1. Introduction
Since the beginning of 2023, dengue outbreaks of significant magnitude have been recorded in the WHO Region of the Americas, with close to three million suspected and confirmed cases of dengue reported so far this year, surpassing the 2.8 million cases of dengue registered for the entire year of 2022. Added to this, all four dengue virus serotypes (DENV1, DENV2, DENV3, and DENV4) are present in the Region of the Americas [
1].
Chikungunya was first detected in the region in 2013, on the island of Saint Martin, and a year later, it had spread to most countries in the region. More than one million cases were reported in the first year after its introduction to the continent. In the first four months of 2023, an increase in the circulation of chikungunya was detected in the region, with more than 214,000 cases reported. [
2]. Despite the global reduction in the disease caused by the Zika virus since 2017, the circulation of this mosquito-transmitted virus has been confirmed in 89 countries worldwide. Although incidence levels remain low, sporadic increases have been observed in recent years [
3]. On the other hand, the evidence that confirmed
Ae.
aegypti was the primary vector in the Americas, and the link between the Zika virus, microcephaly, and other neurological disorders, such as Guillain-Barre syndrome, led the WHO to declare this disease a health emergency of international concern [
4,
5].
Dengue infection is a recurrent cause of hospitalizations, especially in children [
6,
7], in February 2020, the Pan American Health Organization (PAHO) released an epidemiological alert for dengue in the Americas due to an increase in the incidence rate (81.51 cases per 100,000 population), seven times more than reported during the same period in 2019 (11.09 cases per 100,000 population) and mortality related to DHF have increased annually generating a significant economic burden for the health systems of Latin American countries [
8,
9].
Mexico, as in most countries that belong to the subtropical area of the American continent,
Ae.
aegypti is the primary vector of dengue, chikungunya fever, and Zika viruses. Until epidemiological week number 31 of 2023, in our country, there have been 4,554 confirmed cases of dengue without alarm data, 3,468 cases with alarm data, 249 cases of severe dengue, 13 deaths due to dengue infection, and 3 cases of infection by Zika virus [
10]. Likewise, the General Directorate of Epidemiology determined the age groups most affected in 2023 by dengue infections. The group of 10-14 years of age has been the most affected by non-severe dengue, and the groups of 10-14 and 15-19 years were the most affected by severe dengue [
11].
Globally, current efforts to contain the transmission and global spread of dengue, chikungunya fever, and Zika have not proven effective. In Mexico, the Official Standard NOM-032-SSA2-2014 mentions the sustained management of vector control with chemical, biological, and physical control to avoid, to the extent possible, the risk of transmission of one or more vector-borne diseases [
12]. The lack of success in control strategies is likely related to the excessive and widespread use of insecticides that has caused the development of mosquitoes resistant to these products throughout the world [
13] and the tendency to substitute a particular pesticide, for which resistance has been detected, by a new one (susceptible mosquito). However, it presents evidence of harmful effects derived from its use [
14]. In addition to the aforementioned,
Ae.
aegypti is a vector that remains inside houses throughout the day, limiting some of these strategies, such as spatial spraying and using bed nets treated or not with insecticides. However, it is very important to mention that vector control in Mexico is entirely reactive to reports of clinical cases that are delayed and inherent to the passive surveillance system. Additionally, this activity is carried out with limited human, material, and financial resources [
15,
16].
Therefore, it is necessary to reformulate current
Aedes arboviral disease control and prevention strategies. We have learned about the serious impact on disease incidence when primary health care fails to identify suspected cases, affecting confirmatory medical procedures such as laboratory diagnosis, case confirmation, and lack of timely deployment of vector control measures. Surveillance is the systematic collection, analysis, and interpretation of data essential for planning and implementing control activities on adult mosquito populations, before and after outbreaks; It is also the first indicator to evaluate a significant and measurable impact on vector populations and the transmission of these diseases [
17]. That is why this work proposes the design and evaluation of a low-cost attractant-sticky trap that allows monitoring of adult populations of
Ae.
aegypti at the indoor house environments. Future use of field assessment data would support establishing the precise moment and the appropriate strategy to maintain an immediate and sustained reduction in vector and case controls.
2. Materials and Methods
2.1. Development of the UANL Attractant Sticky Trap (UANL Aedes Trap®)
Based on a bibliographic review [
18], we selected the individual compounds (lactic acid, hexanoic acid, ammonium chloride, and linalool) and concentrations showing significant percentages of attraction to
Ae.
aegypti females. Following higher documented results along with our lab experiences, we decided to prepare and run attraction assays with only two blends with the chosen compounds and concentrations, i.e., Blend 1: Linalool 10% (Sigma Aldrich 97%; Merck, St. Louis, USA), Lactic acid 1% (CTR 88.50%; CTR Scientific, Nuevo Leon, Mexico), Hexanoic acid 0.1% (Sigma Aldrich 97%) and Ammonium chloride 0.1% (CTR 99%). Blend 2: Linalool 15%, Lactic acid 10%, Hexanoic acid 1% and Ammonium chloride 1%. We named our prototype UANL
Aedes Trap® to protect University and scientists' property rights.
