Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search strategy
2.2. Inclusion and exclusion criteria
2.3. Study selection and data collection
3. Results
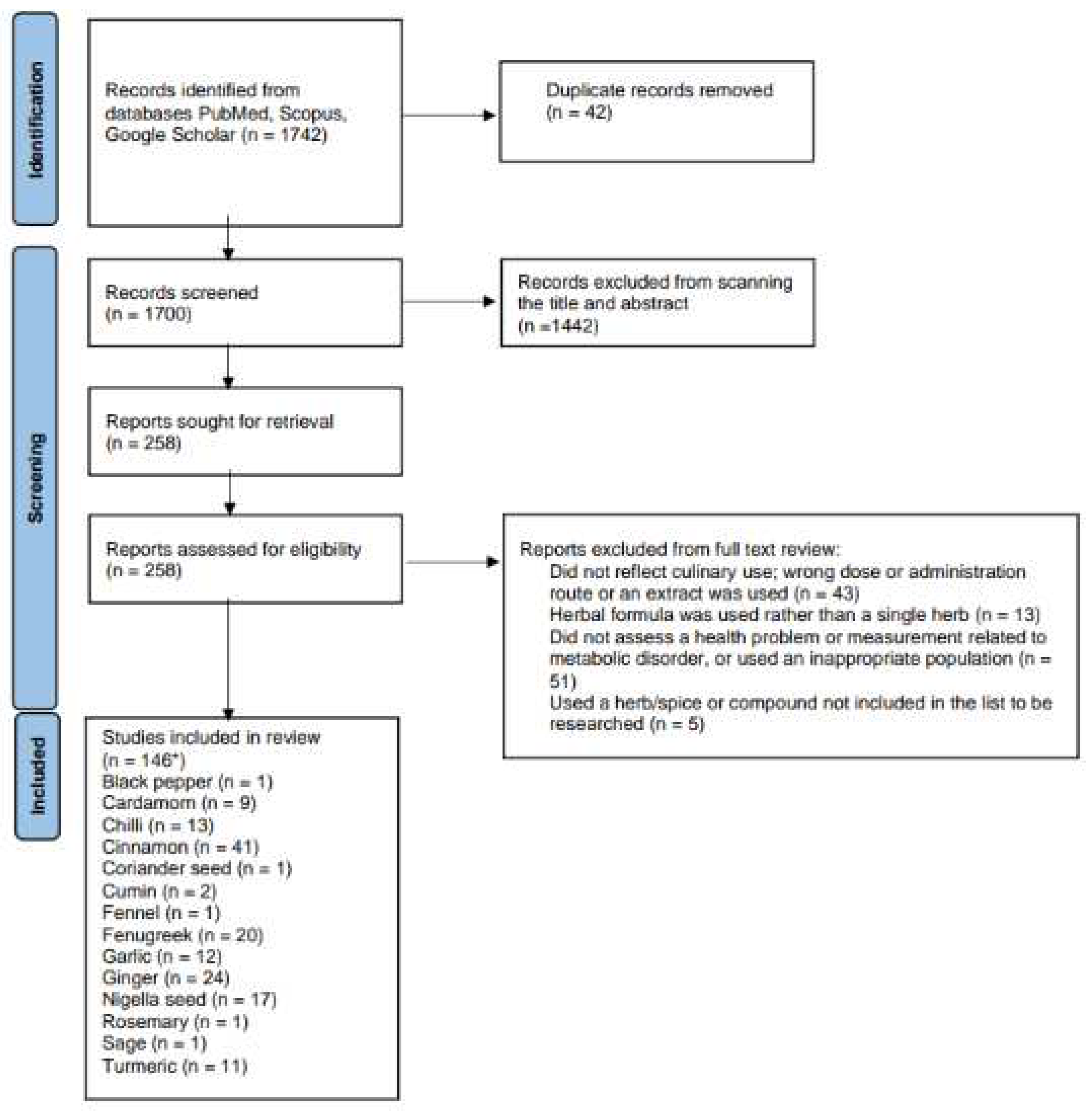
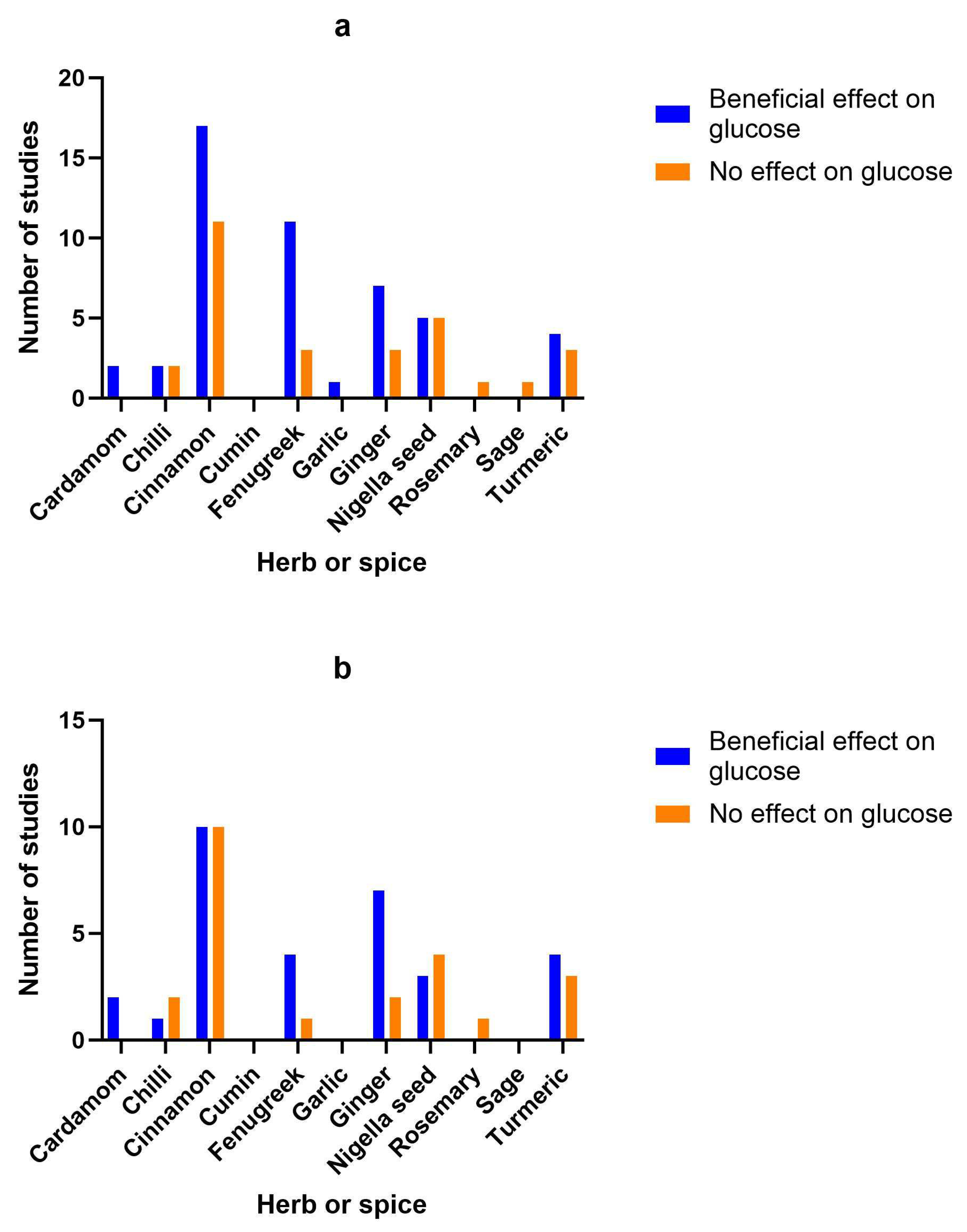
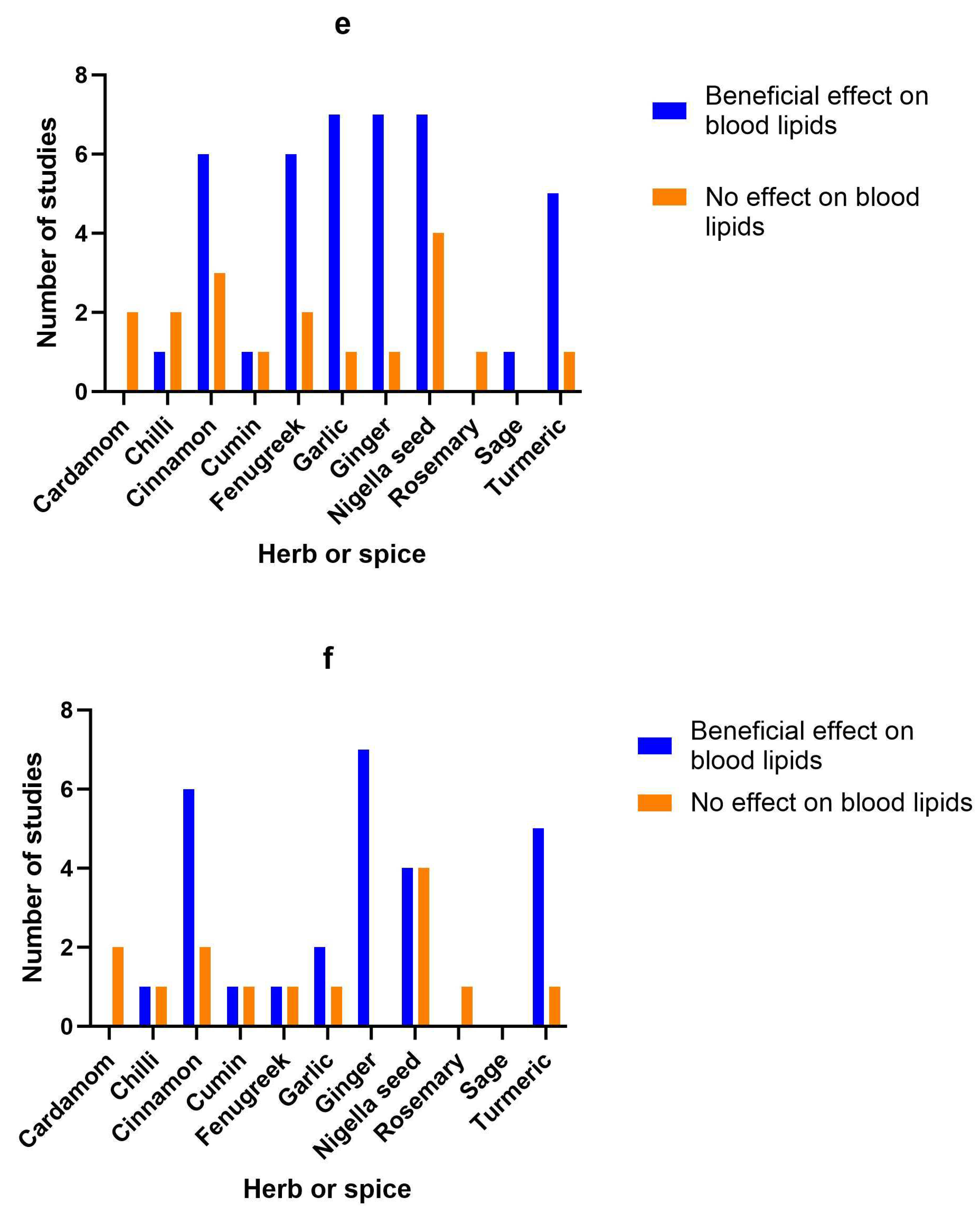
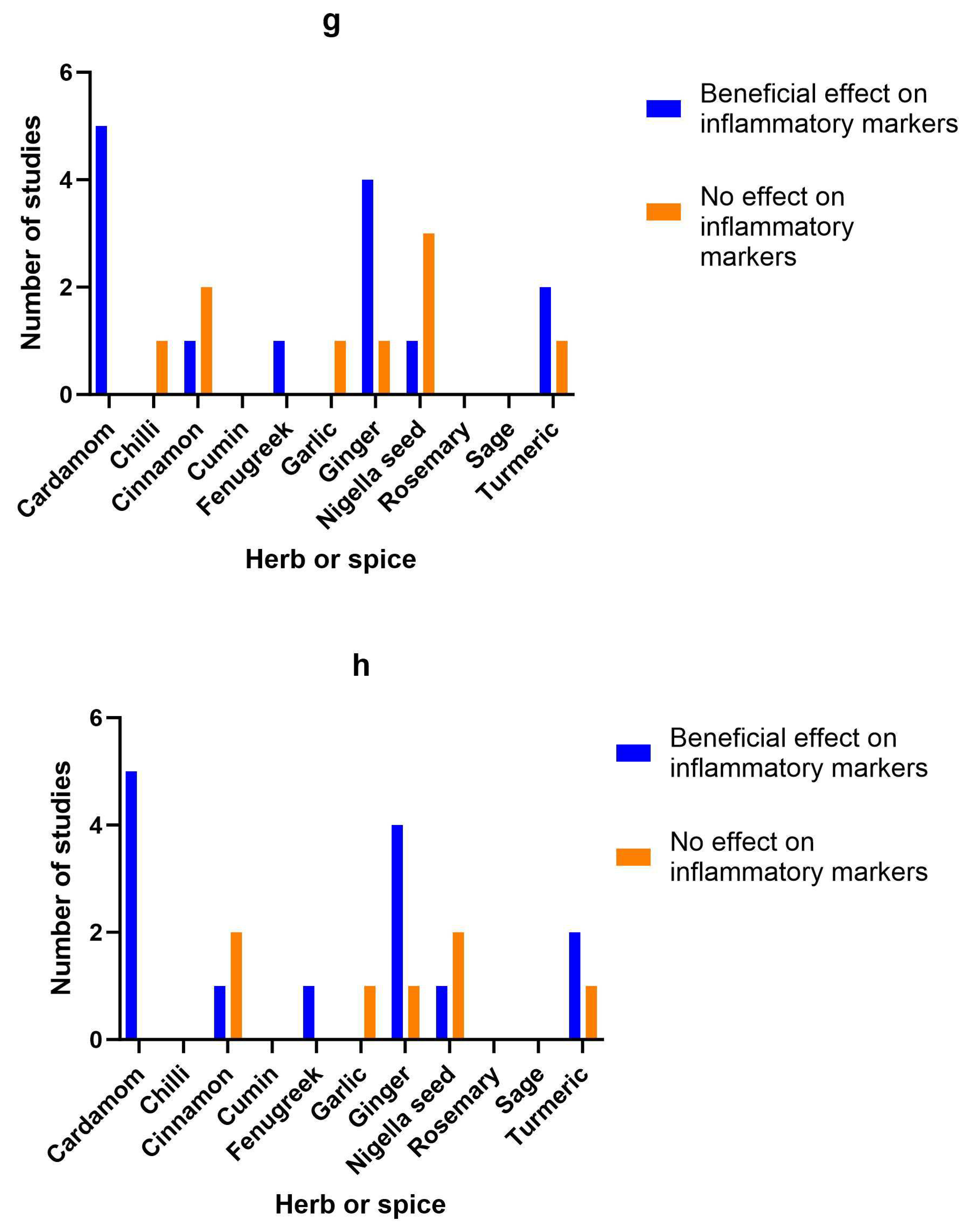
3.1. Black pepper
3.2. Cardamom
3.3. Chilli
3.4. Cinnamon
3.5. Coriander seed
3.6. Cumin
3.7. Fennel
3.8. Fenugreek
3.9. Garlic
3.10. Ginger
3.11. Nigella seeds
3.12. Rosemary
3.13. Sage
3.14. Turmeric
3.15. Herb/spice efficacy
3.16. Adverse effects
3.17. Study quality
4. Discussion
4.1. Limitations and future directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35(3), 414-428.e3.
- Alkhatib DH, Jaleel A, Tariq MNM, Feehan J, Apostolopoulos V, Cheikh Ismail L, et al. The role of bioactive compounds from dietary spices in the management of metabolic syndrome: an overview. Nutrients. 2021, 14(1), 175.
- Jiang TA. Health benefits of culinary herbs and spices. J AOAC International. 2019, 102(2), 395-411.
- Pallauf K, Giller K, Huebbe P, Rimbach G. Nutrition and healthy ageing: calorie restriction or polyphenol-rich "MediterrAsian" diet? Oxid Med Cell Longev. 2013, 2013:707421.
- Bahadoran Z, Golzarand M, Mirmiran P, Saadati N, Azizi F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J Hum Nutr Diet. 2013, 26 Suppl 1, 145-53.
- Dzah CS, Asante-Donyinah D, Letsyo E, Dzikunoo J, Adams ZS. Dietary polyphenols and obesity: a review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis, and starch digestibility and absorption. Plant Foods Hum Nutr. 2022, 78, 1–12.
- Haldar S, Chia SC, Henry CJ. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur J Clin Nutr. 2018, 72(2), 297-300.
- Huang Y, Tsai MF, Thorat RS, Xiao D, Zhang X, Sandhu AK, et al. Endothelial function and postprandial glucose control in response to test-meals containing herbs and spices in adults with overweight/obesity. Front Nutr. 2022, 9, 811433.
- Kroff J, Hume DJ, Pienaar P, Tucker R, Lambert EV, Rae DE. The metabolic effects of a commercially available chicken peri-peri (African bird's eye chilli) meal in overweight individuals. Br J Nutr. 2017, 117(5), 635-644.
- McCrea CE, West SG, Kris-Etherton PM, Lambert JD, Gaugler TL, Teeter DL, et al. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: results of a randomized crossover study and in vitro experiments. J Transl Med. 2015, 13, 7.
- Nakayama H, Tsuge N, Sawada H, Masamura N, Yamada S, Satomi S, et al. A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial. Nutr J. 2014, 13, 67.
- Oh ES, Petersen KS, Kris-Etherton PM, Rogers CJ. Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: a 3-period, crossover, randomized controlled trial. J Nutr. 2020, 150(6), 1600-1609.
- Petersen KS, Rogers CJ, West SG, Proctor DN, Kris-Etherton PM. The effect of culinary doses of spices in a high-saturated fat, high-carbohydrate meal on postprandial lipemia and endothelial function: a randomized, controlled, crossover pilot trial. Food Funct. 2020, 11(4), 3191-3200.
- Petersen KS, Davis KM, Rogers CJ, Proctor DN, West SG, Kris-Etherton PM. Herbs and spices at a relatively high culinary dosage improves 24-hour ambulatory blood pressure in adults at risk of cardiometabolic diseases: a randomized, crossover, controlled-feeding study. Am J Clin Nutr. 2021, 114(6), 1936-1948.
- Zanzer YC, Plaza M, Dougkas A, Turner C, Östman E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: a randomized crossover study in healthy subjects. Food Funct. 2018, 9(5), 2774-2786.
- Peters MDJ, Marnie C, Colquhoun H, Garritty CM, Hempel S, Horsley T, et al. Scoping reviews: reinforcing and advancing the methodology and application. Syst Rev. 2021, 10, 263.
- Cacchione PZ. The evolving methodology of scoping reviews. Clinical Nursing Research. 2016, 25(2), 115-119.
- Agarwal AK. Spice up your life: adipose tissue and inflammation. J Lipids. 2014, 2014, 182575.
- Akhter S. Low to no cost remedies for the management of diabetes mellitus; global health concern. J Diabetes Metab Disord. 2021, 20(1), 951-962.
- Bower A, Marquez S, de Mejia EG. The health benefits of selected culinary herbs and spices found in the traditional mediterranean diet. Crit Rev Food Sci Nutr. 2016, 56(16), 2728-46.
