Submitted:
27 October 2023
Posted:
30 October 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Treatment of corneal neovascularization
Topical treatment
Steroids
Nonsteroidal anti-inflammatory drugs (NSAIDs)
Cyclosporin A
Inhibitors of vascular endothelial growth factor (anti-VEGF)
Tissue inhibitors of metalloproteinases(TIMP)
Other drugs
Other potential therapeutic targets
Nitric oxide synthase (NOS)
Galectin-3 inhibitors
Pigment Epithelium-Derived Factor (PEDF)
PDGFreceptor inhibitor
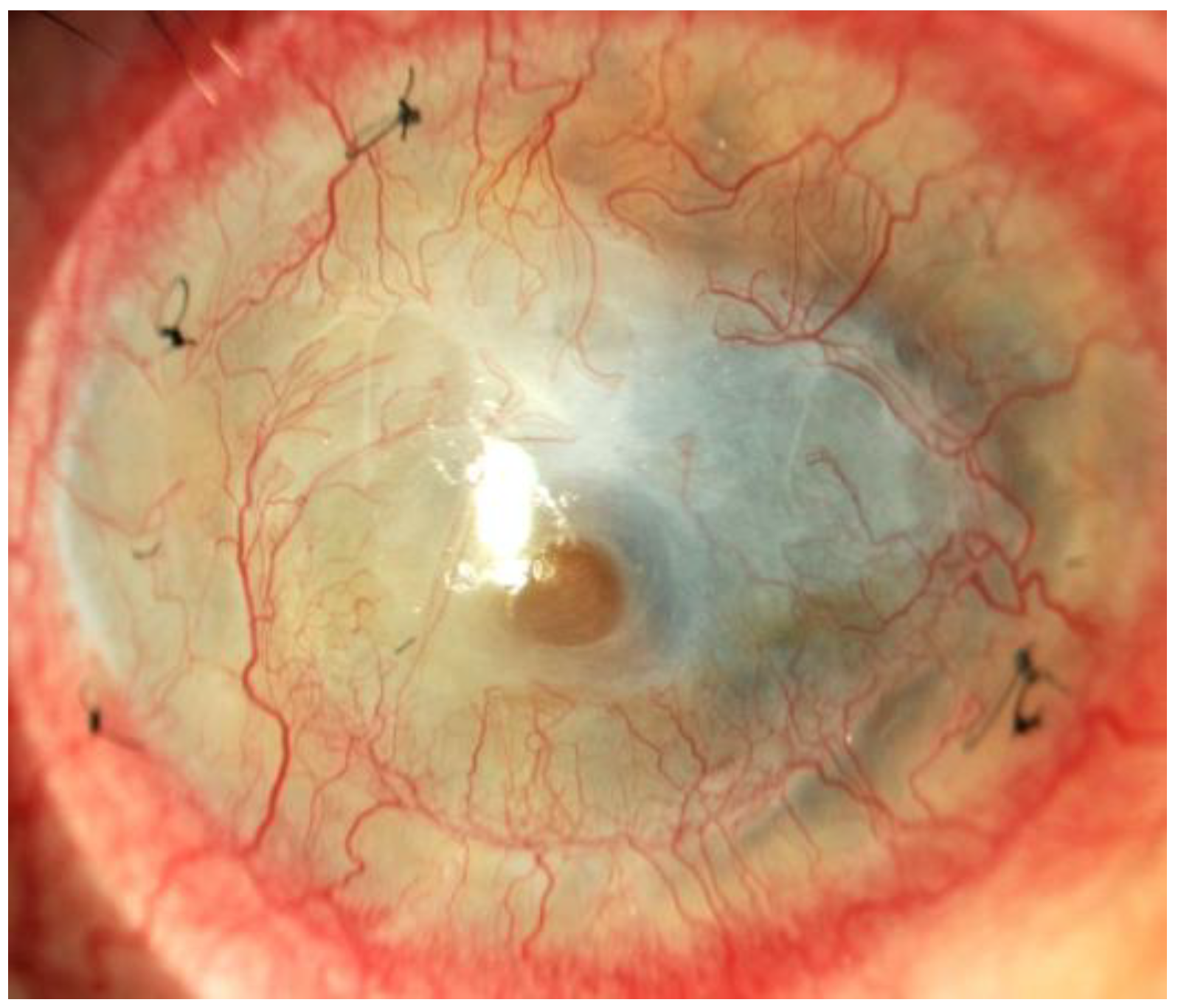
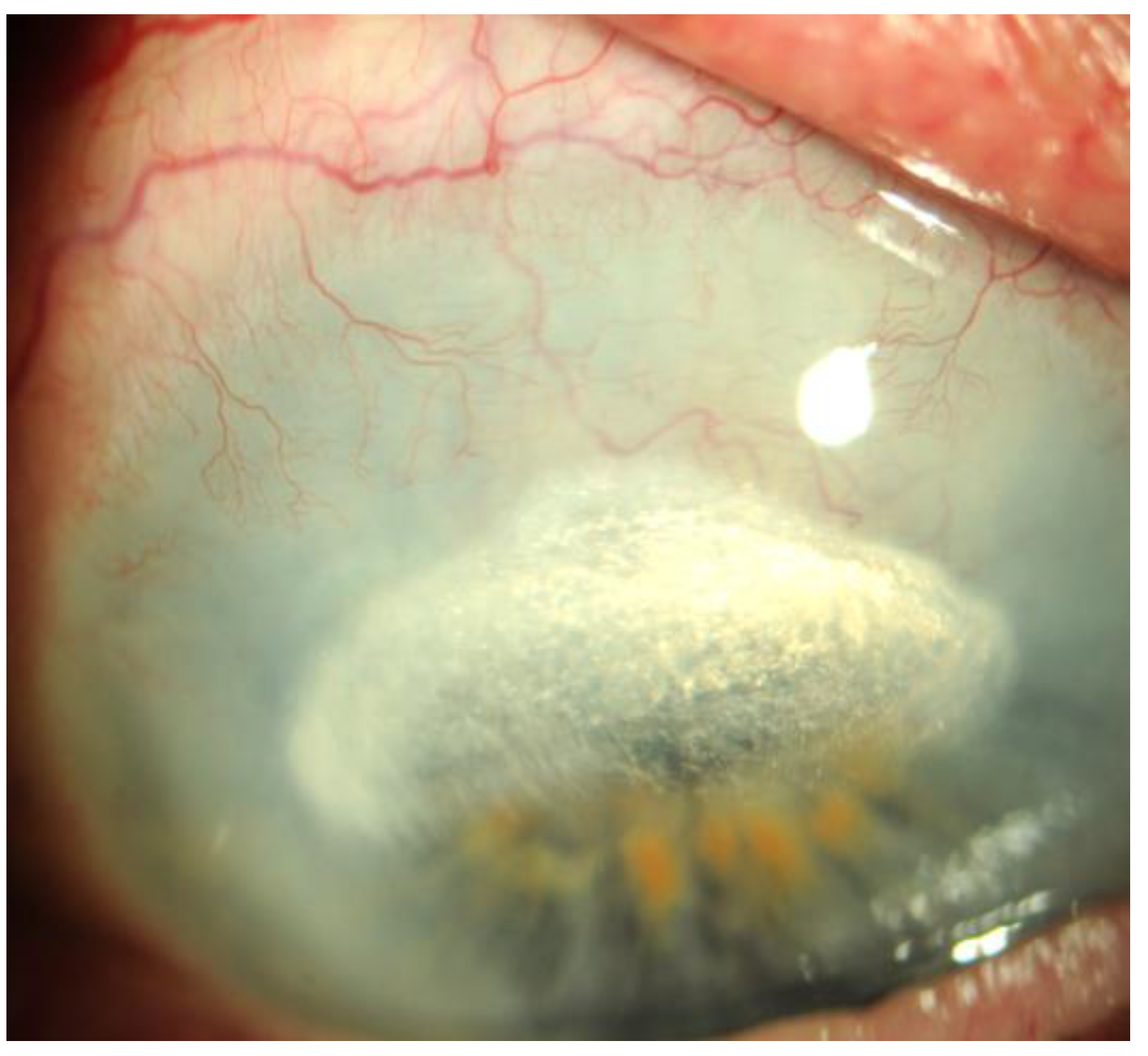
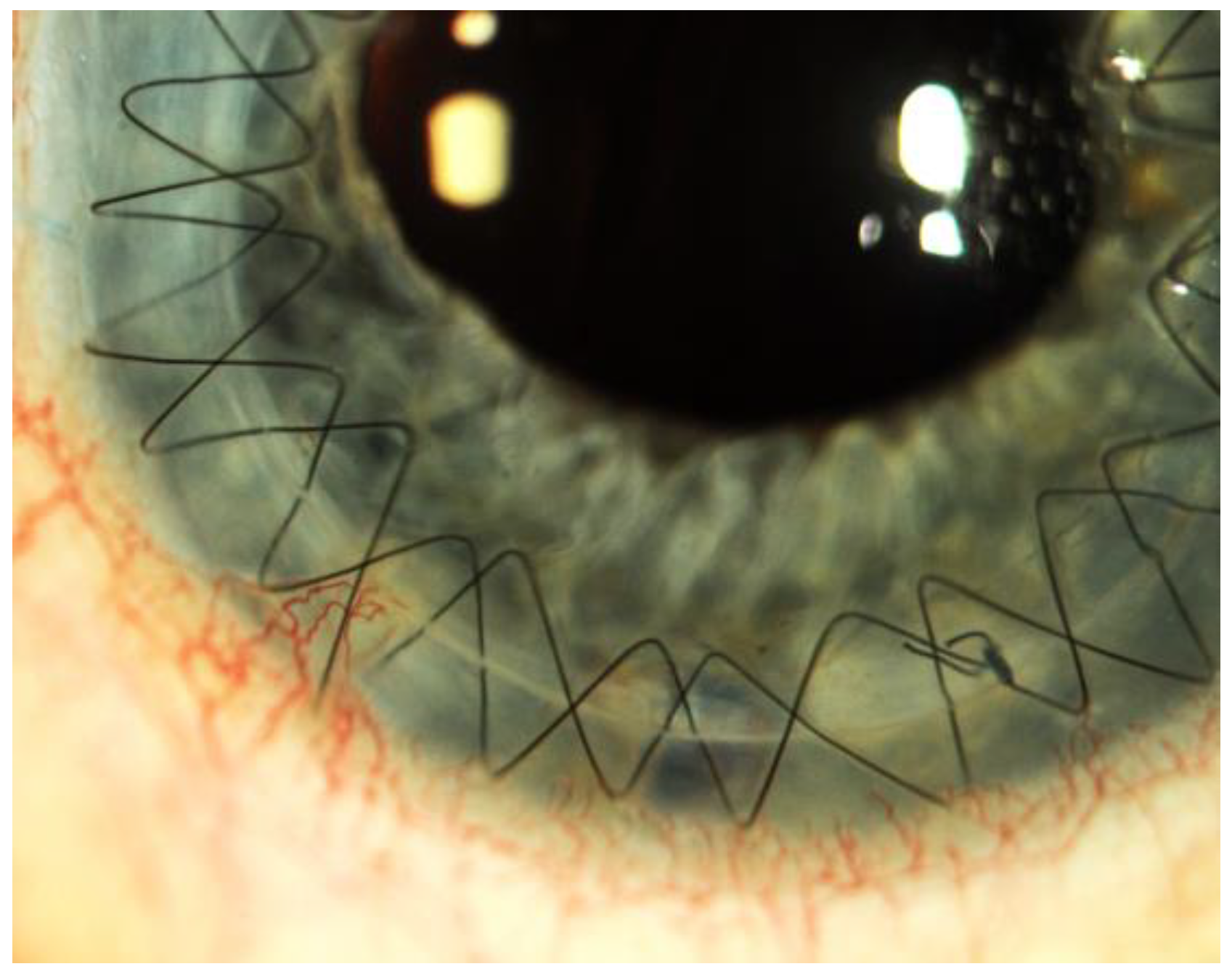
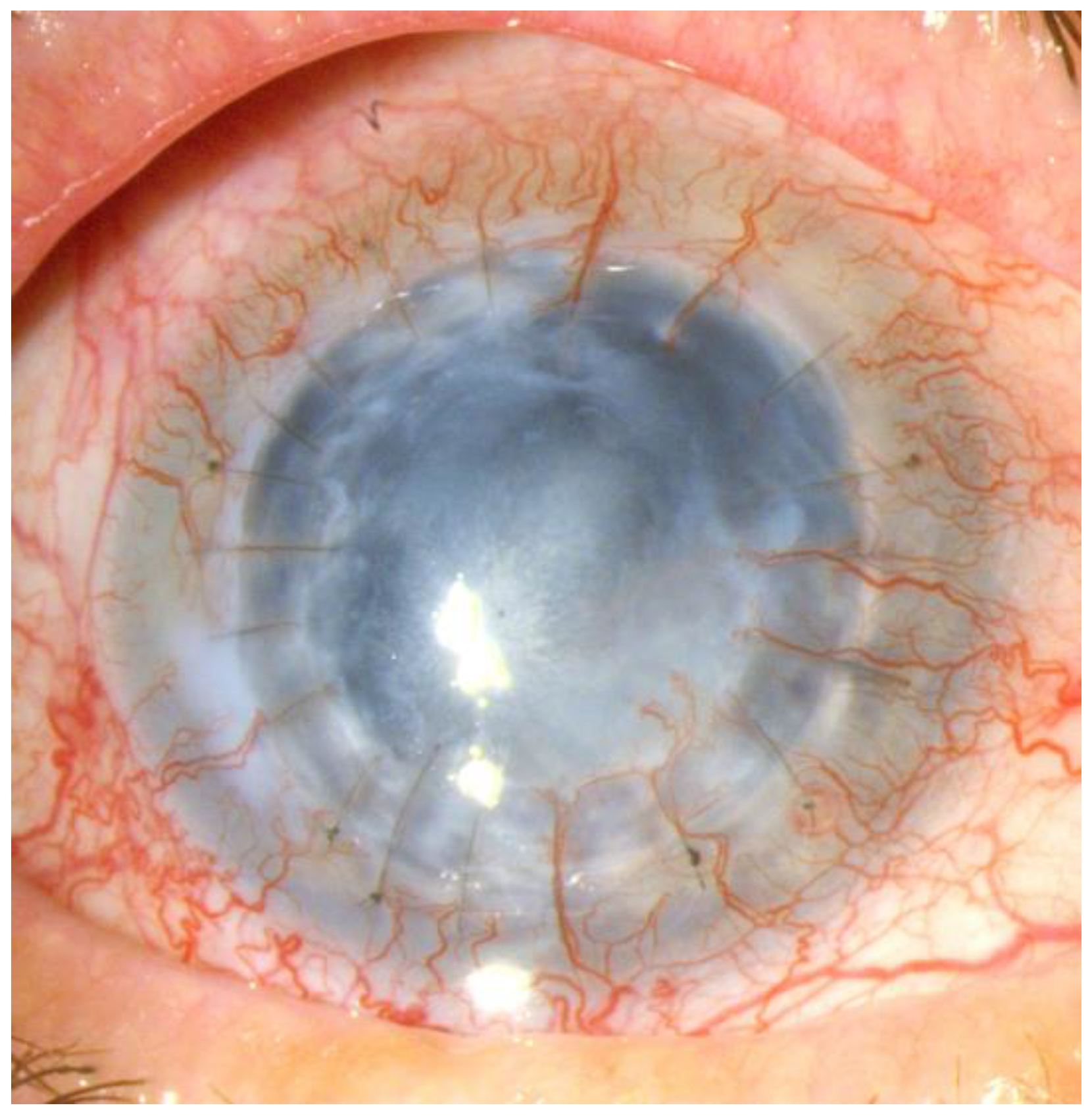
Surgical treatment
Fine needle diathermy
Laser therapy
Amniotic membrane transplantation
Superficial keratectomy
Corneal transplantation
Combination therapies
Summary
References
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal neovascularization: updates on pathophysiology, investigations & management. Romanian J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [CrossRef]
- Gupta, D. , Illingworth, C. Treatments for corneal neovascularization: a review. Cornea 2011, 30, 927–938. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Wenkel, H.; Martus, P.; Langenbucher, A.; Nguyen, N.X.; Seitz, B.; Kuchle, M.; Naumann, G.O. Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2001, 239, 514–521. [Google Scholar] [CrossRef]
- Gallemore, R.; Sharareh, B.; Wallsh, J. Therapeutic effect of dexamethasone implant in retinal vein occlusions resistant to anti-VEGF therapy. Clin. Ophthalmol. 2016, 10, 947–954. [Google Scholar] [CrossRef]
- Rezar-Dreindl, S.; Eibenberger, K.; Pollreisz, A.; Bühl, W.; Georgopoulos, M.; Krall, C.; Dunavölgyi, R.; Weigert, G.; Kroh, M.; Schmidt-Erfurth, U.; et al. Effect of intravitreal dexamethasone implant on intra-ocular cytokines and chemokines in eyes with retinal vein occlusion. Acta Ophthalmol. 2016, 95, e119–e127. [Google Scholar] [CrossRef] [PubMed]
- Mukwaya, A.; Mirabelli, P.; Lennikov, A.; Xeroudaki, M.; Schaupper, M.; Peebo, B.; Lagali, N. Genome-wide expression datasets of anti-VEGF and dexamethasone treatment of angiogenesis in the rat cornea. Sci. Data 2017, 4, 170111–170111. [Google Scholar] [CrossRef]