2.2. Attraction Bioassay Screening in the Laboratory
Aedes Aegypti eggs were obtained from the Insectary of the Regional Center for Research in Public Health (CRISP). For the bioassays, females (4 to 7 days old), maintained at 27 ± 2 ºC, 80 ± 10% RH with a photoperiod of 12:12 light/dark, were used. Mosquitoes were maintained only with 10% sugar solution soaked in cotton swabs. For the attraction tests of candidate compounds, the High-Throughput Research Processing System (HITSS) was used for the initial screening. The system has a modular design that allows the evaluation of contact irritation, spatial repellency, and toxicity of the products [
19]. Whatman no. 2 filter papers (4 × 2 cm) impregnated with 50 µL of the two testing blends were individually placed in the treatment cylinder. Once the treatment cylinder was prepared, it was attached to the plexiglass cylinder at one end of the HITSS system, and the negative control cylinder (Whatman No. 2 (4 × 2 cm) impregnated with 50 µL acetone (Binden 97%) was attached to the other end. The butterfly valves were kept closed at the junction with both cylinders. Fifteen mosquitoes were placed in the central compartment, and the entire system was completely covered with a dark cloth to prevent the passage of light. The mosquitoes remained in the plexiglass chamber for acclimatization for 30 seconds, and then the valves were opened simultaneously and exposed to the treatments for 20 min. Four repetitions were carried out for each blend, including the BG trap bait (lure) considered golden standard as positive control (0.50 g of the BG-Sentinel bait), and negative control. The system was cleaned with acetone and aerated for 30 min between bioassays with different products or concentrations. Blend yielding the higher attractiveness results was selected for an additional larger flying volume evaluation under insectary conditions.
2.2.1. Evaluation of Selected Blend 2 Consistency in Larger Flying Entomological Cages
Two experiment series were carried out to validate the attraction consistency of Blend 2 in larger flying spaces: in the first, individual stripes of Whatman no. 2 filter paper (4 × 2 cm) were impregnated with 100 µL of the treatments (Blend 1, Blend 2, negative control: acetone or positive control: 5 g BG bait placed in Petri dish lids), and 20 mosquitoes were introduced into a metal cage (90 × 90 × 90 cm); they were allowed to acclimatize for 5 min. After 30 minutes, the mosquitoes approaching or landing on the impregnated stripes were counted and removed. In the second type of experiment, the stripes impregnated were introduced, in addition to the use of an electricity 110-V fan (speed 1.0 m/s, 8" diameter Steren S.A. de C.V., Mexico City, Mexico) to favor the dispersion of the blend; similarly, 20 mosquitoes were introduced and allowed to acclimatize for 5 min. After 30 min, the mosquitoes that approached or landed on the stripes were counted and removed. Each experiment lasted three hours, with observations and the introducing of new mosquitoes every 30 min. Each treatment stripe with and without fan was repeated 5 days in the insectary. The laboratory was ventilated for 24 hours between bioassays with different treatments.
2.3. Prototype Design of UANL Aedes Trap for Assays under Semi-Field and Field Conditions
Based on the genetic traits of
Ae. aegypti flying and host-seeking behavior [
20], three elements were incorporated in our UANL
Aedes trap: firstly, a white color cardboard pyramid mold with entrance windows or “holes” near the floor edge (13 × 24 cm, with four 5 × 3 cm side windows; Mod 42 × 24, white; Multi Empaque Monterrey S.A de C.V, Nuevo Leon, Mexico), secondly, the interior walls of the trap were lined with odorless adhesive paper Sku Travo l0001 (Tetengo S. de R.L de C.V., Nuevo Leon, Mexico), and the floor of the trap with adhesive paper type cat paper Eco Company S.A., Cartago, Costa Rica). Thirdly, a second variant was integrated with a small fan (4”, 12 V, 1.44 W, 150 mAm current consumption, version 1.3, silent function: 29.8 dBA; Electronica Steren SA. De C.V,) which was placed on the upper part of the trap to facilitate the suction of the mosquitoes and the dispersal of the attractant blend (
Figure 1).
2.3.1. Attractant Blend 2 Cartridge
Using an Eppendorf tube (1.5 mL), 0.5 g of polyacrylate hydrogel (Green Forest Mexico©, Puebla, Mexico) was activated with water, and 1 mL of our attractant lure (Blend 2) was added. The blend was prepared 24 hours before the bioassays and placed inside the trap 5 min before starting each test. Regarding mosquitoes for assays, Field collected larvae of Ae. aegypti and reared to adult stages in the CRISP/INSP insectary (F1-F5), from 4 to 7 days old without blood-feeding, were used.
