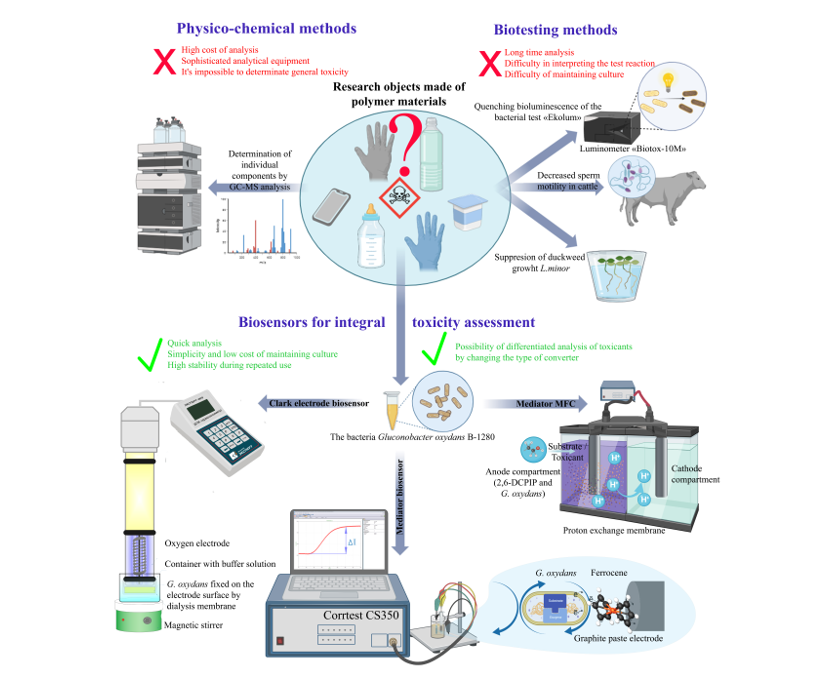
1. Introduction
Increasing production rates and the emergence of new materials are leading to an increase in demand for early testing of products for the presence of substances that cause a cascade of toxic effects leading to human health problems and a decrease in biodiversity [
1,
2,
3,
4,
5]. Among the main toxicants in polymer materials are organic compounds used as plasticizers, fillers and dyes [
6], and heavy metals that can accumulate in the human body through bioaccumulation [
7].
Various physicochemical methods are used to detect individual toxicants [
8,
9], However, it is advisable to determine the integral toxicity index in the test sample based on the reactions of living organisms to assess the synergistic effects of toxicants and identify the toxic effects of unknown compounds that are decomposition products. Often, poorly reproducible results and the duration of standard analysis methods that use biological test systems (ciliates, daphnia, fish, algae, duckweed) do not allow for prompt and reliable determination of the toxicity of the studied samples.
In the last decade, biosensor systems that include a biological recognition element and a physicochemical transducer, have been actively developed to detect pollutants, due to their sensitivity, selectivity, portability, and the possibility of miniaturization [
10,
11,
12]. The similarity of the physiological reactions and cellular organization of microorganisms with higher organisms that are exposed to chemical contamination allows us to obtain more accurate and reliable results. There are a number of commercial biosensors for assessing toxicity based on bioluminescent bacteria (Microtox (AzurEnvironmental, USA), LUMIStox (Beckman Instruments, UK), Tox-Alert (Merck, USA), ToxScreen (ChekLight Ltd., China), Biotox-10M (Nera-S , Russia)) [
13]. Since these devices are based on quenching the bioluminescence of bacteria (
Aliivibrio fischeri (formerly
Photobacterium phosphoreum),
Photobacterium leiognathi,
Vibrio qinhaiensis and genetically engineered
Escherichia coli), their use is limited for the analysis of solutions of increased turbidity and color, with non-optimal pH and temperature [
13,
14]. In addition, the genetically modified microorganisms used in them are quite expensive.
Therefore, microbial electrochemical biosensors of various design (including biofuel cells (BFC), biosensors based on the analysis of oxygen consumption or the use of artificial electron acceptors) are attracting more attention. The selection of electrode material and bioreceptor system can lead to the development of highly sensitive and selective detection of individual toxicants being analyzed, or the overall toxic reaction of the test object can be assessed by reducing oxidative activity [
15,
16,
17]. Review and experimental articles in recent years present whole-cell biosensor systems of various types of detection for the determination of highly toxic heavy metals (lead, mercury, cadmium), phenolic and organophosphorus compounds [
18,
19,
20,
21,
22,
23].
The design features of the biosensor are of decisive importance when developing biosensors for assessing toxicity. The amperometric biosystem is the easiest to operate and cheapest for determining toxicity. This system is based on the Clark oxygen electrode with microbial cells immobilized on the surface [
24,
25]. Due to these advantages, such a system can be easily standardized, validated and implemented in analytical laboratories for daily routine analysis. The use of mediators in second-generation biosensors makes it possible to significantly increase the current value by facilitating electron transfer from the biomaterial to the electrode and thereby improve the sensitivity of electrochemical biosensors in detecting toxic compounds such as phenols [
4,
26] and heavy metal ions (Pb
2+, Cd
2+,Cu
2+,Zn
2+) [
4,
26,
27,
28,
29]. A potential alternative for detecting various toxicants are microbial biofuel cells (MFCs), in which the output signal (voltage, current) depends on changes in environmental conditions for microorganisms in the anode chamber. Such systems can be effectively used for the detection of heavy metals [
30,
31], organic substances [
32,
33,
34] and antibiotics [
35,
36]. However, integral toxicity analysis using MFC still remains problematic due to the problem of low sensitivity compared to mediator microbial biosensors, which is associated with high internal electrical resistance [
22]. Therefore, depending on the electrode material, membrane type, operating mode and configuration of biosensor cells, the efficiency of determining integral toxicity will vary significantly.
Current scientific literature does not describe a study of the effectiveness of determining integral toxicity using electrochemical biosensors with different types of signal recording based on one microbial strain as a biorecognition element (
Figure 1). The bacteria
Gluconobacter oxydans BKM-1280 was used as a test object in the presented work for the development of microbial biosensors. This microorganism is characterized by structural features of the bacterial cell and its metabolism, determined by the periplasmic orientation of the active centers of membrane-bound enzymes [
37,
38], which ensures accessibility to substrates.
Therefore, the purpose of this work is to compare the sensitivity and stability of biosensors based on the bacteria G. oxydans in the composition of an oxygen electrode, a MFC and a mediator-type electrode, intended to determine the integral toxicity of polymer materials.
2. Materials and Methods
2.1. Reagents and Materials
To prepare the solutions, chemicals of analytical purity and deionized water prepared using the Aqualab AL-1 Double system (Aqualab LLC, Moscow, Russia) were used. Yeast extract, agar-agar, trichloroacetic and salicylic acids, inorganic salts (Dia-M, Moscow, Russia), D-sorbitol, phenol, 2,4-dinitrophenol (HIMMED, Moscow, Russia), D-glucose, acetonitrile (Scharlab, Spain), ferrocene, sodium 2,6-dichlorophenolindophenolate (2,6-DCPIP), cellulose dialysis membrane D9777 (pore size 12 kDa) (Sigma-Aldrich Chemicals, Germany), graphite powder and mineral oil (Fluka, Germany). To form MFCs, graphite rods (NIIEI, Russia) and a proton-selective membrane MF-4SK (Plastopolimer, St-Petersburg, Russia) were used.