- Novack, G.D.; Howes, J.; Crockett, R.S.; Sherwood, M.B. Change in Intraocular Pressure During Long-Term Use of Loteprednol Etabonate. Eur. J. Gastroenterol. Hepatol. 1998, 7, 266–269. [Google Scholar] [CrossRef]
- Hosseini, K.; Gollamudi, S.; Reiser, H.; Walters, T.; Lindstrom, R.L. 0.2% Betamethasone Sodium Phosphate: A Multicenter, Randomized, Double-Masked Study to Compare Its Ocular Safety, Tolerability, and Efficacy to Vehicle in Cataract Surgery Subjects. Clin. Ophthalmol. 2023, ume 17, 2219–2230. [Google Scholar] [CrossRef]
- Fong, R.; Cavet, M.E.; DeCory, H.H.; Vittitow, J.L. Loteprednol etabonate (submicron) ophthalmic gel 0.38% dosed three times daily following cataract surgery: integrated analysis of two Phase III clinical studies. Clin. Ophthalmol. 2019, 13, 1427–1438. [Google Scholar] [CrossRef]
- Jonas, J.B.; Kreissig, I.; Degenring, R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog. Retin. Eye Res. 2005, 24, 587–611. [Google Scholar] [CrossRef]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jiao, J.; Li, X.-Y.; et al. Dexamethasone Intravitreal Implant in Patients with Macular Edema Related to Branch or Central Retinal Vein Occlusion: Twelve-Month Study Results. Ophthalmology 2011, 118, 2453–2460. [Google Scholar] [CrossRef]
- Ayalasomayajula, S.P.; Ashton, P.; Kompella, U.B. Fluocinolone Inhibits VEGF Expression via Glucocorticoid Receptor in Human Retinal Pigment Epithelial (ARPE-19) Cells and TNF-α–Induced Angiogenesis in Chick Chorioallantoic Membrane (CAM). J. Ocul. Pharmacol. Ther. 2009, 25, 97–104. [Google Scholar] [CrossRef]
- Syed, Y.Y. Fluocinolone Acetonide Intravitreal Implant 0.19 mg (ILUVIEN((R))): A Review in Diabetic Macular Edema. Drugs 2017, 77, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.R.; Maguire, L.J.; Salomão, D.R. Bilateral Corneal Deposits 1 Week After Starting Oral Prednisone Therapy. JAMA Ophthalmol 2018, 136, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, U.; Ameen, B.S.; Nie, C.; Nechi, D.; Mazhar, I.J.; Yasir, M.; Sarfraz, S.; Shlaghya, G.; Narayana, S.H.; Khan, S. Association Between the Use of Systemic Steroids and Ocular Hypertension as a Side Effect in Pediatric Population: A Systematic Review. Cureus 2023, 15, e42112. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Nakagami, T.; Mochizuki, M.; Hata, N.; Tsuchiya, T.; Hotta, Y. Three Cases of Corneal Melting After Instillation of a New Nonsteroidal Anti-Inflammatory Drug. Cornea 2006, 25, 224–227. [Google Scholar] [CrossRef]
