Submitted:
30 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Treatment Intensification
2.1. RT alone
2.2. RT + ADT
2.2.1. RT + STADT vs RT alone
2.2.2. RT + LTADT vs RT alone
2.2.3. RT + STADT vs RT + LTADT
2.2.4. RT + NADT vs RT alone
2.2.5. Conformal RT + NADT
2.3. RT + ADT + additional drugs
2.4. Use of a radiosensitizer
| Trials | Status | Study period | Treatment |
|---|---|---|---|
| NCT00684905: Leuprolide, bicalutamide, and implant radiation therapy for patients with locally recurrent prostate cancer after external beam radiation therapy | Completed | 2000–2005 | Leuprolide + Bicalutamide + Brachytherapy + Leuprolide |
| NCT00016913: Chemotherapy, hormone therapy, and radiation therapy for patients with locally advanced prostate cancer | Completed | 2001–2008 | Paclitaxel + Estramustine + Carboplatin + Gonadotropin-releasing hormonal therapy (goserelin/leuprolide) + RT |
| NCT02135445: Safety and efficacy of TAK-385 for patients with localized prostate cancer | Completed | 2014–2015 | TAK-385 + RT vs Degarelix + RT |
| NCT00193856: RADAR (randomized androgen deprivation and radiotherapy) trial | Completed | 2003–2017 | 6-Month Leuprorelin acetate + RT 6-Month Leuprorelin acetate + Zoledronic acid + RT 18-Month Leuprorelin acetate + RT 18-Month Leuprorelin acetate + Zoledronic acid + RT |
| NCT00223665: Effects of IAS in men with localized biochemical relapse prostate cancer (IAS) | Completed | 1997–2012 | RT + Intermittent androgen suppression + Flutamide + Leuprolide Acetate |
| NCT00002597: Radiation therapy with or without antiandrogen therapy for patients with stage I or II prostate cancer | Completed | 1994–2018 | Zoladex + Flutamide + RT |
| NCT02057939: Salvage therapeutic radiation with enzalutamide and ADT in men with recurrent prostate cancer (STREAM) | Completed | 2014–2019 | Enzalutamide + ADT + RT |
| NCT02300389: Comparing hypofractionated radiotherapy boost to conventionally fractionated (HYPOPROST) | Completed | 2011–2019 | Hypofractionated IMRT boost + ADT vs Conventional IMRT boost + ADT |
| NCT02472275: PLX3397, radiation therapy, and antihormone therapy for patients with intermediate- or high-risk prostate cancer | Completed | 2015–2019 | PLX3397 + RT + ADT (leuprolide acetate, goserelin acetate, or degarelix) |
| NCT02229734: Fairly brief androgen suppression and stereotactic radiotherapy for high-risk prostate cancer – Protocol 2 (FASTR-2) | Completed | 2014–2021 | SBRT + LHRH (leuprolide) |
| NCT03311555: A salvage trial of AR inhibition with ADT and apalutamide with radiation therapy followed by docetaxel in men with PSA recurrent prostate cancer after radical prostatectomy (STARTAR) | Completed | 2018–2022 | Apalutamide + ADT + RT (salvage radiation therapy) + Docetaxel |
| NCT03649841: Antiandrogen therapy, abiraterone acetate, and prednisone with or without neutron radiation therapy for patients with prostate cancer | Terminated (due to low accrual) |
2020–2023 | ADT + Abiraterone + Prednisone + RT vs ADT + Abiraterone + Prednisone |
| NCT01439542: Fairly brief androgen suppression and stereotactic radiotherapy for high-risk prostate cancer (FASTR) | Terminated (higher than expected Gr3 GU/GI toxicity) |
2011–2017 | Stereotactic radiotherapy + LHRH agonist |
| NCT02508636: Trial of radiotherapy with leuprolide and enzalutamide in high-risk prostate cancer | Terminated (due to low accrual) |
