1. Introduction
The global transition from fossil fuels to cleaner and more sustainable energy sources is an imperative response to the growing environmental concerns associated with the use of conventional hydrocarbon-based fuels [
1]. The detrimental impacts of greenhouse gas emissions, climate change, and resource depletion have accelerated the need for innovative solutions to reduce our reliance on fossil fuels and mitigate their environmental footprint [
1,
2]. In this era of sustainable energy, high-performance energy storage systems play an important role in ensuring the reliability and efficiency of renewable energy sources [
3,
4]. These systems bridge the gap between energy generation and consumption, enabling the effective utilization of intermittent sources like wind and solar power while enhancing grid stability and resilience [
3,
4].
In the landscape of energy storage, solid-state batteries (SSBs) are increasingly recognized as a transformative alternative to traditional liquid electrolyte-based lithium-ion batteries, promising unprecedented advancements in energy density, safety, and longevity [
5,
6,
7]. These benefits stem from the incorporation of advanced electrode materials and solid-state electrolytes, which together enable heightened energy storage capacities. Notably, the absence of flammable liquid electrolytes in SSBs mitigates the risk of thermal runaway, a paramount safety concern, especially in applications like electric vehicles (EVs) and portable electronics [
8,
9,
10,
11]. Beyond safety, SSBs, with their augmented energy densities, champion the development of more compact, energy-efficient devices [
11,
12,
13,
14,
15,
16]. Their resilience against dendrite formation and extended cycle life further accentuates their suitability for applications demanding sustained reliability and long-term energy storage [
15,
16,
17,
18].
However, despite the remarkable promise of SSBs, their commercialization remains constrained by specific challenges, with the cathode being a pivotal component significantly affecting battery performance [
19,
20]. This comprehensive review aims to synthesize the state-of-the-art advancements in solid-state battery cathodes, shedding light on both material chemistry and engineering techniques that contribute to enhanced performance metrics. Through an in-depth exploration of cathode materials, structural optimization, electrical properties, thermal characteristics, and practical considerations, this review provides a holistic perspective on the development of SSB cathodes. The following sections will explore into traditional cathode materials and emerging alternatives, discuss strategies for structural optimization, address practical integration aspects, conduct a comparative evaluation of cathode materials, and finally, identify existing research gaps and propose future directions in the development of SSB cathodes.
2. Traditional Cathode Materials
Lithium cobalt oxide (LiCoO
2) has been a foundation in the development of lithium-ion batteries. It offers high energy density but comes with challenges such as safety risks and limited thermal stability [
21]. The original ideas for LiCoO
2 as a cathode material were inspired by interdisciplinary research in solid-state physics and chemical structure bonding [
21]. A study by Wei
et al. [
22] demonstrated that porous LiCoO
2 fabricated from metal–organic frameworks (see
Figure 1) shows excellent stability and superior rate capability, delivering a reversible capacity of 106.5 mAhg
−1 at 2C with stable capacity retention of 96.4% even after 100 cycles.
LiCoO
2 has a theoretical capacity of 274 mAhg
-1 but often fails to deliver more than half of this due to structural deformation [
23]. The material's high energy density makes it suitable for applications requiring compact and lightweight batteries, such as mobile devices and certain types of electric vehicles. A recent study by Cherkashinin
et al. [
24] investigated into the intrinsic stability of LiCoO
2 and found that the material exhibits fully reversible electronic properties after the first electrochemical cycle, providing insights into the development of doping strategies to enhance its electronic conductivity.
Lithium manganese oxide (LiMn
2O
4) is considered an environmentally friendly and cost-effective alternative to LiCoO
2. It has a theoretical capacity of 148 mAhg
-1 and can deliver more than 95% of its theoretical capacity [
23]. However, it suffers from low conductivity, manganese dissolution in the electrolyte, and structural distortion at elevated temperatures [
23]. Various strategies have been employed to improve LiMn
2O
4's performance, including structure doping with single or multiple cations and anions, and surface modification by coating with materials like carbonaceous compounds, oxides, and phosphates [
25]. Doping stabilizes the LiMn
2O
4 spinel structure and reduces the amount of electrochemically active Mn
3+, which is responsible for manganese dissolution into the electrolyte [
26]. A study by Choi
et al. [
27] compared the performance of LiMn
2O
4, with nickel-cobalt manganese (LiNiMnCoO
2), and lithium-iron phosphate (LiFePO
4), and found that it exhibits good thermal stability and capacity retention under various driving cycles.
Lithium iron phosphate (LiFePO
4) is another alternative cathode material known for its robustness and safety [
28]. It has a theoretical capacity of 170 mAhg
-1 and is particularly stable during charge and discharge cycles [
28]. LiFePO
4 is often used in applications where safety and long cycle life are more critical than energy density, such as in large-scale energy storage systems and certain electric vehicles. In a study focusing on the temperature's effect on different cathode materials, LiFePO
4 was found to have optimal performance in a temperature range of 20-50°C [
29]. The study also highlighted that the state of charge (SOC) has a significant impact on the internal resistance of the battery, affecting its overall performance [
30]. Vogt
et al. [
31] explored the production and characterization of fiber-reinforced all-solid-state electrodes using LiFePO
4 and found that the material demonstrated good utilization of the active material with capacities of up to 139 mAhg
-1 (see
Figure 2).
3. Emerging Cathode Materials
Sulfide-based cathode materials have gained significant attention in the realm of all-solid-state lithium batteries (ASSLBs) [
32,
33,
34]. These materials offer promising attributes, especially when paired with solid-state sulfide electrolytes (SSSEs). A recent study [
34] introduced a novel Li
2.96P
0.98S
3.92O
0.06-Li
3N glass-ceramic electrolyte (GCE) where O and N substitution produced unique functional units that enabled superior ionic conductivity at room temperature1. Notably, these units in the Li
2.96P
0.98S
3.92O
0.06-Li
3N effectively prevent structural degradation against moisture. Furthermore, this GCE addresses key challenges and showcases potential for use in high-energy ASSLBs.
Oxide-based cathode materials, when paired with solid electrolytes, can significantly enhance the energy density of ASSLBs. The transition from liquid-based Li-ion batteries (LIBs) to ASSLBs has been driven by the potential advantages of oxide and sulfide-based solid electrolytes [
35]. A strategic approach to this transition involves analyzing the pairing of solid electrolytes with oxide cathode materials and the Li metal anode [
35]. The chemical, electrochemical, and mechanical properties of these solid electrolytes play a crucial role in determining the performance of the resultant ASSLBs [
35]. A study made by Ban
et al. [
35] prepared a poly(ethylene oxide) (PEO)-LiClO
4−Li
1.3Al
0.3Ti
1.7(PO
4)
3 (LATP) composite solid electrolyte (CSE) membrane using a solution-casting method. The results showed high Li ion conductivity (∼1 × 10
−3 Scm
−1) and excellent mechanical and electrochemical performances. The authors explain that the solid-state battery showed, after 500 cycles, a capacity of 109.3 mAhg
−1 at 1C, providing a promising strategy for the design of high-performance solid-state batteries (see
Figure 3).
Air-based cathodes, specifically in the context of solid oxide fuel cells (SOFCs), have been recognized as potential candidates to meet the increasing energy demand. SOFCs are known for their high energy conversion efficiencies, low pollution exhaust, fuel flexibility, and environmental friendliness, making them a promising alternative to conventional electricity generators [
36]. However, the high operating temperatures of SOFCs (typically between 800°C to 1000°C) have been a significant challenge. To address this, there is a push towards developing thin film-based SOFCs (TF-SOFCs) which can potentially reduce the operating temperature to the 450°C to 600°C range [
36]. Noh
et al. [
36] fabricated a large-area thin-film solid oxide fuel cells (TF-SOFC) using a commercially viable vapor deposition technology. By using a 2-inch sputtering system, a multi-scale-architecture platform consisting of a nanostructured NiO-yttria-stabilized zirconia (YSZ) anode and an approximately 750 nm-thick YSZ/gadolinia doped ceria (GDC) bilayer was fabricated over a 5 × 5 cm NiO-YSZ anode support. The authors claim that an open cell voltage (OCV) of 1.1 V and a peak power density exceeding 1.2 Wcm
−2 at 600°C were obtained. They further present that the total power output at 0.7 V from the 5-cm-by-5 cm TF-SOFC reached 15.52 W at 600°C and 9.76 W at 550°C. The total maximum power outputs were 19.52 and 14.08 W at 600°C and 550°C, respectively.
Emerging cathode materials present a myriad of advantages and challenges that influence their potential in next-generation energy storage systems. While they offer benefits such as fuel flexibility and environmental friendliness, they also come with inherent limitations that need to be addressed for optimal performance.
Table 1 presents some of the advantages and limitations of emerging cathode materials.
The compatibility of cathode materials with solid electrolytes is a critical factor in the performance of ASSLBs. For instance, lithium aluminum germanium phosphate (LAGP) is a solid electrolyte that has gained significant attention due to its stability in air and good ionic conductivity [
37]. However, challenges like poor interface compatibility with Li anodes and slow Li-ion conduction in thick pellets have been identified [
37]. Advanced interface engineering strategies, such as introducing a functional interlayer, have been proposed to address these challenges [
37].
4. Structural Optimization of Cathode Materials
4.1. Nanostructuring
The structural optimization of cathode materials in SSBs plays a crucial role in enhancing their electrochemical performance. Nanostructuring is a promising approach that involves reducing the dimensions of cathode materials to the nanoscale [
38]. This strategy offers several advantages in terms of improved conductivity and enhanced surface area for electrochemical reactions [
38,
39,
40].
