Submitted:
27 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
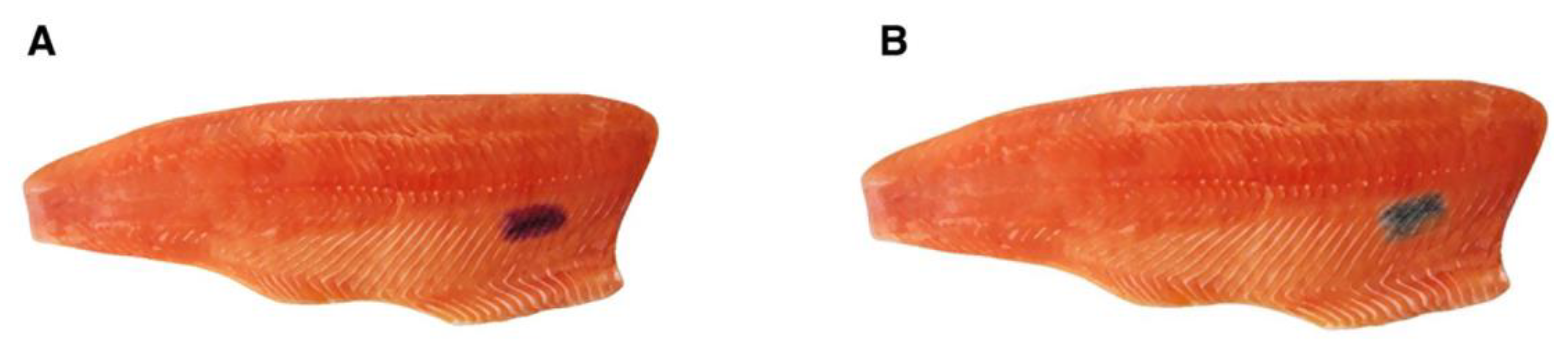
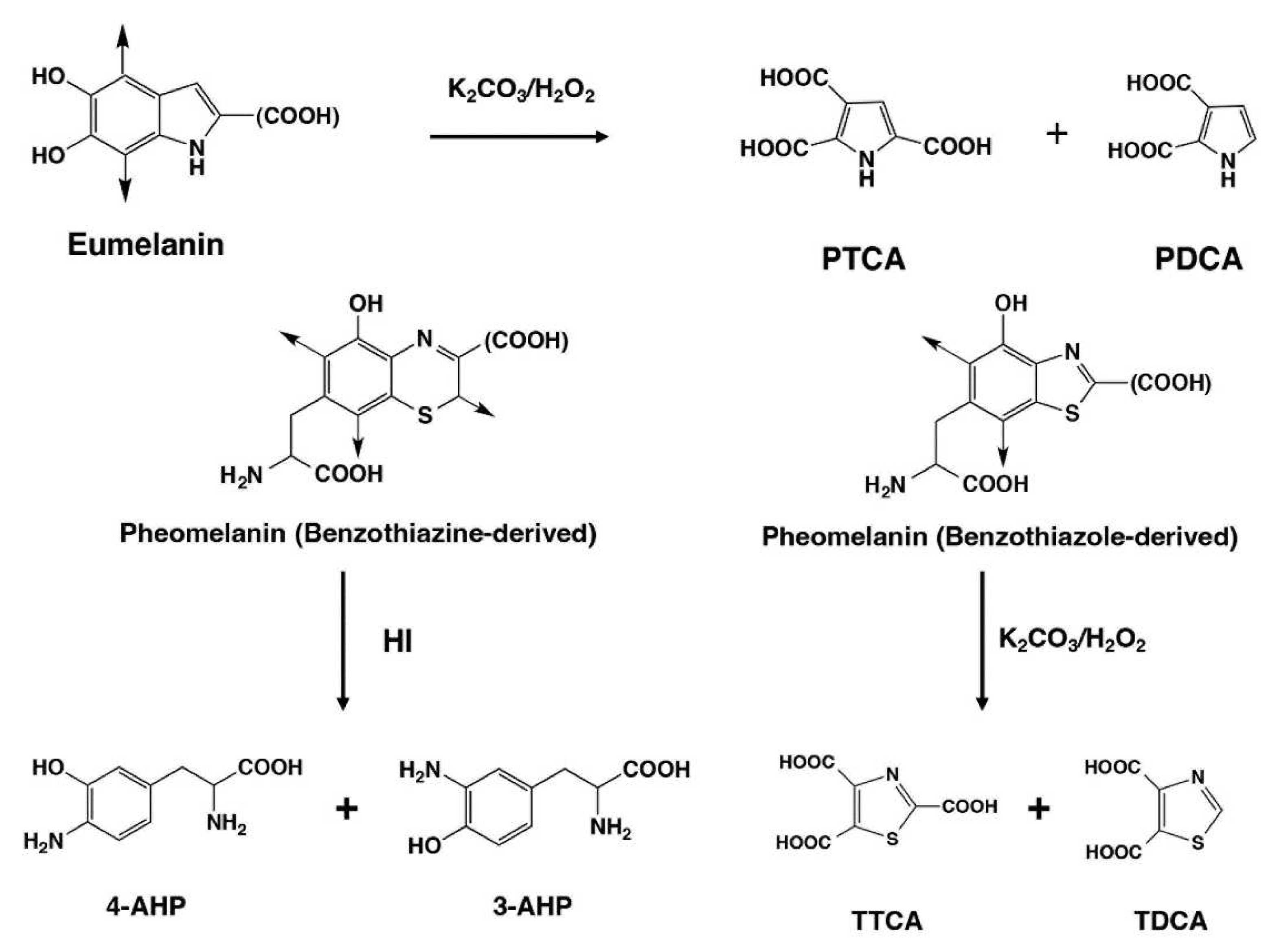
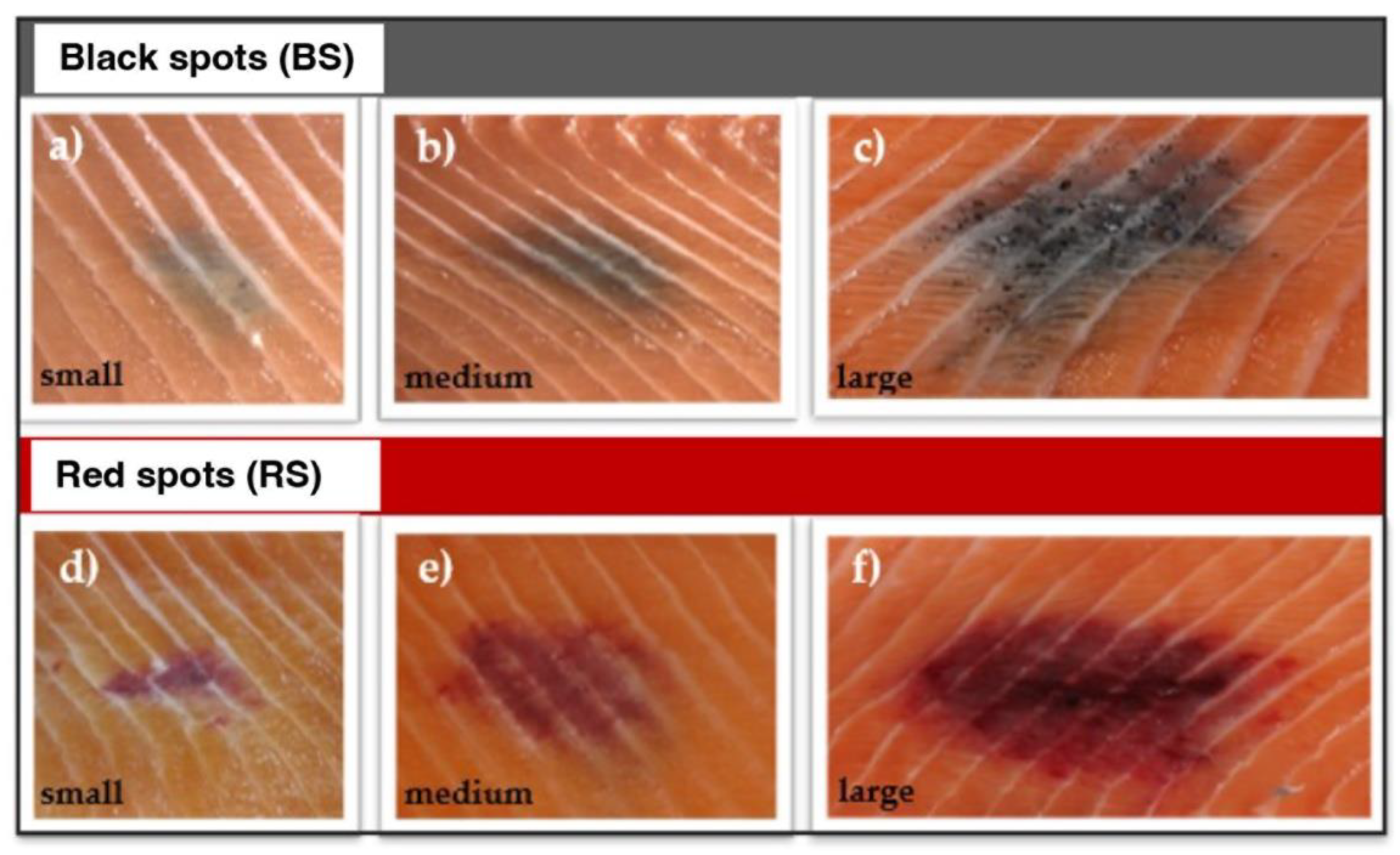
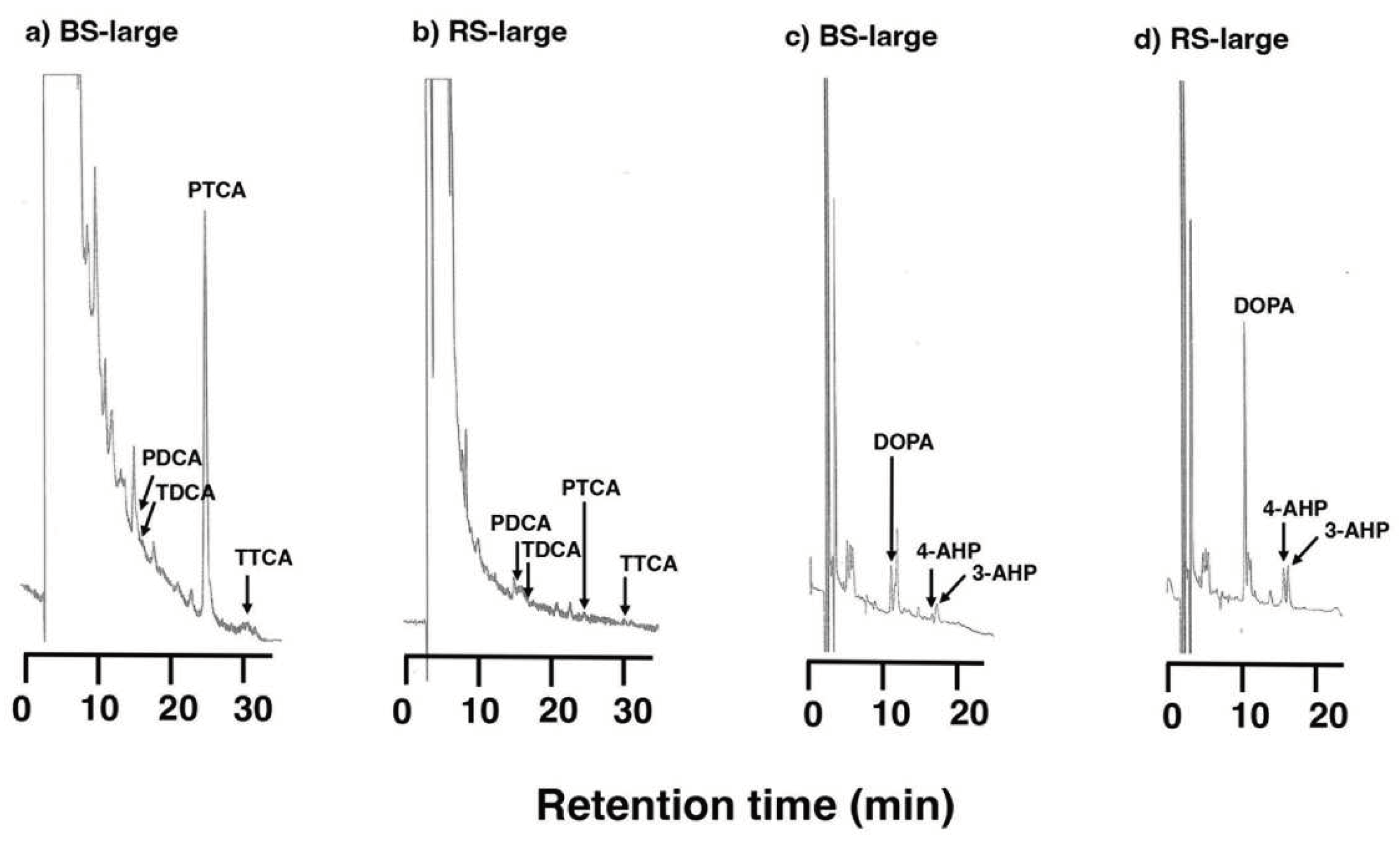
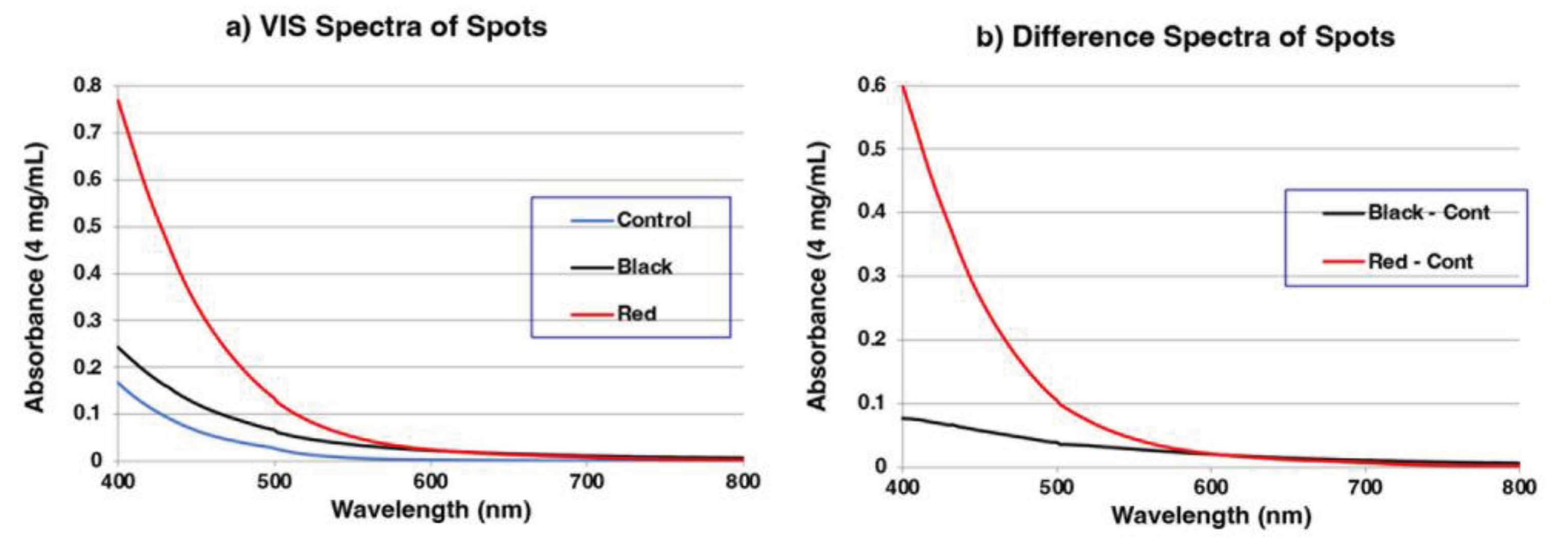
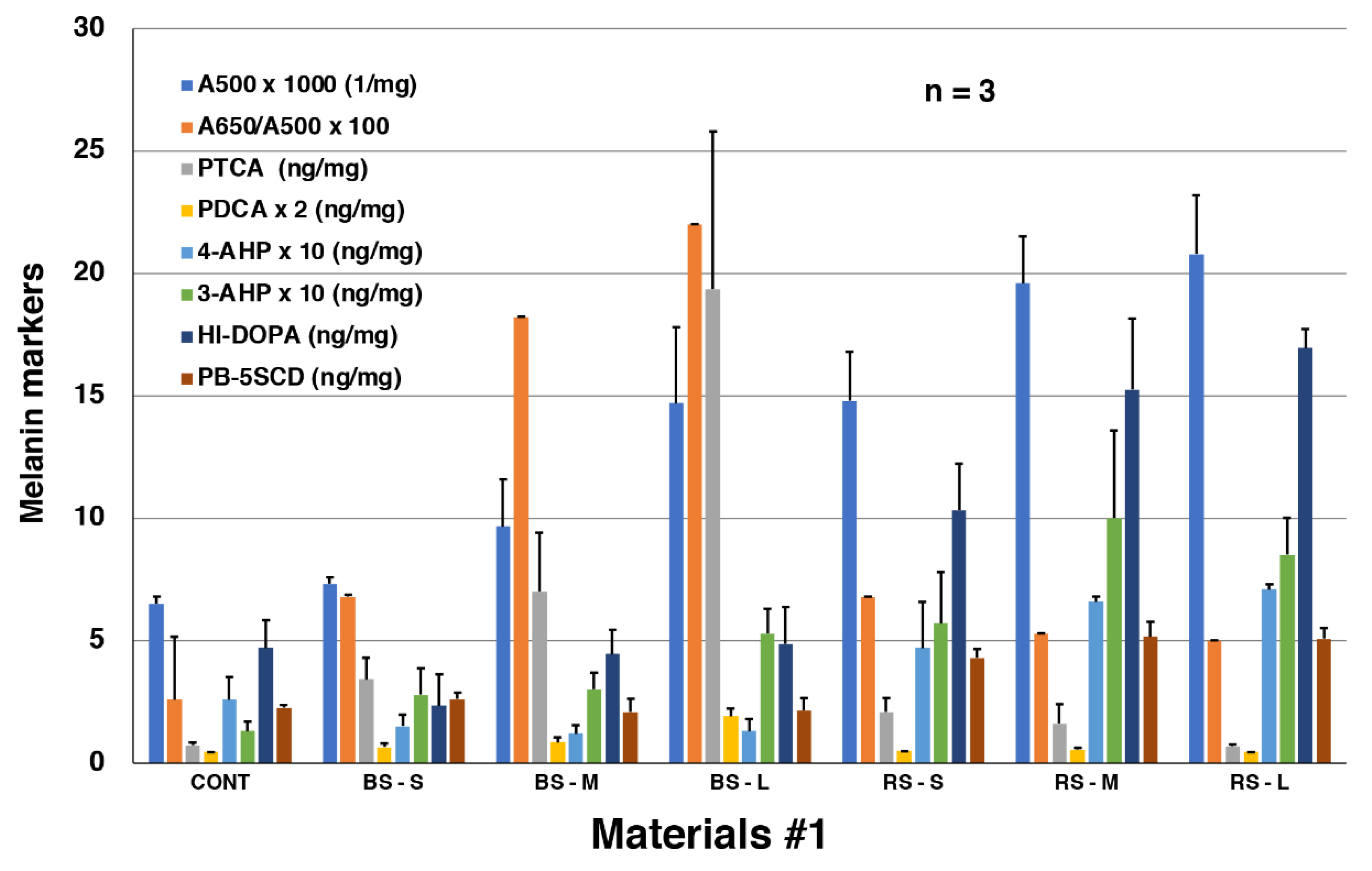