- Raj, N.; Panigrahi, A.; Alam, M.; Gupta, N. Bromfenac-induced neurotrophic keratitis in a corneal graft. BMJ Case Rep. 2022, 15, e249400. [Google Scholar] [CrossRef]
- Song, L.; Liu, X.; Chen, N.; Liu, J.; Nie, A.; Li, W. The Effect of Bromfenac Sodium Nanopolymer Used in Anterior Segment of the Eye on Corneal Neovascularization. Cell. Mol. Biol. 2022, 68, 330–338. [Google Scholar] [CrossRef]
- Matsumura, T.; Iwasaki, K.; Arimura, S.; Takeda, R.; Takamura, Y.; Inatani, M. Topical bromfenac reduces multiple inflammatory cytokines in the aqueous humour of pseudophakic patients. Sci. Rep. 2021, 11, 6018. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Noriega, K.; Butrón-Valdez, K.; Vazquez-Galvan, J.; Mohamed-Noriega, J.; Cavazos-Adame, H.; Mohamed-Hamsho, J. Corneal Melting after Collagen Cross-Linking for Keratoconus in a Thin Cornea of a Diabetic Patient Treated with Topical Nepafenac: A Case Report with a Literature Review. Case Rep. Ophthalmol. 2016, 7, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhu, Y.; Xing, C.; Li, S.; Bao, Z.; Lei, L.; Lin, D.; Wang, Y.; Chen, H.; Xu, X. An injectable thermosensitive hydrogel for dual delivery of diclofenac and Avastin(R) to effectively suppress inflammatory corneal neovascularization. International journal of pharmaceutics 2022, 625, 122081. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, B.; Beden, U.; Erkan, D. Regression of severe corneal stromal neovascularization with topical cyclosporine 0.05% after penetrating keratoplasty for fungal corneal ulcer. Int. Ophthalmol. 2007, 29, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, D.M.; Kahraman, N.; Balcioglu, E.; Duru, Z. Comparison of the inhibitory effect of topical cyclosporine A 0.1% and topical anti-VEGF application in an experimental model of corneal neovascularization. Arq. Bras. de Oftalmol. 2021, 85, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cejka, C.; Cejkova, J.; Trosan, P.; Zajicova, A.; Sykova, E.; Holan, V. Transfer of mesenchymal stem cells and cyclosporine A on alkali-injured rabbit cornea using nanofiber scaffolds strongly reduces corneal neovascularization and scar formation. Histology and histopathology 2016, 31, 969–980. [Google Scholar] [PubMed]