Nanostructuring of cathode materials has been demonstrated in various studies. For instance, Sun
et al. [
38] highlighted the benefits of using a Li
2WO
4-coated LiCoO
2 cathode with a sulfide Li
6PS
5Cl solid electrolyte to boost the electrochemical performance of all-solid-state batteries. Similarly, Liu
et al. [
40] modified the interface between the superionic conductor and polymer electrolyte (polyvinylidene fluoride) by
in situ synthesis of a pyrochlore-type La
2Sn
2O
7 (LSO) ceramic layer on the surface of Li
6.4La
3Zr
1.4Ta
0.6O
12 (LLZTO). The authors explain that synthesis of LSO consumes La in LLZTO, increasing the concentration of Li ions in LLZTO, and significantly improving the conductivity of the composite electrolyte (LLZTO@LSO-CSE). Compared with the pristine sample (3.15 × 10
−5 Scm
−1), the conductivity of LLZTO@0.9%LSO-CSE was improved by an order of magnitude (as high as 1.30 × 10
−4 Scm
−1). Furthermore, the authors explain that after 400 cycles, the discharge capacity of the LiFePO
4|LLZTO@0.9%LSO-CSE|Li remained at 110.6 mAhg
−1, the Coulomb efficiency was 99.4%, and the capacity retention rate was 72%, compared to 75.5 mAhg
−1, 98, and 49% for the pristine one, respectively (see
Figure 4).
4.2. Surface coatings
Surface coatings represent another avenue for improving cathode materials in SSBs. Coating cathode particles with conductive and protective materials can mitigate issues related to structural instability, reactivity with electrolytes, and enhance overall electrode performance [
41,
42]. Recent research by Liang
et al. [
43] focused on surface coating strategies for LiNi
0.6Mn
0.2Co
0.2O
2 (NMC) cathode materials. They demonstrated that a gradient oxy-thiophosphate coating improved the structural stability of NMC cathodes, reducing capacity fading and improving cycling stability. Additionally, surface coatings can enhance the safety of SSBs by preventing the formation of harmful solid-electrolyte interphase (SEI) layers [
44,
45].
4.3. Composite approaches
Composite cathode materials are designed by integrating various components to leverage their complementary properties. This approach combines the advantages of multiple materials to address the challenges associated with low conductivity and structural instability in SSB cathodes [
46,
47]. One notable example is the development of composite cathodes using conductive polymers. Researchers, such as Liu
et al. [
40], have successfully incorporated various strategies to improve the interface of cathode materials in solid-state lithium batteries. The resulting approaches exhibited improved electronic conductivity and ion diffusivity, leading to enhanced rate capability and cycling stability.
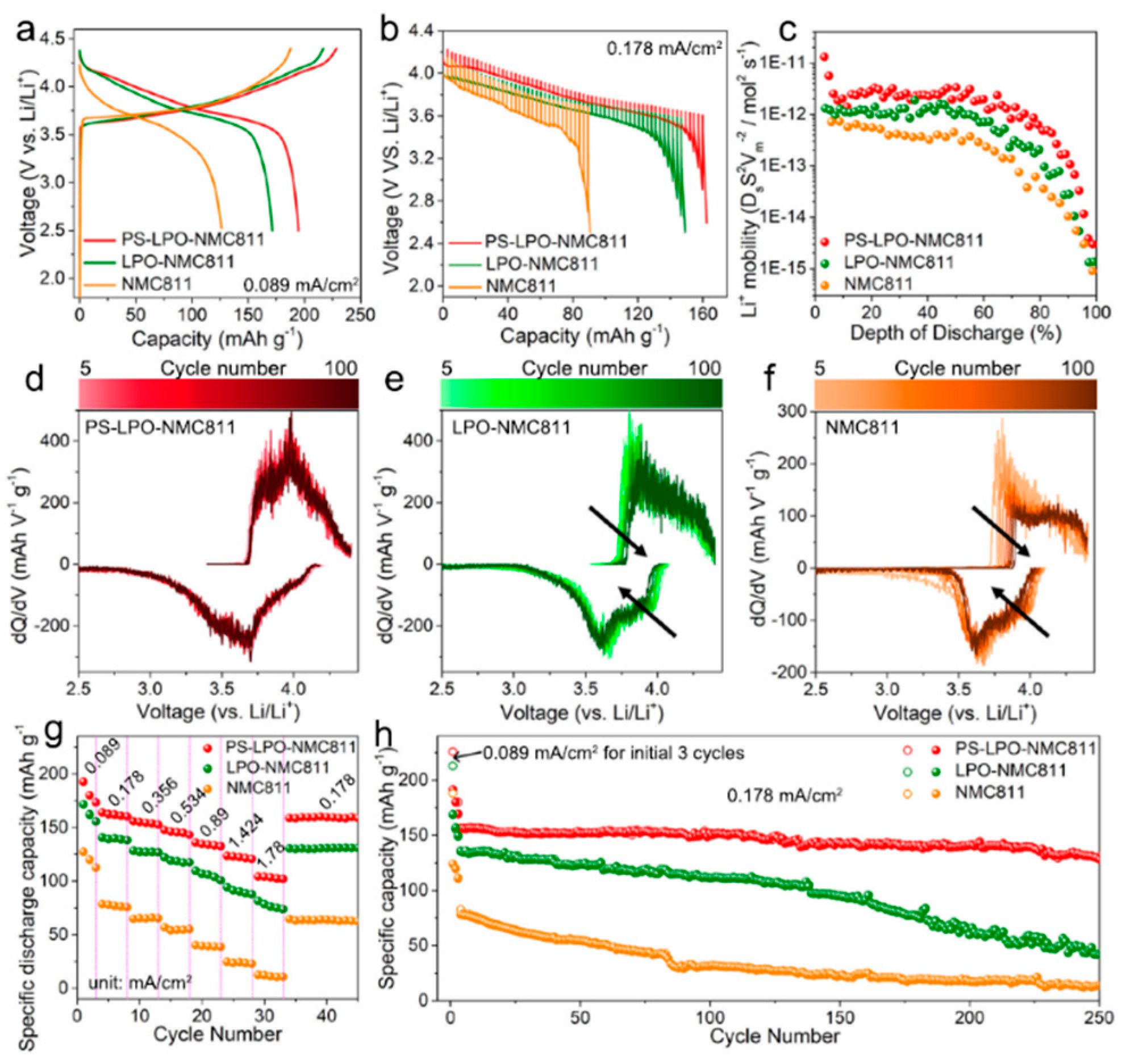
Composite approaches also extend to incorporating ionic conductors within the cathode matrix. Liang
et al. [
44] explain that high-energy Ni-rich layered oxide cathode materials such as LiNi
0.8Mn
0.1Co
0.1O
2 (NMC811) suffer from detrimental side reactions and interfacial structural instability when coupled with sulfide solid-state electrolytes in all-solid-state lithium-based batteries. To circumvent this issue, the research group proposed a gradient coating of the NMC811 particles with lithium oxythiophosphate (Li
3P
1+xO
4S
4x) via atomic layer deposition of Li
3PO
4 and subsequent
in situ formation of a gradient Li
3P
1+xO
4S
4x coating. The tailored surface structure and chemistry of NMC811 hinder the structural degradation associated with the layered-to-spinel transformation in the grain boundaries and effectively stabilize the cathode|solid electrolyte interface during cycling. When tested in combination with an indium metal negative electrode and a Li
10GeP
2S
12 solid electrolyte, the gradient oxy-thiophosphate-coated NCM811-based positive electrode enabled the delivery of a specific discharge capacity of 128 mAhg
−1 after almost 250 cycles at 0.178 mAcm
−2 and 25°C (see
Figure 5).
4.4. Addressing issues like low conductivity and structural instability
The structural optimization of SSB cathodes aims to mitigate two fundamental challenges: low electrical conductivity and structural instability. Various strategies, including those discussed above, are employed to address these issues [
40,
41,
42,
43,
44,
45,
46,
47]. For example, doping cathode materials with elements like niobium (Nb) or aluminum (Al) can enhance the electronic conductivity while maintaining structural integrity [
38,
40,
44]. Additionally, the utilization of advanced characterization methodologies, such as in situ electron microscopy, enabled researchers, as demonstrated by Wang et al. [
39], to observe and address structural alterations during cycling, offering invaluable insights for material refinement.
4.5. Role of electrical properties (electronic and ionic conductivity)
The performance of SSBs is heavily influenced by the electrical properties of their cathode materials. These properties, specifically electronic and ionic conductivity, play a pivotal role in determining the efficiency of charge and ion transport within the battery. Electronic conductivity refers to the ability of a material to conduct electrons. In the context of SSBs, high electronic conductivity ensures that electrons can move freely from the cathode to the anode during the discharge process, and vice versa during charging [
48]. Nanostructuring is a technique that has been employed to enhance this property. By reducing the size of the cathode material to the nanoscale, the pathways for electron diffusion become shorter, leading to improved electronic conductivity [
49].
On the other hand, ionic conductivity pertains to the movement of ions (in this case, Li ions) within the battery. A high ionic conductivity ensures that Li ions can move swiftly from the anode to the cathode during charging and in the reverse direction during discharging. One way to boost ionic conductivity is by incorporating solid-state electrolytes within the cathode structure. This facilitates faster Li-ion transport, leading to a more efficient battery [
49].
A recent advancement in the field of SSBs is the development of the Chevrel Phase Mo
6S
8 nanosheets [
50]. These nanosheets are characterized by their high electronic conductivity, rapid ion transport capability, and strong affinity for lithium polysulfides. The unique feature of these nanosheets is their reversible electrochemical Li-ion intercalation. This means that during the battery's operation, the Mo
6S
8 nanosheets can dynamically enhance their ionic conductivity by undergoing a reversible process of lithium-ion intercalation, forming Li
xMo
6S
8. This dynamic enhancement suppresses the undesirable shuttling effect and accelerates the conversion kinetics, leading to superior battery performance in terms of cycling stability, high-rate capability, and low-temperature performance [
50].