2015–2020 | Enzalutamide + Leuprolide + IMRT |
| NCT01811810: Proton therapy for high-risk prostate cancer | Withdrawn (unable to recruit) |
2013–2014 | XRT + ADT vs XRT + chemotherapy + short-term ADT |
| NCT01517451: Radiation and androgen ablation for prostate cancer | Active | 2013–2026 | ADT + SBRT |
| NCT01952223: A phase III study of cabazitaxel and pelvic radiotherapy in localized prostate cancer and high-risk features of relapse (PEACE2) | Active | 2013–2041 | ADT + Pelvic RT ADT + Cabazitaxel + Prostate RT ADT + Cabazitaxel + Pelvic RT ADT + Prostate RT |
| NCT01546987: Hormone therapy, radiation therapy, and steroid 17alpha-monooxygenase TAK-700 for patients with high-risk prostate cancer | Active | 2012–2029 | ADT + RT vs TAK-700 + ADT + RT |
| NCT04489745: Stereotactic body radiotherapy (SBRT) for localized prostate cancer | Active | 2016–2025 | ADT + SBRT |
| NCT03541850: Stereotactic body radiation therapy for patients with localized prostate cancer that have undergone surgery | Active | 2019–2024 | ADT + SBRT |
| NCT02346253: High-dose brachytherapy for patients with prostate cancer | Active | 2015–2026 | HDR brachytherapy + Bicalutamide + Leuprolide acetate + Goserelin acetate + Triptorelin pamoate + Degarelix |
| NCT00936390: Radiation therapy with or without androgen deprivation therapy for patients with prostate cancer | Active | 2009–2025 | EBRT vs EBRT + ADT |
| NCT01436968: Phase 3 study of ProstAtak® immunotherapy with standard radiation therapy for localized prostate cancer (PrTK03) | Active | 2011–2024 | ProstAtak® + RT +/- ADT |
| NCT02594072: Androgen suppression with stereotactic body or external beam radiation therapy (ASSERT) | Active | 2016–2024 | SABR + Zoladex® vs EBRT + Zoladex® |
| NCT02446444: Enzalutamide in androgen deprivation therapy with radiation therapy for high-risk, clinically localized prostate cancer (ENZARAD) | Active | 2014–2025 | Enzalutamide + LHRHA + EBRT |
| NCT01420250: Cabazitaxel with radiation and hormone therapy for prostate cancer | Active | 2011–2023 | Cabazitaxel + IMRT + Bicalutamide + LHRH agonist |
| NCT02531516: An efficacy and safety study of JNJ-56021927 (Apalutamide) in high-risk prostate cancer subjects receiving primary radiation therapy: ATLAS | Active | 2015–2026 | Apalutamide + Bicalutamide Placebo + GnRH (agonist) + RT |
| NCT03070886: Antiandrogen therapy and radiation therapy with or without docetaxel for patients with prostate cancer that has been removed via surgery | Active | 2017–2031 | ADT + EBRT vs ADT + EBRT + Docetaxel |
| NCT05003752: Hypo-Combi trial: Hypofractionated EBRT plus HDR-BT boost for prostate cancer | Active | 2021–2026 | Hypofractionated EBRT + HDR-BT boost |
| NCT04947254: Androgen ablation therapy with or without niraparib after radiation therapy for the treatment of high-risk localized or locally advanced prostate cancer | Recruiting | 2021–2026 | Apa + ADT + XRT Apa + ADT + XRT + AAP + Niraparib |
| NCT04298983: Abemaciclib in combination with androgen deprivation therapy for locally advanced prostate cancer (RAD 1805) | Recruiting | 2021–2026 | Abemaciclib + ADT + RT |
| NCT05753566: Rezvilutamide in patients with biochemical recurrence after radical prostatectomy for prostate cancer | Recruiting | 2023–2028 | Rezvilutamide + ADT + SRT Rezvilutamide + ADT |
| NCT02303327: Comparative study of radiotherapy treatments for high-risk prostate cancer patients | Recruiting | 2015–2029 | ADT + EBRT + HDR brachytherapy boost ADT + Hypofractionated dose-escalation RT |