- Krizova, D.; Vokrojova, M.; Liehneova, K.; Studeny, P. Treatment of Corneal Neovascularization Using Anti-VEGF Bevacizumab. J. Ophthalmol. 2014, 2014, 178132. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Cheng, S.-F.; Dastjerdi, M.H.; Ferrari, G.; Dana, R. Corneal Neovascularization and the Utility of Topical VEGF Inhibition: Ranibizumab (Lucentis) Vs Bevacizumab (Avastin). Ocul. Surf. 2012, 10, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Bucher, F.; Parthasarathy, A.; Bergua, A.; Onderka, J.; Regenfuß, B.; Cursiefen, C.; Bock, F. Topical Ranibizumab inhibits inflammatory corneal hem- and lymphangiogenesis. Acta Ophthalmol. 2012, 92, 143–148. [Google Scholar] [CrossRef]
- Ferrari, G.; Dastjerdi, M.H.; Okanobo, A.; Cheng, S.-F.; Amparo, F.; Nallasamy, N.; Dana, R.M. Topical Ranibizumab as a Treatment of Corneal Neovascularization. Cornea 2013, 32, 992–997. [Google Scholar] [CrossRef]
- Liarakos, V.S.; Papaconstantinou, D.; Vergados, I.; Douvali, M.; Theodossiadis, P.G. The Effect of Subconjunctival Ranibizumab on Corneal and Anterior Segment Neovascularization: Study on an Animal Model. Eur. J. Ophthalmol. 2014, 24, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.B.; Sakimoto, T.; Javier, J.A.; Azar, D.T.; Wiegand, S.J.; Jain, S.; Chang, J.H. VEGF Trap(R1R2) suppresses experimental corneal angiogenesis. European journal of ophthalmology 2010, 20, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Choi, H.; Rho, C.R. Differential Effects of Bevacizumab, Ranibizumab, and Aflibercept on the Viability and Wound Healing of Corneal Epithelial Cells. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 2016, 32, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Sella, R.; Ben Ishai, M.; Livny, E.; Nahum, Y.; Bahar, I. Subconjunctival Aflibercept for the Treatment of Formed Corneal Neovascularization. Eye Contact Lens: Sci. Clin. Pr. 2020, 47, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S. Treatment of corneal neovascularization with topical aflibercept in a case of exposure keratopathy following cerebellar astrocytoma surgery. Indian J. Ophthalmol. 2019, 67, 145–147. [Google Scholar] [CrossRef]