4.6. Role of Thermal Characteristics (Heat Dissipation and Thermal Stability) in Safety
Thermal characteristics are paramount for the safe operation of SSBs, particularly in high-demand applications such as electric vehicles and large-scale energy storage systems. The inherent risks associated with the thermal behavior of batteries, especially during charging, have led to numerous fire incidents in electric vehicles [
51]. Solid-state polymer electrolytes (SPEs) have emerged as a promising solution due to their unique characteristics [
52]. Unlike their liquid counterparts, SPEs are not prone to leakage and exhibit low flammability, excellent processability, good flexibility, high safety levels, and superior thermal stability [
52]. However, the challenge remains in ensuring that these electrolytes can maintain their stability and performance under various thermal conditions. One of the critical aspects of ensuring thermal safety is understanding and mitigating the heat generated during the battery's operation. For instance, during the charging process, irreversible heat is a significant contributor to temperature rise. Nonetheless, as the battery transitions from a normal charge to an overcharged state, other heat sources, such as reactions due to Mn dissolution and Li deposition, become dominant [
51]. Furthermore, the thermal stability of cathode materials is of utmost importance. A study on nickel-based layered cathode materials using
in situ soft XAS measurements revealed that NiO-type rock salt structures formed at the surface at temperatures above 200°C, indicating potential thermal degradation pathways [
53]. To ensure the safety of SSBs, it is crucial to have a comprehensive understanding of the thermal behavior of all components. Advanced characterization techniques, such as
in situ soft XAS and impedance spectroscopy, provide valuable insights into the thermal and electrochemical stability of battery materials [
53]. By addressing these thermal challenges, researchers and manufacturers can pave the way for safer and more efficient solid-state batteries.
5. Integration of Advanced Cathode Materials into SSBs
5.1. Scalable manufacturing techniques
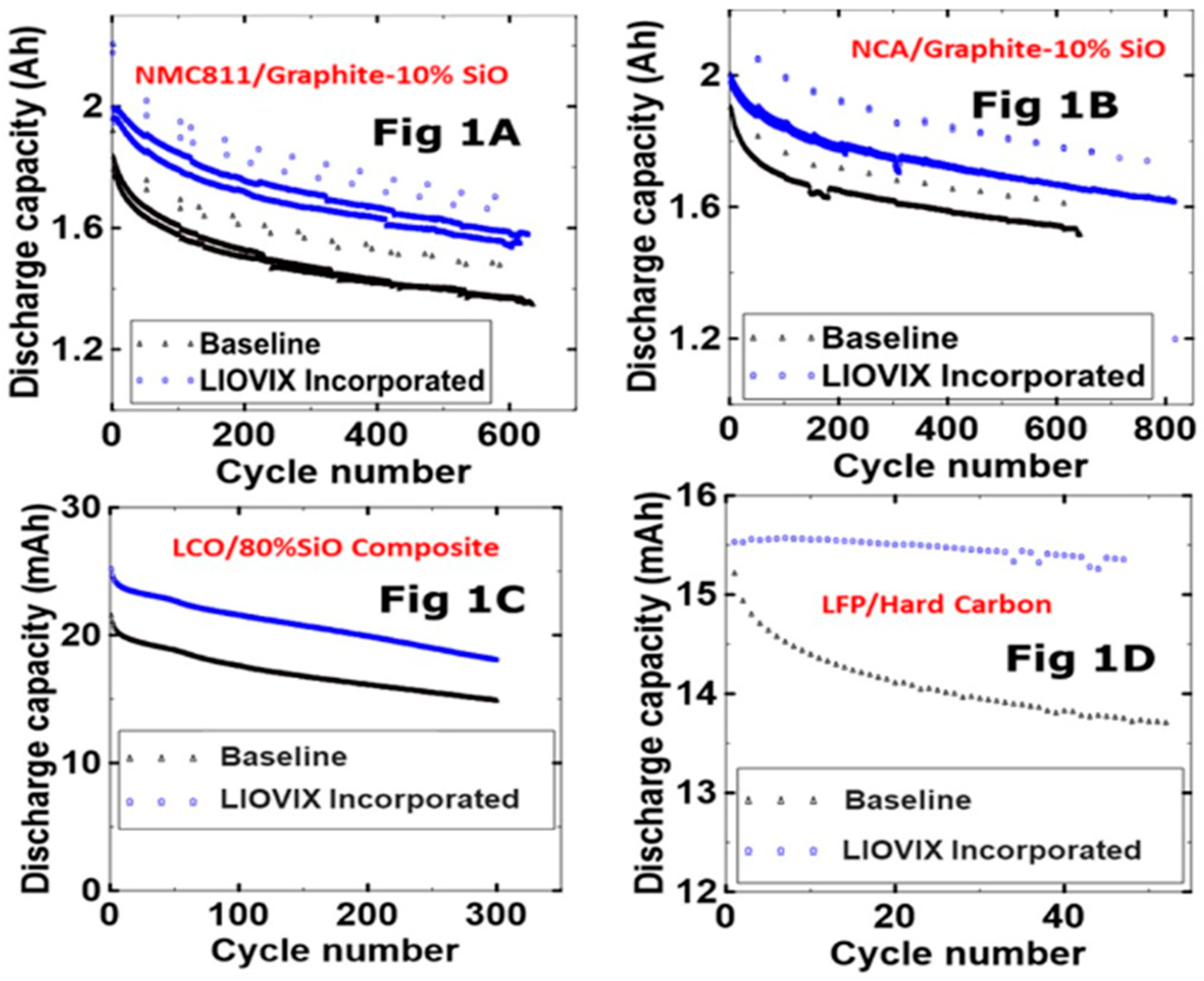
The industry is continuously seeking innovative and sustainable solutions to enhance battery performance, safety, and manufacturing efficiency. One such innovation is LIOVIX™, a proprietary lithium metal product [
54]. This unique printable formulation of lithium metal and other specialty materials can enhance lithium-ion battery performance, reduce manufacturing costs, and pave the way for next-generation battery technology. LIOVIX™ can be printed on a battery's anode during electrode manufacturing in a process called pre-lithiation. This process employs an external source of lithium to offset the first cycle loss of lithium due to SEI formation, allowing for more efficient use of lithium from the cathode and increasing the cell energy density. Importantly, LIOVIX™ technology is scalable using industry-standard coating and printing equipment and can be adapted to various anode or cathode chemistries [
54].
Figure 6 shows the cycle performance for commercially available electrode materials with anode loading >4 mAhcm
−2.
5.2. Electrode-electrolyte interfaces
All-solid-state batteries have the potential for high energy density and enhanced safety due to their nonflammable solid electrolytes. However, a significant challenge is the sluggish ion transmission at the cathode-electrolyte (solid/solid) interface, which results in high resistance at the contact [
55]. This limits the practical implementation of these materials in real-world batteries. Several methods have been proposed to enhance the kinetic condition of ion migration between the cathode and the solid electrolyte. One such method is a composite strategy that mixes active materials and solid electrolytes for the cathode, aiming to decrease the ion transmission barrier at the interface [
55].
5.3. Current collectors
In the realm of SSBs, the role of current collectors is paramount for the effective integration of advanced cathode materials [
56]. Current collectors serve as the conduit for electron flow, ensuring efficient charge and discharge processes within the battery. However, their significance extends beyond mere electron transport. Recent research [
56,
57] has highlighted the challenges associated with the interface of current collectors in SSBs, particularly concerning charge transfer kinetics. Deng
et al. [
56] emphasized that poor rate capability remains a substantial hurdle for the practical application of inorganic all-solid-state lithium-ion batteries (ASSLIBs). They identified that the charge transfer kinetics at the interface of current collectors is crucial for high-rate capacity. To address this, the study introduced a graphene-like carbon (GLC) coating for the modification of aluminum current collectors. This innovative approach not only prevented side reactions at the interface but also resulted in improved cycling stability and high-rate capacity in the LiCoO
2/Li
3InCl
6 (LCO/LIC) ASSLIBs [
56].
Furthermore, the integration of advanced cathode materials in SSBs often necessitates a re-evaluation of the current collector's compatibility and performance [
57]. The interplay between the cathode material and the solid electrolyte can introduce challenges at the microstructural, (chemo-)mechanical, and (electro-)chemical levels. Minnmann
et al. [
57] provided insights into the required properties and potential challenges for inorganic cathode active materials (CAMs) employed in SSBs. They emphasized the importance of tailoring CAMs to address challenges arising from the cathode, particle, and interface levels, particularly concerning the interaction of CAMs with solid electrolytes [
57].