| NCT05781217: Short versus long-term androgen deprivation therapy with salvage radiotherapy in prostate cancer (URONCOR 06-24) | Recruiting | 2023–2032 | STADT + RT LTADT + RT |
| NCT05100472: A study of shorter-course hormone therapy and radiation for high-risk prostate cancer | Recruiting | 2021–2024 | ADT + Brachytherapy + Hypofractionated pelvic external beam radiation |
| NCT05361798: T-cell clonality after stereotactic body radiation therapy alone and in combination with the immunocytokine M9241 in localized high- and intermediate-risk prostate cancer treated with androgen deprivation therapy | Recruiting | 2023–2024 | De-escalating dose of M9241 + SBRT High tolerated dose of M9241 + SBRT SBRT |
| NCT01985828: CyberKnife® as monotherapy or boost SBRT for intermediate- or high-risk localized prostate cancer | Recruiting | 2013–2026 | ADT + CyberKnife + IMRT |
| NCT04943536: Bicalutamide implants (Biolen) with radiation therapy in patients with localized prostate cancer | Recruiting | 2021–2024 | Biolen + RT |
| NCT04894188: Neoadjuvant hormone and radiation therapy followed by radical prostatectomy in patients with high-risk prostate cancer | Recruiting | 2022–2041 | Neoadjuvant ADT & RT + Radical prostatectomy Neoadjuvant ADT + Radical prostatectomy |
| NCT05568550: Pembro with radiation with or without olaparib | Recruiting | 2023–2029 | Pembrolizumab + Olaparib + ADT + RT Pembrolizumab + ADT + RT |
| NCT04349501: Biomarker monitoring of prostate cancer patients with RSI MRI (ProsRSI) | Recruiting | 2020–2026 | RSI-MRI + ADT + RT |
| NCT04136353: Darolutamide augments standard therapy for localized very-high-risk prostate cancer (DASL-HiCaP) | Recruiting | 2020–2028 | Darolutamide + LHRHA + EBRT |
| NCT02102477: Surgery versus radiotherapy for locally advanced prostate cancer (SPCG-15) | Recruiting | 2014–2045 | Radical prostatectomy + RT vs RT + Adjuvant ADT |
| NCT04093375: Radical prostatectomy versus radical radiotherapy for locally advanced prostate cancer | Not yet recruiting | _ | Radical prostatectomy +/- ADT vs RT + Adjuvant ADT |
|
NCT04176081: Study of radiation therapy in combination with darolutamide + degarelix in intermediate-risk prostate cancer (SChLAP/IDC) |
Not yet recruiting |
_ - |
RT vs RT + Darolutamide + Degarelix |
3. TI vs AS
4. Complications
5. Role of precision medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wani, M.; Madaan, S. What Is New in the Management of High-Risk Localized Prostate Cancer? J Clin Med 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Wasim, S.; Lee, S.Y.; Kim, J. Complexities of Prostate Cancer. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Kinsella, N.; Helleman, J.; Bruinsma, S.; Carlsson, S.; Cahill, D.; Brown, C.; Van Hemelrijck, M. Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol 2018, 7, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Tolis, G.; Ackman, D.; Stellos, A.; Mehta, A.; Labrie, F.; Fazekas, A.T.; Comaru-Schally, A.M.; Schally, A.V. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A 1982, 79, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Koka, K.; Verma, A.; Dwarakanath, B.S.; Papineni, R.V.L. Technological Advancements in External Beam Radiation Therapy (EBRT): An Indispensable Tool for Cancer Treatment. Cancer Manag Res 2022, 14, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Isaacs, J.T. A history of prostate cancer treatment. Nat Rev Cancer 2002, 2, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, M.A.; Kaplan, H.S.; Sagerman, R.H. Linear Accelerator Supervoltage Radiotherapy. Vii. Carcinoma of the Prostate. Radiology 1965, 85, 121–129. [Google Scholar] [CrossRef]