- Koenig, Y.; Bock, F.; Horn, F.; Kruse, F.; Straub, K.; Cursiefen, C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 2009, 247, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-S.; Hu, F.-R.; Yang, C.-M.; Yeh, P.-T.; Chen, Y.-M.; Hou, Y.-C.; Chen, W.-L. Subconjunctival Injection of Bevacizumab in the Treatment of Corneal Neovascularization Associated With Lipid Deposition. Cornea 2011, 30, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-I.; Chung, J.L.; Hong, J.P.; Min, K.; Seo, K.Y.; Kim, E.K. Bevacizumab Application Delays Epithelial Healing in Rabbit Cornea. Investig. Opthalmology Vis. Sci. 2009, 50, 4653–4659. [Google Scholar] [CrossRef]
- de Redín, I.L.; Boiero, C.; Recalde, S.; Agüeros, M.; Allemandi, D.; Llabot, J.M.; García-Layana, A.; Irache, J.M. In vivo effect of bevacizumab-loaded albumin nanoparticles in the treatment of corneal neovascularization. Exp. Eye Res. 2019, 185, 107697. [Google Scholar] [CrossRef]
- Al-Debasi, T.; Al-Bekairy, A.; Al-Katheri, A.; Al Harbi, S.; Mansour, M. Topical versus subconjunctival anti-vascular endothelial growth factor therapy (Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization. Saudi journal of ophthalmology : official journal of the Saudi Ophthalmological Society 2017, 31, 99–105. [Google Scholar] [CrossRef]
- Bhatti, N.; Qidwai, U.; Hussain, M.; Kazi, A. Efficacy of sub-conjunctival and topical bevacizumab in high-risk corneal transplant survival. JPMA The Journal of the Pakistan Medical Association 2013, 63, 1256–1259. [Google Scholar] [PubMed]
- Homer, H.C.; Houman, M.S.; Hemmati, D. Treatment of Corneal Neovascularization. EyeNet Magazine October. 2013. [Google Scholar]