- Deekshith C, Jois M, Radcliffe J, Thomas J. Effects of culinary herbs and spices on obesity: a systematic literature review of clinical trials. J Functional Foods. 2021, 81, 104449.
- Gupta K, Testa H, Greenwood T, Kostek M, Haushalter K, Kris-Etherton PM, et al. The effect of herbs and spices on risk factors for cardiometabolic diseases: a review of human clinical trials. Nutr Rev. 2022, 80(3), 400-427.
- Opara EI. Culinary herbs and spices: what can human studies tell us about their role in the prevention of chronic non-communicable diseases? J Sci Food Agric. 2019, 99(10), 4511-4517. [CrossRef]
- Opara EI, Chohan M. Culinary herbs and spices: Their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int J Mol Sci. 2014, 15(10), 19183-202. [CrossRef]
- Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018, 169(7), 467-473.
- Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008, 88(2), 156-75.
- Gregersen NT, Belza A, Jensen MG, Ritz C, Bitz C, Hels O, et al. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br J Nutr. 2013, 109(3), 556-63.
- Verma SK, Jain V, Katewa SS. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J Biochem Biophys. 2009, 46(6), 503-6.
- Aghasi M, Koohdani F, Qorbani M, Nasli-Esfahani E, Ghazi-Zahedi S, Khoshamal H, et al. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric. 2019, 99(8), 3933-3940.
- Aghasi M, Koohdani F, Qorbani M, Nasli-Esfahani E, Ghazi-Zahedi S, Khoshamal H, et al. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric. 2019, 99(8), 3933-3940. [CrossRef]
- Ghazi Zahedi S, Koohdani F, Qorbani M, Nasli-Esfahani E, Aghasi M, Khoshamal H, et al. The effects of Elettaria cardamom supplementation on inflammatory markers and vascular function in patients with type 2 diabetes mellitus: A mechanism -based randomized clinical trial. J Her Med. 2021, 25, 100403.
- Kazemi S, Yaghooblou F, Siassi F, Rahimi Foroushani A, Ghavipour M, et al. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: a randomized double-blind clinical trial. J Sci Food Agric. 2017, 97(15), 5296-5301.
- Cheshmeh S, Ghayyem M, Khamooshi F, Heidarzadeh-Esfahani N, Rahmani N, Hojati N, et al. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: a double-blind randomized clinical trial. Eat Weight Disord. 2022, 27(2), 821-830.
- Cheshmeh S, Elahi N, Ghayyem M, Mosaieby E, Moradi S, Pasdar Y, et al. Effect of green cardamom on the expression of genes implicated in obesity and diabetes among obese women with polycystic ovary syndrome: a double blind randomized controlled trial. Genes Nutr. 2022, 17(1), 17.
- Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, et al. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond). 2018, 15, 63.
- Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, et al. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: a double-blind randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2019, 19(1), 59.
- Ghazi Zahedi S, Koohdani F, Qorbani M, Nasli-Esfahani E, Aghasi M, Khoshamal H, et al. Effects of green cardamom supplementation on serum levels of Hs-CRP, dimethylarginine, nitric oxide and blood pressure in patients with type 2 diabetes: a randomized, double-blind, placebo controlled, clinical trial. J Her Med. 2022, 32, 100555.
- Ahuja KD, Robertson IK, Geraghty DP, Ball MJ. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr. 2006, 84(1), 63-9.
- Ahuja KD, Ball MJ. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br J Nutr. 2006, 96(2), 239-42.
- Ahuja KD, Robertson IK, Geraghty DP, Ball MJ. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur J Clin Nutr. 2007, 61(3), 326-33.
- Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009, 92(1), 108-13.
- Clegg ME, Golsorkhi M, Henry CJ. Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans. Eur J Nutr. 2013, 52(6), 1579-85.
- Janssens PL, Hursel R, Martens EA, Westerterp-Plantenga MS. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One. 2013, 8(7), e67786.
- Janssens PL, Hursel R, Westerterp-Plantenga MS. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite. 2014, 77, 44-9.
- Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab. 2016, 101(12), 4681-4689.
- Reinbach HC, Martinussen T, Møller P. Effects of hot spices on energy intake, appetite and sensory specific desires in humans. Food Quality and Preference. 2010, 21(6), 655–661.