5.4. Practical considerations for implementing advanced cathode materials
The practical implementation of advanced cathode materials in SSBs necessitates a holistic approach that addresses both material-specific challenges and broader system-level considerations. One of the pivotal aspects in this context is the interfacial stability between the cathode active material (CAM) and the solid electrolyte (SE) [
58]. Achieving a stable interface is crucial for ensuring efficient ion transport and minimizing detrimental side reactions that can degrade battery performance. Culver
et al. [
58] highlighted the intrinsic electrochemical instability of high-performance separators in SSBs, emphasizing that the optimization of component material interfaces is essential for achieving high energy and power densities while maintaining device safety and a practical cycle life. They underscored the need for a protective coating on the CAM to inhibit solid electrolyte degradation. However, the challenge lies in devising an economical and scalable approach to fabricate these high-quality protective coatings on CAM particles [
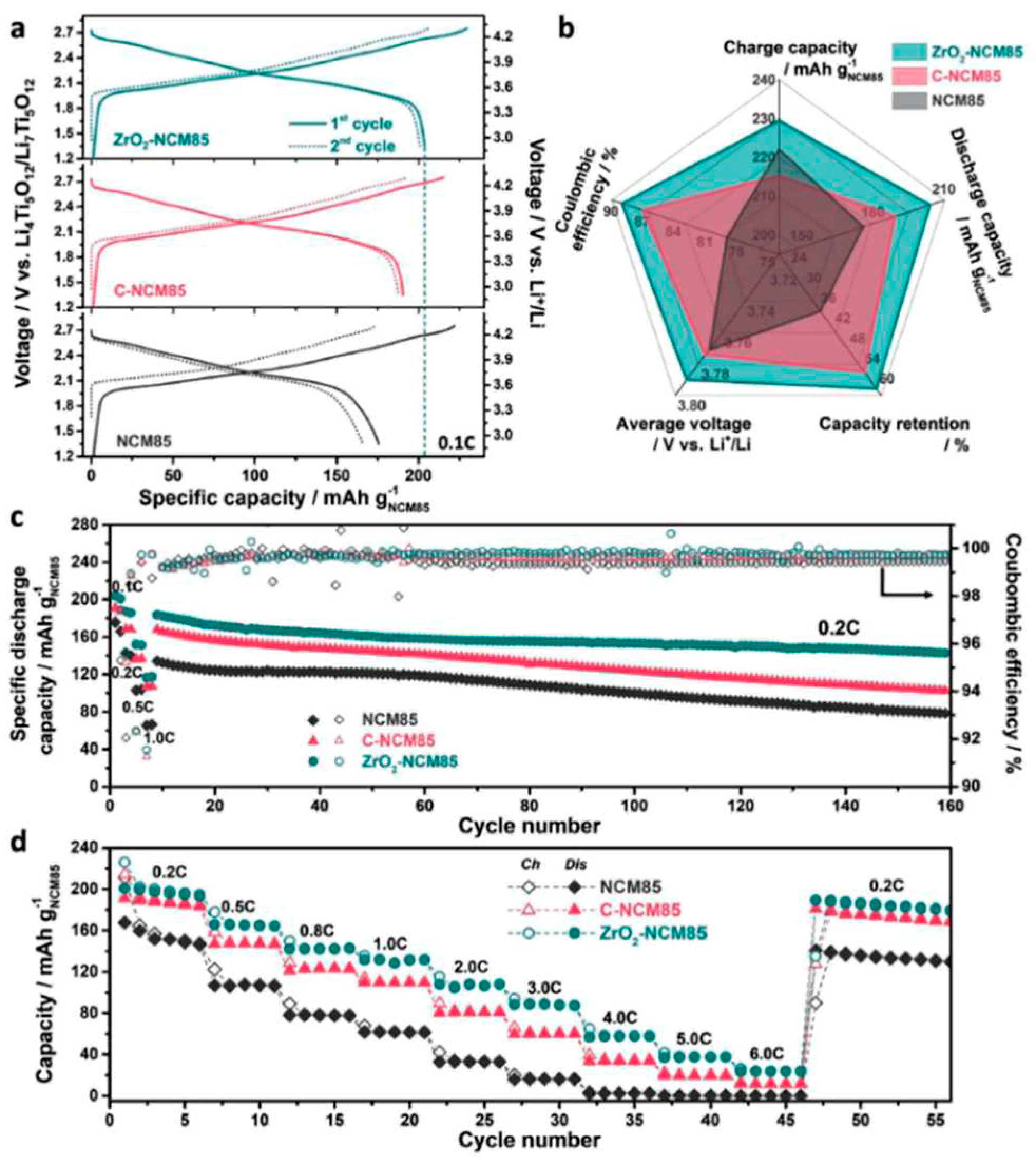
58]. Furthermore, Ma
et al. [
59] introduced a novel wet-coating strategy based on preformed nanoparticles to address this challenge. They demonstrated the application of non-agglomerated nanoparticles of coating material (e.g. ZrO
2) prepared via solvothermal synthesis. After surface functionalization, these nanoparticles were applied to a layered Ni-rich oxide CAM, resulting in a uniform surface layer with a unique structure. When tested in pelletized SSBs with argyrodite Li
6PS
5Cl as SE, the coated CAM exhibited superior lithium-storage properties and good rate capability (see
Figure 7). The key to this improvement was the homogeneity of the coating, which suppressed interfacial side reactions and limited gas evolution during operation. This strategy not only showcased its efficacy in SSBs but also proved beneficial in liquid electrolyte-based Li-ion batteries, highlighting its potential for broader applications [
59].
6. Comparative Evaluation of Cathode Materials
6.1. Key performance indicators
The energy density of a battery is a pivotal metric that quantifies the amount of energy a battery can store relative to its volume or weight [
56]. This parameter is especially crucial for applications where the constraints of space and weight are paramount, such as in electric vehicles and portable electronics. A battery with a high energy density can store more energy in a smaller space, making it more efficient and desirable for these applications. The high voltage spinel material LiMn
1.5Ni
0.5O
4 (LMNO) has emerged as a promising candidate to enhance the energy density of lithium batteries [
52]. This is attributed to its potential to operate at higher voltages, thereby storing more energy. However, like many advanced materials, LMNO is not without its challenges. Its performance can be compromised by long-term cycling and high-temperature stability. These challenges can lead to a decrease in capacity over time, especially when the battery is subjected to repeated charge and discharge cycles or operated at elevated temperatures [
52].
Recent advancements in the field have shown that by partially substituting Mn with Ti in LMNO, the limitations associated with long-term cycling can be effectively addressed [
60]. This modification has been shown to enhance the stability of the material, allowing it to achieve up to 2000 cycles at a high C-rate, which is a measure of the rate at which a battery is discharged relative to its maximum capacity [
60].
6.1.2. Voltage Stability:
Voltage stability is a critical parameter in battery performance, referring to the battery's ability to maintain a consistent voltage during discharge [
28]. A stable voltage is essential as it ensures consistent performance of the battery, preventing potential damage to devices powered by it. A battery that can maintain its voltage during discharge can deliver power more reliably, ensuring that the device it powers operates efficiently and safely.
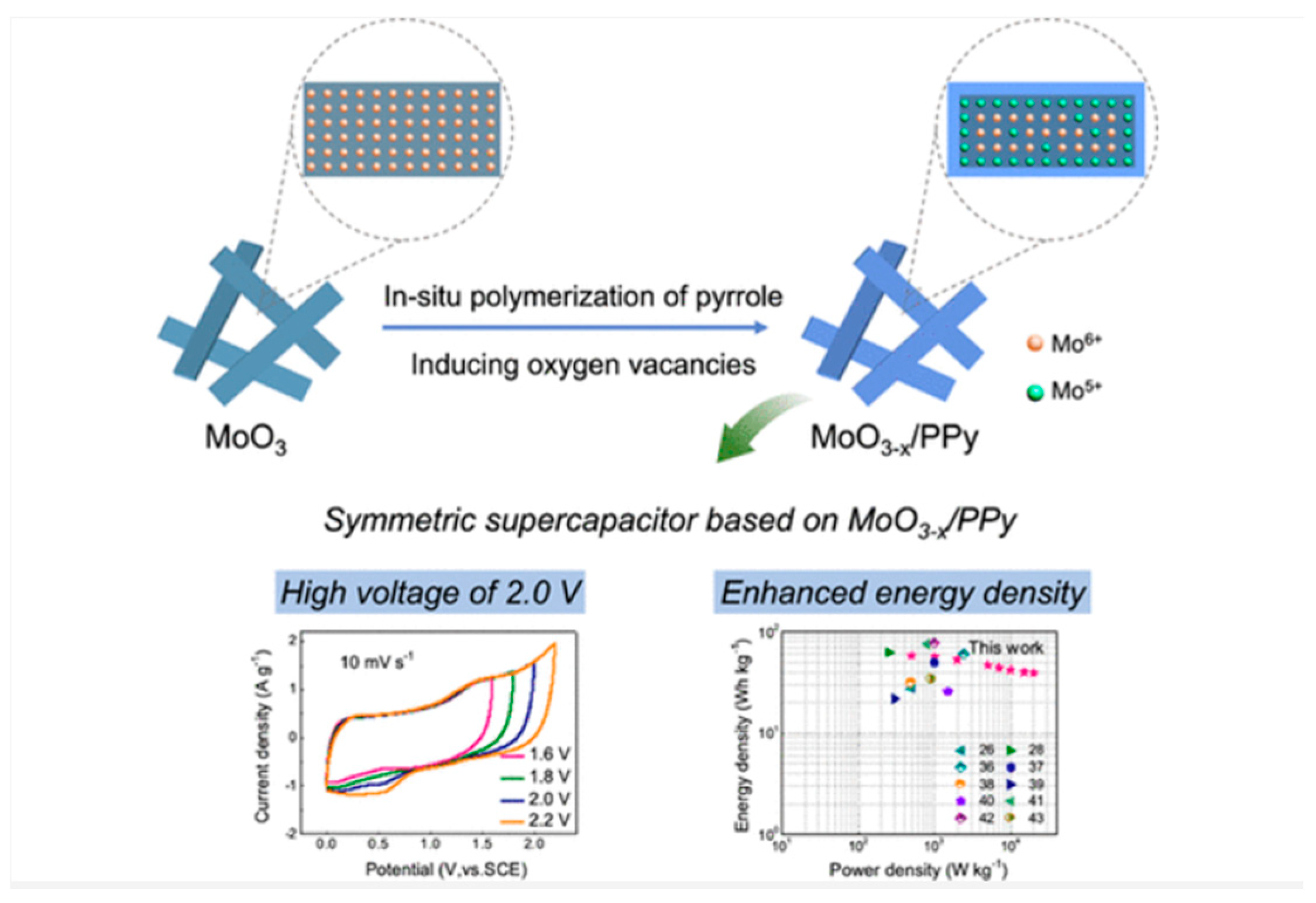
In the domain of energy storage, supercapacitors have emerged as a promising technology due to their high-power density and long-term durability [
61]. A significant advancement in this field is the development of aqueous supercapacitors with extended voltage windows. One such innovation is the development of an aqueous supercapacitor with a high-voltage window of 2.0 V. Liu
et al. [
61] developed an aqueous supercapacitor with a 2.0 V high-voltage window by core–shell MoO
3–x/polypyrrole (MP) nanocomposites as both cathode and anode materials. The authors argue that the ultrathin PPy layer on the MoO
3 core not only improves the conductivity and cycle stability of the nanocomposites but also acted as a reductant, leading to the formation of oxygen vacancies in the MoO
3 core. They further explain that when used as a cathode material, the potential range of the as-obtained MP nanocomposite is up to 1.0 V. The authors conclude that the synthesis of MP nanocomposites is simple, and the electrode performance is significantly enhanced; thus, it is a suitable candidate for high-energy-density aqueous supercapacitors.