2.3.2. Semi-field Assays of UANL Aedes Trap at Greenhouse Scale
The bioassays were carried out in a large 10 × 50 × 5 m long greenhouse of the experimental field station of the Regional Public Health Research Center (CRISP-INSP) located in the Río Florido Ejido, Tapachula, Chiapas, from March to May 2023. During the bioassays, the average ambient temperature was 35.6 ºC and 62% Relative Humidity.
In order to obtain the real capture percentage offered by the UANL
Aedes trap alone and see if there was an improvement in the capture percentages when adding each of the factors that were included in the design (bait and fan), four types of bioassays were carried out in 2 × 2 × 2 m cages. First, the attractant sticky trap alone (AST) was placed, a plant (
Mentha spicata) as a resting refuge, and a container with 10% glucose as food for the mosquitoes. Second, the attractant sticky trap plus our selected attractant bait (Blend 2) (ASTB), a plant (
Mentha spicata), and a container with 10% glucose. Third, the attractive sticky trap with the attractant bait (Blend 2) and the use of a fan (ASTBF), a plant (
Mentha spicata), and a container with 10% glucose. In the fourth, the golden standard or positive control, the BG-Sentinel trap with its integrated BG-bait (BG-SB), a plant (
Mentha spicata), and a container with 10% glucose (
Figure 1).
Once our five-in-a-row-screened cages were set, 100 females of Ae. aegypti were released, and observations were made for 10 min to ensure the acclimatization of the mosquitoes and flight ability. The first and second readings of trapped mosquitoes were taken 24 hours and 7 days after the first release, respectively. Once the 7-day reading was completed, a second release was conducted with 100 fresh female mosquitoes without changing the traps. As a last step, the last reading was taken after 24 hours or day 8. Five daily repetitions were carried out for each AST, ASTB, and ASTBF, although heavy rainfall allowed only 3 repetitions for the BG-SF. The screened cages were ventilated 24 hours a day between bioassays.
2.3.3. Field Evaluation of UANL Aedes Trap in Indoor Household Environments
Based on higher semi-field and field results, we decided to evaluate and compare our UANL Aedes trap (ASTBF) versus standard BG-Sentinel The bioassays were carried out on Jun 2023; these neighborhoods were preselected because, according to data from the Secretary of Health of the state of Chiapas, they are within the areas that each year report the highest number of confirmed dengue cases. Before placing the traps, an informative talk about the design and use of the traps was given to the head of the family or housewife (the number of family members who were willing to attend could participate in the talk); the next step was to request the consent of the head of the family for his house and family to participate in the field evaluation.
Five houses were selected in different neighborhoods of the city of Tapachula, Chiapas (La Primavera, 5 de Febrero, Barrio Nuevo, and Libertad subdivision), in each of them, with the prior authorization and training of the head of the family, one 110-V powered UANL Aedes trap (ASTBF) was placed resting over the living room or kitchen chairs and similar resting surfaces, in a height range of 60-80 cm. The traps were checked out daily for maintenance and without removing trapped mosquitoes until 7 days were counted and identified. Likewise, in three of the selected houses groups (Barrio Nuevo and 5 de Febrero), powered BG-Sentinel traps (BG-SB) were placed on the floor, and they were similarly removed 7 days later for counting and identifying the trapped mosquitoes.
2.4. Statistic Analysis
All data generated were checked out for normality assumptions and variance homogeneity, through the Sapiro Wilks and Levine tests, respectively. The data obtained in the HITSS system, in 90 x 90 × 90 cm entomological cages, the capture percentages obtained in semi-field at 24 hours, 7 days after the first release, and 24 hours after the second release presented normality. All data were analyzed using a one-way ANOVA and a Tukey test to determine the difference between treatments with the AMOVI Statistical Packages. 3.2.21, The Jamovi Project (2023) Sydney, Australia and
https://artofstat.com/web-apps 2022 Bernhard Kllngenberg. For the data obtained in the field, a Welch's T-test was calculated to compare the proportions of mosquforoes captured between the two types of traps and the sex ratios. Likewise, for the data obtained in the HITSS system, the Spatial Activity Index (SAI = ((Nc-Nt) / (Nc+Nt)) × (Nm/N)) was calculated where Nc = number of mosquitoes in the control cylinder, Nt = number of mosquitoes in the cylinder treatment and Nm = total number of mosquitoes. Results closer to -1 indicate greater spatial attraction to the treatment [
19].