- Bagshaw, M.A.; Ray, G.R.; Pistenma, D.A.; Castellino, R.A.; Meares, E.M. External beam radiation therapy of primary carcinoma of the prostate. Cancer 1975, 36, 723–728. [Google Scholar] [CrossRef]
- Hayden, A.J.; Catton, C.; Pickles, T. Radiation therapy in prostate cancer: a risk-adapted strategy. Curr Oncol 2010, 17 Suppl 2, S18–24. [Google Scholar] [CrossRef]
- Kuban, D.A.; Thames, H.D.; Levy, L.B.; Horwitz, E.M.; Kupelian, P.A.; Martinez, A.A.; Michalski, J.M.; Pisansky, T.M.; Sandler, H.M.; Shipley, W.U.; et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys 2003, 57, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Shipley, W.U.; Thames, H.D.; Sandler, H.M.; Hanks, G.E.; Zietman, A.L.; Perez, C.A.; Kuban, D.A.; Hancock, S.L.; Smith, C.D. Radiation therapy for clinically localized prostate cancer: a multi-institutional pooled analysis. JAMA 1999, 281, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Zagars, G.K.; Pollack, A.; von Eschenbach, A.C. Prognostic factors for clinically localized prostate carcinoma: analysis of 938 patients irradiated in the prostate specific antigen era. Cancer 1997, 79, 1370–1380. [Google Scholar] [CrossRef]
- Dal Pra, A.; Cury, F.L.; Souhami, L. Combining radiation therapy and androgen deprivation for localized prostate cancer-a critical review. Curr Oncol 2010, 17, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, C.; Jereczek-Fossa, B.A.; Marvaso, G.; Dicuonzo, S.; Rojas, D.P.; Cattani, F.; Starzynska, A.; Ciardo, D.; Surgo, A.; Leonardi, M.C.; et al. Recent advances in radiation oncology. Ecancermedicalscience 2017, 11, 785. [Google Scholar] [CrossRef] [PubMed]
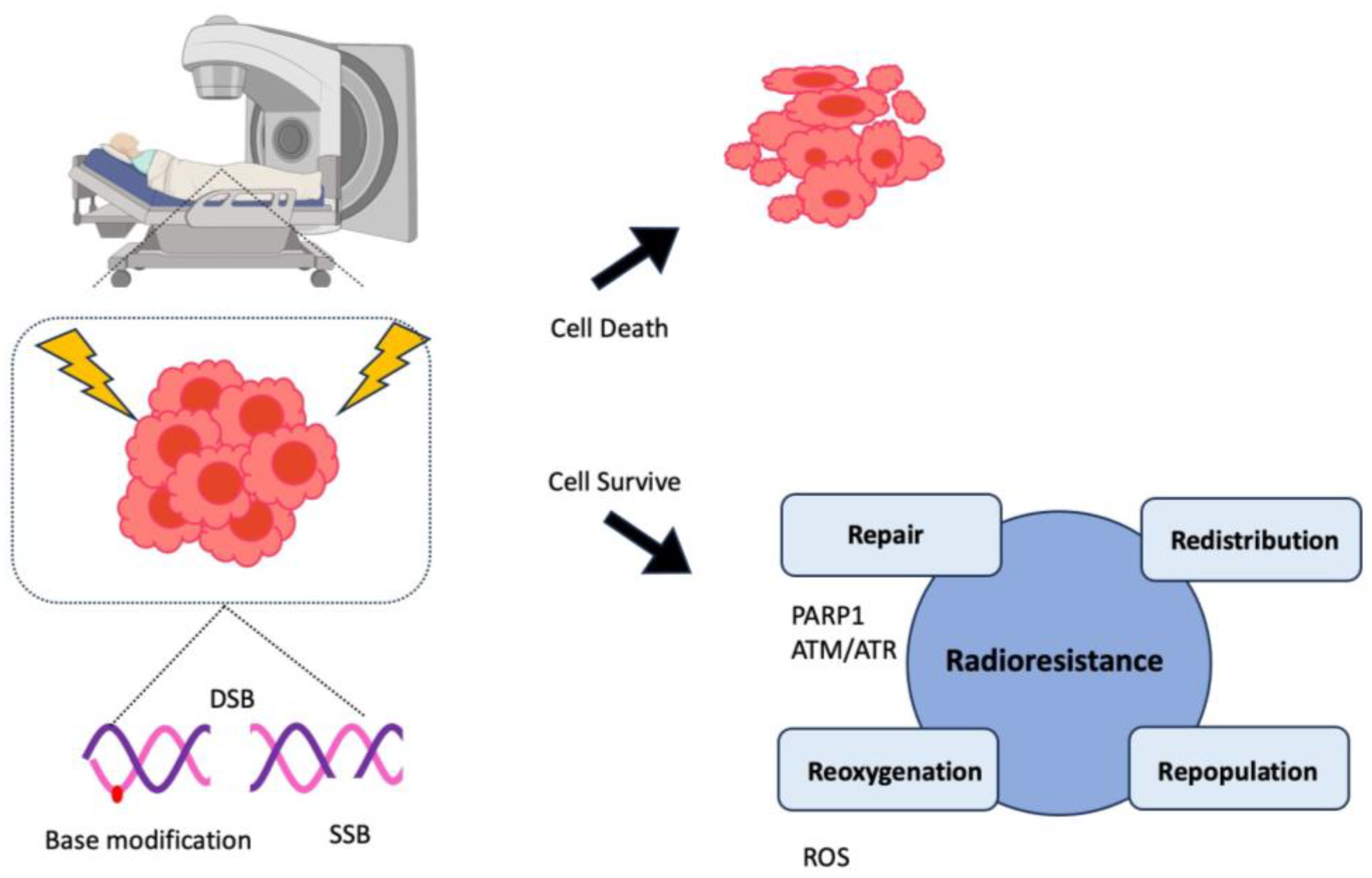
- Mladenov, E.; Magin, S.; Soni, A.; Iliakis, G. DNA double-strand break repair as determinant of cellular radiosensitivity to killing and target in radiation therapy. Front Oncol 2013, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.; Vlashi, E.; McBride, W.H. Radiation resistance of cancer stem cells: the 4 R’s of radiobiology revisited. Stem Cells 2010, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 2000, 355, 1491–1498. [Google Scholar] [CrossRef]
- Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Lancet 1995, 346, 265–269. [Google Scholar] [CrossRef]
- Kuban, D.A.; Tucker, S.L.; Dong, L.; Starkschall, G.; Huang, E.H.; Cheung, M.R.; Lee, A.K.; Pollack, A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008, 70, 67–74. [Google Scholar] [CrossRef]
- Zietman, A.L.; Bae, K.; Slater, J.D.; Shipley, W.U.; Efstathiou, J.A.; Coen, J.J.; Bush, D.A.; Lunt, M.; Spiegel, D.Y.; Skowronski, R.; et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010, 28, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Heemsbergen, W.D.; Al-Mamgani, A.; Slot, A.; Dielwart, M.F.; Lebesque, J.V. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol 2014, 110, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Beckendorf, V.; Guerif, S.; Le Prise, E.; Cosset, J.M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.M.; et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 2011, 80, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014, 15, 464–473. [Google Scholar] [CrossRef]