2.2. Cultivation of Microorganisms
Gluconobacter oxydans strain VKM B-1280 was provided by the All-Russian Collection of Microorganisms of the Institute of Biochemistry and Physiology of Microorganisms of the Russian Academy of Sciences (Pushchino, Russia). G. oxydans bacteria were cultivated on agar containing D-sorbitol (200 g/dm3), yeast extract (20 g/dm3), agar-agar (20 g/dm3), and subcultured monthly. An inoculum of G. oxydans cells was obtained by aerobic cultivation at 28 °C for 24 hours in test tubes filled with 15 ml of medium consisting of D-sorbitol (200 g/dm3) and yeast extract (20 g/dm3) on a BIOSAN ES-20/60 incubator shaker (BioSan, Latvia). The cell biomass was grown in 150 ml of liquid medium in shaking flasks with the resulting inoculum until reaching the late exponential phase (18 h), when the cells contained the most active PQQ-dependent dehydrogenases with the highest yield. After cultivation, the cells were collected by centrifugation for 10 min at 8000 rpm (centrifuge MPW MEDINSTRUMENTS 04-347, Poland) and washed twice with 30 mM Na-phosphate buffer with pH 6.0. The settled cells were resuspended in a new portion of buffer, distributed into Eppendorf microtubes and centrifuged for 10 min at 12,000 rpm (centrifuge MPW MEDINSTRUMENTS 04-347, Poland). The resulting cell sediment was air-dried for an hour and frozen for long-term storage at -18 °C.
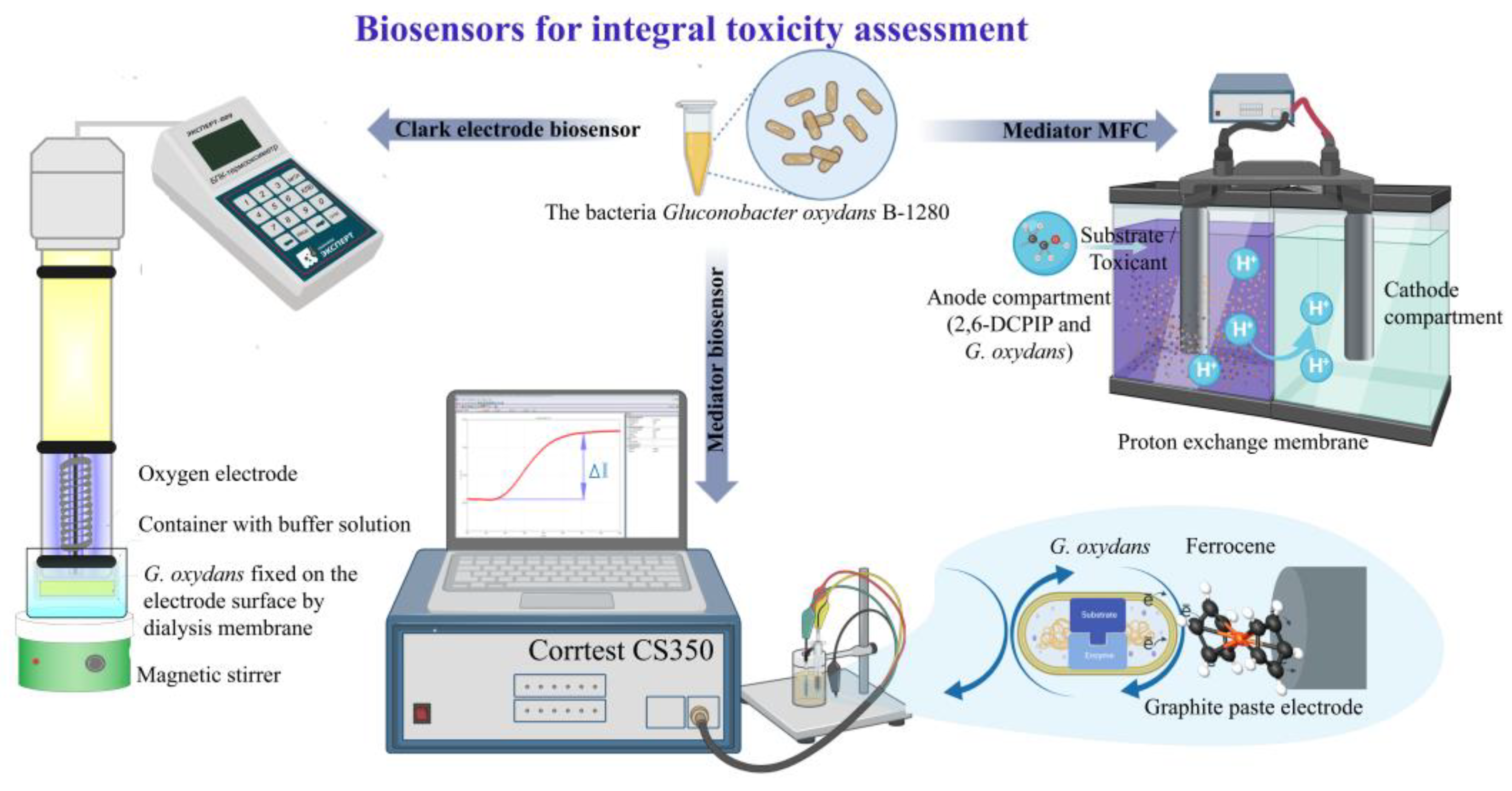
2.3. Formation of Working Electrodes
To form a biorecognition element of a biosensor based on an oxygen electrode, 10 μl of a suspension of bacteria diluted in a 1:1 ratio with 30 mM Na-phosphate buffer solution with pH 6.0 was applied to a fragment of a dialysis membrane measuring 1 × 1 cm (the titer of G. oxydans bacteria is 2.3 × 108 CFU/ml). The biorecognition element was fixed to the electrode using a polymer ring.
To form a mediator biosensor, 10 μl of a bacterial suspension (G. oxydans bacterial titer – 4.5·108 CFU/ml), obtained as described above, was applied to previously prepared working electrodes. To prepare the working electrode, 90 mg of graphite powder, 10 mg of ferrocene, 40 μl of mineral oil and 500 μl of acetone were stirred until the acetone evaporated and the resulting mixture was filled into a plastic tube. The formed working electrodes were left to air dry for 15 minutes, after which the surface of the electrode was covered with a dialysis membrane, which was secured with a plastic ring, to fix the biomaterial and prevent it from being washed out.
In experiments using MFC as a biocatalyst, bacterial cells were used in the anode space (G. oxydans bacteria titer – 8.6 × 108 CFU/g wet biomass), which were stored at room temperature before measurements in the form of a suspension at a concentration of 300 mg/cm3 in 30 mM Na-phosphate buffer pH 6.0.