3. Results
3.1. Tests in the HITSS Screening System
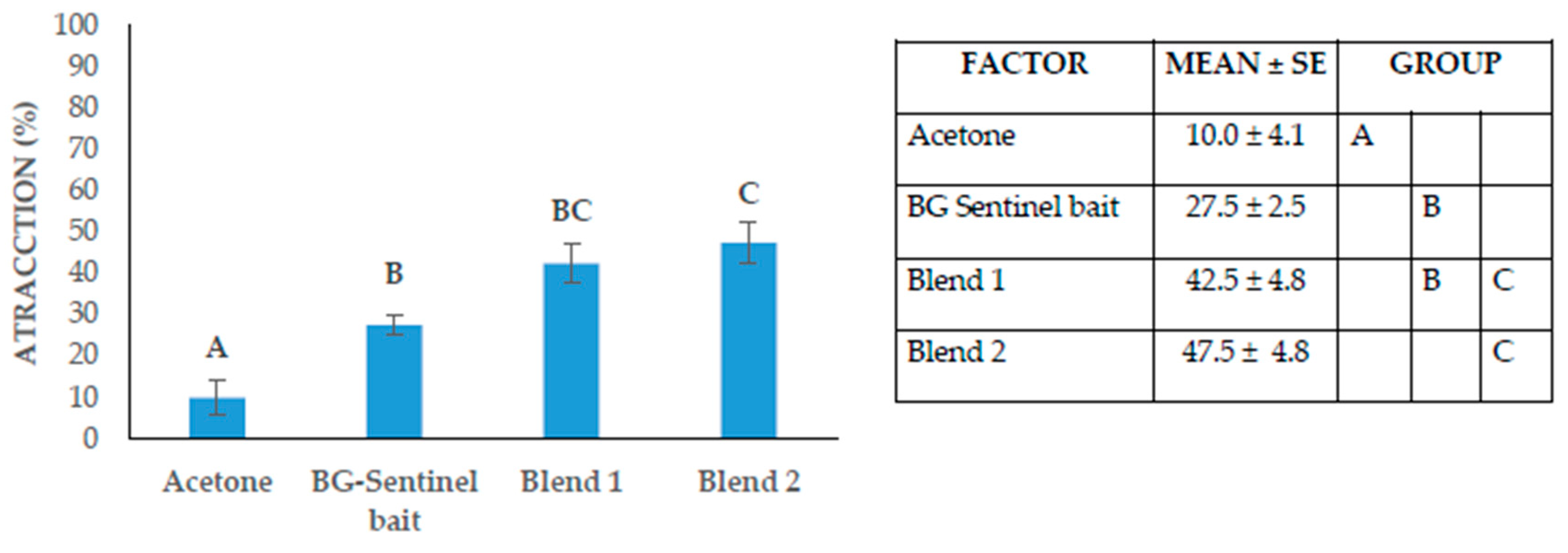
As seen in
Figure 2, there were significant differences in the attraction percentages obtained in the HITSS system. The mean attraction percentage for the negative control was 10.0 ± 4.1%, 27.5 ± 2.5% for the BG-Sentinel bait positive control, 42.5 ± 4.8% for Blend 1, and the highest 47.5 ± 4.8% for Blend 2 (
F = 16.6,
df = 3,
p = < .001). According to the SAI calculations, the negative control obtained a Spatial Activity Index of -0.03 ± 0.0, the positive control -0.18 ± 0.05, 0.2 ± 0.0 for Blend 1 ± and -0.45 ± 0.05 for Blend 2. With the data obtained in the HITSS system, Blend 2 showed the highest percentage of attraction and SAI.
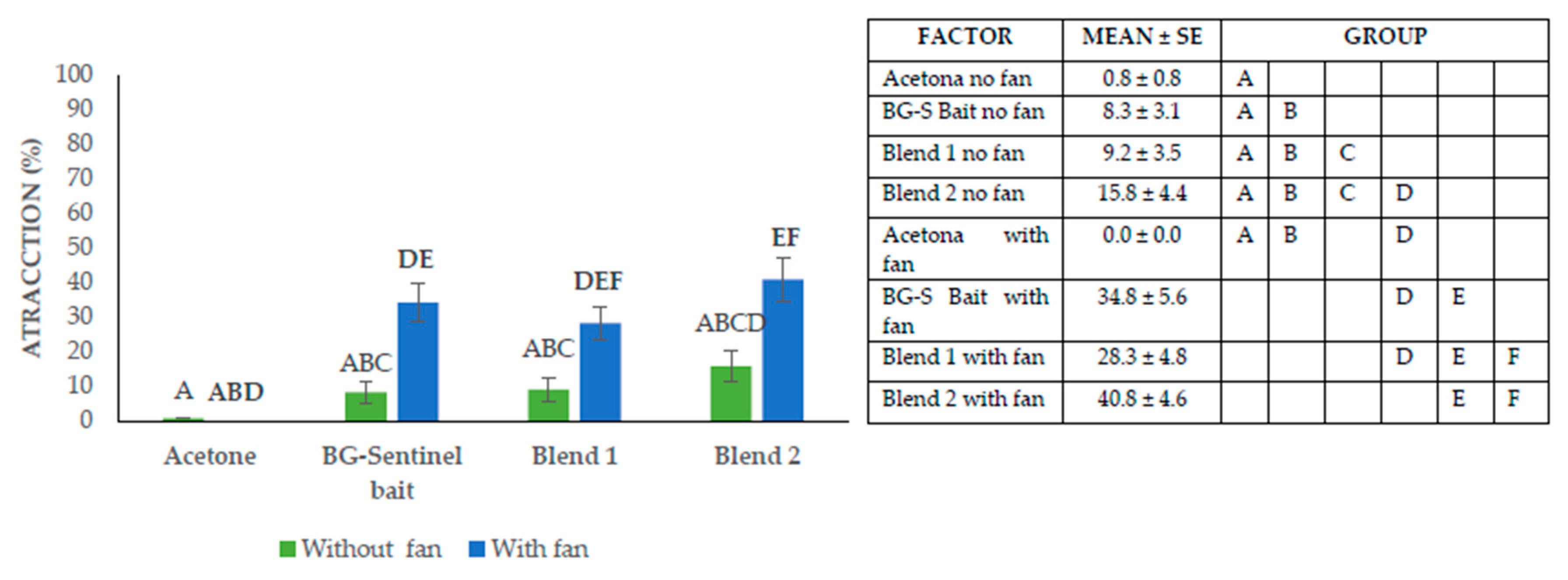
3.2. Consistency of Blend 2 Attraction in Larger Flying Entomological Cages
Figure 3 showed that in the tests without a fan, the attraction mean was reduced, as recorded for the negative control made of acetone stripes, showing the lowest result of 0.83 ± 0.8%. Similarly, whereas the positive control BG-Sentinel bait stripe was 8.3 ± 3.1%, Blend 1 obtained 9.2 ± 3.5%, and Blend 2 showed the highest performance, 15.8 ± 4.4%, under lack of fan air dispersion. Conversely, in the tests where a fan was used, the negative control yielded 0.0 ± 0.0% attraction, the positive control 34.8 ± 5.6%, Blend 1 produced 28.3 ± 4.8%, whereas Blend 2 yielded the highest mean as 40.8 ± 4.6% (
F = 34.9,
d f= 5,
p = < .0001). With the data obtained in an open monitoring system provided by the 90 x 90 cm × cages, it was concluded that Blend 2, helped by the fan, was the mix that presented the best percentage of attraction, followed by the gold standard, the BG-Sentinel bait with the integrated fan.
3.3. Semi-Field Evaluation of UANL Aedes Trap at Greenhouse Scale
Larger 2 × 2 × 2 m screened cages changed results on the side of the BG-SB golden standard trap. The mean capture percentages in the semi-field tests 24 hours after the first release of 100 female mosquitoes were 9.6 ± 2.7% for AST, 21.6 ± 3.5% for Blend 2 or ASTB, 43.2 ± 4.0% for ASTBF, and the highest result 63.7 ± 11.1% for the BG-SB with fan system (
