology, G.M., P.A.M. and A.Z.; validation G.M., V.S. and A.Z.; formal analysis, G.M., G.L.; V.S., F.Z. and P.A.M.; statistical analysis, A.Z.; investigation, A.Z., G.L,F.Z., G.M. and V.S.; data curation, F.Z., G.M., G.L. and V.S.; writing—original draft preparation, A.Z. and P.A.M.; writing—review and editing, A.Z. and P.A.M.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Scudo Technologies PRP, Italy, and by FEASR—Programma di Sviluppo Rurale 2014–2020 Misura 16.1 project MOOH.
Conflicts of Interest
The authors declare no conflicts of interest. The study was partially funded by Scudo Technologies PRP, Italy, but the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18-31. [CrossRef]
- Hogeveen, H.; Steeneveld, W.; Wolf, C.A. Production Diseases Reduce the Efficiency of Dairy Production: A Review of the Results, Methods, and Approaches Regarding the Economics of Mastitis. Annual Review of Resource Economics 2019, 11, 289-312. [CrossRef]
- Luo, T.; Steeneveld, W.; Nielen, M.; Zanini, L.; Zecconi, A. Linear Mixed-Effects Model to Quantify the Association between Somatic Cell Count and Milk Production in Italian Dairy Herds. Animals 2023, 13, 80. [CrossRef]
- Meroni, G.; Sora, V.M.; Martino, P.A.; Sbernini, A.; Laterza, G.; Zaghen, F.; Soggiu, A.; Zecconi, A. Epidemiology of Antimicrobial Resistance Genes in Streptococcus agalactiae Sequences from a Public Database in a One Health Perspective. Antibiotics 2022, 11, 1236. [CrossRef]
- Crestani, C.; Forde, T.L.; Lycett, S.J.; Holmes, M.A.; Fasth, C.; Persson-Waller, K.; Zadoks, R.N. The fall and rise of group B Streptococcus in dairy cattle: reintroduction due to human- to- cattle host jumps? Microb. Genomics 2021, 7, 10. [CrossRef]
- Zecconi, A. Contagious mastitis control. FIL-IDF Bulletin 2007, 416, 34-40.
- Zaghen, F.; Sora, V.M.; Meroni, G.; Laterza, G.; Martino, P.A.; Soggiu, A.; Bonizzi, L.; Zecconi, A. Epidemiology of Antimicrobial Resistance Genes in Staphyloccocus aureus Isolates from a Public Database in a One Health Perspective; Sample Characteristics and Isolates; Sources. Antibiotics 2023, 12, 1225. [CrossRef]
- Sora, V.M.; Panseri, S.; Nobile, M.; Di Cesare, F.; Meroni, G.; Chiesa, L.M.; Zecconi, A. Milk Quality and Safety in a One Health Perspective: Results of a Prevalence Study on Dairy Herds in Lombardy (Italy). Life 2022, 12, 786. [CrossRef]
- Pennone, V.; Prieto, M.; Alvarez-Ordonez, A.; Cobo-Diaz, J.F. Antimicrobial Resistance Genes Analysis of Publicly Available Staphylococcus aureus Genomes. Antibiotics-Basel 2022, 11, 19. [CrossRef]
- Latorre, A.A.; Pacha, P.A.; Gonzalez-Rocha, G.; San Martin, I.; Quezada-Aguiluz, M.; Aguayo-Reyes, A.; Bello-Toledo, H.; Oliva, R.; Estay, A.; Pugin, J.; et al. On-Farm Surfaces in Contact with Milk: The Role of Staphylococcus aureus-Containing Biofilms for Udder Health and Milk Quality. Foodborne Pathogens and Disease 2020, 17, 44-51. [CrossRef]
- Mein, G.A. The Role of the Milking Machine in Mastitis Control. Veterinary Clinics of North America-Food Animal Practice 2012, 28, 307-+. [CrossRef]
- Du, B.Y.; Meng, L.; Liu, H.M.; Zheng, N.; Zhang, Y.D.; Guo, X.D.; Zhao, S.G.; Li, F.D.; Wang, J.Q. Impacts of Milking and Housing Environment on Milk Microbiota. Animals 2020, 10. [CrossRef]
- Zecconi, A.; Piccinini, R.; Fox, K.L. Epidemiologic study of intramammary infections with Staphylococcus aureus during a control program in nine commercial dairy herds. JAVMA 2003, 223, 684-688. [CrossRef]
- Scheib, S.; Leimbach, S.; Avramidis, G.; Bellmann, M.; Nitz, J.; Ochs, C.; Tellen, A.; Wente, N.; Zhang, Y.C.; Viöl, W.; et al. Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens 2023, 12, 14. [CrossRef]
- Tenhagen, B.A.; Heuwieser, W. Cluster disinfection to reduce new intramammary infections in lactating dairy cattle. Tierarztl. Umsch. 2007, 62, 364-+.