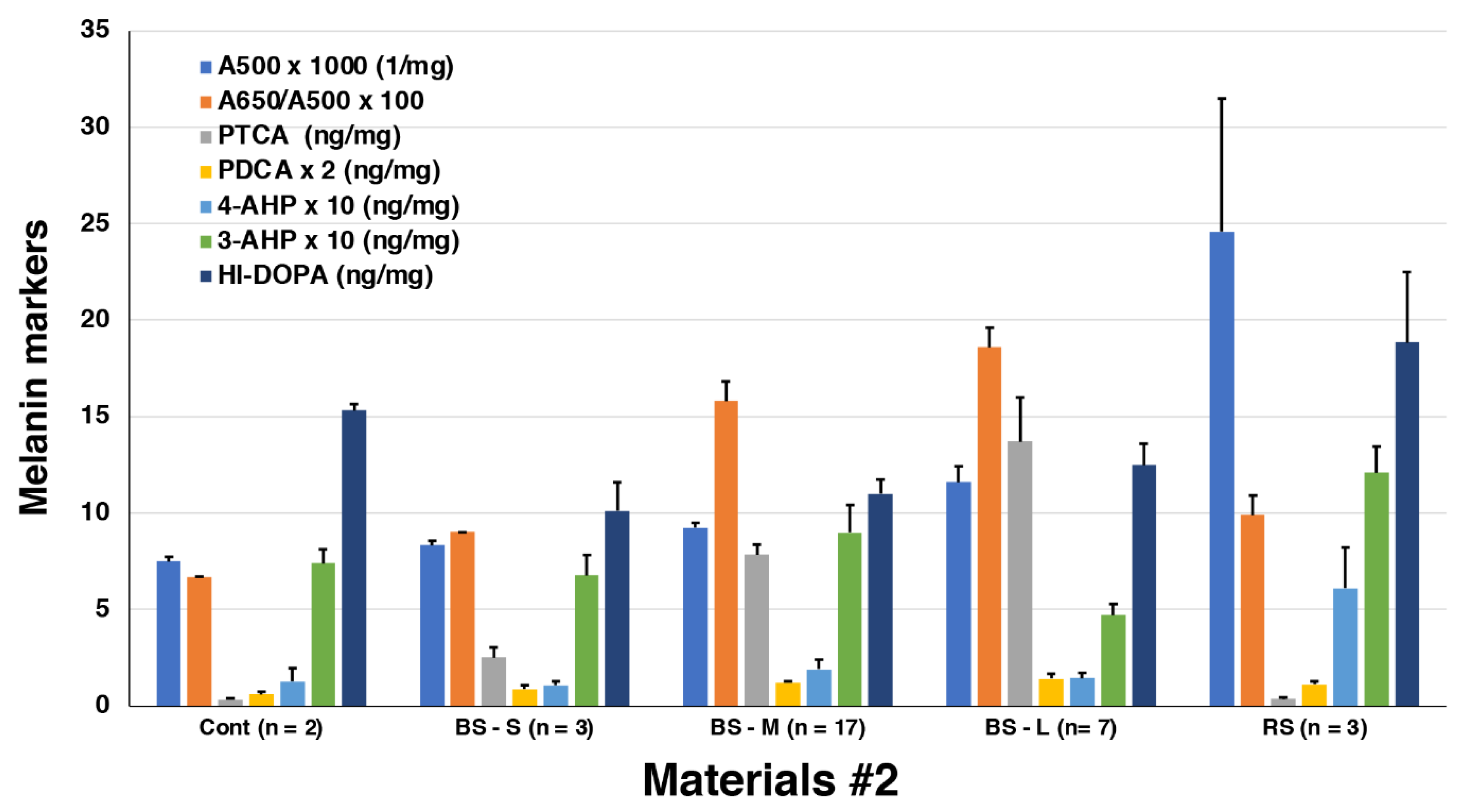
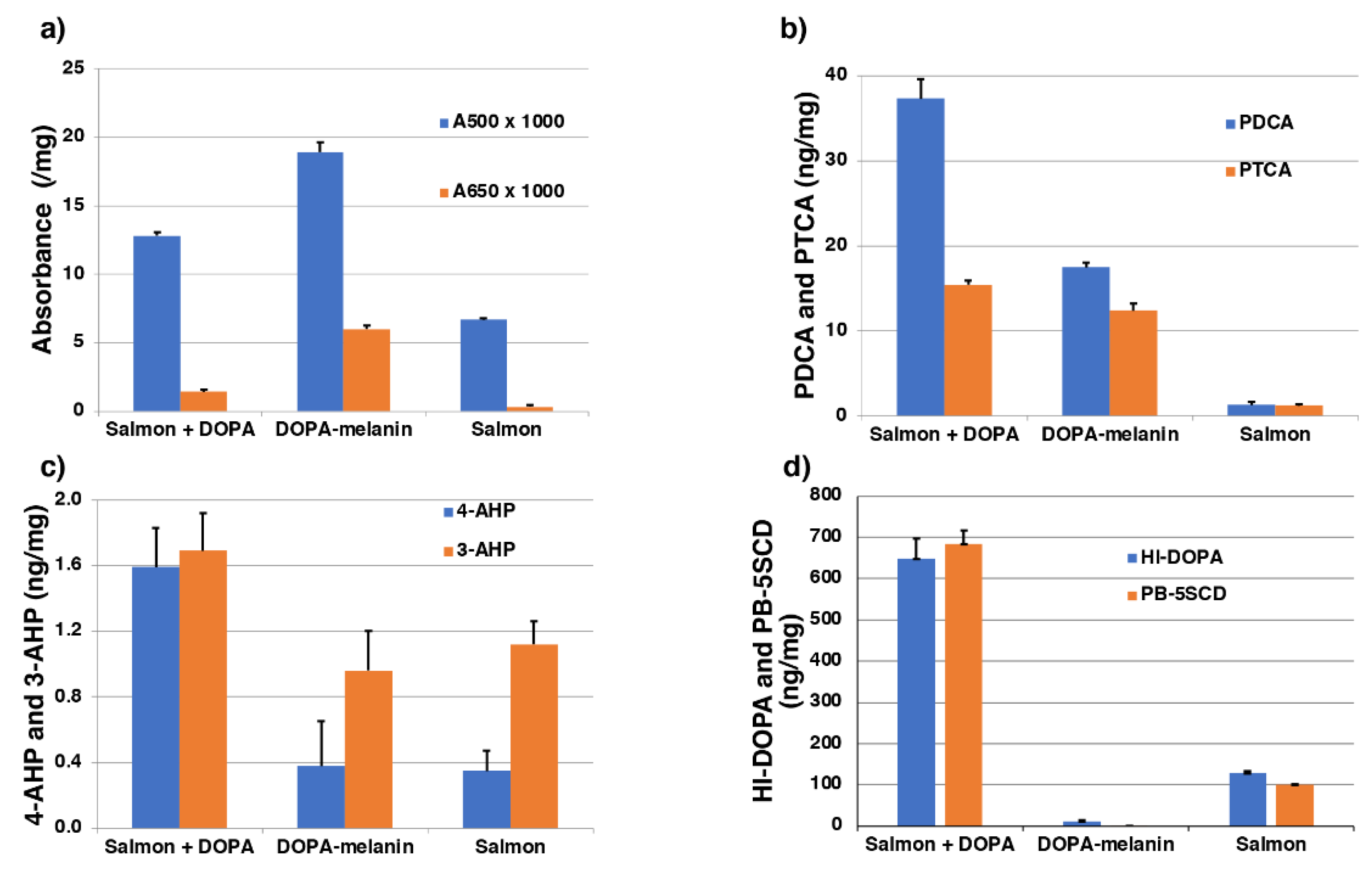
2.1. Chemical characterization by AHPO and HI hydrolysis of MFC and RFC on salmon fillets
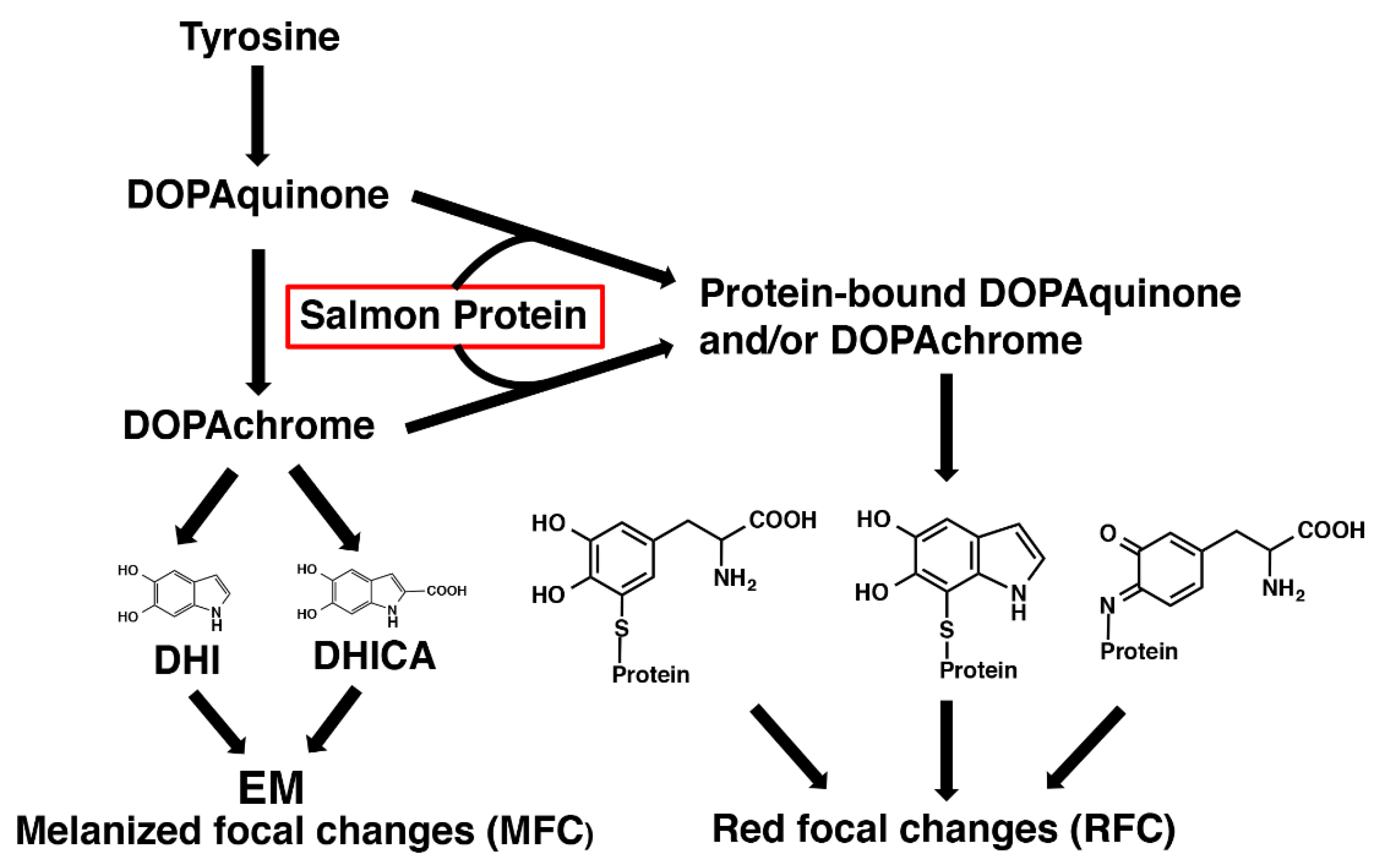
2.2. RFC are suggested to include melanogenic metabolites derived from oxidized proteins produced by DOPAquinone and/or DOPAchrome binding to salmon proteins