- Yuan LJ, Qin Y, Wang L, Zeng Y, Chang H, Wang J, et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin Nutr. 2016, 35(2), 388-393.
- Shi Z, Riley M, Taylor AW, Page A. Chilli consumption and the incidence of overweight and obesity in a Chinese adult population. Int J Obes (Lond). 2017, 41(7), 1074-1079.
- Shi Z, Riley M, Brown A, Page A. Chilli intake is inversely associated with hypertension among adults. Clin Nutr ESPEN. 2018, 23, 67-72.
- Atkinson FS, Stockmann KS, Ek KL, Brand-Miller JC. Cassia but not cinnamon reduces postprandial glucose and insulin responses to oatmeal in lean, young adults. Asia Pacific J Clin Nutr. 2008, 17, S137–S137.
- Bernardo MA, Silva ML, Santos E, Moncada MM, Brito J, Proença L, et al. Effect of cinnamon tea on postprandial glucose concentration. J Diabetes Res. 2015 2015, 913651.
- Hlebowicz J, Darwiche G, Björgell O, Almér LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007, 85(6), 1552-6.
- Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, et al. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009, 89(3), 815-21.
- Magistrelli A, Chezem JC. Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. J Acad Nutr Diet. 2012, 112(11), 1806-9.
- Markey O, McClean CM, Medlow P, Davison GW, Trinick TR, Duly E, et al. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc Diabetol. 2011, 10, 78.
- Mettler S, Schwarz I, Colombani PC. Additive postprandial blood glucose-attenuating and satiety-enhancing effect of cinnamon and acetic acid. Nutr Res. 2009, 29(10), 723-7.
- Soares APDC, de Faria NC, Graciano GF, Dos Santos ALS, Valenzuela VDC, Toulson Davisson Correia MI, et al. Cinnamon infusion reduces satiety and increases energy intake: a randomized crossover trial. Ann Nutr Metab. 2022, 78(5), 265-272.
- Solomon TP, Blannin AK. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes Metab. 2007, 9(6), 895-901.
- Solomon TP, Blannin AK. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur J Appl Physiol. 2009, 105(6), 969-76.
- Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010, 27(10), 1159-67.
- Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care. 2007, 30(4), 813-6.
- Azimi P, Ghiasvand R, Feizi A, Hosseinzadeh J, Bahreynian M, Hariri M, et al. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016, 25(3), 133-40.
- Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007, 30(9), 2236-7.
- Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009, 22(5), 507-12. [CrossRef]
- Davari M, Hashemi R, Mirmiran P, Hedayati M, Sahranavard S, Bahreini S, et al. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and sirtuin-1 (SIRT1) in type 2 diabetes: a randomized, double blind, and controlled clinical trial. Nutr J. 2020, 19(1), 1.
- Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003, 26(12), 3215-8. [CrossRef]
- Lira Neto JCG, Damasceno MMC, Ciol MA, de Freitas RWJF, de Araújo MFM, Teixeira CRS, et al. Efficacy of cinnamon as an adjuvant in reducing the glycemic biomarkers of type 2 diabetes mellitus: a three-month, randomized, triple-blind, placebo-controlled clinical trial. J Am Nutr Assoc. 2022, 41(3), 266-274.
- Mirfeizi M, Mehdizadeh Tourzani Z, Mirfeizi SZ, Asghari Jafarabadi M, Rezvani HR, Afzali M. Controlling type 2 diabetes mellitus with herbal medicines: a triple-blind randomized clinical trial of efficacy and safety. J Diabetes. 2016, 8(5), 647-56.
- Mirmiran P, Davari M, Hashemi R, Hedayati M, Sahranavard S, Bahreini S, et al. A randomized controlled trial to determining the effect of cinnamon on the plasma levels of soluble forms of vascular adhesion molecules in type 2 diabetes mellitus. Eur J Clin Nutr. 2019, 73(12), 1605-1612.
- Mirmiranpour H, Huseini HF, Derakhshanian H, Khodaii Z, Tavakoli-Far B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J Diabetes Metab Disord. 2019, 19(1), 53-60.
- Sahib AS. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: a randomized, placebo-controlled clinical trial. J Intercult Ethnopharmacol. 2016, 5(2), 108-13.
- Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thai. 2006, 89 Suppl 3, S200-5.
- Talaei B, Amouzegar A, Sahranavard S, Hedayati M, Mirmiran P, Azizi F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients. 2017, 9(9), 991.
- Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006, 136(4), 977-80.
- Wainstein J, Stern N, Heller S, Boaz M. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. J Med Food. 2011, 14(12), 1505-10.
- Zahedifar A, Khodashenas M, Bijari B, Zahedifar F. Effects of cinnamon on fasting blood sugar and hemoglobin A1C in patients with type II diabetes mellitus: a randomized clinical trial. [In Persian]. J Mazandaran Univ Med Sci. 2018, 27(156), 80-88.
- Zahmatkesh M, Fallah Huseini H, Hajiaghaee R, Heidari M, Mehrafarin A, Tavakoli-far B. The effects of Cinnamomum zeylanicum J. Presl on blood glucose level in patients with type 2 diabetes, a double-blind clinical trial. J Med Plants. 2012, 11 (SUPPL. 8), 258-263.
- Zare R, Nadjarzadeh A, Zarshenas MM, Shams M, Heydari M. Efficacy of cinnamon in patients with type II diabetes mellitus: a randomized controlled clinical trial. Clin Nutr. 2019, 38(2), 549-556.
- Borzoei A, Rafraf M, Niromanesh S, Farzadi L, Narimani F, Doostan F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J Tradit Complement Med. 2017, 8(1), 128-133.
- Borzoei A, Rafraf M, Asghari-Jafarabadi M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac J Clin Nutr. 2018, 27(3), 556-563.
- Dastgheib M, Barati-Boldaji R, Bahrampour N, Taheri R, Borghei M, Amooee S, et al. A comparison of the effects of cinnamon, ginger, and metformin consumption on metabolic health, anthropometric indices, and sexual hormone levels in women with poly cystic ovary syndrome: A randomized double-blinded placebo-controlled clinical trial. Front Nutr. 2022, 9, 1071515.
- Hajimonfarednejad M, Nimrouzi M, Heydari M, Zarshenas MM, Raee MJ, Jahromi BN. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother Res. 2018, 32(2), 276-283.
- Kort DH, Lobo RA. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am J Obstet Gynecol. 2014 211(5), 487.e1-6.
- Khan AA, Begum W. Efficacy of Darchini in the management of polycystic ovarian syndrome: A randomized clinical study. J Her Med. 2019, 15, 100249.
- Gupta Jain S, Puri S, Misra A, Gulati S, Mani K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: a randomized double -blind control trial. Lipids Health Dis. 2017, 16(1), 113.
- Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res. 2014, 34(2), 143-8.
- Wickenberg J, Lindstedt S, Berntorp K, Nilsson J, Hlebowicz J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br J Nutr. 2012, 107(12), 1845-9.
- Gutierrez JL, Bowden RG, Willoughby DS. Cassia cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. J Diet Suppl. 2016, 13(4), 461-71.
- Pishdad S, Nadjarzadeh A, Salehi Abargouei A, Karimi Nazari E, Papoli M. Effect of cumin and cinnamon on lipid profile in middle-aged women with dyslipidemia: a double blind, randomized controlled clinical trial. Progr Nutr [Internet]. 2018, 20(2-S), 232-7.
- Lee MS, Wahlqvist ML, Chou YC, Fang WH, Lee JT, Kuan JC, et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac J Clin Nutr. 2014, 23(4), 581-91.
- Zeb F, Safdar M, Fatima S, Khan S, Alam S, Muhammad M, et al. Supplementation of garlic and coriander seed powder: Impact on body mass index, lipid profile and blood pressure of hyperlipidemic patients. Pak J Pharm Sci. 2018, 31(5), 1935-1941.
- Zare R, Heshmati F, Fallahzadeh H, Nadjarzadeh A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement Ther Clin Pract. 2014, 20(4), 297-301.
- Bae J, Kim J, Choue R, Lim H. Fennel (Foeniculum vulgare) and fenugreek (Trigonella foenum-graecum) tea drinking suppresses subjective short-term appetite in overweight women. Clin Nutr Res. 2015, 4(3), 168-74.
- Gopalpura PB, Jayanthi C, Dubey S. Effect of Trigonella foenum-graecum seeds on the glycemic index of food: A clinical evaluation. Int J Diab Dev Ctries. 2007, 27(2), 41-45.
- Kiss R, Szabó K, Gesztelyi R, Somodi S, Kovács P, Szabó Z, et al. Insulin-sensitizer effects of fenugreek seeds in parallel with changes in plasma MCH levels in healthy volunteers. Int J Mol Sci. 2018, 19(3), 771.
- Robert SD, Ismail AA, Rosli WI. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. Eur J Nutr. 2016, 55(7), 2275-80.
- Bhadauria SS, Kushwah A. Fenugreek seeds as a therapeutic supplement for patients with noninsulin dependent diabetes mellitus: a cross-sectional study. J Clin Diagnostic Res. 2021, 15(4), BC21–BC23. [CrossRef]
- Geberemeskel GA, Debebe YG, Nguse NA. Antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J Diabetes Res. 2019, 2019, 8507453.
- Hadi A, Arab A, Hajianfar H, Talaei B, Miraghajani M, Babajafari S, Marx W, Tavakoly R. The effect of fenugreek seed supplementation on serum irisin levels, blood pressure, and liver and kidney function in patients with type 2 diabetes mellitus: a parallel randomized clinical trial. Complement Ther Med. 2020, 49, 102315.
- Hassani SS, Arezodar FF, Esmaeili SS, Gholami-Fesharaki M. The effect of combined therapy with fenugreek and nutrition training based on iranian traditional medicine on FBS, HGA1C, BMI, and waist circumference in type 2 diabetic patients: a randomized double-blinded clinical trial. J Advances Med Biomed Res. 2019a, 27(120), 37-42.
- Hassani SS, Fallahi Arezodar F, Esmaeili SS, Gholami-Fesharaki M. Effect of fenugreek use on fasting blood glucose, glycosylated hemoglobin, body mass index, waist circumference, blood pressure and quality of life in patients with type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled clinical trials. Galen Med J. 2019, 8, e1432.
- Kassaian N, Azadbakht L, Forghani B, Amini M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int J Vitam Nutr Res. 2009, 79(1), 34-9.
- Losso JN, Holliday DL, Finley JW, Martin RJ, Rood JC, Yu Y, et al. Fenugreek bread: a treatment for diabetes mellitus. J Med Food. 2009, 12(5), 1046-9.
- Madar Z, Abel R, Samish S, Arad J. Glucose-lowering effect of fenugreek in non-insulin dependent diabetics. Eur J Clin Nutr. 1988, 42(1), 51-4.
- Najdi RA, Hagras MM, Kamel FO, Magadmi RM. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr Health Sci. 2019, 19(1), 1594-1601.
- Rafraf M, Malekiyan M, Asghari-Jafarabadi M, Aliasgarzadeh A. Effect of fenugreek seeds on serum metabolic factors and adiponectin levels in type 2 diabetic patients. Int J Vitam Nutr Res. 2014, 84(3-4), 196-205.
- Sharma RD. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutrition Research. 1986, 6(12), 1353-1364.
- Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr. 1990, 44(4), 301-6.
- Sharma RD, Sarkar A, Hazra DK, Misra B, Singh JB, Maheshwari BB, et al. Hypolipidaemic effect of fenugreek seeds: a chronic study in non-insulin dependent diabetic patients. Phytotherapy Research. 1996, 10, 332-334.
- Tavakoly R, Maracy MR, Karimifar M, Entezari MH. Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur J Integrative Med. 2018, 18, 13–17.
- Bordia A, Verma SK, Srivastava KC. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1997, 56(5), 379-84.
- Sowmya P, Rajyalakshmi P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Foods Hum Nutr. 1999, 53(4), 359-65.