- Riekerink, R.; Ohnstad, I.; van Santen, B.; Barkema, H.W. Effect of an automated dipping and backflushing system on somatic cell counts. J. Dairy Sci. 2012, 95, 4931-4938. [CrossRef]
- Deng, Z.J.; Koop, G.; Hogeveen, H.; Fischer, E.A.J.; van den Borne, B.H.P.; van der Tol, R.; Lam, T. Transmission dynamics of Staphylococcus aureus and Streptococcus agalactiae in a Dutch dairy herd using an automatic milking system. Prev. Vet. Med. 2021, 192, 7. [CrossRef]
- Bonestroo, J.; Fall, N.; Hogeveen, H.; Emanuelson, U.; Klaas, I.C.; van der Voort, M. The costs of chronic mastitis: A simulation study of an automatic milking system farm. Prev. Vet. Med. 2023, 210, 13. [CrossRef]
- Skarbye, A.P.; Krogh, M.A.; Ostergaard, S.R. Retrospective cohort study of management procedures associated with dairy herd-level eradication of Streptococcus agalactiae in the Danish surveillance program. J. Dairy Sci. 2021, 104, 5988-5997. [CrossRef]
- Sukthavorn, K.; Nootsuwan, N.; Jongrungruangchok, S.; Veranitisagul, C.; Koonsaeng, N.; Laobuthee, A. Effect of nano-silver coated carbon black on curing, mechanical, antimicrobial, and electrical properties of natural rubber composite. J. Appl. Polym. Sci. 2022, 139, 9. [CrossRef]
- Tomacheski, D.; Pittol, M.; Ribeiro, V.F.; Santana, R.M.C. Efficiency of silver-based antibacterial additives and its influence in thermoplastic elastomers. J. Appl. Polym. Sci. 2016, 133, 10. [CrossRef]
- Tomacheski, D.; Pittol, M.; Ribeiro, V.F.; Santana, R.M.C. Efficiency of silver-based antibacterial additives and its influence in thermoplastic elastomers. J. Appl. Polym. Sci. 2016, 133. [CrossRef]
- Peddinti, B.S.T.; Scholle, F.; Ghiladi, R.A.; Spontak, R.J. Photodynamic Polymers as Comprehensive Anti-Infective Materials: Staying Ahead of a Growing Global Threat. Acs Applied Materials & Interfaces 2018, 10, 25955-25959. [CrossRef]
- Kaczor, P.; Bazan, P.; Kuciel, S. Bioactive Polyoxymethylene Composites: Mechanical and Antibacterial Characterization. Materials 2023, 16, 16. [CrossRef]
- Przybylek, M.; Bakar, M.; Mendrycka, M.; Kosikowska, U.; Malm, A.; Worzakowska, M.; Szymborski, T.; Kedra-Krlik, K. Rubber elastomeric nanocomposites with antimicrobial properties. Materials Science and Engineering C-Materials for Biological Applications 2017, 76, 269-277. [CrossRef]
- Zecconi, A.; Dell’Orco, F.; Rizzi, N.; Vairani, D.; Cipolla, M.; Pozzi, P.; Zanini, L. Cross-sectional study on the prevalence of contagious pathogens in bulk tank milk and their effects on somatic cell counts and milk yield. Ital J Anim Sci 2019, 19, 66-74. [CrossRef]
- Holmoy, I.H.; Toftaker, I.; Kirkeby, C.; Osteras, O.; Jorgensen, H.J.; Nodtvedt, A. A cohort study of the effect of Streptococcus agalactiae on milk yield and somatic cell count in Norwegian dairy cows. J. Dairy Sci. 2019, 102, 8385-8399. [CrossRef]
- Cuny, C.; Friedrich, A.; Kozytska, S.; Layer, F.; Nübel, U.; Ohlsen, K.; Strommenger, B.; Walther, B.; Wieler, L.; Witte, W. Emergence of methicillin-resistant Staphylococcus aureus (MRSA) in different animal species. International Journal of Medical Microbiology 2010, 300, 109-117. [CrossRef]
- Svennesen, L.; Nielsen, S.S.; Mahmmod, Y.S.; Kromker, V.; Pedersen, K.; Klaas, I.C. Association between teat skin colonization and intramammary infection with Staphylococcus aureus and Streptococcus agalactiae in herds with automatic milking systems. J. Dairy Sci. 2019, 102, 629-639. [CrossRef]
- Skarbye, A.P.; Krogh, M.A.; Denwood, M.; Bjerring, M.; Ostergaard, S. Effect of enhanced hygiene on transmission of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in dairy herds with automatic milking systems. J. Dairy Sci. 2021, 104, 7195-7209. [CrossRef]