3. Discussion
4. Materials and Methods
4.1. Fish material
4.2. HPLC conditions
4.3. Preparative isolation of PTCA from MFC
4.4. Experiment mimicking the production of RFC in salmon fillets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malik, M. S.; Bjørgen, H.; Nyman, I. B.; Wessel, Ø.; Koppang, E. O.; Dahle, M. K.; Rimstad, E. PRV-1 infected macrophages in melanized focal changes in white muscle of Atlantic salmon (Salmo salar) correlates with a pro-inflammatory environment. Front. Immunol. 2021, 12, 664624. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, H.; Wessel, Ø.; Fjelldal, P.G.; Hansen, T.; Sveier, H.; Sæbø, H.R.; Enger, K.B.; Monsen, E.; Kvellestad, A.; Rimstad, E.; Koppang, E.O. Piscine orthoreovirus (PRV) in red and melanised foci in white muscle of Atlantic salmon (Salmo salar). Vet. Res. 2015, 46, 89. [Google Scholar] [CrossRef] [PubMed]
- Mørkøre, T.; Larsson, T.; Kvellestad, A.S.; Koppang, E.O.; Åsli, M.; Krasnov, A.; Gannestad, K.H. Mørke flekker i laksefilet. Kunnskapsstatus og tiltak for å begrense omfanget (Melanised changes in salmon fillet. Knowledge status and efforts to limit their occurrence). NOFIMA report. 2015, Retrieved from https://brage.bibsys.no/xmlui/handle/11250/2368359.