F = 17.2,
df = 3,
p = 0.002) (
Table 1). Capture means recorded on 7 days after the first release, 12.1 ± 3.3% accounted for the AST; 27.0 ± 5.8% for the ASTB, and 64.3 ± 11.6% for the BG-SB (
F = 13.7,
df = 2,
p = 0.001) (
Table 1). Due to adverse weather conditions causing potential damage to the traps, the capture percentages were not recorded for 7 days after the first release for the ASTBF trap. Finally, the capture percentages in the semi-field tests 24 hours after the second release were 2.4 ± 0.8% for the AST, 10.0 ± 1.8 % for the ASTB; 40.8 ± 2.9% for the ASTBF and 65.3 ± 7.5% for the BG-SB (
F = 13.77,
df = 3,
p = 0.0028) (
Table 1). With the data obtained in the semi-field tests, it is concluded that the BG-Sentinel trap with its bait and integrated fan was the one that presented the highest attraction percentages 24-h and 7 days, after the first release, respectively, and 24-h after the second release.
3.4. Field Evaluation of UANL Aedes Trap
Only our ASTBF trap was evaluated in the field and placed in the living room or bedroom of the selected houses during a 7-day sampling period. At the same time, a BG-SB was placed in the yard or hallway of the chosen houses. In total, five of our ASTBF captured 28 mosquitoes with a mean of 6.0 ± 1.5 (64.3% females and 37.7% males), while the BG-SB captured a close number of 30 mosquitoes with a mean of 10 ± 2.6 (63.3% females and 33.7% males). No significant difference in total catches between the UANL
Aedes trap (ASTBF) and the BG-Sentinel trap was observed (
T-Welch = -0.46,
df = 1,
p = 0.65). Likewise, there wasn´t a significant difference in the total captures of females (
T-Welch = -0.24,
d f = 1,
p= 0.81) and males (
T-Welch = -0.27,
df = 1,
p = 0.78) between the UANL
Aedes traps (ASTBF) and the BG-Sentinel traps (
Table 2). With the data obtained in the field tests, we can observe no difference in the number of captures of
Ae. aegypti mosquitoes (regardless of sex) for both traps. Therefore, the UANL
Aedes trap has an attraction percentage equal to the BG-Sentinel gold standard attractant trap.