- Zecconi, A.; Piccinini, R.; Casirani, G.; Binda, E.; Migliorati, L. Effects of automatic milking system on teat tissues, intramammary infections and somatic cell counts. Ital J Anim Sci 2003, 2, 275-282. [CrossRef]
- Bazan, P.; Mazur, K.E.; Rybicka, K.; Kuciel, S. The influence of organic and inorganic antibacterial additives on the strength and biocidal properties of thermoplastic elastomers (TPO). Industrial Crops and Products 2023, 198, 15. [CrossRef]
- Tomacheski, D.; Pittol, M.; Simoes, D.N.; Ribeiro, V.F.; Santana, R.M.C. Influence of natural ageing on mechanical, thermal and antimicrobial properties of thermoplastic elastomers containing silver nanoparticles and titanium dioxide. Polymer Bulletin 2018, 75, 3917-3934. [CrossRef]
Figure 1.
Killing activity of elastomer R1 and its control against S. aureus, S. agalactiae and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 1.
Killing activity of elastomer R1 and its control against S. aureus, S. agalactiae and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 2.
Killing activity of elastomer R2 and its control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 2.
Killing activity of elastomer R2 and its control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 3.
Killing activity of elastomers S3-4 and tits control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points post contact (6h and 24h).
Figure 3.
Killing activity of elastomers S3-4 and tits control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points post contact (6h and 24h).
Figure 4.
Killing activity of elastomers S5-S6 and its control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 4.
Killing activity of elastomers S5-S6 and its control against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL), and at two different time points after contact (6h and 24h).
Figure 5.
Killing activity of elastomers S7-9 and their controls against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL) at two different time points after contact (6h and 24h).
Figure 5.
Killing activity of elastomers S7-9 and their controls against S. aureus, S. agalactiae, and E. coli at two different initial concentrations (103 CFU/mL and 104 CFU/mL) at two different time points after contact (6h and 24h).
Table 1.
Description of receipts for the 2 different rubber elastomers considered. The receipt without additives represented the control.
Table 1.
Description of receipts for the 2 different rubber elastomers considered. The receipt without additives represented the control.
| Main components |
Patented antimicrobial additive (1%) |
Acronym
(in table and figures) |
NBR Acrylonitrile Copolymer ( CAS 9003-18-3) 50-60 %
Carbon black (CAS: 1333-86-4) 3-7%
Plasticizer ( CAS 103-23-1) 6-10%
Calcined kaolin (CAS 92704-41-1) 20-24%
Precipitated silica (112926-00-8) 4-6%
Stearic acid (CAS: 57-11-4) 0.1-1%
TMTM (CAS 97-74-5) 0,1-1%
ZDBC ( CAS 136-23-2) 0,1-1%
Sulfur (CAS 7704-14-9) 0.1-2% |
Zinc oxide (CAS 1314-13-2)
Magnesium oxide (CAS 1309-48-4)
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS- 308069-39-8)
Zinc pyrithione (CAS 13463-41-7) |
R1 |
Butilic rubber (CAS 9010-85-5) 54-57%
Carbon black (CAS: 1333-86-4) 26-29%
Calcium carbonate (CAS 1317-65-3) 9.5-11%
Stearic acid (CAS: 57-11-4) 0.1-1%
ZOEC (CAS 14324-55-1) 0.25-1%
TMDM (CAS 137-26-8) 0.4-1.8%
Sulfur (CAS 7704-14-9) 0.1-2%
|
Zinc oxide (CAS 1314-13-2)
Magnesium oxide (CAS 1309-48-4)
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS 308069-39-8)
Zinc pyrithione (CAS 13463-41-7) |
R2 |
Table 2.