Figure 8.
Graphical abstract of the aqueous supercapacitor with a 2.0 V high-voltage window by core–shell MoO
3–x/polypyrrole (MP) nanocomposites as both cathode and anode materials. (Reprinted with permission from ref. [
61], Copyright 2022, American Chemical Society).
Figure 8.
Graphical abstract of the aqueous supercapacitor with a 2.0 V high-voltage window by core–shell MoO
3–x/polypyrrole (MP) nanocomposites as both cathode and anode materials. (Reprinted with permission from ref. [
61], Copyright 2022, American Chemical Society).
6.1.3. Cycle Life
One of the critical performance metrics for batteries is their cycle life. Cycle life is a measure of a battery's longevity and indicates the number of charge and discharge cycles a battery can undergo before its capacity drops to a predetermined percentage of its original value, typically 80% [
41]. A battery with a longer cycle life is more desirable as it can be used for more extended periods without significant degradation in performance. In the quest for high-energy-density lithium-ion batteries, high-voltage high-nickel low-cobalt lithium layered oxide cathodes have emerged as potential candidates [
62]. These cathodes can store a significant amount of energy, making them suitable for applications that require long-lasting power. However, like many advanced materials, their cycle life can be compromised due to structural deterioration over time. This deterioration can lead to a decrease in capacity and overall battery performance.
Recent research has inquired into strategies to improve the cycle life of these high-nickel cathodes. One such study revealed that doping the cathode material LiNi
0.6Co
0.05 Mn
0.35O
2 with a small dose of titanium (Ti) can significantly enhance the cell's performance. This doping strategy not only improved the structural stability of the cathode but also led to a notable enhancement in its cycle life [
62].
7. Identification of Research Gaps and Challenges
7.1. Existing research gaps in SSB cathodes
ASSLBs are increasingly being recognized for their enhanced safety features. To fully harness their capabilities, the integration of high-voltage cathodes is crucial [
63,
64,
65,
66]. This would elevate the energy density of solid batteries, positioning them as strong contenders against their liquid counterparts. However, the incorporation of high-voltage cathodes is not without its challenges. The stability of the cathode material under high voltages is a significant concern [
65]. For instance, in the range of solid-state sodium-ion batteries (SSSBs), the electrochemical stability between sulfide-based solid electrolytes and high-voltage oxide cathodes has been a limiting factor for their long-term performance [
65].
The chemical stability at the interface between the cathode and the electrolyte is utmost. Recent studies have highlighted the potential of halide-based solid electrolytes, which demonstrate compatibility with cathodes and exhibit high ionic conductivity [
66]. However, even these advanced materials face challenges when it comes to ultra-high voltage operations [
66]. The mechanical integrity at the electrode-electrolyte interface is crucial for maintaining consistent ionic flow and overall battery performance. The generation of gases within the battery can lead to swelling, internal pressure build-up, and potential rupture, compromising the safety and longevity of the battery [
66].
To address these challenges, recent research endeavors have been directed towards enhancing solid-state electrolytes [
67]. This includes the exploration of polymer solid electrolytes, sulfide solid electrolytes, and oxide solid electrolytes. Furthermore, strategies such as coating protection, synthesis modification, and structural improvement of cathode materials have been proposed to bolster the electrochemical performance of these batteries [
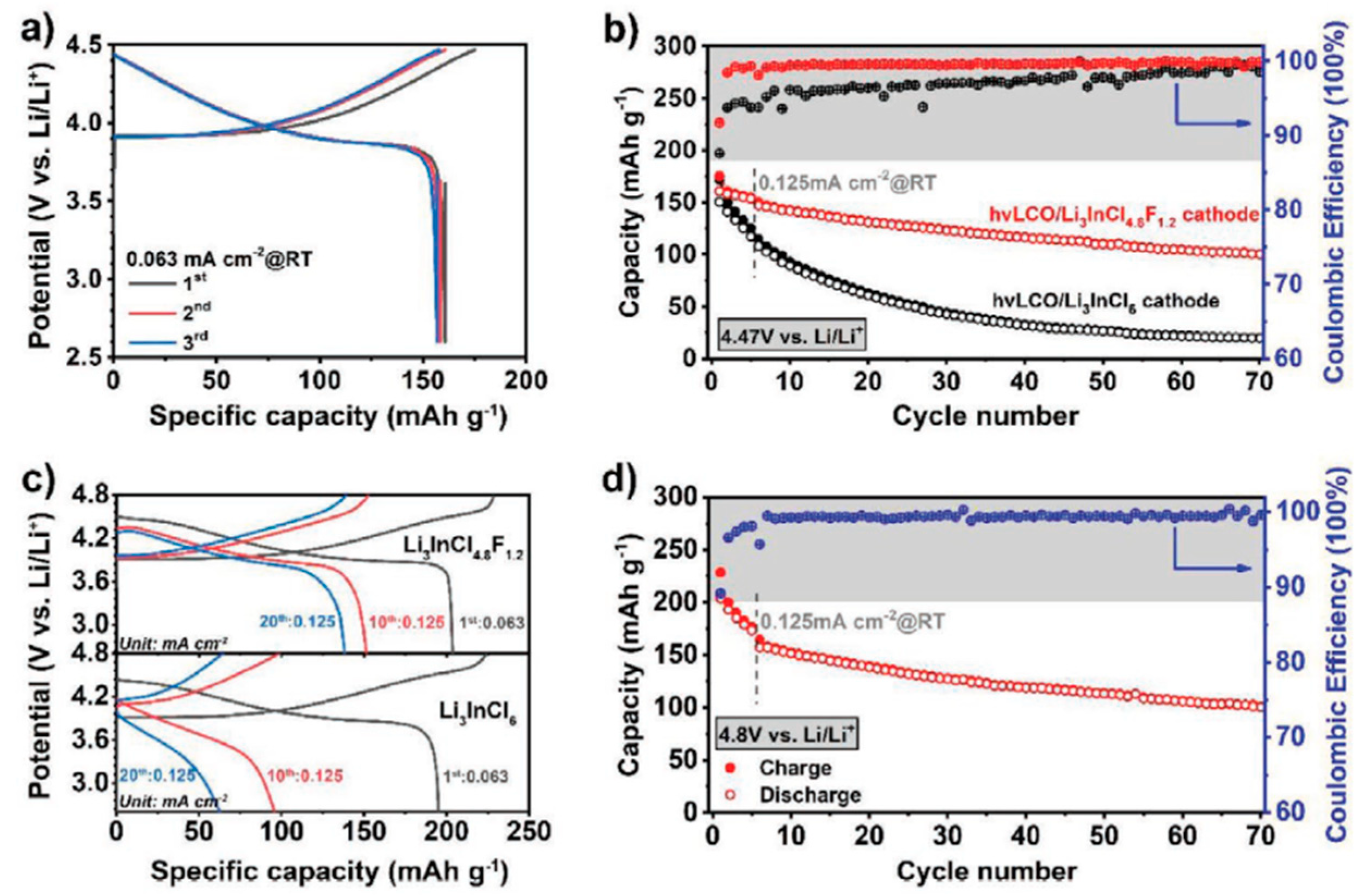
67]. For example, Zhang and group [
67] designed a dual-halogen Li-ion conductor: Li
3InCl
4.8F
1.2. Fluor was demonstrated to selectively occupy a specific lattice site in a solid superionic conductor (Li
3InCl
6) to form a new dual-halogen solid electrolyte (DHSE). With the incorporation of F, the Li
3InCl
4.
8F
1.2 DHSE became dense and maintained a room-temperature ionic conductivity over 10
−4 Scm
−1. Moreover, the authors explain that the Li
3InCl
4.8F
1.2 DHSE exhibited a practical anodic limit over 6 V (vs Li/Li
+), which can enable high-voltage ASSLIBs with decent cycling. The group concluded that their work provides a new design strategy for the fast Li-ion conductors with high oxidation stability and shows great potential to high-voltage ASSLIBs.
Figure 9 shows the electrochemical performance of the full cells using Li
3InCl
4.8F
1.2 and Li
3InCl
6 cathode.