- Yousefi E, Zareiy S, Zavoshy R, Noroozi M, Jahanihashemi H, Ardalani H. Fenugreek: a therapeutic complement for patients with borderline hyperlipidemia: a randomised, double-blind, placebo-controlled, clinical trial. Adv Integ Med. 2017, 4(1), 31–35.
- Qidwai W, Qureshi R, Hasan SN, Azam SI. Effect of dietary garlic (Allium Sativum) on the blood pressure in humans--a pilot study. J Pak Med Assoc. 2000, 50(6), 204-7.
- Zhang S, Liu M, Wang Y, Zhang Q, Liu L, Meng G, et al. Raw garlic consumption is inversely associated with prehypertension in a large-scale adult population. J Hum Hypertens. 2020, 34(1), 59-67.
- Aslani N, Entezari MH, Askari G, Maghsoudi Z, Maracy MR. Effect of garlic and lemon juice mixture on lipid profile and some cardiovascular risk factors in people 30-60 years old with moderate hyperlipidaemia: a randomized clinical trial. Int J Prev Med. 2016, 7, 95.
- Choudhary PR, Jani RD, Sharma MS. Effect of raw crushed garlic (Allium sativum L.) on components of metabolic syndrome. J Diet Suppl. 2018, 15(4), 499-506.
- van Doorn MB, Espirito Santo SM, Meijer P, Kamerling IM, Schoemaker RC, Dirsch V, et al. Effect of garlic powder on C-reactive protein and plasma lipids in overweight and smoking subjects. Am J Clin Nutr. 2006, 84(6), 1324-9.
- Scharbert G, Kalb ML, Duris M, Marschalek C, Kozek-Langenecker SA. Garlic at dietary doses does not impair platelet function. Anesth Analg. 2007, 105(5), 1214-8.
- Bakhsh R, Chughtai MI. Influence of garlic on serum cholesterol, serum triglycerides, serum total lipids and serum glucose in human subjects. Nahrung. 1984, 28(2), 159-63.
- Roberts K, Jahner DKW, Buddington RK. Influence of garlic supplementation on human fecal flora and serum lipid levels. FASEB Journal 1998, 12(5), A876.
- Charron CS, Dawson HD, Albaugh GP, Solverson PM, Vinyard BT, Solano-Aguilar GI, et al. A single meal containing raw, crushed garlic influences expression of immunity- and cancer-related genes in whole blood of humans. J Nutr. 2015, 145(11), 2448-55.
- Sangouni AA, Alizadeh M, Jamalzehi A, Parastouei K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: a randomized clinical trial. Phytother Res. 2021, 35(8), 4433-4441.
- Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement Ther Med. 2020, 51, 102428.
- Sangouni AA, Mohammad Hosseini Azar MR, Alizadeh M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomised controlled clinical trial. Br J Nutr. 2020, 124(4), 450-456.
- Janssen PL, Meyboom S, van Staveren WA, de Vegt F, Katan MB. Consumption of ginger (Zingiber officinale roscoe) does not affect ex vivo platelet thromboxane production in humans. Eur J Clin Nutr. 1996, 50(11), 772-4.
- Miyamoto M, Matsuzaki K, Katakura M, Hara T, Tanabe Y, Shido O. Oral intake of encapsulated dried ginger root powder hardly affects human thermoregulatory function, but appears to facilitate fat utilization. Int J Biometeorol. 2015, 59(10), 1461-74.
- Mansour MS, Ni YM, Roberts AL, Kelleman M, Roychoudhury A, St-Onge MP. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: a pilot study. Metabolism. 2012, 61(10), 1347-52.
- Ebrahimzadeh Attari V, Asghari Jafarabadi M, Zemestani M, Ostadrahimi A. Effect of Zingiber officinale supplementation on obesity management with respect to the uncoupling protein 1 -3826A>G and ß3-adrenergic receptor Trp64Arg polymorphism. Phytother Res. 2015, 29(7), 1032-9.
- Ebrahimzadeh Attari V, Ostadrahimi A, Asghari Jafarabadi M, Mehralizadeh S, Mahluji S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: a RCT. Eur J Nutr. 2016, 55(6), 2129-36.
- Ebrahimzadeh Attari V, Mahluji S, Asghari Jafarabadi M, Ostadrahimi A. Effects of supplementation with ginger (Zingiber officinale Roscoe) on serum glucose, lipid profile and oxidative stress in obese women: a randomized, placebo-controlled clinical trial. Pharm Sci. 2015, 21(4), 184-191.
- Carvalho GCN, Lira-Neto JCG, Araújo MFM, Freitas RWJF, Zanetti ML, Damasceno MMC. Effectiveness of ginger in reducing metabolic levels in people with diabetes: a randomized clinical trial. Rev Lat Am Enfermagem. 2020, 28, e3369.
- Khandouzi N, Shidfar F, Rajab A, Rahideh T, Hosseini P, Mir Taheri M. The effects of ginger on fasting blood sugar, hemoglobin a1c, apolipoprotein B, apolipoprotein a-I and malondialdehyde in type 2 diabetic patients. Iran J Pharm Res. 2015, 14(1), 131-40.
- Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SE. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food Sci Nutr. 2013, 64(6), 682-6.
- Mahluji S, Ostadrahimi A, Mobasseri M, Ebrahimzade Attari V, Payahoo L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Adv Pharm Bull. 2013, 3(2), 273-6.
- Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014, 65(4), 515-20.
- Hajimoosayi F, Jahanian Sadatmahalleh S, Kazemnejad A, Pirjani R. Effect of ginger on the blood glucose level of women with gestational diabetes mellitus (GDM) with impaired glucose tolerance test (GTT): a randomized double-blind placebo-controlled trial. BMC Complement Med Ther. 2020, 20(1), 116.
- Mozaffari-Khosravi H, Talaei B, Jalali BA, Najarzadeh A, Mozayan MR. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. 2014, 22(1), 9-16.
- Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Complement Integr Med. 2015, 12(2), 165-70.
- Zarezadeh M, Saedisomeolia A, Khorshidi M, Kord Varkane H, Makhdoomi Arzati M, Abdollahi M, et al. Asymmetric dimethylarginine and soluble inter-cellular adhesion molecule-1 serum levels alteration following ginger supplementation in patients with type 2 diabetes: a randomized double-blind, placebo-controlled clinical trial. J Complement Integr Med. 2018, 16(2), /j/jcim.2019.16.issue-2/jcim-2018-0019/jcim-2018-0019.xml.
- Mohammadzadeh Honarvar N, Zarezadeh M, Khorshidi M, Makhdoomi Arzati M, Yekaninejad MS, Abdollahi M, et al. The effect of an oral ginger supplementation on NF-κB concentration in peripheral blood mononuclear cells and anthropomorphic data of patients with type 2 diabetes: a randomized double-blind, placebo-controlled clinical trial. Complement Ther Med. 2019, 42, 7-11.
- Alizadeh-Navaei R, Roozbeh F, Saravi M, Pouramir M, Jalali F, Moghadamnia AA. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med J. 2008, 29(9), 1280-4.
- Kamari N, Moradinazar M, Qasemi M, Khosravy T, Samadi M, Abdolahzad H. Combination of the effect of ginger and anti-inflammatory diet on children with obesity with nonalcoholic fatty liver disease: a randomized clinical trial. Food Sci Nutr. 2023, 11(4), 1846-1859.
- Rafie Hosseini SA, Hajiani E, Saki Malehi A, Mard SA. Effect of ginger powder supplementation in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Clin Exper Gastroenterol. 2020, 13, 35–45.
- Ashraf H, Heydari M, Shams M, Zarshenas MM, Tavakoli A, Sayadi M. Efficacy of ginger supplementation in relieving persistent hypothyroid symptoms in patients with controlled primary hypothyroidism: a pilot randomized, double-blind, placebo-controlled clinical trial. Evid Based Complement Alt Med. 2022, 5456855–10.
- Pelegrin S, Galtier F, Chalançon A, Gagnol JP, Barbanel AM, Pélissier Y, et al. Effects of Nigella sativa seeds (black cumin) on insulin secretion and lipid profile: A pilot study in healthy volunteers. Br J Clin Pharmacol. 2019, 85(7), 1607-1611.
- Amin F, Islam N, Anila N, Gilani AH. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome - a double blind randomized controlled trial - TAK-MetS trial. Complement Ther Med. 2015, 23(2), 165-74.
- Datau EA, Wardhana, Surachmanto EE, Pandelaki K, Langi JA, Fias. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010 42(3), 130-4.
- Farhangi MA, Dehghan P, Tajmiri S, Abbasi MM. The effects of Nigella sativa on thyroid function, serum vascular endothelial growth factor (VEGF) - 1, Nesfatin-1 and anthropometric features in patients with Hashimoto's thyroiditis: a randomized controlled trial. BMC Complement Altern Med. 2016, 16(1), 471.
- Farhangi MA, Tajmiri S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto's thyroiditis. Clin Nutr ESPEN. 2020, 37, 207-212.
- Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, et al. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull. 2014, 4(1), 29-33.
- Mohtashami A, Mahaki B, Azadbakht L, Entezari MH. Effects of bread with Nigella sativa on lipid profiles, apolipoproteins and inflammatory factor in metabolic syndrome patients. Clin Nutr Res. 2016, 5(2), 89-95.
- Mohtashami A. Effects of bread with Nigella Sativa on blood glucose, blood pressure and anthropometric indices in patients with metabolic syndrome. Clin Nutr Res. 2019, 8(2), 138-147.
- Shirazi M, Khodakarami F, Feizabad E, Ghaemi M. The effects of Nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine. 2020, 69(1), 49-52.
- Ibrahim RM, Hamdan NS, Mahmud R, Imam MU, Saini SM, Rashid SN, et al. A randomised controlled trial on hypolipidemic effects of Nigella sativa seeds powder in menopausal women. J Transl Med. 2014, 12, 82.
- Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009, 15(6), 639-44.
- Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med Arch. 2012, 66(3), 198-200.
- Badar A, Kaatabi H, Bamosa A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: nonrandomized clinical trial. Ann Saudi Med. 2017, 37(1), 56-63.
- Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015, 10(2), e0113486.
- Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010, 54(4), 344-54.
- Darand M, Darabi Z, Yari Z, Saadati S, Hedayati M, Khoncheh A, et al. Nigella sativa and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: results from a randomized, double-blind, placebo-controlled, clinical trial. Complement Ther Med. 2019, 44, 204-209.
- Darand M, Darabi Z, Yari Z, Hedayati M, Shahrbaf MA, Khoncheh A, et al. The effects of black seed supplementation on cardiovascular risk factors in patients with nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2019, 33(9), 2369-2377.
- Akbari S, Sohouli MH, Ebrahimzadeh S, Ghanaei FM, Hosseini AF, Aryaeian N. Effect of rosemary leaf powder with weight loss diet on lipid profile, glycemic status, and liver enzymes in patients with nonalcoholic fatty liver disease: a randomized, double-blind clinical trial. Phytother Res. 2022, 36(5), 2186-2196.
- Sá CM, Ramos AA, Azevedo MF, Lima CF, Fernandes-Ferreira M, Pereira-Wilson C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int J Mol Sci. 2009, 10(9), 3937-3950.
- Maithili Karpaga Selvi N, Sridhar MG, Swaminathan RP, Sripradha R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian J Clin Biochem. 2015, 30(2), 180-6.
- Darmian MA, Hoseini R, Amiri E, Golshani S. How combined and separate aerobic training and turmeric supplementation alter lipid profile and glycemic status? A clinical trial in middle-aged females with type 2 diabetes and hyperlipidemia. Int. Cardiovasc. Res. J. 2021, 15(3), e118791, 111-118.
- Darmian MA, Hoseini R, Amiri E, Golshani S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: a clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21(1), 275-283.
- Adab Z, Eghtesadi S, Vafa MR, Heydari I, Shojaii A, Haqqani H, et al. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother Res. 2019, 33(4), 1173-1181.