- Bjørgen, H.; Haldorsen, R.; Oaland, Ø.; Kvellestad, A.; Kannimuthu, D.; Rimstad, E.; Koppang, E.O. Melanized focal changes in skeletal muscle in farmed Atlantic salmon after natural infection with Piscine orthoreovirus (PRV). J. Fish Dis. 2019, 42, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.A.; Austbø, L.; Mørkøre, T.; Thorsen, J.; Hordvik, I.; Fischer, U.; Jirilo, E.; Rimstad, E.; Koppang, E.O. Pigment-producing granulomatous myopathy in Atlantic salmon: a novel inflammatory response. Fish Shellfish Immunol. 2012, 33, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Macchi, G. J.; Romanol, A.; Christiansen, H.E. Melanomacrophage centres in white-mouth croaker Micropogonias fuerney, as biological indicators of environmental changes. J. Fish Biol. 1992, 40, 971–973. [Google Scholar] [CrossRef]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Barni, S. Mechanisms of changes to the liver pigmentary component during the annual cycle (activity and hibernation) of Rana esculenta. L. J. Ant. 2002, 200, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Henninger, J.M.; Beresford, W.A. Is it coincidence that iron and melanin coexist in hepatic and other melanomacrophages? Histol. Histopathol. 1990, 5, 457–459. [Google Scholar] [PubMed]
- Ribeiro, H. J.; Procópio, M.S.; Gomes, J.M.; Vieira, F.O.; Russo, R.C.; Balzuweit, K.; Chiarini-Garcia, H.; Castro, A.C.S.; Rizzo, E.; Corrêa, J. D. Jr. Functional dissimilarity of melanomacrophage centres in the liver and spleen from females of the teleost fish Prochilodus argenteus. Cell Tissue Res. 2001, 346, 417–425. [Google Scholar] [CrossRef]
- Dubey, S.; Roulin, A. Evaluation and biomedical consequences of internal melanins. Pigment Cell Melanoma Res. 2014, 27, 327–338. [Google Scholar] [CrossRef]
- Guida, G.; Gallone, A.; Maida, I. , Boffoli, D.; Cicero, R. Tyrosinase gene expression in the Kupffer cells of Rana esculenta L. Pigment Cell Res. 2000, 13, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Purrello, M.; Scalia, M.; Corsaro, C.; Di Pietro, C.; Piro, S.; Sichel, G. Melanosynthesis, differentiation, and apoptosis in Kupffer cells from Rana esculenta. Pigment Cell Res. 2001, 14, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Scalia, M.; Di Pietro, C.; Poma, M.; Ragusa, M.; Sichel, G.; Corsaro, C. The spleen pigment cells in some Amphibia. Pigment Cell Res. 2004, 17, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Sichel, G.; Scalia, M.; Corsaro, C. Amphibia Kupffer cells. Microsc. Res. Tech. 2002, 57, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, H.; Kumar, S.; Gunnes, G.; Press, C.M.; Rimstad, E.; Koppang, E. O. Immunopathological characterization of red focal changes in Atlantic salmon (Salmo salar) white muscle. Vet. Immunol. Immunopathol. 2020, 222, 110035. [Google Scholar] [CrossRef]
- Krasnov, A.; Moghadam, H.; Larsson, T.; Afanasyev, S.; Mørkøre, T. Gene expression profiling in melanised sites of Atlantic salmon fillets. Fish Shellfish Immunol. 2016, 55, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Agius, C. The melano-macrophage centres of fish: A review. In M.J. Manning & M.F. Tatner (Eds.), Fish Immunology, 1985, 85-105. Academic Press. [CrossRef]
- Brimsholm, M.; Fjelldal, P.G.; Hansen, T.; Fraser, T.K.W.; Solberg, M.; Glover, K.; Koppang, E.O.; Bjørgen, H. Red and melanized focal changes in white skeltal muscle in Atlantic salmon (Salmo sala): Comaprative analysis of farmed, wild and hybrid reared under identical conditions. J. Fish Dis. 2023. [Google Scholar] [CrossRef]
- Gallone, A.; Sagliano, A.; Guida, G.; Ito, S.; Wakamatsu, K.; Capozzi, V.; Perna, G.; Zanna, P.; Cicero, R. The melanogenic system of the liver pigmented macrophages of Rana esculenta L. – Tyrosinase activity. Histol. Histopathol. 2007, 22, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, J.; Høyheim, B.; Koppang, E.O. Isolation of the Atlantic salmon tyrosinase gene family reveals heterogenous transcripts in a leukocyte cell line. Pigment Cell Res. 2006, 19, 327–336. [Google Scholar] [CrossRef]
- Mackintosh, J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef]
- McNamara, M.E.; Rossi, V.; Slater, T.S.; Rogers, C.S.; Ducrest, A.-L.; Dubey, S.; Roulin, A. Decoding the evolution of melanin in vertebrates. Trends Ecol. Evol. 2021, 36, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kato, K.; Wakamatsu, K.; Ito, S.; Ishimaru, K.; Hirata, T.; Murata, O.; Kumai. H. The histological analysis, colorimetric evaluation, and chemical quantification of melanin content in ‘suntanned’ fish. Pigment Cell Res. 2005, 18, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M. Reactivities of quinone methides versus o-quinones in catecholamine metabolism and eumelanin biosynthsis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J-C. ; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; Simon, J.D.; Ito, S. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M. L.; Panzella, L. The multifaceted opportunities provides by the pheomelanin-inspired 1,4-benzothiazine chromophore: a still-undervalued issue. Molecules, 2023, 28, 6237. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S. Melanins in Vertebrates. 2021, pp 45-89, In: Hashimoto H., Goda M., Futahashi R., Kelsh R., Akiyama T. (eds). Pigments, Pigment Cells and Pigment Patterns. Springer, Singapore. [CrossRef]