- Zagars, G.K.; Johnson, D.E.; von Eschenbach, A.C.; Hussey, D.H. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: long-term results of a prospective randomized study. Int J Radiat Oncol Biol Phys 1988, 14, 1085–1091. [Google Scholar] [CrossRef]
- Zumsteg, Z.S. Local versus systemic treatment intensification: what is the optimal strategy for localized prostate cancer? Prostate Cancer Prostatic Dis 2022, 25, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Markovic, D.; Patel, J.; Juarez, J.E.; Ma, T.M.; Shabsovich, D.; Nickols, N.G.; Reiter, R.E.; Elashoff, D.; Rettig, M.B.; et al. Radiation therapy dose and androgen deprivation therapy in localized prostate cancer: a meta-regression of 5-year outcomes in phase III randomized controlled trials. Prostate Cancer Prostatic Dis 2022, 25, 126–128. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol 2018, 4, e180039. [Google Scholar] [CrossRef]
- Nabid, A.; Carrier, N.; Martin, A.G.; Bahary, J.P.; Lemaire, C.; Vass, S.; Bahoric, B.; Archambault, R.; Vincent, F.; Bettahar, R.; et al. Duration of Androgen Deprivation Therapy in High-risk Prostate Cancer: A Randomized Phase III Trial. Eur Urol 2018, 74, 432–441. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Chen, M.H.; Renshaw, A.A.; Loffredo, M.; Kantoff, P.W. Androgen suppression and radiation vs radiation alone for prostate cancer - A randomized trial. Jama-J Am Med Assoc 2008, 299, 289–295. [Google Scholar] [CrossRef]
- Jones, C.U.; Hunt, D.; McGowan, D.G.; Amin, M.B.; Chetner, M.P.; Bruner, D.W.; Leibenhaut, M.H.; Husain, S.M.; Rotman, M.; Souhami, L.; et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011, 365, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Nabid, A.; Carrier, N.; Vigneault, E.; Van Nguyen, T.; Vavassis, P.; Brassard, M.A.; Bahoric, B.; Archambault, R.; Vincent, F.; Bettahar, R.; et al. Androgen deprivation therapy and radiotherapy in intermediate-risk prostate cancer: A randomised phase III trial. Eur J Cancer 2021, 143, 64–74. [Google Scholar] [CrossRef]
- Dubray, B.M.; Beckendorf, V.; Guerif, S.; Le Prise, E.; Reynaud-Bougnoux, A.; Levi, J.M.H.; Nguyen, T.D.; Hennequin, C.; Cretin, J.; Fayolle-Campana, M.; et al. Does short-term androgen depletion add to high-dose radiotherapy (80 Gy) in localized intermediate-risk prostate cancer? Intermediary analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). Journal of Clinical Oncology 2011, 29. [Google Scholar] [CrossRef]
- Dubray, B.M.; Salleron, J.; Guerif, S.G.; Le Prise, E.; Reynaud-Bougnoux, A.; Hannoun-Levi, J.-M.; Nguyen, T.D.; Hennequin, C.; Cretin, J.; Fayolle-Campana, M. Does short-term androgen depletion add to high dose radiotherapy (80 Gy) in localized intermediate risk prostate cancer? Final analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). 2016.
- Kapoor, A.; Hotte, S.J. Localized prostate cancer. Can Urol Assoc J 2016, 10, S138–S139. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; Van Tienhoven, G.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; Billiet, I.; et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010, 11, 1066–1073. [Google Scholar] [CrossRef]