- Srinivasan A, Selvarajan S, Kamalanathan S, Kadhiravan T, Prasanna Lakshmi NC, Adithan S. Effect of Curcuma longa on vascular function in native Tamilians with type 2 diabetes mellitus: A randomized, double-blind, parallel arm, placebo-controlled trial. Phytother Res. 2019, 33(7), 1898-1911.
- Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010, 9, 43.
- Nieman DC, Cialdella-Kam L, Knab AM, Shanely RA. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: a metabolomics approach. Plant Foods Hum Nutr. 2012, 67(4), 415-21.
- Uchio R, Okuda-Hanafusa C, Saji R, Kawasaki K, Muroyama K, Murosaki S, et al. A hot water extract of Curcuma longa L. improves fasting serum glucose levels in participants with low-grade inflammation: reanalysis of data from two randomized, double-blind, placebo-controlled trials. Nutrients. 2022, 14(18), 3763.
- Jarhahzadeh M, Alavinejad P, Farsi F, Husain D, Rezazadeh A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetol Metab Syndr. 2021, 13(1), 112.
- Navekar R, Rafraf M, Ghaffari A, Asghari-Jafarabadi M, Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J. Am. Coll. Nutr. 2017, 36(4), 261-267.
- Kandikattu HK, Rachitha P, Jayashree GV, Krupashree K, Sukhith M, Majid A, et al. Anti-inflammatory and anti-oxidant effects of cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS. Biomed Pharmacother. 2017, 91, 191-201.
- Yahyazadeh R, Ghasemzadeh Rahbardar M, Razavi BM, Karimi G, Hosseinzadeh H. The effect of Elettaria cardamomum (cardamom) on the metabolic syndrome: narrative review. Iran J Basic Med Sci. 2021, 24(11), 1462-1469.
- Panchal SK, Bliss E, Brown L. Capsaicin in metabolic syndrome. Nutrients. 2018, 10(5), 630.
- Akilen R, Tsiami A, Devendra D, Robinson N. Cinnamon in glycaemic control: systematic review and meta analysis. Clin Nutr. 2012, 31(5), 609-15.
- Sharma S, Mandal A, Kant R, Jachak S, Jagzape M. Is cinnamon efficacious for glycaemic control in type-2 diabetes mellitus? J. Pak. Med. Assoc. 2020, 70(11), 2065-2069.
- Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: a review. Nutrients. 2022, 14(13), 2773.
- Yu T, Lu K, Cao X, Xia H, Wang S, Sun G, et al. The effect of cinnamon on glycolipid metabolism: a dose-response meta-analysis of randomized controlled trials. Nutrients. 2023, 15(13), 2983.
- Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013, 11(5), 452-9.
- Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 2008, 31(1), 41-3.
- Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J. Med. Food. 2011, 14(9), 884-9.
- Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, et al. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: a meta-analysis and meta-regression. Diabetes Res. Clin. Pract. 2019, 156, 107815.
- Jamali N, Kazemi A, Saffari-Chaleshtori J, Samare-Najaf M, Mohammadi V, Clark CCT. The effect of cinnamon supplementation on lipid profiles in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2020, 55, 102571.
- Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst. Rev. 2012, 2012(9), CD007170.
- Ranasinghe P, Jayawardana R, Galappaththy P, Constantine GR, de Vas Gunawardana N, Katulanda P. Efficacy and safety of 'true' cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis. Diabet. Med. 2012, 29(12), 1480-92.
- Heydarpour F, Hemati N, Hadi A, Moradi S, Mohammadi E, Farzaei MH. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: a systematic review and meta-analysis. J. Ethnopharmacol. 2020, 254, 112741.
- Kutbi EH, Sohouli MH, Fatahi S, Lari A, Shidfar F, Aljhdali MM, et al. The beneficial effects of cinnamon among patients with metabolic diseases: a systematic review and dose-response meta-analysis of randomized-controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62(22), 6113-6131.
- Wu T, Huang W, He M, Yue R. Effects of cinnamon supplementation on lipid profiles among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis. Complement Ther Clin Pract. 2022, 49, 101625.
- Krittanawong C, Isath A, Scott CZ, Wang Z, Kaplin S, Jneid H, et al. Association between cinnamon consumption and risk of cardiovascular health: a systematic review and meta-analysis. Am J Med. 2022, 135(1), 110-117.
- Maierean SM, Serban MC, Sahebkar A, Ursoniu S, Serban A, Penson P, et al. The effects of cinnamon supplementation on blood lipid concentrations: a systematic review and meta-analysis. J. Clin. Lipidol. 2017, 11(6), 1393-1406.
- Valussi M. Functional foods with digestion-enhancing properties. Int J Food Sci Nutr. 2012, 63 Suppl 1, 82-9.
- Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr. J. 2014, 13, 7.
- Askarpour M, Alami F, Campbell MS, Venkatakrishnan K, Hadi A, Ghaedi E. Effect of fenugreek supplementation on blood lipids and body weight: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2020, 253, 112538.
- Shabil M, Bushi G, Bodige PK, Maradi PS, Patra BP, Padhi BK, et al. Effect of fenugreek on hyperglycemia: a systematic review and meta-analysis. Medicina (Kaunas). 2023, 59(2), 248.
- Amini MR, Payandeh N, Sheikhhossein F, Pourreza S, Ghalandari H, Askarpour M, et al. The effects of fenugreek seed consumption on blood pressure: a systematic review and meta-analysis of randomized controlled trials. High Blood Press. Cardiovasc. Prev. 2023, 30(2), 123-133.
- Correia AGDS, Alencar MB, Dos Santos AN, da Paixão DCB, Sandes FLF, Andrade B, et al. Effect of saffron and fenugreek on lowering blood glucose: a systematic review with meta-analysis. Phytother Res. 2023, 37(5), 2092-2101.
- Fakhr L, Chehregosha F, Zarezadeh M, Chaboksafar M, Tarighat-Esfanjani A. Effects of fenugreek supplementation on the components of metabolic syndrome: a systematic review and dose-response meta-analysis of randomized clinical trials. Pharmacol Res. 2023, 187, 106594.
- Gong J, Fang K, Dong H, Wang D, Hu M, Lu F. Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: a meta-analysis. J. Ethnopharmacol. 2016, 194, 260-268.
- Heshmat-Ghahdarijani K, Mashayekhiasl N, Amerizadeh A, Teimouri Jervekani Z, Sadeghi M. Effect of fenugreek consumption on serum lipid profile: a systematic review and meta-analysis. Phytother Res. 2020, 34(9), 2230-2245.
- Khodamoradi K, Khosropanah MH, Ayati Z, Chang D, Nasli-Esfahani E, Ayati MH, et al. The effects of fenugreek on cardiometabolic risk factors in adults: a systematic review and meta-analysis. Complement. Ther. Med. 2020, 52, 102416.
- Gadidala SK, Johny E, Thomas C, Nadella M, Undela K, Adela R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: A systematic review and meta-analysis. Phytother Res. 2023, 37(6), 2242-2254.
- Schwingshackl L, Missbach B, Hoffmann G. An umbrella review of garlic intake and risk of cardiovascular disease. Phytomedicine. 2016, 23(11), 1127-33.
- Varshney R, Budoff MJ. Garlic and Heart Disease. J. Nutr. 2016, 146(2), 416S-421S.
- Sobenin IA, Myasoedova VA, Iltchuk MI, Zhang DW, Orekhov AN. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 2019, 17(10), 721-728.
- Westerterp-Plantenga M, Diepvens K, Joosen AM, Bérubé-Parent S, Tremblay A. Metabolic effects of spices, teas, and caffeine. Physiol. Behav. 2006, 89(1), 85-91.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3(5), 337-52.
- Dalli M, Bekkouch O, Azizi SE, Azghar A, Gseyra N, Kim B. Nigella sativa L. phytochemistry and pharmacological activities: a review (2019-2021). Biomolecules. 2021, 12(1), 20.
- Salehi B, Quispe C, Imran M, Ul-Haq I, Živković J, Abu-Reidah IM, et al. Nigella plants - traditional uses, bioactive phytoconstituents, preclinical and clinical studies. Front. Pharmacol. 2021, 12, 625386.
- Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement. Ther. Med. 2017, 35, 6-13.
- Sahebkar A, Soranna D, Liu X, Thomopoulos C, Simental-Mendia LE, Derosa G, et al. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J. Hypertens. 2016, 34(11), 2127-35.
- Askari G, Rouhani MH, Ghaedi E, Ghavami A, Nouri M, Mohammadi H. Effect of Nigella sativa (black seed) supplementation on glycemic control: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 33(5), 1341-1352.
- Hallajzadeh J, Milajerdi A, Mobini M, Amirani E, Azizi S, Nikkhah E, et al. Effects of Nigella sativa on glycemic control, lipid profiles, and biomarkers of inflammatory and oxidative stress: a systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2020, 34(10), 2586-2608.
- Kavyani Z, Musazadeh V, Golpour-Hamedani S, Moridpour AH, Vajdi M, Askari G. The effect of Nigella sativa (black seed) on biomarkers of inflammation and oxidative stress: an updated systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2023, 31(3), 1149-1165.
- Montazeri RS, Fatahi S, Sohouli MH, Abu-Zaid A, Santos HO, Găman MA, et al. The effect of Nigella sativa on biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J. Food Biochem. 2021, 45(4), e13625.
- Mousavi SM, Sheikhi A, Varkaneh HK, Zarezadeh M, Rahmani J, Milajerdi A. Effect of Nigella sativa supplementation on obesity indices: a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2018, 38, 48-57.
- Namazi N, Larijani B, Ayati MH, Abdollahi M. The effects of Nigella sativa L. on obesity: a systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173-181.
- Tang G, Zhang L, Tao J, Wei Z. Effect of Nigella sativa in the treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35(8), 4183-4193.
- Tavakoly R, Arab A, Vallianou N, Clark CCT, Hadi A, Ghaedi E, Ghavami A. The effect of Nigella sativa L. supplementation on serum C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 149-155.
- Rolfe V. Mackonochie M, Mills S, MacLennan E. Turmeric/curcumin health outcomes: A meta-review of systematic reviews. Eur. J. Integ. Med. 2020, 40, 101252.
- Dei Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential. Nutrients. 2019, 11(9), 2147.
- Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. 2014, 28(5), 633-42.
- Sakkas H, Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27(3), 429-438.
- El-Kased RF, El-Kersh DM. GC-MS profiling of naturally extracted essential oils: antimicrobial and beverage preservative actions. Life (Basel). 2022, 12(10), 1587.
- Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112(2), 203-13.
- Ajazuddin, Alexander A, Qureshi A, Kumari L, Vaishnav P, Sharma M, et al. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia. 2014, 97, 1-14.
- Chiavaroli L, Lee D, Ahmed A, Cheung A, Khan TA, Blanco S, et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ. 2021, 374, n1651.
- Mensink RP, Aro A, Den Hond E, German JB, Griffin BA, ter Meer H-U, et al. PASSCLAIM – Diet-related cardiovascular disease. Eur. J. Nutr. 2003, 42(Suppl 1), 1/6-1/27.
- Edmands WM, Ferrari P, Rothwell JA, Rinaldi S, Slimani N, Barupal DK, et al. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am. J. Clin. Nutr. 2015, 102(4), 905-13.
- Shahwan M, Alhumaydhi F, Ashraf GM, Hasan PMZ, Shamsi A. Role of polyphenols in combating type 2 diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567-579.