2.4. Electrochemical Measurements
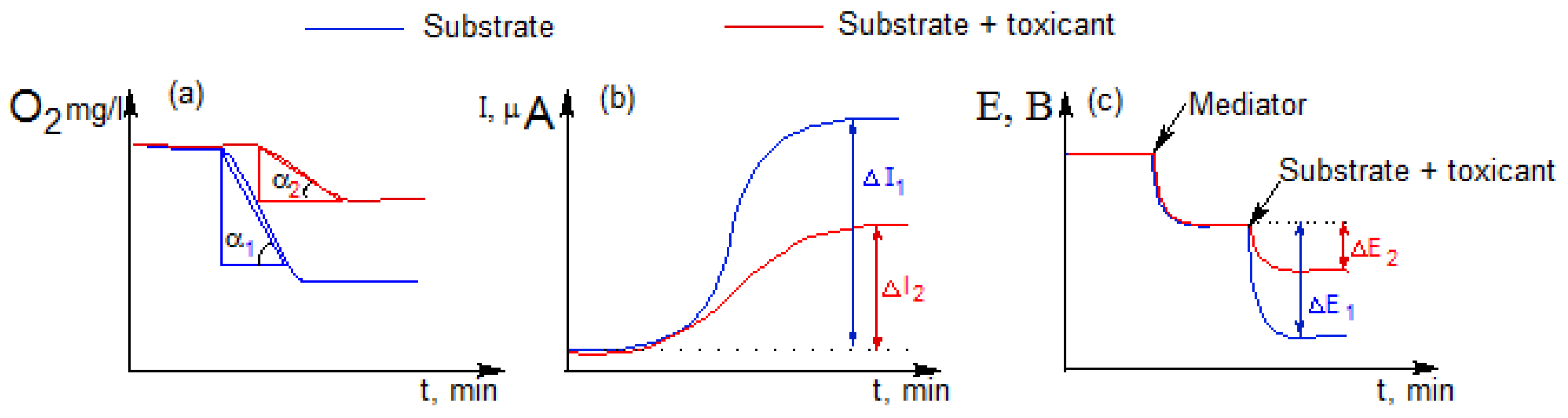
Biosensor measurements on a Clark-type oxygen electrode (DKTP-02) with immobilized bacteria were carried out using the EXPERT-001 analyzer (Econix-Expert, Moscow, Russia) interfaced with a personal computer running specialized software EXP2PR (Econix-Expert, Moscow, Russia). A substrate (glucose solution 1 mol/dm
3) was added to a measuring cell with a volume of 5 cm
3 with constant stirring with a magnetic stirrer (250 rpm) to 4 cm
3 of Na-phosphate buffer pH 6.0. A decrease in oxygen concentration in the near-electrode space was recorded with a measuring sensor as a result of an enzymatic reaction. The biosensor signal was the maximum rate of change in oxygen concentration upon addition of substrates (mgO
2/dm
3 s
-1) (
Figure 2a).
An electrochemical station “CORRTEST” (Corrtest Instruments, China) was used to carry out measurements on the mediator biosensor and MFC. In the case of a mediator biosensor, the electrochemical signal was recorded using a two-electrode system consisting of an Ag/AgCl electrode (reference electrode) and a working graphite-paste electrode. The measurements were carried out at a constant potential of 0.25 V relative to the silver chloride electrode. After establishing a stationary current value, an aliquot of the substrate (glucose solution 1 mol/dm
3) was added to the cuvette (volume 5 cm
3) and waited for the next stationary state. The amplitude of the change in current strength before and after introducing the substrate into the measuring cell (∆I, μA) was taken as the response of the biosensor (
Figure 2b).
The biofuel cell consisted of anode and cathode chambers of the same volume (5 cm
3), separated by a proton-selective membrane, spectral graphite rods with a diameter of 8 mm were used as electrodes (immersion depth 10 mm). The electrodes were washed until the potential value was 0 mV. A suspension of bacteria (3 mg/ml) and 2,6-DCPIP (concentration 150 μM) was added with constant stirring with a magnetic stirrer (400 rpm) into the anode compartment to a working solution with a volume of 3 cm
3 (30 mMNa-phosphate buffer solution with pH 6.0). After the stationary potential value was established, a glucose solution (concentration in the anode chamber 10 mM) was introduced into the anode chamber. In this way, the response to the addition of the substrate was recorded as the amplitude of the generated potential difference (∆E, mV) (
Figure 2c).
2.5. Sampling and Sample Preparation for Biotesting Methods
6 samples of consumer goods made of polymer materials were studied. 3 samples suggested contact with food products (water bottle, food container, baby bottle with pacifier) and 3 samples suggested contact with human skin (phone case, dousing gloves, medical gloves). Sample preparation for biotesting methods involved the preparation of aqueous extracts of the materials under study. All samples were cut into pieces measuring 2 mm × 2 mm with a thickness of no more than 5 mm. A crushed sample of products (1±0.01 g) was placed in a vessel with a ground-in stopper, filled with a 50-fold volume (50 cm3) of distilled water (pH 6.8–7.4), mixed thoroughly, ensuring complete wetting of the sample with water, and thermostated at 40 °C for 24 hours (dry air thermostat TV-80-1, Kasimovsky Instrument Plant, Kasimov, Russia).
2.6. Extraction of Samples for Chromatography
The crushed sample material was placed in glass containers (grinding was carried out as described above). 20 cm
3 of acetonitrile was added to the samples and sonicated in an ultrasonic bath (Guangzhou Hanker Electronic Technology Co., Ltd., China) for 1 hour at room temperature according to the method [
39] to extract toxic substances. Acetonitrile was transferred into clean glass vials and immediately used for analysis
2.7. Chemical Analysis of Samples
Extracts in acetonitrile were analyzed using a Kristall-4000M gas chromatograph with a Maestro-AMS mass detector (MSD) (injection volume 1 μl) on a ZB-5ms column measuring 30 m × 0.25 mm (stationary phase - 5% phenyl-arylene 95% methylpolysiloxane, phase thickness 0.25 µm). Chromatography conditions: carrier gas – helium (total flow 80.6 cm3/min), initial thermostat temperature – 60 °C, final thermostat temperature – 300 °C, temperature increase rate – 20 °C/min, evaporator temperature – 300 °C C, MSD time range – from 2.5 to 15 min, solvent pass – 2.5 min. Identification of compounds was carried out by comparison of mass spectra with the NIST 14 mainlib/replib library (score ≥ 60%).
2.8. Determination of Toxicity Using the Ekolum Test System
Rehydration of the lyophilized bacterial test system Ecolum, control of the error of the toxicological analysis technique and the biotesting procedure using the Biotox-10M device were carried out according to the method attached to the device. For each sample, three control–experiment pairs were measured sequentially. The toxicity of the sample was assessed by the relative difference in the intensity of bioluminescence of the control and experimental samples and the calculation of the toxicity index (T) using the formula:
where I
к is the luminescence intensity of the control sample of bacteria, and I
o is the luminescence intensity of bacteria after adding an aqueous extract of the test sample.
2.9. Standard Biotest Method Based on the Test Object L. minor
The toxicity of aqueous extracts of the studied polymeric materials was assessed by the inhibition of the yield of duckweed (
L. minor), expressed as a percentage (I
y) (ISO 20079:2005). Containers with control and experimental samples were kept for 7 days under a fluorescent lamp. The average percent yield inhibition was calculated as follows:
where I
y is the percentage of yield inhibition; b
C is the final biomass in the control; b
T is the final biomass in the experimental sample.
If the inhibition level was 20% or more, the sample was considered toxic.
2.10. Bioassay Using Bovine Sperm
The safety assessment of products made from polymer and textile materials was carried out using cattle sperm as a test object (Russian national standard GOST 53485-2009). The mobility index (It) was measured using an AT-05 image analyzer (BMK-INVEST, Kaluga, Russia). A sample was considered toxic if the obtained index value was not within the interval 70%< It<120%.
3. Results and Discussion
3.1. Development of Electrochemical Biosensors with Different Types of Signal Recording for Assessing Integral Toxicity
The bacteria
G. oxydans was chosen as an effective biocatalyst. Previously,
G. oxydans was successfully used for environmental monitoring in bioelectrochemical systems of all types (based on oxygen electrode [
24], mediator type [
40,
41] and MFC [
42,
43,
44,
45]). Earlier, during the development of a biosensor based on an oxygen electrode to assess integral toxicity, the sensitivity of bacteria to both the toxic effects of organic pollutants and heavy metals was established [
24].