- Bolla, M.; Collette, L.; Blank, L.; Warde, P.; Dubois, J.B.; Mirimanoff, R.O.; Storme, G.; Bernier, J.; Kuten, A.; Sternberg, C.; et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002, 360, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Pilepich, M.V.; Winter, K.; Lawton, C.A.; Krisch, R.E.; Wolkov, H.B.; Movsas, B.; Hug, E.B.; Asbell, S.O.; Grignon, D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys 2005, 61, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Bae, K.; Hanks, G.E.; Porter, A.; Grignon, D.J.; Brereton, H.D.; Venkatesan, V.; Lawton, C.A.; Rosenthal, S.A.; Sandler, H.M.; et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 2008, 26, 2497–2504. [Google Scholar] [CrossRef]
- Keating, N.L.; O’Malley, A.J.; Smith, M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006, 24, 4448–4456. [Google Scholar] [CrossRef]
- Bolla, M.; de Reijke, T.M.; Van Tienhoven, G.; Van den Bergh, A.C.; Oddens, J.; Poortmans, P.M.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009, 360, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Segundo, C.; Cabeza Rodriguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; San-Segundo, C.G.; Rodriguez, M.A.C.; Sole, J.M.; Olive, A.P.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy and risk-adapted androgen deprivation in localised prostate cancer (DART 01/05): 10-year results of a phase 3 randomised, controlled trial. Lancet Oncol 2022, 23, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Bae, K.; Hanks, G.E.; Porter, A.; Grignon, D.J.; Brereton, H.D.; Venkatesan, V.; Lawton, C.A.; Rosenthal, S.A.; Sandler, H.M.; et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. Journal of Clinical Oncology 2008, 26, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.A.F.; Lin, X.; Hanks, G.E.; Lepor, H.; Grignon, D.J.; Brereton, H.D.; Bedi, M.; Rosenthal, S.A.; Zeitzer, K.L.; Venkatesan, V.M.; et al. Duration of Androgen Deprivation in Locally Advanced Prostate Cancer: Long-Term Update of NRG Oncology RTOG 9202. Int J Radiat Oncol Biol Phys 2017, 98, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Dignam, J.J.; Hamstra, D.A.; Lepor, H.; Grignon, D.; Brereton, H.; Currey, A.; Rosenthal, S.; Zeitzer, K.L.; Venkatesan, V.M.; Horwitz, E.M.; et al. Time Interval to Biochemical Failure as a Surrogate End Point in Locally Advanced Prostate Cancer: Analysis of Randomized Trial NRG/RTOG 9202. Journal of Clinical Oncology 2019, 37, 213. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Joseph, D.; Lamb, D.S.; Spry, N.A.; Duchesne, G.; Matthews, J.; Atkinson, C.; Tai, K.H.; Christie, D.; Kenny, L.; et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): an open-label, randomised, phase 3 factorial trial. Lancet Oncol 2014, 15, 1076–1089. [Google Scholar] [CrossRef]
- Denham, J.W.; Joseph, D.; Lamb, D.S.; Spry, N.A.; Duchesne, G.; Matthews, J.; Atkinson, C.; Tai, K.H.; Christie, D.; Kenny, L.; et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol 2019, 20, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Steigler, A.; Lamb, D.S.; Joseph, D.; Turner, S.; Matthews, J.; Atkinson, C.; North, J.; Christie, D.; Spry, N.A.; et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 2011, 12, 451–459. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Kuo, M.T. Improving radiotherapy in cancer treatment: Promises and challenges. Oncotarget 2017, 8, 62742–62758. [Google Scholar] [CrossRef]