Description of the receipts for the 7 different silicones considered. The receipt without additives represented control.
Table 2.
Description of the receipts for the 7 different silicones considered. The receipt without additives represented control.
| Main components |
Patented antimicrobial additive |
Acronym
(in table and figures) |
Silicon rubber (CAS: 63394-02-5) 95-99%
50 % Dicumyl peroxide (CAS 80-43-3)– Organic peroxides, H242
50%2.5-Dimethyl-2,5-di(tert-butylperox) hexane. (CAS 78-63-7)– Organic peroxides, H24 |
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS 308069-39-8)
Zinc pyrithione (CAS 13463-41-7) |
S3 |
Zinc oxide (CAS 1314-13-2)
Magnesium oxide (CAS 1309-48-4)
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS 308069-39-8)
Zinc pyrithione (CAS 13463-41-7 R2) |
S4 |
Magnesium oxide (CAS 1309-48-4)
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS 308069-39-8)
Zinc pyrithione (CAS 13463-41-7) |
S5 |
Zinc oxide (CAS 1314-13-2)
Magnesium oxide (CAS 1309-48-4)
Silver chloride (CAS 7783-90-6)
Silver phosphate glass (CAS 308069-39-8) |
S6 |
2% Silver phosphate glass (CAS 308069-39-8)
Zinc pyrithione (CAS 13463-41-7) |
S7 |
| Same as above but at 3% concentration |
S8 |
| Same as above but at 4% concentration |
S9 |
Table 3.
Comparison of median survival rates calculated using Kaplan-Mayer method for the additivated R1 elastomer vs. control against the three major mastitis pathogens.
Table 3.
Comparison of median survival rates calculated using Kaplan-Mayer method for the additivated R1 elastomer vs. control against the three major mastitis pathogens.
| Pathogen |
Median survival rate (%) |
Control vs PAA Added
P= |
| Control |
R1 |
| S. aureus |
T1a
|
T6 |
T24 |
T1 |
T6 |
T24 |
|
| 103 CFU/ml |
82 |
81 |
0 |
70 |
50 |
0 |
<0.0001 |
|
104 CFU/ml |
95 |
75 |
14 |
78 |
50 |
0 |
<0.0001 |
| S.agalactiae |
|
|
|
|
|
|
|
| 103 CFU/ml |
96 |
61 |
0 |
70 |
50 |
0 |
<0.0001 |
| 104 CFU/ml |
97 |
86 |
2 |
67 |
50 |
0 |
<0.0001 |
| E. coli |
|
|
|
|
|
|
|
| 103 CFU/ml |
79 |
53 |
7 |
67 |
50 |
0 |
<0.0001 |
| 104 CFU/ml |
80 |
56 |
3 |
67 |
50 |
0 |
<0.0001 |
Table 4.
Comparison of median survival rates calculated using Kaplan-Mayer method for the additivated R2 elastomer vs control against the three major mastitis pathogens.
Table 4.
Comparison of median survival rates calculated using Kaplan-Mayer method for the additivated R2 elastomer vs control against the three major mastitis pathogens.
| Pathogen |
Median survival rate (%) |
Control vs PAA added
P= |
| Control |
R2 |
| S. aureus |
T1a
|
T6 |
T24 |
T1 |
T6 |
T24 |
|
| 103 CFU/ml |
100 |
84 |
12 |
95 |
71 |
2 |
<0.0001 |
| 104 CFU/ml |
92 |
78 |
12 |
86 |
65 |
0 |
<0.0001 |
| S. agalactiae |
|
|
|
|
|
|
|
| 103 CFU/ml |
79 |
63 |
0 |
75 |
0 |
0 |
<0.0001 |
| 104 CFU/ml |
85 |
55 |
0 |
73 |
0 |
0 |
<0.0001 |
| E. coli |
|
|
|
|
|
|
|
| 103 CFU/ml |
88 |
57 |
0 |
90 |
85 |
44 |
<0.0001 |
| 104 CFU/ml |
88 |
54 |
0 |
94 |
82 |
28 |
<0.0001 |
Table 4.
Comparison of median survival rates calculated by the Kaplan-Mayer method for the additivated S3-S4 elastomers and control against the three major mastitis pathogens (differences between PAA-added and control elastomers were all significant at P=0.001).
Table 4.