The use of different bioelectrochemical systems (using an oxygen electrode, a mediator graphite-paste electrode and MFC) will identify the most sensitive test system for assessing toxicity, since in these three systems the reaction at the electrode is caused by different biochemical pathways of electron transfer from the bacteria
G. oxydans. In the presence of a toxicant in a microbial cell, some functions of cellular metabolism are disrupted, the rate of oxygen consumption during substrate oxidation decreases, which serves as an indicator of inhibition in a system based on an oxygen electrode. In mediator systems, the final acceptor of electrons in the microbial respiratory chain are redox-active substances (mediators) that remove electrons at different stages of the electron transfer chain (ETC), which leads to a change in the test reaction of bacteria to the same toxicant. Ferrocene [
4] and 2,6-DCPIP [
46] were used respectively to obtain a biosensor signal (
Figure 2) independent of oxygen partial pressure and improve the efficiency of electron transport from the bacterial cell to the electrode surface in the mediator biosensor and MFC.
The difference in the generated current/potential in the systems "immobilized bacteria - oxygen electrode", "immobilized bacteria - ferrocene - graphite-paste electrode" and "suspension of bacteria - 2,6-DCPIP - graphite electrode" as a result of oxidation of the substrate by the enzymatic system of bacteria in the presence and in the absence of toxicants in the analyzed sample will be the inhibition value (toxicity index T, %) (3):
where R
substrate is an electrochemical signal for the introduction of a substrate into the system in the absence of a toxicant; R
substrate+toxicant is an electrochemical signal for the introduction of a substrate into the system in the presence of a toxicant.
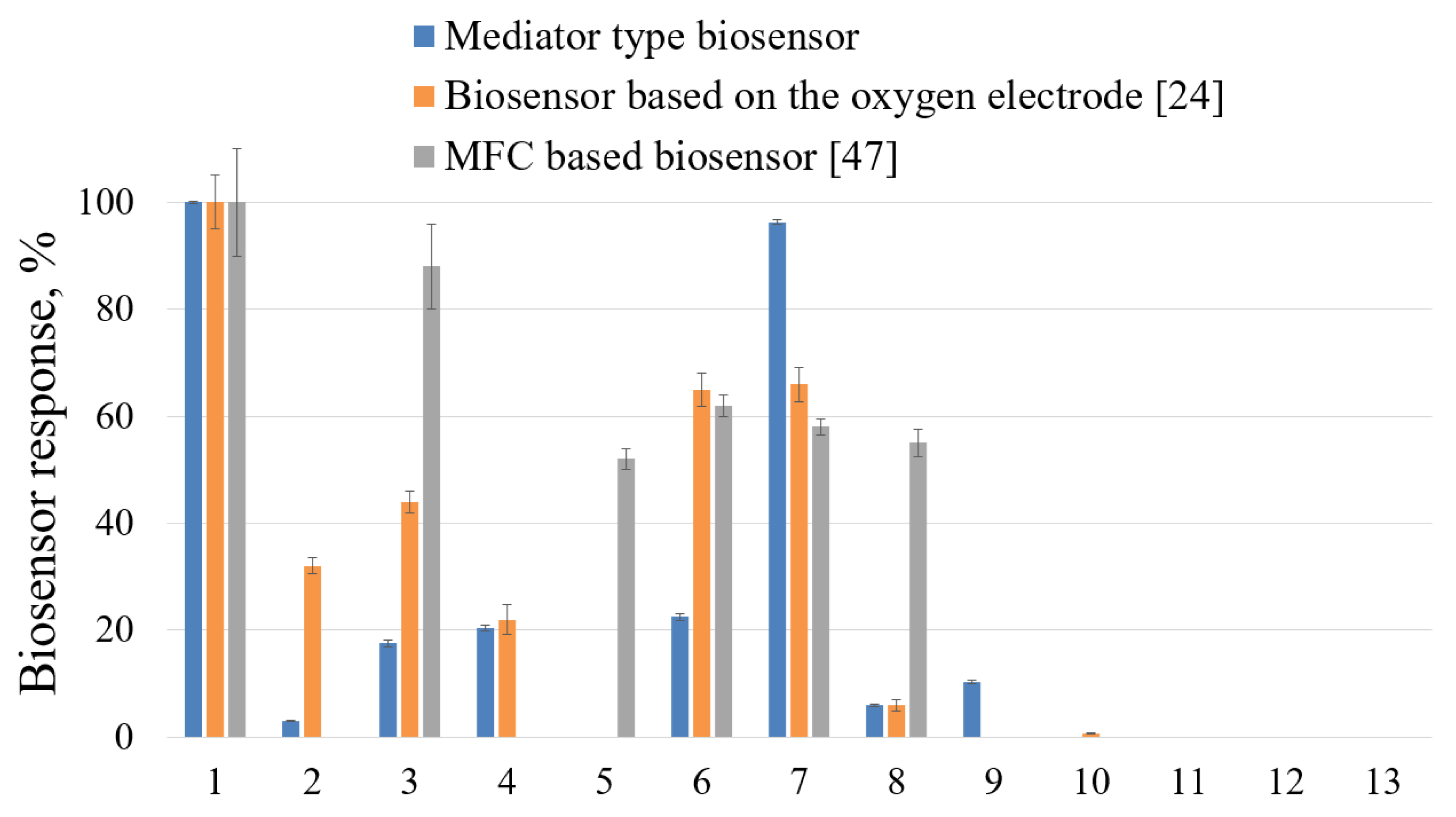
The spectra of oxidized substrates by
G. oxydans bacteria were assessed as part of biosensors based on an oxygen electrode (substrates no. 11–13) [
24], MFC [
47] and a mediator type to identify substances that may have a negative effect. In this work, the oxidative activity of microorganisms was additionally studied by adding substrates № 11–13 (oxygen electrode) and № 9–13 (MFC) to the measuring cell. Data on the spectra of substrates oxidized by
G. oxydans bacteria are presented in percentage terms relative to the maximum response of the biosensor to glucose in the diagram (
Figure 3).
G. oxydans bacteria do not oxidize phenol, 4-nitrophenol, 2,4-dinitrophenol, trichloroacetic and salicylic acids; therefore, these substances can be selected as model toxicants. For the biosensor based on Clark's electrode, the absence of responses to formaldehyde was recorded, and in the mediator biosensor - to phenol. This change in the spectrum of oxidizable substrates of the bacteria G. oxydans is explained by the peculiarities of the reduction of the mediator during its interaction with the CPE of microorganisms.
The toxic effect of substances that are not subject to metabolic transformation by G. oxydans bacteria was assessed by a decrease in the analytical signal for glucose, as the most intensively oxidized substrate. The low probability of the presence of glucose in the composition of the studied samples of polymeric materials will not introduce an analytical error when determining their toxic effect on the conformation of the active center of glucose oxidase, which is characterized by absolute specificity.
3.2. Main Characteristics of Biosensors for Determining Toxicity
The stability (in the absence and under the influence of a toxicant (Zn
2+, EC
50)) and sensitivity of the electrochemical system were studied to assess the possibility of using a biosensor with a certain type of signal recording based on the bacteria
G. oxydans to determine integral toxicity. The study of the influence of the pollutant on the operational and long-term stability of the sensors was carried out in the presence of zinc (II) ions. This toxicant has a negative effect on the oxidative activity of microorganisms in all types of biosensors and is also used as a reference for monitoring the error of the toxicological analysis method based on the Ecolum bacterial test. When the oxidative activity of bacteria decreased by 50% of the maximum, the biosensor was considered unsuitable for measurements.
Table 1 presents the main characteristics of biosensors based on the bacteria
G. oxydans for toxicity assessment.
Electrochemical systems are designed to establish the duration of stable operation in the absence of exposure to pollutants on biomaterial, and are also characterized by high long-term stability (31 and 25 days). Toxic exposure leads to a reduction in the period of stable functioning of biosensors by almost half, which is associated with irreversible changes in the conformation of the active centers of microbial cell enzymes (accumulation of toxic effects). This must be taken into account in future studies when preparing a standardized method for determining integral toxicity. The relative standard deviation calculated to characterize operational stability does not exceed 15% in all cases and satisfies the criterion for using biosensors. However, it is worth noting that MFC showed the lowest convergence of results, which limits its use.