- Zapata, G.; Racca, L.; Tau, J.; Berra, A. Topical Use of Rapamycin in Herpetic Stromal Keratitis. Ocul. Immunol. Inflamm. 2012, 20, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, J.; Shi, Y.; Liu, M. Rapamycin inhibits corneal inflammatory response and neovascularization in a mouse model of corneal alkali burn. Exp. Eye Res. 2023, 233, 109539. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Joo, C.-K.; Chung, S.K. Comparative Study of Tacrolimus and Bevacizumab on Corneal Neovascularization in Rabbits. Cornea 2015, 34, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, L.; Gil, J.; Costa, E.; Tavares, C.; Rosa, A.; Quadrado, M.J.; Murta, J. Topical tacrolimus in high-risk corneal transplants. Eur. J. Ophthalmol. 2023, 34, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, O.A.; Marinho, D.R.; Kwitko, S. Topical 0.03% tacrolimus preventing rejection in high-risk corneal transplantation: a cohort study. Br. J. Ophthalmol. 2013, 97, 1395–1398. [Google Scholar] [CrossRef]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain research reviews 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, L.; Liu, G.; Zhang, X.; Mukaida, N. Enhanced experimental corneal neovascularization along with aberrant angiogenic factor expression in the absence of IL-1 receptor antagonist. Investigative ophthalmology & visual science 2009, 50, 4761–4768. [Google Scholar]
- Amparo, F.; Dastjerdi, M.H.; Okanobo, A.; Ferrari, G.; Smaga, L.; Hamrah, P.; Jurkunas, U.; Schaumberg, D.A.; Dana, R. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA ophthalmology 2013, 131, 715–723. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, G.-Q.; Lu, P.-R. Potential involvement of nitric oxide synthase but not inducible nitric oxide synthase in the development of experimental corneal neovascularization. JAMA ophthalmology 2011, 4, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Cao, Z.; Leffler, H.; Nilsson, U.J.; Panjwani, N. Galectin-3 Inhibition by a Small-Molecule Inhibitor Reduces Both Pathological Corneal Neovascularization and Fibrosis. Investig. Opthalmology Vis. Sci. 2017, 58, 9–20. [Google Scholar] [CrossRef]
- Mori, K.; Gehlbach, P.; Ando, A.; McVey, D.; Wei, L.; A Campochiaro, P. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Investigative ophthalmology & visual science 2002, 43, 2428–2434. [Google Scholar]