- Palacios, D.A.; Miyake, M.; Rosser, C.J. Radiosensitization in prostate cancer: mechanisms and targets. BMC Urol 2013, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, S.; Walker, A.J.; Gandhi, N.; Narang, A.; Wild, A.T.; Hales, R.K.; Herman, J.M.; Song, D.Y.; Deweese, T.L.; Antonarakis, E.S.; et al. Molecularly targeted agents as radiosensitizers in cancer therapy--focus on prostate cancer. Int J Mol Sci 2013, 14, 14800–14832. [Google Scholar] [CrossRef]
- Komorowska, D.; Radzik, T.; Kalenik, S.; Rodacka, A. Natural Radiosensitizers in Radiotherapy: Cancer Treatment by Combining Ionizing Radiation with Resveratrol. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int J Nanomedicine 2021, 16, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, G.; Joensuu, T.; Nokisalmi, P.; Reddy, C.; Isola, J.; Ruutu, M.; Kouri, M.; Kupelian, P.A.; Collan, J.; Pesonen, S.; et al. A phase I/II trial of gefitinib given concurrently with radiotherapy in patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys 2010, 78, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Vuky, J.; Pham, H.T.; Warren, S.; Douglass, E.; Badiozamani, K.; Madsen, B.; Hsi, A.; Song, G. Phase II study of long-term androgen suppression with bevacizumab and intensity-modulated radiation therapy (IMRT) in high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012, 82, e609–615. [Google Scholar] [CrossRef]
- Ahmad, I.U.; Forman, J.D.; Sarkar, F.H.; Hillman, G.G.; Heath, E.; Vaishampayan, U.; Cher, M.L.; Andic, F.; Rossi, P.J.; Kucuk, O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer 2010, 62, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: the enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Chendil, D.; Ranga, R.S.; Meigooni, D.; Sathishkumar, S.; Ahmed, M.M. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 2004, 23, 1599–1607. [Google Scholar] [CrossRef]
- Veeraraghavan, J.; Natarajan, M.; Herman, T.S.; Aravindan, N. Curcumin-altered p53-response genes regulate radiosensitivity in p53-mutant Ewing’s sarcoma cells. Anticancer Res 2010, 30, 4007–4015. [Google Scholar]
- Takahashi, J.; Misawa, M.; Iwahashi, H. Combined treatment with X-ray irradiation and 5-aminolevulinic acid elicits better transcriptomic response of cell cycle-related factors than X-ray irradiation alone. Int J Radiat Biol 2016, 92, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Tanaka, N.; Hori, S.; Ohnishi, S.; Takahashi, H.; Fujii, T.; Owari, T.; Ohnishi, K.; Iida, K.; Morizawa, Y.; et al. Dual benefit of supplementary oral 5-aminolevulinic acid to pelvic radiotherapy in a syngenic prostate cancer model. Prostate 2019, 79, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Semenas, J.; Dizeyi, N.; Persson, J.L. Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer. Drug Des Devel Ther 2013, 7, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Wang, J.; Freeman, M.L.; Kirschner, A.N. Radiosensitization by enzalutamide for human prostate cancer is mediated through the DNA damage repair pathway. PLoS One 2019, 14, e0214670. [Google Scholar] [CrossRef]
- de, V., II; Luiting, H.B.; Roobol, M.J. Active Surveillance for Prostate Cancer: Past, Current, and Future Trends. J Pers Med 2023, 13. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2023, 388, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Alvarez, J.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; McCollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef]