3.3. Quantitative Assessment of the Toxic Effects of Pollutants on the Bacteria G. oxydans as Part of Biosensors with Different Types of Signal Recording
To form an effective biosensor for determining integral toxicity, it is necessary to quantify the toxic effect of individual substances on the metabolic activity of microbial cells. To determine the negative impact on the bacteria G. oxydans, organic substances that are not oxidized by these bacteria (
Figure 3) and are used in the production of polymers as structural components or polymerization catalysts (phenol, 2,4-dinitrophenol, salicylic acid) were used as model toxicants). In addition, a number of heavy metal ions were used (iron (III), cadmium (II), manganese (II), chromium (III) and zinc (II)), the toxic effects of which have been studied in other bioassay methods [
48,
49,
50,
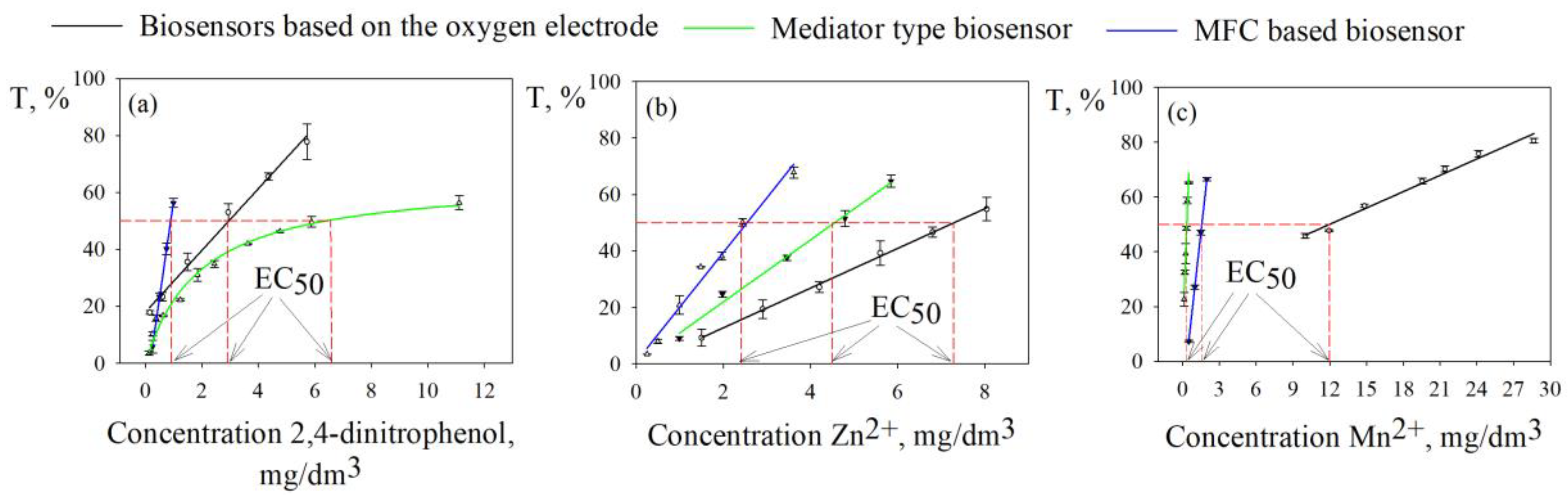
51]. A solution of an organic substance or a heavy metal salt was added to the microbial cells in a cuvette, a stationary current/potential value was waited for (exposure time of at least 5 minutes) and after adding an aliquot of the substrate, the response of the biosensor was recorded. Thus, under conditions of incubation of microbial cells in a medium with a toxicant, the sensitivity of bacteria was assessed by the value of T, calculated by formula (3). The inhibition curves of the oxidative activity of G. oxydans bacteria as part of different types of biosensors by manganese (II), zinc (II) ions and 2,4-dinitrophenol are presented in
Figure 4.
The intensity of the inhibitory effect of toxicants in various bioassay methods was assessed by comparing the values of the half-maximal effective concentration EC50 of the pollutant, which was determined with a 50% decrease in the oxidative activity of bacteria. Based on the obtained dependences of the index on the concentration of Mn2+ ions, it can be noted that the highest EC50 value was obtained using a biosystem based on an oxygen electrode and is 12 mg/dm3, which indicates the least sensitivity of G. oxydans bacteria in this type of sensor to the presence in the environment manganese(II). The EC50 values of 2,4-dinitrophenol and zinc(II) ions were determined using different types of biosensors, correlate with each other and are values of the same order.
Inhibition curves of the oxidative activity of G. oxydans bacteria as part of different electrochemical systems were constructed for all model toxicants studied. The EC
50 values of inhibitors of the oxidative activity of biorecognition elements of biosensors presented in
Table 2 were compared with the results characterizing the sensitivity of known electrochemical systems
[4,24,26,30,52,53,54,55,56] and test objects used in standard methods [
48,
49,
50,
51] to negative effects studied toxicants.
The bacteria G. oxydans exhibit high sensitivity to the toxic effects of pollutants of organic and inorganic nature, being part of electrochemical installations with different principles of signal recording. A biosensor based on an oxygen electrode and G. oxydans bacteria is more sensitive to zinc (II) ions in comparison with analogues
[4,52]. However, the EC
50 of toxicants such as cadmium (II) ions and phenol in the case of an oxygen biosensor is orders of magnitude lower than that of a mediator sensor. The system “G. oxydans bacteria – ferrocene – graphite-paste electrode” is superior in sensitivity to biosensors formed using an oxygen electrode and MFC. The mediator biosensor is characterized by greater sensitivity to the presence of heavy metals in the environment (to Cr
3+, Mn
2+ ions at the level of maximum permissible concentrations for water bodies of drinking and cultural water use), which is also observed when compared with similar electrochemical systems and biotesting methods, described in the literature
[4,24,26,52,55,56]. Using MFC the EC
50 of phenol, 2,4-dinitrophenol and Mn
2+, Zn
2+, Cd
2+ ions were determined at a level not inferior to other types of biosensors based on the bacteria G. oxydans and known prototypes in terms of sensitivity
[30,53,54]. This MFC configuration is promising for determining integral toxicity due to its high sensitivity, but to obtain consistent results it is necessary to use a biocatalyst of the same activity, which can be achieved by immobilizing microbial cells in the anode space.
Despite the low EC
50 values of model toxicants obtained using different types of biosensors based on the bacteria G. oxydans, standard test objects (Daphnia magna [
49] and Lemna minor [
50,
51]) are superior to biosensor analyzers in sensitivity to the toxic effect of heavy metals . However, the use of electrochemical systems based on microbial cells remains an urgent task due to their portability, rapidity and the possibility of repeated use of the test object for analysis. This indicates the promise of using a mediator biosensor based on the bacteria G. oxydans to determine integral toxicity.
3.4. Toxicity Analysis of Polymer Samples
For analysis of integral toxicity, 6 samples of consumer goods made of polymer materials were taken. The gas chromatography-mass spectrometry (GC-MS) method was used to identify the presence of individual toxic substances in their composition. Sample extracts were prepared using acetonitrile, since this extractant did not dissolve the polymers under study and is effective in extracting chemical compounds of polar and non-polar nature (phthalates, pesticides) [
57,
58]. Therefore, as a result of analyzing the extract of the “phone case” sample, a chromatogram was obtained and mass spectra of 6 constituent components of the studied material, identified using the NIST14 library with a score of ≥ 60% (
Figure 5)).