4. Discussion
It is documented that the chemical attractants evaluated in our study have demonstrated significant percentages of attraction for the
Ae.
aegypti, both individually and in blends [
18]. The attraction results obtained in the laboratory and insectary varied depending on the attractant concentration and the fan's use. Blend 2 was the treatment selected to be used as attractant bait for our trap since it presented the highest attraction rates in the HITSS system (47.5 ± 4.8%), spatial activity index (-0.45 ± 0.05), as well as replicating these results consistency under larger flying spaces in entomological cages (40.8 ± 4.6%) including when using a fan to help attractant plumes dispersion.
Volatility is the tendency of molecules to evaporate; the chemical nature of the attractant also explains it [
21]. Several authors have added battery or powered fans to enhance odor dispersal to exploit the property. In our 90 × 90 × 90 cm insectary cages, we noted that when a fan blows the wind, better attraction percentages were confirmed in our even larger 2 × 2 × 2 semi-field assays. Similarly, without a fan where only the UANL
Aedes trap® (Blend 2: linalool 15%, lactic acid 10%, hexanoic acid 1%, and ammonium chloride 1%) was used, a capture percentage of 21.6 ± 3.5% was recorded 24 hours after the first release; however, and an increase of 50% was recorded (43.2 ± 4.0%) with the fan. How volatile attractants are released depends on the compound's diffusion method, which is affected by the diffusion coefficient of the active ingredient and the product's chemical properties. To ensure proper dispersion and protection of the product, techniques like microencapsulation, a suitable matrix, and fans are employed. These techniques consider the characteristics of the active ingredient, releaser, and environmental factors [
21].
In the semi-field assays, we found the BG-Sentinel trap showed the best capture percentages in the three reading times (above 60%) but not in the laboratory tests, where only a 2 × 4 cm Whatman paper with its bait was exposed (8.3%) and with the fan (34.8%). This was confirmatory for us about the importance of proper bait formulation, proper trap design, and fan integration. On the other hand, semi-field assays placing only the pyramid cardboard body, the attractant sticky trap (AST), capture percentages between 10% and 40% were shown for ASTB and ASTBF. We hypothesize that these percentages could be increased through a slow-release matrix of the attractant bait. A second factor to consider besides the fan is to improve trap performance. However, a few complaining comments from dwellers about the trap's electricity consumption were recorded. Future trap designs should avoid using fans to lower prices through electricity consumption and to receive better acceptance by homeowners. On the other hand, although our results in the semi-field tests showed significant differences between BG-SB and UANL Aedes trap (ASTBF), and in favor of the golden standard BG-Sentinel, in the field tests, there was no statistically evident difference in the capture rates of Ae. aegypti; i.e., our UANL Aedes trap (ASTBF trap) captured the same number of mosquitoes as the gold standard BG-SB in 7 nights. The optimistic results motivate us to consider the possibility of a more extensive cluster randomized field trial and establishing new variables such as different seasons of the year and locations.
Vector population traps, such as GAT and BG-Sentinel, can be used to surveillance
Aedes species before and after outbreaks. Johnson et al. compared the use of these traps for gravid
Aedes females and concluded that using them both complementarily is better [
22]. GAT is more versatile and cost-effective than BG-Sentinel, which is more expensive and only sometimes well-accepted. Our study showed the price difference between BG-Sentinel and our low-cost sticky trap made with over-the-counter products. Although this study has promising results, it is essential to consider a few things when conducting field tests. During a case-control study, Parra et al. faced many obstacles, including losing 660 traps and changing the location of 40 during a 36-week study. The leading causes of loss were inaccessible homes, holidays, and rainy days. Despite these setbacks, their traps captured 6,024
Aedes and 1,333
Culex mosquitoes. Similarly, our semi-field tests were affected by harsh weather conditions, but we could still conclude the study successfully. [
23].
During field assays, our UANL
Aedes traps were well-accepted by families. In contrast, the BG-Sentinel had to be relocated due to discomfort caused by its smell. Our trap showed encouraging results for intra-home monitoring of
Ae. aegypti. However, the design needs improvement for outdoor functionality. Our proposed low-cost entomological monitoring system can help surveillance
Aedes mosquito populations indoors- and outdoors. This is crucial because standardized protocols for dengue surveillance rely on Aedic indices that are considered weekly estimators of transmission risk. Sometimes the surveillance can be complemented with the use of ovitraps, which are sensitive to detect the presence of the vector, especially in areas where the level of infestation of
Ae. aegypti is low or evaluate pupal indices as they are considered more accurate because most emerge as adults [
24]. However, despite their great usefulness, larval indices do not always show a good correlation with the abundance of adult mosquitoes because a female can distribute her eggs in more than one breeding site. Pupal indices are rarely used due to the practical difficulties and labor of counting pupae, particularly those found in large containers [
25]. According to Parra et al., traditional methods of measuring the risk of dengue infection in an area should not rely solely on immature stages of the mosquito, as only adult females can spread the virus through their bite. Instead, they suggest evaluating the entomological index obtained from an adult trap and comparing it with the cost of monitoring using the Breteau index (BI). Their results showed that the entomological index was positively correlated with the incidence of dengue, particularly during intervals with less intense vector control measures. Additionally, the operating costs of the adult index were lower than those of the BI, requiring 71.5% fewer human resources [
23,
25].