| Herb or spice | Reference | Study type | Population | Dose and formulation | Length of intervention | Findings | Study Quality |
|---|---|---|---|---|---|---|---|
| Cardamom | 28 | Single-blind clinical trial | 20 individuals with hypertension | 3g/day in capsules | 12 weeks | BP significantly decreased and fibrinolytic activity increased. Fibrinogen and lipid levels did not change. | Low |
| 32 | Double-blind RCT | 80 prediabetic subjects | 3g/day in capsules | 8 weeks | Inflammatory markers CRP, CRP:IL-6 ratio and oxidative stress marker MDA were all decreased significantly | High | |
| 29 | Double-blind RCT | 83 overweight or obese diabetic patients | 3g/day in capsules | 10 weeks | There was an improvement in HbA1c, insulin control and TG and an increase in Sirt1. Cholesterol levels did not change. | High | |
| 36 | Double-blind RCT | 87 overweight or obese patients with NAFLD | 3g/day in capsules | 12 weeks | Cardamom improved fatty liver grade, blood glucose, lipids and irisin, but BMI, total cholesterol and FBG were not changed. | High | |
| 37 | Double-blind RCT | 83 pts with type 2 diabetes | 3g/day in capsules | 10 weeks | Cardamom led to a sig decrease in CRP and systolic BP, and an increase in serum NO. Serum ADMA and diastolic BP did not change. | High | |
| 31 | Double-blind RCT | 83 overweight pts with type 2 diabetes | 3g/day in capsules | 10 weeks | VCAM, ICAM, E-selectin and IL-6 were sig decreased in the cardamom group. MMP-9 and CD163 levels were unchanged. | High | |
| 35 | Double-blind RCT | 87 pts with NAFLD | 3g/day in capsules | 12 weeks | Cardamom sig increased Sirt1 and decreased inflammatory markers hs-CRP, IL-6, TNFα and liver marker ALT, as well as improving the degree of fatty liver. Weight, BMI and AST did not change. | High | |
| 34 | Double-blind RCT | 194 obese women with PCOS | 3g/day in capsules | 16 weeks | Anthropometric indices decreased. Glycemic indices and androgen hormones improved. | High | |
| 33 | Double-blind RCT | 194 obese women with PCOS | 3g/day in capsules | 16 weeks | LH, androstenedione and dehydroepiandrosterone were decreased. FSH increased. Inflammatory markers TNFα, IL-6 and CRP were decreased | High | |
| Chilli | 42 | Cross-over clinical trial | 7 healthy volunteers | 30g fresh chilli | Single dose | A combination of chilli and medium-chain TG sig increased diet-induced thermogenesis | Low |
| 38 | Randomized cross-over study | 36 healthy participants | 30g/day chilli in food | 8 weeks | When participants with a BMI >26 consumed a chilli-containing meal after 4 weeks of daily chilli, there was reduced C-peptide and insulin and higher hepatic clearance of insulin. But blood glucose and energy expended were not sig changed. | High | |
| 39 | Randomized cross-over study | 27 healthy adults | 30g/day chilli in food | 8 weeks | Consumption of chilli increased the resistance of lipoproteins to oxidation, but had no effect on serum lipids, lipoproteins and total antioxidant score. | Low | |
| 40 | Randomized cross-over study | 36 healthy participants | 30g/day chilli in food | 8 weeks | There were no effects on metabolic or vascular parameters (glucose, lipids, BP, insulin). However, in men, chilli decreased resting heart rate and increased myocardial perfusion time. | High | |
| 9 | Randomized cross-over study | 34 healthy overweight volunteers | Meal containing chilli with 5.82mg total capsaicinoids | Single dose | Chilli decreased post-prandial insulin when added to a meal. Metabolic rate, core temperature, CRP and microvascular reactivity were unchanged. | Low | |
| 46 | Cross-over clinical trial | 40 healthy adults | 0.6g in food | Single dose | Eating a meal with chilli in increased the desire to eat sweet food, but had no impact on energy intake | Low | |
| 45 | Randomized cross-over study | 12 healthy adults | 10mg capsaicinoids/day in capsules | 5 weeks | There was no change in anthropometric and metabolic measurements from chilli consumption. Chilli increased the firmicutes/bacteroidetes ratio and faecalibacterium abundance that coincided with the increase of plasma levels of GLP-1 and GIP and the decrease of plasma ghrelin level. Benefits were linked to gut enterotypes. | Low | |
| 47 | Double-blind RCT | 42 pregnant women with gestational diabetes | 1.25g/day added to food | 4 weeks | Postprandial glucose, insulin and insulin resistance were sig reduced by chilli. Serum cholesterol and triglyceride were also reduced by chilli. Serum calcitonin gene-related peptide was sig increased by chilli. When the babies were born, chilli had sig reduced the incidence of large-for-gestational age newborns. | High | |
| 49 | Prospective cohort study | 12970 healthy adults | <20g/day up to >50g/day in food | 9 years | Chilli intake was inversely associated with risk of hypertension in Chinese adults | ||
| 48 | Prospective cohort study | 12970 healthy adults | <20g/day up to >50g/day in food | 9 years | There was a positive association between chilli intake and energy intake, however, chilli consumption was inversely associated with incidence of overweight/obesity | ||
| 41 | Cross-over clinical trial | 12 healthy adults | 5g in capsules | Single dose | When 5g of chilli was administered in a capsule after a glucose challenge, plasma glucose levels were sig lower after 30 and 45 mins than those in the placebo group. Insulin levels in the chilli group were sig higher than in the placebo group at 1 and 2 hours after glucose challenge. | Low | |
| 43 | Single-blind, randomized, cross-over trial | 14 healthy volunteers | 3.09g/day in food | 36 hours | Chilli increased fat oxidation and prevented reductions in sleeping metabolic rate, diet-induced thermogenesis or resting energy expenditure that were caused by restricting food intake. This indicates a potential beneficial effect in dieting individuals. There was no effect of chilli on BP | Low | |
| 44 | Single-blinded, randomized, cross-over design | 15 healthy adults | 3.09g/day in food | 36 hours | Chilli significantly decreased the desire to eat and increased satiety and fullness, particularly when participants under-ate. | Low | |
| Cinnamon | 59 | Single-blind randomized cross-over study | 8 sedentary, healthy males | 3g/day in capsules | 2 weeks | Cinnamon reduced glucose response to a glucose challenge and improved insulin sensitivity, but effects were not long-lasting once cinnamon consumption ceases. | High |
| 74 | Double-blind RCT | 25 post-menopausal women with type 2 diabetes | 1.5g/day in capsules | 7 weeks | Cinnamon did not improve fasting plasma glucose or insulin concentrations, whole-body oral glucose tolerance, or blood lipid profiles | Low | |
| 58 | Randomized cross-over study | 7 lean, healthy adults | 5g in capsules | Single dose | Cinnamon reduced plasma glucose responses to glucose tolerance tests and improved insulin sensitivity | Low | |
| 53 | Randomized cross-over study | 15 healthy adults | 1 or 3g/day in food | Single dose | Ingestion of 3 g cinnamon reduced postprandial serum insulin and increased GLP-1 concentrations without significantly affecting blood glucose, GIP, the ghrelin concentration, satiety, or GER in healthy subjects. 1g did not have an effect | High | |
| 55 | Single-blind randomized cross-over study | 9 healthy young adults | 3g in capsules | Single dose | 3 g cinnamon did not alter the postprandial response to a high-fat test meal. No change in gastric emptying, glucose response, arterial function, oxidative stress or appetite. | High | |
| 87 | Double-blind cross-over RCT | 10 individuals with impaired glucose tolerance | 6g in capsules | Single dose | No sig differences in glucose or insulin responses compared with placebo | High | |
| 88 | Double-blind cross-over RCT | 10 young, sedentary obese women | 5g in capsules | Single dose | Peak blood glucose was lower in the cinnamon group, but blood insulin and insulin sensitivity/resistance were not affected. | High | |
| 71 | Double-blind RCT | 26 pts with type 2 diabetes | 1g/day in capsules | 12 weeks | Cinnamon sig reduced FBG by 6 weeks that this was maintained for the whole 12 weeks of the study. The decrease in HbA1c was not significant. Serum glutathione and superoxide dismutase were sig increased by cinnamon at 12 weeks, while MDA was sig reduced, indicating an overall antioxidant effect. | Low | |
| 54 | Randomized cross-over study | 30 healthy obese or normal weight individuals | 6g powder in food | Single dose | Cinnamon sig reduced blood glucose in obese and healthy weight individuals | Low | |
| 51 | Randomized clinical trial | 30 healthy adults | 100ml cinnamon tea | Single dose | Cinnamon sig decreased postprandial maximal glucose level | Low | |
| 52 | Crossover clinical study | 14 healthy individuals | 6g powder in food | Single dose | Cinnamon sig reduced the postprandial glucose response and the gastric emptying rate. | Low | |
| 50 | Randomized crossover study | 10 healthy individuals | 6g in food | Single dose | Cassia cinnamon, but not Ceylon cinnamon, reduced postprandial insulin and glucose responses | Low | |
| 83 | Double-blind RCT | 45 women with PCOS | 1.5g/day in capsules | 24 weeks | Menstrual cyclicity improved for women taking cinnamon with no effect on insulin resistance or serum androgens | High | |
| 64 | Single-blind RCT | 109 adults with diabetes | 1g/day in capsules | 12 weeks | Cinnamon lowered HbA1c | High | |
| 72 | Single-blind RCT | 60 patients with type 2 diabetes | 1.5g/day | 12 weeks | Cinnamon had no impact on fasting plasma glucose, HbA1c, or serum lipids | ||
| 63 | Double-blind RCT | 43 individuals with diabetes | 1g/day in capsules | 12 weeks | Cinnamon produced no significant change in fasting glucose, lipid, A1C, or insulin levels | High | |
| 86 | Double-blind RCT | 50 pts with NAFLD | 1.5g/day in capsules | 12 weeks | significant decreases in HOMA index, fasting blood glucose, total cholesterol, triglyceride, ALT, AST, GGT, and high-sensitivity CRP with cinnamon | High | |
| 73 | Double-blind RCT | 39 adults with diabetes | 3g/day in capsules | 8 weeks | Cinnamon had no effect on glycaemic and inflammatory markers | High | |
| 69 | Double-blind RCT | 44 adults with diabetes | 3g/day in capsules | 8 weeks | Cinnamon had no effect on soluble vascular adhesion molecules | High | |
| 84 | Single-blind RCT | 40 women with PCOS | 1.5g of Ceylon cinnamon in capsules | 8 weeks | Sig improvement in cyclicity with cinnamon, which was equivalent to metformin. No change in fasting blood glucose or serum progesterone or androgen levels. | High | |
| 65 | Double-blind RCT | 39 adults with diabetes | 3g/day in capsules | 8 weeks | Cinnamon had no effect on inflammatory markers | High | |
| 61 | Double-blind RCT | 57 adolescents with diabetes | 1g/day in capsules | 12 weeks | Cinnamon had no effect on A1c or insulin sensitivity | High | |
| 60 | Double-blind RCT | 58 patients with type 2 diabetes | 2g/day in capsules | 12 weeks | Intake of 2g of cinnamon significantly reduced the HbA1c, SBP and DBP among poorly controlled type 2 diabetes patients | High | |
| 75 | Double-blind RCT | 59 adults with type 2 diabetes | 1.2g/day in capsules | 12 weeks | There was no significant change in SBP from baseline when cinnamon was compared with placebo | High | |
| 76 | Double-blind RCT | 136 individuals with type 2 diabetes | 1.5g/day in capsules | 90 days | HbA1c was sig reduced by cinnamon, but there was no effect on FBG. | High | |
| 77 | Double-blind RCT | 61 pts with type 2 diabetes | 2g/day in capsules | 8 weeks | Cinnamon did not sig improve FBG, HbA1c, blood lipids | High | |
| 79 | Double-blind RCT | 84 overweight individuals with PCOS | 1.5g/day in capsules | 8 weeks | Cinnamon increased serum antioxidant capacity, and improved total cholesterol, LDL and HDL | High | |