- Land, E.J.; Ramsden, C.A.; Riley, P.A. Tyrosinase autoactivation and the chemistry of ortho-quinone amines. Acc. Chem. Res. 2003, 36, 300–308. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Körner, A.M.; Bergstrom, A.; Bologna, J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature, 1980, 286, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef] [PubMed Central]
- Kroumpouzos, G.; Urabe, K.; Kobayashi, T.; Sakai, C.; Hearing, V.J. Functional analysis of the slaty gene product (TRP2) as dopachrome tautomerase and the effect of a point mutation on its catalytic function. Biochem. Biophys. Res. Commun. 1994, 202, 1060–1068. [Google Scholar] [CrossRef]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral PH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent advances and progress on melanin: from source to application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of peheomelanin. Pigment Cell Res. 2002, 15, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline peroxde oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanin. Pigment Cell Res. 2011, 24, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kato, T.; Maruta, K.; Jimbow, K.; Fujita, K. “Total”acidic metabolites of catecholamines in urine as determined by hydrolysis with hydriodoc acid and liquid chromatograohy: application to patients with neuroblastoma and melanoma. Clin. Chem. 1985, 31, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kato, T.; Fujita, K. Covalent binding of catechols to proteins through the sulphydryl group. Biochem. Pharmacol. 1988, 37, 1707–1710. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinoid products formed tyrosinase and phenoloxidae action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kato, T.; Shinpo, K.; Fujia, K. Oxidation of tyrosine residues in proteins by tyrosinase. Formation of protein-bonded 3,4-dihydroxyphenylalanine and 5-S-cysteinyl-3,4-dihydroxyphenylalanine. Biochem. J. 1984, 222, 407–411. [Google Scholar] [CrossRef]
- Sathival, S.; Smiley, S.; Prinyawiwatkul, W.; Bechtel, P.J. Functional and nutritional properties of red salmon (Oncorhynchus nerka) enzymatic hydrolysates. J. Food Sci. 2005, 70, C401–C406. [Google Scholar] [CrossRef]
- Razzaq, W.; Masood, Z.; Hassan, H.U.; Benzar, S.; Nadeem, K.; Arai, T. An investigation on protein and amino acid contents in scales and muscles of pomfret Parastromateus niger (Bloch, 1795) and Pampus argenteus (Eupharasen, 1788). Brazilian J. Biol. 2024, 84, e258880. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Shono, S.; Toda, K. Phenotypic expression in pigment cell. In Pigment Cell, M. Seiji, ed. (Tokyo, Japan: University of Tokyo Press), 1981, pp. 263-268.
- Sissener, N.H.; Waagbø, R.; Rosenlund, G.; Tvenning, L.; Susort, S.; Lea, T.B.; Oalamd, Ø.; Chen, L.; Breck, O. Reduced n-3 long chain fatty acid levels in feed for Atlantic salmon (Salmo salar L.) do not reduced growth, robustness or product quality through an entire full scale commercial production cycle in seawater. Aquac. 2016, 464, 236–245. [Google Scholar] [CrossRef]
- Lutfi, E.; Berge, G.M.; Bæverfjord, G.; Sigholt, T.; Bou, M.; Larsson, T.; Mørkøre, T.; Evensen, Ø.; Sissener, N.H.; Rosenlund, G.; Sveen, L.; Østbye, T-K. ; ruyter, B. Increasing dietary levels of the n-3 long-chain PUFA, EPA and DHA, improves the growth, welfare, rubustness and fillet quality of Atlantic salmon in sea cages. Br. J. Nut. 2023, 129, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Balcos, M.C.; Kim, S.Y.; Jeong, H-S. ; Yun, H-Y.; Baek, K.J.; Kwon, N.S.; Park, K-C.; Kim, D-S. Docosahexaenoic acid inhibits melanin synthesis in murine melanoma cells in vitro through increasing tyrosinase degradation. Acta Pharmacol. Sin. 2014, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Terao, A.; Ueda, S.; Namiki, M. Red pigment formation by the reaction of oxidized adcorbic acid and protein in a food model system of low moisture content. Agric. Biol. Chem. 1985, 49, 3139–3144. [Google Scholar] [CrossRef]
- Alayash, A.I.; Patel, R.P.; Cashon, R.E. Redox reactions of hemoglobin and myoglobin: Biological and toxicological implications. Antioxid. Redox Signal. 2001, 3, 313–327. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W.; Alayash, A.I.; Belcher, J.D.; Vercellotti, G.M. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood 2013, 121, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Guerrero, R.; Baeverfjord, G.; Evensen, Ø.; Hamre, K.; Larsson, T.; Dessen, J-E. ; Gannestad, K-H.; Mørkøre, T. Rib abnormalities and their association withfocal dark spots in Atlantic salmon fillets. Aquac. 2022, 561, 738697. [Google Scholar] [CrossRef]
- Ito, S.; Del Bino, S.; Hirobe, T.; Wakamatsu, K. Improved HPLC conditions to determine eumelanin and pheomelanin in biological samples using an ion pair reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