As a result, alternative methods to estimate the abundance of adults or improvements to the available techniques are beginning to grow [
26]. Actual vector demographic data is needed to monitor the study area and sub-areas such as city blocks. The adult rates of
Ae. aegypti described in the work of Parra et al., and Ong et al., as well as the use of attractive sticky traps such as the one developed in this study, can be applied at various levels of spatial aggregation for complete monitoring of the study area. However, although these indices and the use of adult traps are suitable for predicting dengue risk, they need to be tested and validated in various settings before routine use [
23,
26].
5. Conclusions
There is an urgent need to develop low-cost traps for surveillance of adult populations of mosquitoes, both indoors and outdoors, in Latin America and other areas. The mosquito species Ae. aegypti is attracted to humans and their environment, which makes it essential to monitor its abundance and distribution. Developing innovative technologies with affordable prices and high sensitivity and specificity is essential to highlight this issue in vector and disease surveillance activities since most countries still rely on the doubtful entomological index for their dengue management recommendations.
Author Contributions
Keila Elizabeth Paiz-Moscoso: Experimental design, laboratory work, semi-field work, fieldwork, manuscript writing, manuscript correction. Jorge J. Rodríguez-Rojas: laboratory work, revision of the manuscript, translation of the manuscript. Rogelio Danís-Lozano: Design and planning of semi-field and fieldwork, correction of the manuscript. Luis Alberto Cisneros-Vázquez: Design and planning of laboratory, semi-field and fieldwork, data analysis, correction of the manuscript. Eduardo A. Rebollar-Téllez: Experimental design, data analysis, manuscript revision. Rosa Maria Sánchez-Casas: Laboratory work, correction of the manuscript, translation of the manuscript. Ildefonso Fernández-Salas: Experimental design, writing of the manuscript, revision of the manuscript, translation of the manuscript.
Funding
This research was funded by Programa de Apoyo a la Ciencia, Tecnología e Innovación Científica ProACTI de la UANL, Proyecto 14-BQ-2023.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in the section "MDPI Research Data Policies" at
https://www.mdpi.com/ethics.
Acknowledgments
We would like to thank the field technicians Crescencio Díaz (CRISP/INSP) and Sandra Luz Robles (CRISP/INSP) for their support in the establishment and maintenance of the mosquito colonies; and their work in semi-field and field tests.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Dengue: the region of The Americans. 2023. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON475.
- WHO. Chikungunya. 2023. https://www.paho.org/es/noticias/4-5-2023-ante-aumento-casos-expertos-analizan-propagacion-chikungunya-americas#:~:text=En%20el%20primer%20cuatrimestre%20de,la%20magnitud%20y%20el%20momento.
- WHO. Zika. 2023. https://www.paho.org/es/noticias/1-9-2023-zika-virus-silencioso-que-requiere-mayor-vigilancia-control#:~:text=Washington%2C%20DC%2C%201%20de%20setiembre,89%20pa%C3%ADses%20alrededor%20del%20mundo.
- WHO. PAHO. 2023. https://iris.paho.org/handle/10665.2/34331?locale-attribute=es.
- Gonzalez-Escobar, G.; Valadere, A.M.; Adams, R.; Polson-Edwards, K.; Hinds, A. Q.; Misir, A.; Hospedales, C.J. Prolonged Zika virus viremia in a patient with Guillain-Barré syndrome in Trinidad and Tobago. Rev. Pana. Salud Publica. 2018, 41, e136. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.W.; Thein, T.L.; Ng, Y.; Boudville, I.C.; Chia, P.Y.; Lee, V.J.M.; Leo, Y.S. A 15-year review of dengue hospitalizations in Singapore: Reducing admissions without adverse consequences, 2003 to 2017. PLoS Negl. Trop. Dis. 2019, 13, e0007389. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.N.S. Changing epidemiology of dengue fever in children in South America. Current Opinion in Pediatrics. 2023, 35, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Junior, J.B.S.; Massad, E.; Lobao-Neto, A.; Kastner, R.; Oliver, L.; Gallagher, E. Epidemiology and costs of dengue in Brazil: a systematic literature review. IJID 2022. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Barbosa, H.; Medina-Moreno, S.; Zapata, J. C.; Chua, J. V. Dengue infections in Colombia: epidemiological trends of a hyperendemic country. TropicalMed. 2020, 5, 156. [Google Scholar] [CrossRef] [PubMed]
- Secretaria de Salud. Direccion General de epidemiologia. Sistema Nacional de Vigilancia Epidemiologica. Sistema Unico de Informacion. Boletin Epidemiológico. Semana 31. 2023. https://www.gob.mx/cms/uploads/attachment/file/849667/sem31.pdf.