Unidentified peaks corresponded to contaminants migrating into the sample as a result of erosion of the chromatographic column sorbent (m/z 73, 207, 281, 355) [
59]. In the case of separation of the extract of the “medical gloves” and “pouring gloves” samples, the largest number of peaks (more than 40) were identified, which made it possible to identify only 4 unique chemical compounds. This is explained by the complex formulation of polymer materials, which includes various additives and fillers, which, during the production and storage of the polymer, can be converted into products of an unknown chemical structure with a specific effect on the body. Thus, using the GC-MS method, substances used as plasticizers and stabilizers in the production of polymer materials, giving them characteristic properties (diphenylmethane-4,4'-diisocyanate, triphenyl phosphate, polyethylene adipate, dimethyl phthalate, bis( 2-ethylhexyl) phthalate), and isomers of meso- and terephthalic acids (4-(1-hydroxy-1-methylethyl)acetophenone, α,α'-dihydroxy-1,3-diisopropylbenzene) (
Table 3).
The presence in the analyzed samples of phthalates and isocyanates with proven toxic effects on humans [
60,
61,
62,
63] and test organisms [
64,
65,
66] indicates a potential manifestation of the inhibitory effect of the materials under study on the reactions of the test objects used.
Aqueous extracts of samples of industrially produced goods made from polymeric materials were studied for integral toxicity using biosensors with different types of signal recording based on the bacteria G. oxydans and standard biotesting methods (test objects: biosensor Ecolum, Lemna minor and cattle sperm). To determine toxicity using the formed electrochemical systems, the response of the biosensor to glucose was assessed (the concentration corresponded to the middle of the linear dependence of the sensor signal on glucose content) in the absence and presence of the test sample (the minimum possible dilution of the test aqueous extract in a measuring cuvette with 30mM Na-phosphate buffer pH 6.0 1:3).T was calculated using formula (3) taking into account dilution. The toxicity of the sample was recorded when T exceeded 50%. The summary results of testing samples of industrially produced goods for toxicity are presented in
Table 4.
A correlation was established for samples No. 2 (toxic) and No. 6 (non-toxic) using biosensors and standard biotesting methods. Dimethyl phthalate in sample No. 6 is not a substance of increased toxicity (
Table 3) [
67], which is confirmed by the absence of a negative effect on test organisms. However, highly toxic diphenylmethane-4,4'-diisocyanate [
68] and components of unknown structure in sample No. 2 led to a significant decrease in the oxidative activity of G. oxydans bacteria, the luminescence of the Ecolum test-system, and the motility of cattle sperm.
The different levels of reaction inhibition in the study of samples No. 1–5 are associated with the unequal sensitivity of the test objects to the toxicants contained. Thus, a higher sensitivity to toxic effects is observed in unicellular organisms compared to duckweed, which is explained by the reduced influence of volatile pollutants on Lemna minor due to their migration into the air over a long period of analysis (7 days). From the toxicity results of samples No. 3 and No. 4, it can be noted that the bacteria G. oxydans are more sensitive to the toxic effects of bis(2-ethylhexyl) phthalate than the Ecolum biosensor and sperm.
The sensitivity of the mediator biosensor turned out to be comparable to a biosensor based on MFC and higher than the sensitivity of the electrochemical system based on an oxygen electrode. This is consistent with the hypothesis about the transport of electrons by a mediator from different parts of the CPE of a bacterial cell, which contributes to the emergence of a specific toxic effect in microorganisms within electrochemical systems of various types due to disruption of biochemical processes.
5. Conclusions
Thus, the use of electrochemical systems with different types of signal recording based on the bacteria G. oxydans is a promising biotesting method that allows quantitative assessment of the integral toxicity of various materials. The advantage of such microbial sensors is the early warning of the toxic effects of industrially produced products on human health. The rapidity and low cost of these types of biosensors will complement traditional methods of toxicological analysis with sophisticated analytical equipment and comprehensively assess the safety of the materials under study.
The developed biosensors using an oxygen electrode, MFC and a mediator type graphite-paste electrode based on the bacteria G. oxydans are comparable in sensitivity to the commercial biosensor Ecolum and standard biotesting methods, which allows them to be used to assess the toxicity index of consumer goods.
Author Contributions
Conceptualization, N.Yu.Yu. and T.N.K.; methodology, N.Yu.Yu., S.V.A. and T.N.K.; software, N.Yu.Yu.; validation, N.Yu.Yu. and T.N.K.; formal analysis, T.N.K., D.A.B. and M.M.K.; investigation, T.N.K., D.A.B. and M.M.K.; resources, S.V.A. and T.N.K.; data curation, N.Yu.Yu. and T.N.K.; writing—original draft preparation, T.N.K.; writing—review and editing, N.Yu.Yu. and V.A.A.; visualization, N.Yu.Yu.; supervision, S.V.A. and V.A.A.; project administration, T.N.K. and N.Yu.Yu.; funding acquisition, T.N.K., S.V.A. and N.Yu.Yu. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was funded by a grant from the Government of the Tula Region in the field of science and technology in 2022 under agreement DS/133 dated July 22, 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sree, C.G.; Buddolla, V.; Lakshmi, B.A.; Kim, Y.-J. Phthalate Toxicity Mechanisms: An Update. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109498. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Choi, K.-C. Adverse Effects of Pesticides on the Functions of Immune System. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 235, 108789. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.S.; Arlyapov, V.A.; Turovskaya, A.D.; Shvets, V.I.; Reshetilov, A.N. A Mediator Microbial Biosensor for Assaying General Toxicity. Enzyme Microb. Technol. 2020, 132, 109435. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. - Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Campanale; Massarelli; Savino; Locaputo; Uricchio A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public. Health 2020, 17, 1212. [CrossRef]