7.2. Challenges in the development and commercialization of SSB cathodes:
7.2.1. Metal-Chalcogen Batteries (MCBs)
MCBs are increasingly recognized as potential successors in the realm of energy storage solutions [69]. Their cost-effectiveness, impressive theoretical capacity, and environmental compatibility make them stand out. However, the road to their commercialization is riddled with challenges. During the charge and discharge cycles, the electrodes in MCBs undergo significant volume changes. This can lead to mechanical degradation and reduced cycle life of the battery [69]. The dissolution and migration of soluble intermediates, particularly lithium polysulfides, can result in a phenomenon known as the shuttle effect. This not only reduces the battery's efficiency but also its overall lifespan [69]. The intermediate conversions in MCBs often suffer from sluggish reaction kinetics, which can impede the battery's performance [70]. The growth of uncontrolled dendrites on alkali metal anodes can pose serious safety risks, including short-circuiting and potential battery failure [70]. To address these challenges, the integration of metal-organic framework (MOF)-based materials into MCBs has been proposed. MOFs, with their unique properties such as high porosity, low density, expansive surface area, regular pore channels, adjustable pore size, and topological diversity, can effectively mitigate the aforementioned issues. For instance, the anionic Bio-MOF-100 and its derived single-atom zinc catalyst have been shown to simultaneously inhibit lithium dendrite growth and the shuttle effect, leading to enhanced battery performance [71].
8. Future Directions in SSB Cathode Development
The evolution of ASSBs is pivoted around the inception of pioneering materials, cell designs, and fabrication techniques. One of the key strategies to amplify the rate capability, elongate the cycle life, and curtail the interfacial resistance of an ASSB cell is by coating the Cathode Active Material (CAM). In this context, Atomic Layer Deposition (ALD) and Physical Vapor Deposition (PVD) have emerged as promising techniques. Specifically, these methods have been employed to coat NCM811 particles with lithium niobate, which has shown potential in enhancing the overall performance of ASSBs [72].
8.1. Laser Sintering of Ceramic-Based Solid-State Battery Materials
Laser sintering stands out as a promising technique for the fabrication of ceramic-based solid-state battery materials. This method is characterized by its short interaction durations, which translate to elevated heating rates. Such high heating rates can curtail diffusion processes, thereby preserving the intrinsic crystal structure of the materials. Research spotlighting the impact of varying interaction times on the crystal structure and adhesion during the laser sintering of LLZO and LCO micro particle layers is gaining momentum. For instance, the garnet-type Li7La3Zr2O12 (LLZO) solid electrolyte, when processed using advanced techniques, showcases high ionic conductivity and compatibility with high-voltage cathode materials [73]. Moreover, the lithium aluminum titanium phosphate (LATP) ceramic, when subjected to specific sintering conditions, exhibits promising characteristics for its application in solid-state batteries [74]. Another study highlighted the potential of lithium-rich antiperovskites (LiRAPs) as sintering aids, which can significantly lower the sintering temperature of ceramic-based batteries [75].
9. Conclusions
In the global shift from fossil fuels to cleaner energy alternatives, the demand for high-performance energy storage systems has become paramount. Solid-State Batteries stand out as a potential successor to conventional lithium-ion batteries, boasting enhanced energy density, superior safety profiles, and extended service life. Foundational cathode materials such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), and lithium iron phosphate (LiFePO4) have paved the way for SSB advancements. Yet, the interplay between these traditional cathodes and SSB performance remains complex.
Novel cathode materials, spanning sulfides, oxides, and air-based cathodes, bring forth distinct advantages and inherent challenges. Their synergistic interaction with solid electrolytes is vital for the optimal operation of SSBs. To amplify the efficacy of these cathodes, strategies like nanostructuring, surface modifications, and composite formulations are under investigation, targeting issues such as limited conductivity and structural vulnerabilities. Factors like electronic and ionic conductivity, as well as thermal attributes encompassing heat dissipation and thermal robustness, are integral in dictating battery safety and efficiency.
The incorporation of these advanced cathode materials into SSBs necessitates scalable fabrication methods, with a keen focus on the intricacies of electrode-electrolyte interfaces and current collectors. Practicality remains at the forefront when deploying these sophisticated materials. A holistic assessment of cathode materials, benchmarked against performance metrics like energy density, voltage stability, and cycle longevity, offers a comprehensive understanding of their potential and areas necessitating refinement.
Through our meticulous review of the literature, it is evident that while there have been significant strides in SSB development, the journey ahead is extensive. Persistent research gaps and challenges in SSB cathode evolution need to be bridged and surmounted for their successful market introduction. The trajectory of SSB cathode research is undoubtedly optimistic, teeming with prospective research avenues and potential breakthroughs in materials and methodologies. As the global momentum shifts towards sustainable energy paradigms, the continual refinement and innovation in SSBs will be pivotal in sculpting an eco-friendly future.
Author Contributions
Conceptualization, A.M. and F.M.; methodology, A.M. and F.M.; writing—original draft preparation, A.M. and F.M.; writing—review and editing, A.M. and F.M.; supervision, F.M.; funding acquisition, A.M. and F.M. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from NSF Center for the Advancement of Wearable Technologies-CAWT (Grant 1849243) is gratefully acknowledged.
Data Availability Statement
The data is contained in the article and is available from the corresponding authors on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Q.; Feng, Q.; Lei, Y.; Tang, S.; Xu, L.; Xiong, Y.; Fang, G.; Wang, Y.; Yang, P.; Liu, J.; Liu, W.; Xiong, X. Quasi-solid-state Zn-air batteries with an atomically dispersed cobalt electrocatalyst and organohydrogel electrolyte. Nat. Commun. 2022, 13, 3689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Z.; Wu, Y.; Ji, S.; Yuan, Z.; Liu, J.; Zhu, M. In Situ Construction a Stable Protective Layer in Polymer Electrolyte for Ultralong Lifespan Solid-State Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104277. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.-J.; Yao, J.; Zhang, L.; Xu, R.; Wang, S.; Yan, X.; Yu, C.; Wang, L. Promoting favorable interfacial properties in lithium-based batteries using chlorine-rich sulfide inorganic solid-state electrolytes. Nat. Commun. 2022, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Rong, X.; Gao, A.; Liu, Y.; Li, J.; Mao, M.; Qi, X.; Chai, G.; Zhang, Q.; Suo, L.; Gu, L.; Li, H.; Huang, X.; Chen, L.; Liu, B.; Hu, Y.-S. Rational design of a topological polymeric solid electrolyte for high-performance all-solid-state alkali metal batteries. Nat. Commun. 2022, 13, 4181. [Google Scholar] [CrossRef] [PubMed]
- Minnmann, P.; Strauss, F.; Bielefeld, A.; Ruess, R.; Adelhelm, P.; Burkhardt, S.; Dreyer, S.L.; Trevisanello, E.; Ehrenberg, H.; Brezesinski, T.; Richter, F.H.; Janek, J. Designing Cathodes and Cathode Active Materials for Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2201425. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, F.; Shen, L.; Deng, S.; Wang, Z.; Li, M.; Yao, X. 20 μm-Thick Li6.4La3Zr1.4Ta0.6O12-Based Flexible Solid Electrolytes for All-Solid-State Lithium Batteries. Energy Mater. Adv. 2022, 2022, 9753506. [Google Scholar] [CrossRef]