- Secretaria de Salud. Direccion General de Epidemiologia. Panomara Epidemiologico de Dengue. Semana Epidemiologica 31. 2023. https://www.gob.mx/cms/uploads/attachment/file/848245/Pano_dengue_31_2023.pdf.
- Secretaria de Salud. Norma Oficial Mexicana NOM-032-SSA2-2014, para la vigilancia epidemiológica, promoción, prevención y control de las enfermedades transmitidas por vectores. 2014. https://www.dof.gob.mx/nota_detalle.php?codigo=5389045&fecha=16/04/2015#gsc.tab=0.
- Saavedra-Rodriguez, K.; Maloof, F.V.; Campbell, C.L.; Garcia-Rejon, J.; Lenhart, A.; Penilla, P.; Black, W. C. Parallel evolution of vgsc mutations at domains IS6, IIS6 and IIIS6 in pyrethroid resistant Aedes aegypti from Mexico. Scientific Reports 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Solis-Santoyo, F.; Rodriguez, A.D.; Penilla-Navarro, R.P.; Sanchez, D.; Castillo-Vera, A.; Lopez-Solis, A.D.; Saavedra-Rodriguez, K. Insecticide resistance in Aedes aegypti from Tapachula, Mexico: Spatial variation and response to historical insecticide use. PLoS Negl. Trop. Dis. 2021, 15, e0009746. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.L.; Connelly, C.R. A review of the control of Aedes aegypti (Diptera: Culicidae) in the continental United States. J.M.l entomology. 2021, 58, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Dalpadado, R.; Gunathilaka, N.; Amarasinghe, D.; Udayanaga, L. A challenge for a unique dengue vector control programme: assessment of the spatial variation of insecticide resistance status amongst Aedes aegypti and Aedes albopictus populations in Gampaha District, Sri Lanka. BioMed Research International. 2021. [Google Scholar] [CrossRef] [PubMed]
- Correa Morales, F. Desarrollo de un método rápido de rociado residual intradomiciliario para el control de Aedes aegypti (L) vector de los virus dengue, chikungunya y zika en México. Diss. Universidad Autónoma de Nuevo León. 2021.
- Satoto, T.B.T.; Hartini, S.; Tontowi, A.E. Systematic review: Effectiveness of combination of lactic acid attractants for control of dengue vector: Aedes spp. J. Vector. Borne. Dis. 2021, 2021 58, 99–105. [Google Scholar] [CrossRef]
- Paiz-Moscoso, K.E.; Fernández-Salas, I.; Grieco, J.P.; Achee, N.L.; Torres-Estrada, J.L. Response of Aedes aegypti L. (Diptera: Culicidae) to transfluthrin and linalool impregnated in different of fabric types. Salud Públ. Méx. 2020, 62, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Clements, A. N. (2011). The biology of mosquitoes: viral, arboviral and bacterial pathogens (Vol. 3). Cabi.
- Tay, J.W.; Choe, D.H.; Mulchandani, A.; Rust, M. K. Hydrogels: from controlled release to a new bait delivery for insect pest management. J. Econ. Entomol. 2020, 113, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Hurst, T.; Quoc, H.L.; Unlu, I.; Freebairn, C.; Faraji, A.; Ritchie, S.A. Field comparisons of the Gravid Aedes Trap (GAT) and BG-Sentinel trap for monitoring Aedes albopictus (Diptera: Culicidae) populations and notes on indoor GAT collections in Vietnam. J. Med. Entomol. 2017, 54, 340–348. [Google Scholar] [PubMed]
- Parra, M.C.P.; Favaro, E.A.; Dibo, M.R.; Mondini, A.; Eiras, A.E.; Kroon, E.G.; Chiaravalloti-Neto, F. Using adult Aedes aegypti females to predict areas at risk for dengue transmission: A spatial case-control study. Acta Tropica. 2018, 182, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Aik, J.; Ng, L.C. Adult Aedes abundance and risk of dengue transmission. PLoS Negl. 2021, 15, e0009475. [Google Scholar]
- Kumar, K.R.; Bhoopathy, K.; Samuel, P. Pupal index (PI) as a reliable tool for assessing adult population and prediction of dengue epidemic (DF/DHF). 2022.
- Ong, J.; Chong, C.S.; Yap, G.; Lee, C.; Abdul Razak, M.A.; Chiang, S.; Ng, L.C. Gravitrap deployment for adult Aedes aegypti surveillance and its impact on dengue cases. PLoS NTD. 2020, 14, e0008528. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).