| 80 | Double-blind RCT | 84 overweight individuals with PCOS | 1.5g in capsules | 8 weeks | Cinnamon significantly decreased serum FBG, insulin, homeostatic model assessment for insulin resistance, total cholesterol and LDL-cholesterol and weight and increased HDL-cholesterol compared with placebo | High | |
| 68 | Triple-blinde RCT | 105 pts with type 2 diabetes | 1g/day in capsules | 12 weeks | Cinnamon sig improved glucose control and reduced BMI | High | |
| 70 | Double-blind RCT | 115 pts with type 2 diabetes | 0.5g/day in capsules | 12 weeks | Cinnamon sig reduced FBG, HbA1c and hepatic enzymes. Probiotics were also effective. | High | |
| 66 | Double-blind RCT | 60 people with type 2 diabetes | 1, 3, or 6g/day in capsules | 40 days | Intake of 1, 3, or 6g of cinnamon per day reduces serum glucose, triglyceride, LDL-cholesterol, and total cholesterol inpeople with type 2 diabetes | High | |
| 85 | Double-blind RCT | 116 Asian Indians with metabolic syndrome | 3g/day | 16 weeks | FBG, HbA1c, waist circumference, and BMI were significantly reduced by cinnamon. Waist-hip ratio, BP, serum total cholesterol, LDL-cholesterol, serum triglycerides, and HDL-cholesterol were also sig improved. | High | |
| 67 | Triple-blind RCT | 160 people with type 2 diabetes | 3g/day | 12 weeks | Cinnamon reduced HbA1c and blood glucose | High | |
| 78 | Triple-blind RCT | 140 patients with diabetes | 1g/day | 12 weeks | Cinnamon supplementation led to improvement of all anthropometric (BMI, body fat, and visceral fat), glycemic (FBG, 2hpp, HbA1C, fasting insulin, and insulin resistance), and lipids (cholesterol, LDL-c and HDL-c) outcomes (except for triglycerides) | High | |
| 82 | Double-blind RCT | 59 women with PCOS | 1.5g/day | 12 weeks | fasting insulin, HOMA-IR, LDL and HDL were sig reduced in the cinnamon group. Changes in blood sugar, serum androgen levels and anthropometric measures were not significant. | High | |
| 57 | Open, randomized, cross-over clinical trial | 21 healthy volunteers | 2g in 200ml hot water | Single dose | No sig difference in energy expenditure, dietary-induced thermogenesis, hunger fullness and desire to eat. However, cinnamon tea decreased satiety and increased food intake in the subsequent meal. | Low | |
| 56 | Randomized cross-over study | 18 healthy adults | 4g | Single dose | Cinnamon decreased blood glucose and satiety 15 min after test meal, but didn't decrease blood sugar overall. | Low | |
| Cinnamon and ginger | 81 | Double-blind RCT | 83 women with PCOS | 1.5g of cinnamon or ginger | 8 weeks | Cinnamon and ginger both sig decreased weight and BMI. Insulin resistance decreased, but only in the cinnamon group. FSH and LH sig decreased in the ginger group, while testosterone was sig reduced in the cinnamon group. | High |
| Cinnamon, cardamom, saffron, ginger | 62 | Single-blind RCT | 208 pts with type 2 diabetes | 3g | 8 weeks | No significant difference in BP, serum soluble (s)ICAM-1 concentrations and anthropometric measures | High |
| Cumin | 92 | Randomized clinical trial | 88 overweight/obese women | 6g/day | 12 weeks | Cumin powder reduced serum levels of fasting cholesterol, triglyceride, and LDL and increased HDL. Weight, BMI, waist circumference, fat mass and its percentage significantly reduced | High |
| Cumin and cinnamon | 89 | Double-blind RCT | 99 women with dyslipidemia | 3g/day | 8 weeks | Cumin and cinnamon both sig reduced total cholesterol compared with placebo. Differences in triglycerides, HDL and LDL were not significant. | High |
| Fennel and fenugreek | 93 | Single-blinded cross-over trial | 9 healthy women | 2g fennel infused in 250ml of water and strained. 24g of fenugreek, infused in 250ml of water and strained. | Single dose (with 1-week washout between each arm of the study) | Both fennel and fenugreek increased feelings of fullness and decreased desire to eat food, however, there were no changes in amount of food consumed after drinking either tea compared with placebo tea. | Low |
| Fenugreek | 112 | Clinical trial | 20 adults with hypercholesterolemia | 12.5-18g/day | 4 weeks | Total cholesterol and LDL cholesterol sig decreased at both doses. | Low |
| 108 | Clinical trial | pts with type 1 diabetes | 100g/day | 10 days | Fenugreek sig reduced fasting blood sugar, improved glucose tolerance, and reduced LDL, total cholesterol and triglycerides | ||
| 104 | Clinical trial | Type 2 diabetics | 15g | Single dose | Postprandial glucose was sig decreased, but there was no impact on insulin or lipids | ||
| 95 | Double-blind RCT | 13 healthy volunteers | 3g/day | 10 days | Fenugreek improved glucose tolerance and insulin sensitivity (as shown by reduction in melanin-concentrating hormone) | High | |
| 94 | Double-blind cross-over RCT | 10 healthy volunteers and 6 pts with type 2 diabetes | Bread with 10% fenugreek | Single dose | Adding fenugreek (1 part to 9 parts of wheat flour) reduced the glycaemic response and GI of bread in both healthy volunteers and diabetics | Low | |
| 102 | Clinical trial | 18 pts with type 2 diabetes | 10g/day | 8 weeks | FBS, TG and VLDL-C decreased significantly (25 %, 30 % and 30.6 % respectively) after taking fenugreek seed soaked in hot water whereas there were no significantly changes in lab parameters in cases who consumed it mixed with yoghurt | Low | |
| 103 | Double-blind RCT | 8 pts with diabetes | 5.6g in bread | Single dose | Blood glucose was not changed, but total insulin concentration decreased | High | |
| 96 | Randomized cross-over study | 10 healthy adults | Bread with 10% fenugreek | Single dose | Adding fenugreek reduced the glycaemic response and GI of bread | Low | |
| 105 | Randomized clinical trial | 12 pts with uncontrolled diabetes | 2g/day | 12 weeks | Blood glucose was not changed, but fasting insulin level increased significantly. The ratio of HDL:LDL sig decreased | Low | |
| 110 | Parallel randomized study | 48 pts with type 2 diabetes | 15g/day fenugreek powder | 8 weeks | Fenugreek sig decreased CRP and increased superoxide dismutase. There was no effect on glutathione peroxidase activity, total antioxidant capacity, IL-6 or TNFα | High | |
| 109 | Clinical trial | 60 type 2 diabetics | 25g/day | 24 weeks | Serum cholesterol and triglyceride were sig reduced | Low | |
| 97 | Cross-sectional observational study | 25 patients with type 2 diabetes | 5g/day | 12 weeks | Fasting blood glucose was decreased significantly by month 2. Postprandial blood glucose level was sig lower by month 3. | ||
| 99 | Parallel randomized study | 50 pts with type 2 diabetes | 15g/day fenugreek powder | 8 weeks | Fenugreek sig decreased fasting blood glucose, and liver enzymes, serum ALT and alkaline phosphatase, compared with baseline. Compared with control group, SBP, AST and irisin (a marker of metabolic health) were decreased. | High | |
| 98 | RCT | 114 pts with type 2 diabetes | 50g/day | 4 weeks | Fenugreek improved lipid metabolism | Low | |
| 113 | Double-blind RCT | 56 adults with borderline hyperlipidemia | 8g/day | 8 weeks | TG, LDL, total cholesterol and FBG were sig decreased by fenugreek | High | |
| 106 | Triple-blind RCT | 88 pts with type 2 diabetes | 10g/day | 8 weeks | Fenugreek seeds significantly decreased FBG and HbA1c, serum levels of insulin, HOMA-IR, total cholesterol and TG and increased serum levels of adiponectin | High | |
| 100 | Double-blind RCT | 125 pts with type 2 diabetes | 10g/day | 8 weeks | Fenugreek alone and fenugreek combined with nutrition training sig decreased FBG, HbA1c, BMI and waist circumference compared with placebo. | High | |
| 101 | Double-blind RCT | 62 pts with type 2 diabetes | 10g/day fenugreek powder | 8 weeks | Fenugreek sig improved mean FBG, HgA1C, BMI, waist circumference, DBP, and quality of life | High | |
| 107 | Randomized cross-over study | 8 healthy individuals | 25g | single dose | Fenugreek seeds sig reduced the rise in blood glucose and insulin caused by a meal. | Low | |
| Garlic | 117 | Clinical trial | 40 patients with metabolic syndrome | 100mg/kg bodyweight crushed garlic | 4 weeks | Raw crushed garlic significantly reduced waist circumference, SBP and DBP, TG, FBG and significantly increased serum HDL cholesterol. There was no significant difference found in BMI | Low |
| 120 | Clinical trial | 4 healthy adults | 40g fresh garlic | 1 week | Garlic sig reduced the serum cholesterol and triglycerides when consumed with a high-fat diet | Low | |
| 121 | Clinical trial | 20 healthy individuals | 3g/day | 90 days | Garlic sig reduced total cholesterol and LDL, but had no impact on toal bacterial faecal count | ||
| 119 | Single-blind randomized cross-over study | 18 healthy volunteers | 4.2g | 1 week | Baseline values of platelet function were within normal range in all volunteers. Platelet function was not impaired by single and repeated oral consumption of Greek tsatsiki containing raw garlic | Low | |
| 116 | Randomized clinical trial | 112 hyperlipidemic patients | 20g/day | 8 weeks | Garlic and a combination of garlic and lemon sig reduced blood lipids (total cholesterol, TG and LDL) and BP, while increasing HDL. | Low | |
| 114 | Retrospective cohort study | 101 healthy adults | There was a significant association between people who eat higher levels of garlic in the diet and those with lower SBP. 232g garlic/month = <100mmHg vs 148g garlic/month = >120mmHg | ||||
| 118 | Double-blind RCT | 90 overweight smokers | 2.1g/day | 12 weeks | Garlic had no significant effect on inflammatory biomarkers, endothelial function, or lipid profile in normolipidemic subjects with risk factors for CVD | High | |
| 125 | Double-blind RCT | 90 pts with NAFLD | 1.6g/day | 12 weeks | Garlic decreased hepatic steatosis, liver enzymes and blood lipids (total cholesterol, TG, HDL and LDL) | High | |
| 124 | Double-blind RCT | 90 pts with NAFLD | 1.6g/day | 12 weeks | Waist circumference, body fat, FBG, insulin and insulin resistance sig improved. Skeletal muscle mass increased and antioxidant capacity increased. | High | |
| 123 | Double-blind RCT | 90 pts with metabolic syndrome | 1.6g/day | 12 weeks | Garlic increased HDL. Sig decreases in waist circumference, BP, TG, insulin and appetite. | High | |
| 115 | Cross-sectional cohort study | 22812 adults | Raw garlic | There was an inverse association between higher garlic intake and prehypertension | |||
| Garlic and coriander seed | 91 | Single-blind RCT | 80 pts with hyperlipidemia | 2g/day | 40 days | Garlic and coriander improved BMI, total cholesterol, HDL and LDL. Garlic powder was more effective than coriander | Low |
| Ginger | 143 | Double-blind RCT | 160 obese children with NAFLD | 1g/day | 12 weeks | Serum FBG and CRP, BMI, waist circumference, AST, hepatic steatosis, total cholesterol and LDL sig decreased with ginger. | High |
| 127 | Placebo-controlled study | 23 healthy male volunteers | 1g | Single dose | Ginger had no effect on thermoregulatory function, but increased fat utilisation in the morning | High | |
| 126 | Randomized cross-over study | 18 healthy volunteers | 15g raw ginger or 40g cooked ginger | 2 weeks | Ginger did not affect thromboxane production | High | |
| 128 | Randomised cross-over study | 10 healthy men | 2 g | 2 days | Ginger significantly enhanced thermogenesis and reduced hunger and food intake. | Low | |
| 139 | Double-blind RCT | 20 60-year-old patients with diabetes | 3 g | 12 weeks | Significant improvements found in blood glucose, insulin resistance, inflammatory and oxidative markers (CRP and MDA). | High | |