- Turner, A.; Filella, M. Hazardous Metal Additives in Plastics and Their Environmental Impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Jalili Ghazizadeh, A.; Hashemi, P.; Afkhami, A.; Arduini, F.; Bagheri, H. An Overview to Electrochemical Biosensors and Sensors for the Detection of Environmental Contaminants. J. Iran. Chem. Soc. 2020, 17, 2429–2447. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, Monitoring and Biodegradation of the Fungicide Carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Microbial-Derived Biosensors for Monitoring Environmental Contaminants: Recent Advances and Future Outlook. Process Saf. Environ. Prot. 2019, 124, 8–17. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors. 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, A.; Arlyapov, V.; Medvedeva, A.; Lepikash, R.; Melnikov, P.; Reshetilov, A. Mediator Microbial Biosensor Analyzers for Rapid Determination of Surface Water Toxicity. Sensors. 2022, 22, 8522. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Piñas, F.; Rodea-Palomares, I.; Leganés, F.; González-Pleiter, M.; Angeles Muñoz-Martín, M. Evaluation of the Ecotoxicity of Pollutants with Bioluminescent Microorganisms. In Bioluminescence: Fundamentals and Applications in Biotechnology - Volume 2; Thouand, G., Marks, R., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; Volume 145, pp. 65–135. ISBN 978-3-662-43618-9. [Google Scholar]
- Yu, D.; Li, R.; Rong, K.; Fang, Y.; Liu, L.; Yu, H.; Dong, S. A Novel, Environmentally Friendly Dual-Signal Water Toxicity Biosensor Developed through the Continuous Release of Fe3+. Biosens. Bioelectron. 2023, 220, 114864. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell Immobilization Strategies for Biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Kamanina, O.; Arlyapov, V.; Rybochkin, P.; Lavrova, D.; Podsevalova, E.; Ponamoreva, O. Application of Organosilicate Matrix Based on Methyltriethoxysilane, PVA and Bacteria Paracoccus Yeei to Create a Highly Sensitive BOD. 3 Biotech. 2021, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts. 2018, 8, 92. [Google Scholar] [CrossRef]
- Moraskie, M.; Roshid, M.H.O.; O’Connor, G.; Dikici, E.; Zingg, J.-M.; Deo, S.; Daunert, S. Microbial Whole-Cell Biosensors: Current Applications, Challenges, and Future Perspectives. Biosens. Bioelectron. 2021, 191, 113359. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Voyvodic, P.L.; Zúñiga, A.; Bonnet, J. Microbially Derived Biosensors for Diagnosis, Monitoring and Epidemiology. Microb. Biotechnol. 2017, 10, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Gao, G.; Yang, Y.; Wang, Y.; Gao, L.; Zhi, J. Redox Mediator-Based Microbial Biosensors for Acute Water Toxicity Assessment: A Critical Review. ChemElectroChem. 2020, 7, 2513–2526. [Google Scholar] [CrossRef]
- Cui, Y.; Lai, B.; Tang, X. Microbial Fuel Cell-Based Biosensors. Biosensors 2019, 9, 92. [Google Scholar] [CrossRef]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial Fuels Cell-Based Biosensor for Toxicity Detection: A Review. Sensors. 2017, 17, 2230. [Google Scholar] [CrossRef]
- Perchikov, R.N.; Provotorova, D.V.; Kharkova, A.S.; Arlyapov, V.A.; Medvedeva, A.S.; Machulin, A.V.; Filonov, A.E.; Reshetilov, A.N. Bioanalytical System for Determining the Phenol Index Based on Pseudomonas Putida BS394(pBS216) Bacteria Immobilized in a Redox-Active Biocompatible Composite Polymer “Bovine Serum Albumin–Ferrocene–Carbon Nanotubes. ” Polymers. 2022, 14, 5366. [Google Scholar] [CrossRef]
- Yudina, N.Yu.; Zaitsev, M.G.; Arlyapov, V.A.; Alferov, V.A.; Ponamoreva, O.N.; Reshetilov, A.N. A Biosensor for Express Assessment of Integral Toxicity of Polymer- and Textile-Based Products. Biotechnology. 2021, 37, 119–128. [Google Scholar] [CrossRef]
- Buckova, M.; Licbinsky, R.; Jandova, V.; Krejci, J.; Pospichalova, J.; Huzlik, J. Fast Ecotoxicity Detection Using Biosensors. Water. Air. Soil Pollut. 2017, 228, 166. [Google Scholar] [CrossRef]
- Gao, G.; Fang, D.; Yu, Y.; Wu, L.; Wang, Y.; Zhi, J. A Double-Mediator Based Whole Cell Electrochemical Biosensor for Acute Biotoxicity Assessment of Wastewater. Talanta 2017, 167, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yong, D.; Kim, H.; Zhang, Z.; Ma, S.; Han, X. A Ferricyanide-mediated Activated Sludge Bioassay for Determination of the Toxicity of Water. Electroanalysis 2016, 28, 580–587. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Shen, J.; Yu, Y.; Zhi, J. A Reagentless Electrochemical Biosensor Based on Thionine Wrapped E. Coli and Chitosan-Entrapped Carbon Nanodots Film Modified Glassy Carbon Electrode for Wastewater Toxicity Assessment. Electrochimica Acta 2016, 222, 303–311. [Google Scholar] [CrossRef]
- Gao, G.; Qian, J.; Fang, D.; Yu, Y.; Zhi, J. Development of a Mediated Whole Cell-Based Electrochemical Biosensor for Joint Toxicity Assessment of Multi-Pollutants Using a Mixed Microbial Consortium. Anal. Chim. Acta 2016, 924, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity Detection in Water Containing Heavy Metal Ions with a Self-Powered Microbial Fuel Cell-Based Biosensor. Talanta 2017, 168, 210–216. [Google Scholar] [CrossRef]
- Nguyen Tran, P.H.; Thi Luong, T.T.; Thi Nguyen, T.T.; Nguyen, H.Q.; Duong, H.V.; Kim, B.H.; Pham, H.T. Possibility of Using a Lithotrophic Iron-Oxidizing Microbial Fuel Cell as a Biosensor for Detecting Iron and Manganese in Water Samples. Environ. Sci. Process. Impacts 2015, 17, 1806–1815. [Google Scholar] [CrossRef]
- Labro, J.; Craig, T.; Wood, S.A.; Packer, M.A. Demonstration of the Use of a Photosynthetic Microbial Fuel Cell as an Environmental Biosensor. Int. J. Nanotechnol. 2017, 14, 213. [Google Scholar] [CrossRef]
- Chen, Z.; Niu, Y.; Zhao, S.; Khan, A.; Ling, Z.; Chen, Y.; Liu, P.; Li, X. A Novel Biosensor for P-Nitrophenol Based on an Aerobic Anode Microbial Fuel Cell. Biosens. Bioelectron. 2016, 85, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, N.; Zhou, Q. Concentration Responses of Toxicity Sensor with Shewanella Oneidensis MR-1 Growing in Bioelectrochemical Systems. Biosens. Bioelectron. 2013, 43, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, X.; Shi, Y.; Qi, Y.; Huang, D.; Tadé, M.; Wang, S.; Liu, S. FePO4 Based Single Chamber Air-Cathode Microbial Fuel Cell for Online Monitoring Levofloxacin. Biosens. Bioelectron. 2017, 91, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Czeller, M.; Rostás, V.; Kovács, T. Microbial Fuel Cell-Based Diagnostic Platform to Reveal Antibacterial Effect of Beta-Lactam Antibiotics. Enzyme Microb. Technol. 2015, 73–74, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, S.; Chen, J.; Zhou, J. Dehydrogenases of Acetic Acid Bacteria. Biotechnol. Adv. 2022, 54, 107863. [Google Scholar] [CrossRef]
- Schenkmayerová, A.; Bertóková, A.; Šefčovičová, J.; Štefuca, V.; Bučko, M.; Vikartovská, A.; Gemeiner, P.; Tkáč, J.; Katrlík, J. Whole-Cell Gluconobacter Oxydans Biosensor for 2-Phenylethanol Biooxidation Monitoring. Anal. Chim. Acta 2015, 854, 140–144. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Reshetilov, A.N.; Plekhanova, Yu.V.; Tarasov, S.E.; Arlyapov, V.A.; Kolesov, V.V.; Gutorov, M.A.; Gotovtsev, P.M.; Vasilov, R.G. Effect of Some Carbon Nanomaterials on Ethanol Oxidation by Gluconobacter Oxydans Bacterial Cells. Appl. Biochem. Microbiol. 2017, 53, 123–129. [Google Scholar] [CrossRef]