Comparison of median survival rates calculated by the Kaplan-Mayer method for the additivated S3-S4 elastomers and control against the three major mastitis pathogens (differences between PAA-added and control elastomers were all significant at P=0.001).
| Pathogen |
Median survival rate (%) |
| Control |
S3 |
S4 |
| S. aureus |
T1a
|
T6 |
T24 |
T1 |
T6 |
T24 |
T1 |
T6 |
T24 |
| 103 CFU/ml |
98 |
79 |
6 |
87 |
62 |
3 |
95 |
68 |
3 |
| 104 CFU/ml |
86 |
77 |
8 |
80 |
40 |
0 |
78 |
51 |
0 |
| S.agalactiae |
|
|
|
|
|
|
|
|
|
| 103 CFU/ml |
79 |
71 |
2 |
90 |
45 |
0 |
85 |
0 |
0 |
| 104 CFU/ml |
81 |
79 |
50 |
93 |
50 |
0 |
100 |
0 |
0 |
| E.coli |
|
|
|
|
|
|
|
|
|
| 103 CFU/ml |
88 |
84 |
84 |
98 |
81 |
0 |
91 |
76 |
3 |
| 104 CFU/ml |
92 |
93 |
99 |
87 |
64 |
0 |
89 |
61 |
1 |
Table 5.
Comparison of median survival rates calculated by the Kaplan-Mayer method for the additivated S5 and S6 elastomer and control against the three major mastitis pathogens. (differences between PAA additivated and control elastomers were all significant at P=0.001).
Table 5.
Comparison of median survival rates calculated by the Kaplan-Mayer method for the additivated S5 and S6 elastomer and control against the three major mastitis pathogens. (differences between PAA additivated and control elastomers were all significant at P=0.001).
| Pathogen |
Median survival rate (%) |
| Control |
S5 |
S6 |
| S. aureus |
T1a
|
T6 |
T24 |
T1 |
T6 |
T24 |
T1 |
T6 |
T24 |
| 103 CFU/ml |
98 |
79 |
6 |
90 |
57 |
1 |
96 |
67 |
2 |
| 104 CFU/ml |
86 |
77 |
8 |
93 |
68 |
1 |
95 |
67 |
4 |
| S. agalactiae |
|
|
|
|
|
|
|
|
|
| 103 CFU/ml |
79 |
71 |
2 |
100 |
0 |
0 |
100 |
51 |
0 |
| 104 CFU/ml |
81 |
79 |
50 |
100 |
51 |
0 |
100 |
58 |
0 |
| E. coli |
|
|
|
|
|
|
|
|
|
| 103 CFU/ml |
88 |
84 |
84 |
85 |
74 |
2 |
86 |
68 |
4 |
| 104 CFU/ml |
92 |
93 |
99 |
76 |
50 |
1 |
80 |
61 |
1 |
Table 6.
Comparison of median survival rates calculated by Kaplan-Mayer method for the additivated R1 elastomer and control against the three major mastitis pathogens. (differences between PAA additivated and control elastomers were all significant at P=0.001).
Table 6.
Comparison of median survival rates calculated by Kaplan-Mayer method for the additivated R1 elastomer and control against the three major mastitis pathogens. (differences between PAA additivated and control elastomers were all significant at P=0.001).
| Pathogen |
Median survival rate (%) |
| Control |
S7 |
S8 |
S9 |
| S. aureus |
T1 |
T6 |
T24 |
T1 |
T6 |
T24 |
T1 |
T6 |
| 103 CFU/ml |
98a
|
79 |
6 |
99 |
75 |
7 |
95 |
76 |
26 |
98 |
77 |
5 |
| 104 CFU/ml |
86 |
77 |
8 |
95 |
86 |
26 |
96 |
62 |
17 |
93 |
87 |
18 |
| S. agalactiae |
|
|
|
|
| 103 CFU/ml |
79 |
71 |
2 |
91 |
81 |
6 |
98 |
81 |
14 |
94 |
86 |
22 |
| 104 CFU/ml |
81 |
79 |
50 |
99 |
84 |
0.4 |
99 |
72 |
6 |
97 |
84 |
8 |
| E. coli |
|
|
|
|
| 103 CFU/ml |
88 |
84 |
84 |
97 |
93 |
27 |
99 |
71 |
7 |
94 |
78 |
22 |
| 104 CFU/ml |
92 |
93 |
99 |
96 |
93 |
15 |
100 |
98 |
31 |
99 |
95 |
8 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).