- Albero Blanquer, L.; Marchini, F.; Seitz, J.R.; Daher, N.; Bétermier, F.; Huang, J.; Gervillié, C.; Tarascon, J. Optical sensors for operando stress monitoring in lithium-based batteries containing solid-state or liquid electrolytes. Nat. Commun. 2022, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Qiao, L.; Santiago, A.; Judez, X.; Sáenz de Buruaga, A.; Jimenez, G.; Armand, M.; Zhang, H.; Li, C. Perspective of polymer-based solid-state Li-S batteries. Energy Mater. J. 2022, 2, 200003. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Meng, Y.; Jang, J. Scaling up high-energy-density sulfidic solid-state batteries: A lab-to-pilot perspective. Joule 2022, 6, 1755–1769. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Y.; Hao, F.; Kmiec, S.; Dong, H.; Xu, R.; Zhao, K.; Ai, Q.; Terlier, T.; Wang, L.; Zhao, L.; Guo, L.; Lou, J.; Xin, H.L.; Martin, S.W.; Yao, Y.-H. An electrochemically stable homogeneous glassy electrolyte formed at room temperature for all-solid-state sodium batteries. Nat. Commun. 2022, 13, 2854. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.-S.; Kim, S.; Yoon, K.; Han, S.; Lee, M.H.; Ko, Y.; Noh, J.; Kim, W.-Y.; Kang, K. Design of a lithiophilic and electron-blocking interlayer for dendrite-free lithium-metal solid-state batteries. Sci. Adv. 2022, 8, eabq0153. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lai, C.; Chen, K.; Wu, Q.; Gu, Y.; Wu, C.; Li, C. Dual fluorination of polymer electrolyte and conversion-type cathode for high-capacity all-solid-state lithium metal batteries. Nat. Commun. 2022, 13, 7914. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Teo, J.; Walther, F.; Ma, Y.; Zhang, R.; Mazilkin, A.; Tang, Y.; Goonetilleke, D.; Janek, J.; Bianchini, M.; Brezesinski, T. Advanced Nanoparticle Coatings for Stabilizing Layered Ni-Rich Oxide Cathodes in Solid-State Batteries. Adv. Funct. Mater. 2022, 32, 2111829. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Hu, J.; Sun, S.; Yuan, H.; Fu, Z.; Chen, X.; Huang, J.; Ouyang, M.; Zhang, Q. The void formation behaviors in working solid-state Li metal batteries. Sci. Adv. 2022, 8, eadd0510. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, Y.; Cao, M.; Gu, Q.; Wang, H.; Yu, J.; Guo, Z.-H.; Zhou, X. Advanced inorganic/polymer hybrid electrolytes for all-solid-state lithium batteries. J. Adv. Ceram. 2022, 11, 835–861. [Google Scholar] [CrossRef]
- Eckhardt, J.K.; Klar, P.; Janek, J.; Heiliger, C. Interplay of Dynamic Constriction and Interface Morphology between Reversible Metal Anode and Solid Electrolyte in Solid State Batteries. ACS Appl. Mater. Interfaces 2022, 14, 35545–35554. [Google Scholar] [CrossRef]
- Liang, J.; Maas, E.; Luo, J.; Li, X.; Chen, N.; Adair, K.; Li, W.; Li, J.Y.; Hu, Y.; Liu, J.; Zhang, L.; Zhao, S.; Lu, S.-g.; Wang, J.; Huang, H.; Zhao, W.; Parnell, S.; Smith, R.I.; Ganapathy, S.; Wagemaker, M.; Sun, X. A Series of Ternary Metal Chloride Superionic Conductors for High-Performance All-Solid-State Lithium Batteries. Adv. Energy Mater. 2022, 12, 2103921. [Google Scholar] [CrossRef]
- Fu, C.; Homann, G.; Grissa, R.; Rentsch, D.; Zhao, W.; Gouveia, T.; Falgayrat, A.; Lin, R.; Fantini, S.; Battaglia, C. A Polymerized-Ionic-Liquid-Based Polymer Electrolyte with High Oxidative Stability for 4 and 5 V Class Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2200412. [Google Scholar] [CrossRef]
- Payandeh, S.; Brezesinski, T.; Strauss, F.; Mazilkin, A.; Kondrakov, A. Tailoring the LiNbO3 coating of Ni-rich cathode materials for stable and high-performance all-solid-state batteries. Nano Res. 2022, 1, e9120016. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K. Processing and manufacturing of next generation lithium-based all solid-state batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Huang, Y. The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity. Interdiscip. Mater. 2022, 1, 323–329. [Google Scholar] [CrossRef]
- W, H.; Tian, Y.; An, Y.; Feng, J.; Xiong, S.; Qian, Y. Porous lithium cobalt oxide fabricated from metal–organic frameworks as a high-rate cathode for lithium-ion batteries. RSC Advances 2020, 10, 30000–30010. [Google Scholar] [CrossRef] [PubMed]
- Marincaş, A.-H.; Ilea, P. Enhancing Lithium Manganese Oxide Electrochemical Behavior by Doping and Surface Modifications. Coatings 2021, 11, 456. [Google Scholar] [CrossRef]
- Cherkashinin, G.; Eilhardt, R.; Nappini, S.; Cococcioni, M.; Píš, I.; Dal Zilio, S.; Bondino, F.; Marzari, N.; Magnano, E.; Alff, L. Energy Level Alignment at the Cobalt Phosphate/Electrolyte Interface: Intrinsic Stability vs Interfacial Chemical Reactions in 5 V Lithium-Ion Batteries. ACS Applied Materials & Interfaces 2021, 13, 57482–57493. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, X.; Lu, W.; Zhang, J.; Ni, H. The Influence of Temperature on the Capacity of Lithium-Ion Batteries with Different Anodes. Energies 2022, 15, 60. [Google Scholar] [CrossRef]
- Nguyen, Q.; Luu, V.T.; Nguyen, H.L.; Lee, Y.-W.; Cho, Y.; Kim, S.Y.; Jun, Y.-S.; Ahn, W. Li7La3Zr2O12 Garnet Solid Polymer Electrolyte for Highly Stable All-Solid-State Batteries. Frontiers in Chemistry 2021, 8, 619832. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lim, N.-g.; Lee, S.J.; Park, J. Feasibility Study for Sustainable Use of Lithium-Ion Batteries Considering Different Positive Electrode Active Materials under Various Driving Cycles by Using Cell to Electric Vehicle (EV) Simulation. Sustainability 2020, 12, 9764. [Google Scholar] [CrossRef]
- Bal, B.; Ozdogru, B.; Nguyen, D.T.; Li, Z.; Murugesan, V.; Çapraz, Ö.Ö. Probing the Formation of Cathode-Electrolyte Interphase on Lithium Iron Phosphate Cathodes via Operando Mechanical Measurements. ACS Appl. Mater. Interfaces 2023, 15, 42449–42459. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-W.; Kang, S.-W.; Heo, K.; Lee, J.; Kim, M.; Hwang, D.; Kim, S.-J.; Kim, J.; Lim, J. Effect of Nanoparticles in LiFePO4 Cathode Material Using Organic/Inorganic Composite Solid Electrolyte for All-Solid-State Batteries. Langmuir 2023, 39, 45–52. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.; Gao, Y.; Zhao, Y.; Kong, X.; Rong, Q.; Xiong, J.; Yu, D.; Ding, S. Angew. Chem. Int. Ed. 2023, 62, e202307255. [CrossRef]
- Vogt, D.; Michalowski, P.; Kwade, A. Production and Characterisation of Fibre-Reinforced All-Solid-State Electrodes and Separator for the Application in Structural Batteries. Batteries 2022, 8, 55. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Yang, K.; Wang, H.; Yu, C.; Xu, D.; Xu, B.; Wang, L.-M. Superior Blends Solid Polymer Electrolyte with Integrated Hierarchical Architectures for All-Solid-State Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 36886–36896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, R.; Zhang, D.; Liu, J.; Liu, F.; Cui, J.; Lin, Z.; Wu, J.; Zhu, M. Constructing Li-Rich Artificial SEI Layer in Alloy-Polymer Composite Electrolyte to Achieve High Ionic Conductivity for All Solid-State Lithium Metal Batteries. Adv. Mater. 2021, 33, 2004711. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sun, S.; Yu, P.; Yang, W. Design Unique Air-Stable and Li–Metal Compatible Sulfide Electrolyte via Exploration of Anion Functional Units for All-Solid-State Lithium-Metal Batteries. Adv. Funct. Mater. 2022, 32, 2201528. [Google Scholar] [CrossRef]
- Ban, X.; Zhang, W.; Chen, N.; Sun, C. A High-Performance and Durable Poly(ethylene oxide)-Based Composite Solid Electrolyte for All Solid-State Lithium Battery. J. Phys. Chem. C 2018, 122, 9852–9858. [Google Scholar] [CrossRef]
- Noh, H.-S.; Hong, J.; Kim, H.; Yoon, K.J.; Kim, B.-K.; Lee, H.-W.; Lee, J.-H.; Son, J.-W. Scale-Up of Thin-Film Deposition-Based Solid Oxide Fuel Cell by Sputtering, a Commercially Viable Thin-Film Technology. J. Electrochem. Soc. 2016, 163, F613. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Xie, Z.; Qu, W.; Freschi, D.J.; Liu, J. Progress and Perspectives of Lithium Aluminum Germanium Phosphate-Based Solid Electrolytes for Lithium Batteries. Adv. Funct. Mater. 2023, 33, 2300973. [Google Scholar] [CrossRef]
- Sun, Z.; Lai, Y.; lv, N.; Hu, Y.; Li, B.; Jing, S.; Jiang, L.; Jia, M.; Li, J.; Chen, S.; Liu, F. Boosting the Electrochemical Performance of All-Solid-State Batteries with Sulfide Li6PS5Cl Solid Electrolyte Using Li2WO4-Coated LiCoO2 Cathode. Adv. Mater. Interfaces 2021, 8, 2100624. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Tang, L.; Han, F.; Zhang, Y.; Xia, Y.; Wang, L.; Lu, S. Review of the electrochemical performance and interfacial issues of high-nickel layered cathodes in inorganic all-solid-state batteries. Int. J. Miner. Metall. Mater. 2022, 29, 1003–1018. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Feng, W.; Kang, F. Interface Improvement of Li6.4La3Zr1.6Ta0.6O12@La2Sn2O7 and Cathode Transfer Printing Technology with Splendid Electrochemical Performance for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39414–39423. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Zhao, S.; Song, J.; Liao, Y.; Tang, H. Sulfur/carbon cathode composite with LiI additives for enhanced electrochemical performance in all-solid-state lithium-sulfur batteries. Adv Compos Hybrid Mater 2023, 6, 162. [Google Scholar] [CrossRef]
- Kitsche, D.; Tang, Y.; Hemmelmann, H.; Walther, F.; Bianchini, M.; Kondrakov, A.; Janek, J.; Brezesinski, T. Atomic Layer Deposition Derived Zirconia Coatings on Ni-Rich Cathodes in Solid-State Batteries: Correlation Between Surface Constitution and Cycling Performance. Small Sci. 2023, 3, 2200073. [Google Scholar] [CrossRef]