- Jin, J.; Ma, J.-X.; Guan, M.; Yao, K. Inhibition of Chemical Cautery–Induced Corneal Neovascularization by Topical Pigment Epithelium–Derived Factor Eyedrops. Cornea 2010, 29, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Dell, S.; Peters, S.; Mu¨ther, P.; Kociok, N.; Joussen, A.M. The Role of PDGF Receptor Inhibitors and PI3-Kinase Signaling in the Pathogenesis of Corneal Neovascularization. Investig. Opthalmology Vis. Sci. 2006, 47, 1928–1937. [Google Scholar] [CrossRef]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecova, P.; Levy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: the I-CAN study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Bock, F.; Horn, F.K.; Kruse, F.E.; Seitz, B.; Borderie, V.; Früh, B.; Thiel, M.A.; Wilhelm, F.; Geudelin, B.; et al. GS-101 Antisense Oligonucleotide Eye Drops Inhibit Corneal Neovascularization: Interim Results of a Randomized Phase II Trial. Ophthalmology 2009, 116, 1630–1637. [Google Scholar] [CrossRef]
- Natarajan, R.; Ravichandran, S. Fine-needle diathermy for corneal vascularisation. Indian J. Ophthalmol. 2022, 70, 1868–1869. [Google Scholar] [CrossRef]
- Pillai, C.T.; Dua, H.S.; Hossain, P. Fine needle diathermy occlusion of corneal vessels. Investigative ophthalmology & visual science 2000, 41, 2148–2153. [Google Scholar]
- Drzyzga, Ł.M.-K.E. Angiogeneza w rogówce i strategie leczenia antyangiogennego. Okulistyka po Dyplomie 2013, 3. [Google Scholar]
- Le, V.N.H.; Schneider, A.-C.; Scholz, R.; Bock, F.; Cursiefen, C. Fine Needle-Diathermy Regresses Pathological Corneal (Lymph)Angiogenesis and Promotes High-Risk Corneal Transplant Survival. Sci. Rep. 2018, 8, 5707. [Google Scholar] [CrossRef] [PubMed]
- Gerten, G. Bevacizumab (avastin) and argon laser to treat neovascularization in corneal transplant surgery. Cornea 2008, 27, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.G.; Donato, E.; Cordeiro, M.d.C.; de Andrade, M.M.; de Freitas, J.A.H. Treating corneal neovascularization using a combination of anti-VEGF injection and argon laser photocoagulation application - case report. Romanian J. Ophthalmol. 2021, 65, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Bucher, F.; Bi, Y.; Gehlsen, U.; Hos, D.; Cursiefen, C.; Bock, F. Regression of mature lymphatic vessels in the cornea by photodynamic therapy. Br. J. Ophthalmol. 