- Chen, R.C.; Basak, R.; Meyer, A.M.; Kuo, T.M.; Carpenter, W.R.; Agans, R.P.; Broughman, J.R.; Reeve, B.B.; Nielsen, M.E.; Usinger, D.S.; et al. Association Between Choice of Radical Prostatectomy, External Beam Radiotherapy, Brachytherapy, or Active Surveillance and Patient-Reported Quality of Life Among Men With Localized Prostate Cancer. JAMA 2017, 317, 1141–1150. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- Davis, M.K.; Rajala, J.L.; Tyldesley, S.; Pickles, T.; Virani, S.A. The Prevalence of Cardiac Risk Factors in Men with Localized Prostate Cancer Undergoing Androgen Deprivation Therapy in British Columbia, Canada. J Oncol 2015, 2015, 820403. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Zhang, K.W.; Collier, A.; Linch, M.; Calaway, A.C.; Ponsky, L.; Guha, A.; Ghosh, A.K. Cardiovascular Complications of Prostate Cancer Therapy. Curr Treat Opt Card 2020, 22. [Google Scholar] [CrossRef]
- Narayan, V.; Ross, A.E.; Parikh, R.B.; Nohria, A.; Morgans, A.K. How to Treat Prostate Cancer With Androgen Deprivation and Minimize Cardiovascular Risk A Therapeutic Tightrope. Jacc-Cardiooncol 2021, 3, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Tzortzis, V.; Samarinas, M.; Zachos, I.; Oeconomou, A.; Pisters, L.L.; Bargiota, A. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones (Athens) 2017, 16, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Ozguroglu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Quon, H.; McNutt, T.; Lee, J.; Bowers, M.; Jiang, W.; Lakshminarayanan, P.; Cheng, Z.; Han, P.; Hui, X.; Shah, V.; et al. Needs and Challenges for Radiation Oncology in the Era of Precision Medicine. Int J Radiat Oncol Biol Phys 2019, 103, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Hernando Polo, S.; Moreno Munoz, D.; Rosero Rodriguez, A.C.; Silva Ruiz, J.; Rosero Rodriguez, D.I.; Counago, F. Changing the History of Prostate Cancer with New Targeted Therapies. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Genes Dev 2018, 32, 1105–1140. [Google Scholar] [CrossRef]
- Hall, W.A.; Bergom, C.; Thompson, R.F.; Baschnagel, A.M.; Vijayakumar, S.; Willers, H.; Li, X.A.; Schultz, C.J.; Wilson, G.D.; West, C.M.L.; et al. Precision Oncology and Genomically Guided Radiation Therapy: A Report From the American Society for Radiation Oncology/American Association of Physicists in Medicine/National Cancer Institute Precision Medicine Conference. Int J Radiat Oncol Biol Phys 2018, 101, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.E.; Liu, V.Y.T.; Michalski, J.; Davicioni, E.; Berlin, A.; Simko, J.P.; Efstathiou, J.A.; Tran, P.T.; Sandler, H.M.; Hall, W.A.; et al. Genomic Classifier Performance in Intermediate-Risk Prostate Cancer: Results From NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiat Oncol Biol Phys 2023. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Park, W. Radiotherapy in prostate cancer treatment: results of the patterns of care study in Korea. Radiat Oncol J 2017, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Bae, G.H.; Kim, J.H.; Kim, J. Outcomes of prostate cancer patients after robot-assisted radical prostatectomy compared with open radical prostatectomy in Korea. Sci Rep 2023, 13, 7851. [Google Scholar] [CrossRef]
- Heath, E.I.; Dyson, G.E.; Cackowski, F.C.; Hafron, J.; Powell, I. Treatment Intensification Patterns and Utilization in Patients with Metastatic Castration-Sensitive Prostate Cancer. Clin Genitourin Cancer 2022, 20, 524–532. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