- Tian, L.W.; Kim, J.W.; Hong, S.B.; Ryu, H.H.; Kim, U.H.; Sun, Y.K.; Kim, D.W. All-solid-state lithium batteries featuring hybrid electrolytes based on Li+ ion-conductive Li7La3Zr2O12 framework and full-concentration gradient Ni-rich NCM cathode. J. Chem. Eng. 2022, 450, 138043. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, Y.; Li, X.; Luo, J.; Deng, S.; Zhao, Y.; Sun, Y.; Wu, D.; Hu, Y.; Li, W.; Sham, T.K.; Li, R.; Gu, M.; Sun, X. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.X.; Wang, J.J. Solid-state batteries: from fundamental interface characterization to realize sustainable promise. Rare Met. 2020, 39, 743–744. [Google Scholar] [CrossRef]
- Sun, Z.; Lai, Y.; lv, N.; Hu, Y.; Li, B.; Jing, S.; Jiang, L.; Jia, M.; Li, J.; Chen, S.; Liu, F. Boosting the Electrochemical Performance of All-Solid-State Batteries with Sulfide Li6PS5Cl Solid Electrolyte Using Li2WO4-Coated LiCoO2 Cathode. Adv. Mater. Interfaces 2021, 8, 2100624. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, S.; Tang, L.; Han, F.; Xia, Y.; Wang, L.; Lu, S. Review of the electrochemical performance and interfacial issues of high-nickel layered cathodes in inorganic all-solid-state batteries. Int. J. Miner. Metall. Mater. 2022, 29, 1003–1018. [Google Scholar] [CrossRef]
- Gannett, C.N.; Peterson, B.M.; Melecio-Zambrano, L.; Trainor, C.Q.; Fors, B.P.; Abruña, H.D. Performance optimization and fast rate capabilities of novel polymer cathode materials through balanced electronic and ionic transport. J. Mater. Chem. A 2021, 9, 5657–5663. [Google Scholar] [CrossRef]
- Lou, S.; Liu, Q.; Zhang, F. Insights into interfacial effect and local lithium-ion transport in polycrystalline cathodes of solid-state batteries. Nat. Commun. 2020, 11, 5700. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; He, T.; Yan, X.; Yu, J.; Bi, J.; Ye, D.; Yao, W.; Tang, Y.; Zhao, H.; Zhang, J. Chevrel Phase Mo6S8 Nanosheets Featuring Reversible Electrochemical Li-Ion Intercalation as Effective Dynamic-Phase Promoter for Advanced Lithium-Sulfur Batteries. Small 2023, 19, 2300042. [Google Scholar] [CrossRef]
- Bashirpour-Bonab, H. Thermal behavior of lithium batteries used in electric vehicles using phase change materials. Int. J. Energy Res. 2020, 44, 12583–12591. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yu, T.-Y.; Yeh, S.-C.; Wu, N.-L.; Jeng, R.-J. Spiro-Twisted Benzoxazine Derivatives Bearing Nitrile Group for All-Solid-State Polymer Electrolytes in Lithium Batteries. Polymers 2022, 14, 2869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fan, H.; Dang, Y.; Zhuang, Q.; Arandiyan, H.; Wang, Y.; Cheng, N.; Sun, H.; Pérez Garza, H.H.; Zheng, R.; Wang, Z.; Mofarah, S.S.; Koshy, P.; Bhargava, S.; Cui, Y.; Shao, Z.; Liu, Y. Recent advances in in situ and operando characterization techniques for Li7La3Zr2O12-based solid-state lithium batteries. Mater. Horiz. 2023, 10, 1479–1538. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.; Fitch, B.; Xia, J. Industrial Perspectives on Innovation: Sustainability, Safety, Scalability, and Advanced Performance. Meet. Abstr. 2022, MA2022-02, 218. [Google Scholar] [CrossRef]
- Negi, R.S.; Yusim, Y.; Pan, R.; Ahmed, S.; Volz, K.; Takata, R.; Schmidt, F.; Henss, A.; Elm, M.T. A Dry-Processed Al2O3/LiAlO2 Coating for Stabilizing the Cathode/Electrolyte Interface in High-Ni NCM-Based All-Solid-State Batteries. Adv. Mater. Interfaces 2022, 9, 2101428. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, M.; Rao, A.; Lin, X.; Doyle-Davis, K.; Liang, J.; Yu, C.; Li, R.; Zhao, S.; Zhang, L.; Huang, H.; Wang, J.; Singh, C.V.; Sun, X. Fast-Charging Halide-Based All-Solid-State Batteries by Manipulation of Current Collector Interface. Adv. Funct. Mater. 2022, 32, 2200767. [Google Scholar] [CrossRef]
- Minnmann, P.; Strauss, F.; Bielefeld, A.; Ruess, R.; Adelhelm, P.; Burkhardt, S.; Dreyer, S.L.; Trevisanello, E.; Ehrenberg, H.; Brezesinski, T.; Richter, F.H.; Janek, J. Designing Cathodes and Cathode Active Materials for Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2201425. [Google Scholar] [CrossRef]
- Culver, S.P.; Koerver, R.; Zeier, W.G.; Janek, J. On the Functionality of Coatings for Cathode Active Materials in Thiophosphate-Based All-Solid-State Batteries. Adv. Energy Mater. 2019, 9, 1900626. [Google Scholar] [CrossRef]
- Ma, Y.; Teo, J.H.; Walther, F.; Ma, Y.J.; Zhang, R.; Mazilkin, A.; Tang, Y.; Goonetilleke, D.; Janek, J.; Bianchini, M.; Brezesinski, T. Advanced Nanoparticle Coatings for Stabilizing Layered Ni-Rich Oxide Cathodes in Solid-State Batteries. Adv. Funct. Mater. 2022, 32, 2111829. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, T.T.; Kwok, C.Y.; Kim, S.Y.; Assoud, A.; Zhang, Q.; Janek, J.; Nazar, L.F. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 2022, 7, 83–93. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Meng, Y.; Plamthottam, R.; Tjiu, W.W.; Zhang, C.; Liu, T. Ultrathin Polypyrrole Layers Boosting MoO3 as Both Cathode and Anode Materials for a 2.0 V High-Voltage Aqueous Supercapacitor. ACS Appl. Mater. Interfaces, 2022; 14, 4490–4499. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, Y.; Chu, C.; Chang, L.; Wang, Z.; Zhang, D.; Liu, W.; Zhuang, Z.; Wang, L. Stabilizing effects of atomic Ti doping on high-voltage high-nickel layered oxide cathode for lithium-ion rechargeable batteries. Nano Res. 2022, 15, 4091–4099. [Google Scholar] [CrossRef]
- Haruna, A.B.; Mwonga, P.; Barrett, D.; Rodella, C.B.; Forbes, R.P.; Venter, A.; Sentsho, Z.; Fletcher, P.J.; Marken, F.; Ozoemena, K.I. Defect-Engineered β-MnO2−δ Precursors Control the Structure–Property Relationships in High-Voltage Spinel LiMn1.5Ni0.5 O4−δ. ACS Omega 2021, 6, 25562–25573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Meng, Y.; Plamthottam, R.; Tjiu, W.W.; Zhang, C.; Liu, T. Ultrathin Polypyrrole Layers Boosting MoO3 as Both Cathode and Anode Materials for a 2.0 V High-Voltage Aqueous Supercapacitor. ACS Appl. Mat. Interfaces 2022, 14, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yao, X.; Wang, S.; Zhang, D.; Yin, D.; Wang, L.; Cheng, Y. Gospel for Improving the Lithium Storage Performance of High-Voltage High-Nickel Low-Cobalt Layered Oxide Cathode Materials. ACS Appl. Mater. Interfaces 2021, 13, 58871–58884. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.A.; Banerjee, S.; Tang, H.; Richardson, P.M.; Doux, J.M.; Qi, J.; Zhu, Z. A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries. Nat. Commun. 2021, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, F.; Wang, S.; Liang, J.; Wang, J.; Wang, C.; Zhang, H.; Adair, K.; Li, W.; Li, M.; Duan, H.; Zhao, Y.; Yu, R.; Li, R.; Huang, H.; Zhang, L.; Zhao, S.; Lu, S.; Sham, T.-K.; Mo, Y.; Sun, X. Advanced High-Voltage All-Solid-State Li-Ion Batteries Enabled by a Dual-Halogen Solid Electrolyte. Adv. Energy Mater. 2021, 11, 2100836. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Song, H.; Richardson, P.M.; Doux, J.M.; Qi, J.; Zhu, Z. Insights Into the Interfacial Degradation of High-Voltage All-Solid-State Lithium Batteries. Nano-Micro Lett. 2022; 14, 191. [Google Scholar] [CrossRef]
- Li, F.; Zhang, X.; Liu, X.; Zhao, M. Novel Conductive Metal–Organic Framework for a High-Performance Lithium–Sulfur Battery Host: 2D Cu-Benzenehexathial (BHT). ACS Appl. Mater. Interfaces 2018, 10, 15012–15020. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-L.; Li, Z.; Ma, L.-Y.; Li, M.-Z.; Huang, S.-L.; Hong, X.-J.; Cai, Y.; Lan, Y. Single-Atom Zinc and Anionic Framework as Janus Separator Coatings for Efficient Inhibition of Lithium Dendrites and Shuttle Effect. ACS Nano 2021, 15, 13436–13443. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huyan, Y.; Li, N.; Lei, D.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. A Seamless Metal-Organic Framework Interphase with Boosted Zn2+ Flux and Deposition Kinetics for Long-Living Rechargeable Zn Batteries. Nano Lett. 2023, 23, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Weber, S.; Graumann, T.; Zebrowski, S.; Mainusch, N.; Dilger, N.; Cerdas, F.; Zellmer, S. An Environmental and Technical Evaluation of Vacuum-Based Thin Film Technologies: Lithium Niobate Coated Cathode Active Material for Use in All-Solid-State Battery Cells. Energies 2023, 16, 1278. [Google Scholar] [CrossRef]
- Kravchyk, K.; Okur, F.; Kovalenko, M. Break-Even Analysis of All-Solid-State Batteries with Li-Garnet Solid Electrolytes. ACS Energy Lett. 2021, 6, 2202–2207. [Google Scholar] [CrossRef]
- Gunduz, D.C.; Schierholz, R.; Yu, S.; Tempel, H.; Kungl, H.; Eichel, R. Combined quantitative microscopy on the microstructure and phase evolution in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J. Adv. Ceram. 2020, 9, 149–161. [Google Scholar] [CrossRef]
- Feng, W.; Yang, P.; Dong, X.; Xia, Y. A Low Temperature Soldered All Ceramic Lithium Battery. ACS Appl. Mater. Interfaces 2022, 14, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).