- Plekhanova; Tarasov; Bykov; Prisyazhnaya; Kolesov; Sigaev; Signore; Reshetilov Multiwalled Carbon Nanotubes and the Electrocatalytic Activity of Gluconobacter Oxydans as the Basis of a Biosensor. Biosensors 2019, 9, 137. [CrossRef]
- Alferov, S.V.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Biofuel Cell Based on Bacteria of the Genus Gluconobacter as a Sensor for Express Analysis of Biochemical Oxygen Demand. Appl. Biochem. Microbiol. 2018, 54, 689–694. [Google Scholar] [CrossRef]
- Alferov, S.V.; Vozchikova, S.V.; Arlyapov, V.A.; Alferov, V.A.; Reshetilov, A.N. Competition between Redox Mediator and Oxygen in the Microbial Fuel Cell. Appl. Biochem. Microbiol. 2017, 53, 267–272. [Google Scholar] [CrossRef]
- Alferov, S.V.; Minaicheva, P.R.; Arlyapov, V.A.; Asulyan, L.D.; Alferov, V.A.; Ponamoreva, O.N.; Reshetilov, A.N. Bioanode for a Microbial Fuel Cell Based on Gluconobacter Oxydans Immobilized into a Polymer Matrix. Appl. Biochem. Microbiol. 2014, 50, 637–643. [Google Scholar] [CrossRef]
- Tarasov, S.; Plekhanova, Y.; Kashin, V.; Gotovtsev, P.; Signore, M.; Francioso, L.; Kolesov, V.; Reshetilov, A. Gluconobacter Oxydans-Based MFC with PEDOT:PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors 2022, 12, 699. [Google Scholar] [CrossRef]
- Reshetilov, A.; Alferov, S.; Tomashevskaya, L.; Ponamoreva, O. Testing of Bacteria Gluconobacter Oxydans and Electron Transport Mediators Composition for Application in Biofuel Cell. Electroanalysis 2006, 18, 2030–2034. [Google Scholar] [CrossRef]
- Alferov, S.V.; Voevodskaya, O.A.; Nhuen, V.T.; Arlyapov, V.A.; Ponamoreva, O.N.; Reshetilov, A.N. Effectiveness of electronic transport mediators in the processes of electrocatalytic oxidation of substrates by enzyme systems of microorganisms. Sens. Syst. 2010, 25, 346–351. (in Russian). [Google Scholar]
- Mohseni, M.; Abbaszadeh, J.; Maghool, S.-S.; Chaichi, M.-J. Heavy Metals Detection Using Biosensor Cells of a Novel Marine Luminescent Bacterium Vibrio Sp. MM1 Isolated from the Caspian Sea. Ecotoxicol. Environ. Saf. 2018, 148, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute Toxicity of 50 Metals to Daphnia Magna. J. Appl. Toxicol. 2015, 35, 824–830. [Google Scholar] [CrossRef]
- Naumann, B.; Eberius, M.; Appenroth, K.-J. Growth Rate Based Dose–Response Relationships and EC-Values of Ten Heavy Metals Using the Duckweed Growth Inhibition Test (ISO 20079) with Lemna Minor L. Clone St. J. Plant Physiol. 2007, 164, 1656–1664. [Google Scholar] [CrossRef]
- Bošnir, J.; Puntarić, D.; Cvetković, Ž.; Pollak, L.; Barušić, L.; Klarić, I.; Miškulin, M.; Puntarić, I.; Puntarić, E.; Milošević, M. Effects of Magnesium, Chromium, Iron and Zinc from Food Supplements on Selected Aquatic Organisms. Coll. Antropol. 2013, 37, 965–971. [Google Scholar]
- Yang, Y.; Fang, D.; Liu, Y.; Liu, R.; Wang, X.; Yu, Y.; Zhi, J. Problems Analysis and New Fabrication Strategies of Mediated Electrochemical Biosensors for Wastewater Toxicity Assessment. Biosens. Bioelectron. 2018, 108, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jae Sun Lee; Kim, D. S.; Hyeon Jin, Jeon, Park, B.S., Hee Jin, Yang, Hyun, M.S., Eds.; Kim, M. Microbial Fuel Cell as a Biosensor to Monitor Various Toxic Metal Substances in Water. In Proceedings of the 2015 9th International Conference on Sensing Technology (ICST); IEEE: Auckland, New Zealand, December 2015; pp. 416–419. [Google Scholar]
- Farré, M.; Barceló, D. Toxicity Testing of Wastewater and Sewage Sludge by Biosensors, Bioassays and Chemical Analysis. TrAC Trends Anal. Chem. 2003, 22, 299–310. [Google Scholar] [CrossRef]
- Johnson, B.T. Microtox® Toxicity Test System — New Developments and Applications. In Microscale Testing in Aquatic Toxicology; CRC Press: Boca Raton, 2018; pp. 201–218. ISBN 978-0-203-74719-3. [Google Scholar]
- Codina, J.C.; Ascensión Muñoz, M.; Cazorla, F.M.; Pérez-Garcı́a, A.; Moriñigo, M.A.; De Vicente, A. The Inhibition of Methanogenic Activity from Anaerobic Domestic Sludges as a Simple Toxicity Bioassay. Water Res. 1998, 32, 1338–1342. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Palomino-Cabello, C.; Turnes-Palomino, G.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Determination of Phthalate Acid Esters Plasticizers in Polyethylene Terephthalate Bottles and Its Correlation with Some Physicochemical Properties. Polym. Test. 2018, 68, 87–94. [Google Scholar] [CrossRef]
- Tripathy, V.; Saha, A.; Kumar, J. Detection of Pesticides in Popular Medicinal Herbs: A Modified QuEChERS and Gas Chromatography–Mass Spectrometry Based Approach. J. Food Sci. Technol. 2017, 54, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.T. Mass spectrometry in organic chemistry, 2nd ed.; TECHNOSPHERE: Moscow, Russia, 2003; p. 79114, (in Russian). ISBN 978-5-94836-409-4. [Google Scholar]
- Weng, X.; Zhu, Q.; Liao, C.; Jiang, G. Cumulative Exposure to Phthalates and Their Alternatives and Associated Female Reproductive Health: Body Burdens, Adverse Outcomes, and Underlying Mechanisms. Environ. Sci. Technol. 2023, 57, 8189–8212. [Google Scholar] [CrossRef] [PubMed]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Phthalates: European Regulation, Chemistry, Pharmacokinetic and Related Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef]
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin Exposure to Isocyanates: Reasons for Concern. Environ. Health Perspect. 2007, 115, 328–335. [Google Scholar] [CrossRef]
- Schupp, T.; Plehiers, P.M. Absorption, Distribution, Metabolism, and Excretion of Methylene Diphenyl Diisocyanate and Toluene Diisocyanate: Many Similarities and Few Differences. Toxicol. Ind. Health 2022, 38, 500–528. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; You, Y.; Xu, W.; Lv, Z.; Liu, Z.; Chen, W.; Shi, Y.; Wang, J. Response of Pseudomonas Fluorescens to Dimethyl Phthalate. Ecotoxicol. Environ. Saf. 2019, 167, 36–43. [Google Scholar] [CrossRef]
- Cong, B.; Liu, C.; Wang, L.; Chai, Y. The Impact on Antioxidant Enzyme Activity and Related Gene Expression Following Adult Zebrafish (Danio Rerio) Exposure to Dimethyl Phthalate. Animals 2020, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tripathi, M.K.; Yasir, M.; Ranjan, A.; Shrivastava, R. Effects of Carbamate Pesticides Intermediates on Escherichia Coli Membrane Architecture: An in Vitro and in Silico Approach. Environ. Anal. Health Toxicol. 2021, 36, e2021020. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannilli, V.; Passatore, L.; Carloni, S.; Sciacca, G.; Cerasa, M.; Zacchini, M. Ecotoxicological and Genotoxic Effects of Dimethyl Phthalate (DMP) on Lemna Minor L. and Spirodela Polyrhiza (L.) Schleid. Plants under a Short-Term Laboratory Assay. Sci. Total Environ. 2022, 806, 150972. [Google Scholar] [CrossRef]
- Hamada, H.; Liljelind, I.; Bruze, M.; Engfeldt, M.; Isaksson, M.; Jönsson, B.; Tinnerberg, H.; Lindh, C.; Axelsson, S.; Zimerson, E. Assessment of Dermal Uptake of Diphenylmethane-4,4’-Diisocyanate Using Tape Stripping and Biological Monitoring. Eur. J. Dermatol. 2018, 28, 143–148. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).