2014, 98, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kim, M.K.; Seo, K.Y.; Ueta, M.; Yoon, K.C. Effectiveness of photodynamic therapy with verteporfin combined with intrastromal bevacizumab for corneal neovascularization in Stevens–Johnson syndrome. Int. Ophthalmol. 2017, 39, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Al-Torbak, A.A. Photodynamic therapy with verteporfin for corneal neovascularization. Middle East African journal of ophthalmology 2012, 19, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.J.; Ambati, B.K.; Marcus, D.M.; Ratanasit, A. Photodynamic therapy for corneal neovascularisation and lipid degeneration. Br. J. Ophthalmol. 2004, 88, 840–840. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Gehra, A.; Sirohi, N. Role of Frequency Doubled Nd. Yag Laser in Treatment of Corneal Neovascularisation. Journal of clinical and diagnostic research: JCDR 2016, 10, NC01–NC04. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Pi, Y.-L. Effect of amnion membrane transplantation on corneal neovascularization in 10 patients with alkali burn. 4. [CrossRef]
- Yang, L.-L.; Zhou, Q.-J.; Wang, Y.; Gao, Y.; Wang, Y.-Q. Comparison of the therapeutic effects of extracts from Spirulina platensis and amnion membrane on inflammation-associated corneal neovascularization. International journal of ophthalmology 2012, 37, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Scorcia, V.; Giannaccare, G.; Lucisano, A.; Vaccaro, S.; Battaglia, C.; Yu, A.C.; Bovone, C.; Busin, M.; Spena, R. Corneal neovascularisation following deep anterior lamellar keratoplasty for corneal ectasia: incidence, timing and risk factors. Br. J. Ophthalmol. 2021, 106, 1363–1367. [Google Scholar] [CrossRef]
- Hoffart, L.; Matonti, F.; Conrath, J.; Daniel, L.; Ridings, B.; Masson, G.S.; Chavane, F. Inhibition of corneal neovascularization after alkali burn: comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin. Exp. Ophthalmol. 2010, 38, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Reidy, J.J.; Riaz, K.M. Short-term regression of corneal neovascularization with combination therapy of argon green laser photocoagulation and subconjunctival bevacizumab. Int. Med Case Rep. J. 2019, ume 12, 89–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