| 140 | Double-blind RCT | 45 diabetic patients | 2g/day | 10 weeks | Ginger supplementation decreased ADMA serum levels (although this change wasn't sig different to placebo), but had no effect on sICAM-1. | High | |
| 144 | Double-blind RCT | 50 pts with NAFLD | 1.5g/day of ginger | 12 weeks | No sig difference between ginger and placebo for anthropometric measurements or liver markers. However, FBG and insulin resistance were sig improved by ginger. Serum lipids (total cholesterol and LDL) and CRP sig decreased in the ginger group. | High | |
| 141 | Double-blind RCT | 45 diabetic patients | 2g/day | 10 weeks | No effect of ginger on anthropometric measurements or NFκB | High | |
| 135 | Double-blind RCT | 64 pts with type 2 diabetes | 2g/day | 8 weeks | TNFα and hs-CRP were sig reduced by ginger. IL-6 was reduced by ginger compared with baseline, but not sig compared with placebo | High | |
| 134 | Double-blind RCT | 64 pts with diabetes | 2g/day | 8 weeks | Ginger supplementation significantly lowered the levels of insulin, LDL-cholesterol, TG and the HOMA index and increased the QUICKI index, but had no effect on FBG, total cholesterol, HDL-C and HbA1c. | High | |
| 138 | Double-blind RCT | 88 diabetics | 3g/day | 8 weeks | Ginger improved fasting blood glucose, insulin levels and insulin sensitivity | High | |
| 129 | Double-blind RCT | 80 healthy obese women | 2g/day | 12 weeks | Ginger decreased anthropometric measurements and appetite | High | |
| 137 | Double-blind RCT | 70 women with gestational diabetes | 1.5g/day | 6 weeks | Ginger treatment significantly reduced the levels of FBS, serum insulin and HOMA index, but did not affect postprandial blood sugar | High | |
| 131 | Double-blind RCT | 70 Obese women | 2g/day | 12 weeks | Ginger sig reduced blood glucose, total cholesterol, TG and LDL/HDL, while increasing MDA and HDL. Body weight, waist circumference and BMI were also sig reduced without any difference in energy and macronutrient intake between groups. | High | |
| 145 | Pilot double-blind RCT | 60 hypothyroid patients with normal serum TSH | 1g/day | 30 days | Ginger sig reduced the thyroid symptom score. Additionally, weight gain, cold intolerance, constipation, dry skin, appetite, memory loss, concentration disturbance, and feeling giddy or dizzy domains also sig improved. Ginger supplementation also led to a significant decrease in body weight, BMI, waist circumference, serum TSH, FBG, TG, and total cholesterol levels compared to the placebo. | High | |
| 136 | Double-blind RCT | 70 pts with type 2 diabetes | 1.6 g | 12 weeks | Ginger sig reduced multiple markers of metabolic health (blood glucose, insulin, insulin resistance, cholesterol) and inflammation (CRP and prostaglandin). | High | |
| 130 | Double-blind RCT | 80 Obese women | 2g | 12 weeks | Statistically significant reduction in BMI, serum insulin and HOMA-IR. | High | |
| 132 | Double-blind RCT | 103 pts with diabetes | 1.2g/day | 12 weeks | Ginger sig reduced fasting blood glucose and total cholesterol | High | |
| 133 | Double-blind RCT | 41 pts with type 2 diabetes | 2g/day | 12 weeks | Ginger supplementation significantly reduced the levels of fasting blood sugar, HbA1c, apolipoprotein B, apolipoprotein B/apolipoprotein A-I and MDA in ginger group in comparison to baseline, as well as control group, while it increased the level of apolipoprotein A-I | High | |
| 142 | Double-blind RCT | 85 pts with hyperlipidemia | 3g/day | 45 days | TG and cholesterol levels were sig lower in the ginger group than placebo. Changes in LDL and HDL were not significant between the two groups | High | |
| Ginger and fenugreek | 111 | Placebo-controlled study | 30 patients with coronary artery disease and 30 healthy individuals | 4g/day or 10g one-off dose of ginger. 5g/day fenugreek | 12 weeks | Ginger did not affect platelet aggregation when given at 4g/day, but 10g single dose reduced platelet aggregation. Ginger had no effect on blood sugar or blood lipids. Fenugreek had no effect on cholesterol, TG or blood sugar in healthy individuals, but reduced cholesterol and triglycerides in pts with coronary artery disease and diabetes | Low |
| Mustard, black pepper, ginger, horseradish | 27 | Single-blind cross-over trial | 22 young, healthy males | 20g ginger or 1.3g of black pepper | Single dose | Ginger and black pepper had no effect on appetite, energy intake or diet-induced thermogenesis | High |
| Nigella seeds | 153 | Double-blind cross-over RCT | 51 pts with metabolic syndrome | 3g/day | 8 weeks | Nigella had no sig effect on BP, weight, waist circumference, FBG and BMI | High |
| 152 | Double-blind cross-over RCT | 51 pts with metabolic syndrome | 3g/day | 8 weeks | No sig effect of Nigella on blood lipids, apolipoproteins and inflammatory factor | High | |
| 146 | Double-blind RCT | 30 healthy male volunteers | 1g/day | 4 weeks | Nigella seeds had no effect on glycaemia or insulin. Total cholesterol and LDL-cholesterol were decreased with no effect on triglycerides or HDL-cholesterol. | Low | |
| 160 | Clinical trial | 94 pts with type 2 diabetes | 1, 2 or 3g/day | 12 weeks | Sig reductions in FBG, postprandial glucose, HbA1c and insulin resistance from 2g/day. No effect on serum C-peptide or body weight. | Low | |
| 151 | Placebo-controlled study | 35 menopausal women with metabolic syndrome | 1g/day | 8 weeks | No sig change in body weight. Nigella sig reduced FBG. Total cholesterol, TG and LDL were sig reduced. HDL change wasn't sig. | Low | |
| 155 | Randomized controlled trial | 37 menopausal women with moderate risk of hyperlipidemia | 1g/day | 8 weeks | Sig decrease in hyperlipidemia from Nigella | Low | |
| 158 | Single-blind, non-randomized trial | 114 pts with type 2 diabetes | 2g/day | one year | N sativa group had a significant decline in TC, LDL-cholesterol, total cholesterol/HDL-C and LDL/HDL ratios, as well as DBP, mean arterial pressure and heart rate. | High | |
| 156 | Double-blind RCT | 73 adults with hyperlipidemia | 2g/day | 6 weeks | No significant effects were seen for blood sugar or lipids | High | |
| 149 | Double-blind RCT | 40 pts with Hashimoto's thyroiditis | 2g/day | 8 weeks | Nigella sativa supplementation significantly reduced anthropometric variables including weight, BMI, and waist circumference. Serum TSH and anti-TPO concentrations reduced while serum T3 increased in Nigella sativa treated group. VEGF also decreased significantly in Nigella group. | High | |
| 161 | Double-blind RCT | 50 pts with NAFLD | 2g/day | 12 weeks | Levels of CRP and NFκB were sig decreased by Nigella. No sig change in hepatic steatosis or TNFα. | High | |
| 162 | Double-blind RCT | 50 pts with NAFLD | 2g/day | 12 weeks | Sig reduction in glucose, insulin and insulin resistance, but no change to lipid profile. Percentage of hepatic steatosis also decreased | High | |
| 150 | Double-blind RCT | 40 pts with Hashimoto's thyroiditis | 2g/day | 8 weeks | Nigella seeds sig decreased TG, LDL, weight and BMI, and increased SOD and total antioxidant capacity. | High | |
| 159 | Double-blind RCT | 114 pts with type 2 diabetes | 2g/day | one year | No change to BMI. Nigella led to a decrease in FBG, HbA1c and insulin resistance | High | |
| 154 | Double-blind RCT | 140 menopausal women with metabolic syndrome | 500mg/day | 8 weeks | LDL-cholesterol, TG, total cholesterol and FBG decreased significantly | High | |
| 148 | Double-blind RCT | 39 men with obesity | 3g/day | 12 weeks | There was no change in serum free testosterone, FBG, TG and inflammatory markers. However, body weight, waist circumference and BP did improve. | High | |
| 157 | Randomized, placebo-controlled trial | 74 individuals with hypercholesterolemia | 2g/day | 4 weeks | Nigella seed sig lowered triglycerides, LDL and cholesterol compared with baseline, but had no effect on blood glucose or HDL. | High | |
| Rosemary | 163 | Double-blind RCT | 110 pts with NAFLD | 4g/day | 8 weeks | There were no sig effects on liver enzymes, anthropometric measurements, FBG, insulin, insulin resistance and blood lipids from rosemary when compared with placebo. | High |
| Sage | 164 | Non-randomized cross-over trial | 6 healthy female volunteers | 600ml sage tea/day | 4 weeks | No effects on blood glucose, but LDL and total cholesterol decreased, while HDL increased. Lymphocyte hsp70 expression also increased | Low |
| Turmeric | 166 | Single-blinded RCT | 42 women with type 2 diabetes and hyperlipidemia | 2.1g/day | 8 weeks | There was a sig improvement in body composition, lipid profile and glycemic status in the turmeric group and the aerobic training or the aerobic training plus turmeric groups compared with control group. The combined group also had sig lower blood lipids, blood glucose and insulin than turmeric alone group. | High |
| 170 | Randomized cross-over study | 14 healthy volunteers | 6g | Single dose | Turmeric increased postprandial insulin without affecting plasma glucose | High | |
| 165 | Open-label, randomized clinical trial | 60 diabetic pts on metformin | 2g/day | 4 weeks | Turmeric sig reduced fasting plasma glucose, but had no effect on post-prandial glucose. Turmeric also increased glutathione, MDA and CRP compared with baseline. LDL cholesterol was sig decreased compared with baseline. | High | |
| 174 | Double-blind RCT | 46 pts with NAFLD | 3g/day | 12 weeks | Turmeric consumption decreased serum levels of glucose, insulin, HOMA-IR and leptin. Changes in weight, BMI and liver enzymes were not significant | High | |
| 167 | Randomized, single-blinded placebo-controlled trial | 42 hyperlipidemic pts with type 2 diabetes | 2.1g/day | 8 weeks | Turmeric alone and turmeric plus aerobic training sig decreased waist circumference, FBG, TG and BP, while HDL cholesterol increased. Metabolic syndrome Z score and inflammatory markers significantly improved in both turmeric groups. | High | |
| 168 | Double-blind RCT | 80 type 2 diabetes mellitus patients (30-70 years old) | 2.1g turmeric powder | 8 weeks | Turmeric was found to significantly decrease body weight, TG and total cholesterol. | High | |
| 173 | Double-blind RCT | 64 pts with NAFLD | 2g/day | 8 weeks | Turmeric sig reduced liver enzymes AST, ALT and GGT compared with placebo. Triglycerides, LDL, HDL and MDA sig decreased from baseline, but weren't sig different from placebo. | High | |
| 169 | Double-blind RCT | 114 pts with type 2 diabetes | 1.2g/day | 12 weeks | Turmeric sig reduced arterial stiffness. No change was found in markers of endothelial function. | High | |
| Turmeric and Nigella seed | 147 | Double-blind RCT | 250 healthy men with metabolic syndrome | 1.5g/day Nigella, 2.4g/day turmeric | 8 weeks | Nigella seed led to sig improvement in triglycerides, total cholesterol, LDL and HDL, but no change to anthropometric measures, blood glucose, BP or inflammation. Turmeric sig improved cholesterol, LDL and inflammation, but not TG, HDL, anthropometric measures, BP or blood glucose. | High |
| Turmeric and cinnamon | 90 | Double-blind RCT | 48 people >60 years with prediabetes | 1g turmeric or 2g of cinnamon | Single dose | Co-ingestion of turmeric with white bread increases working memory independent of body fatness, glycaemia, insulin, or AD biomarkers. Cinnamon had no impact on working memory. Use of turmeric or cinnamon regularly had no impact on glycaemia or insulin responses to breakfast | High |
| Turmeric and red pepper spice | 171 | Double-blind cross-over RCT | 98 overweight or obese women | 2.8g turmeric/day | 4 weeks | Turmeric does not alter oxidative stress or inflammation in overweight/obese females with systemic inflammation, or cause a significant shift in the global metabolic profile | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





