Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test products
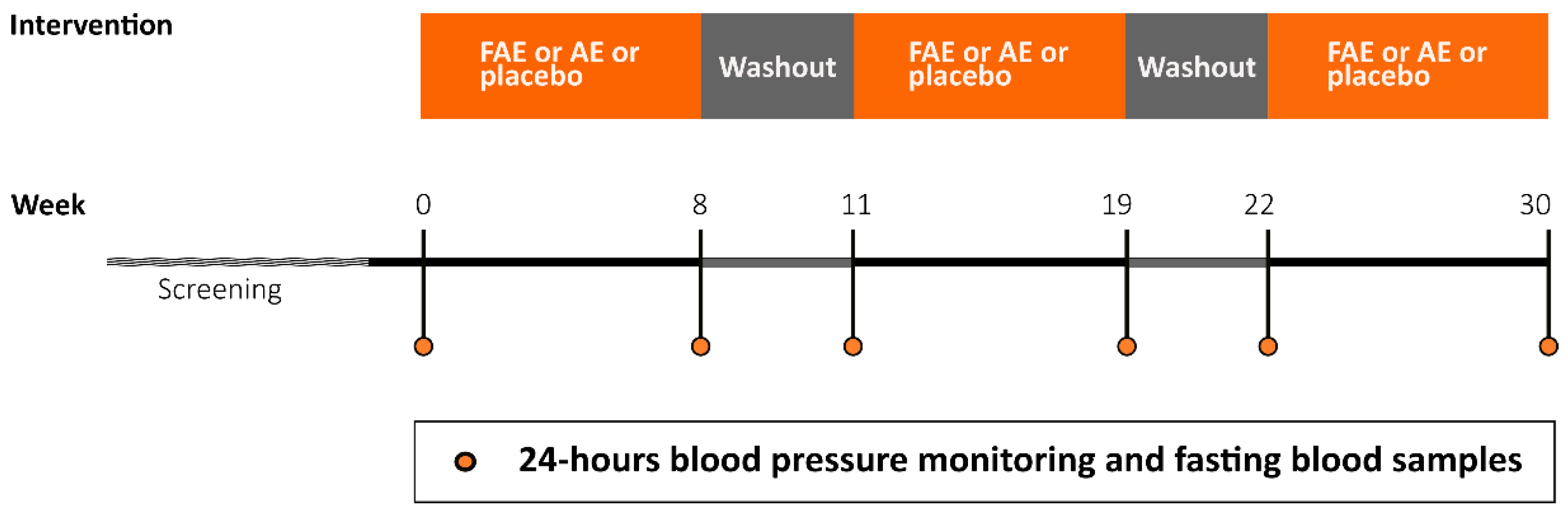
2.2. Study design and participants
2.4. Blood analyses
2.4.1. Hs-CRP and adiponectin
2.4.2. Lipids
2.5. Power calculation
2.6. Statistics
3. Results
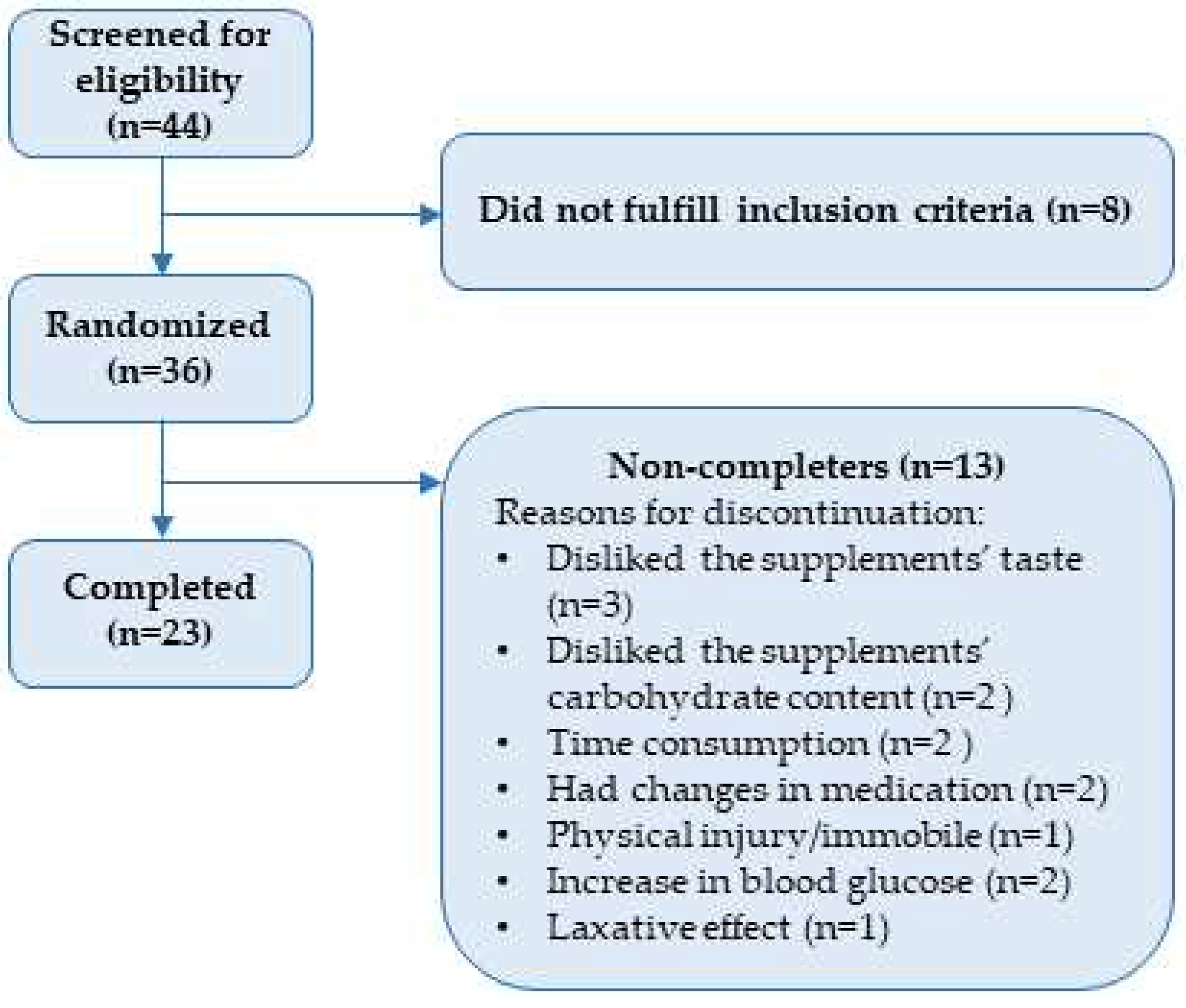
3.1. Baseline characteristics
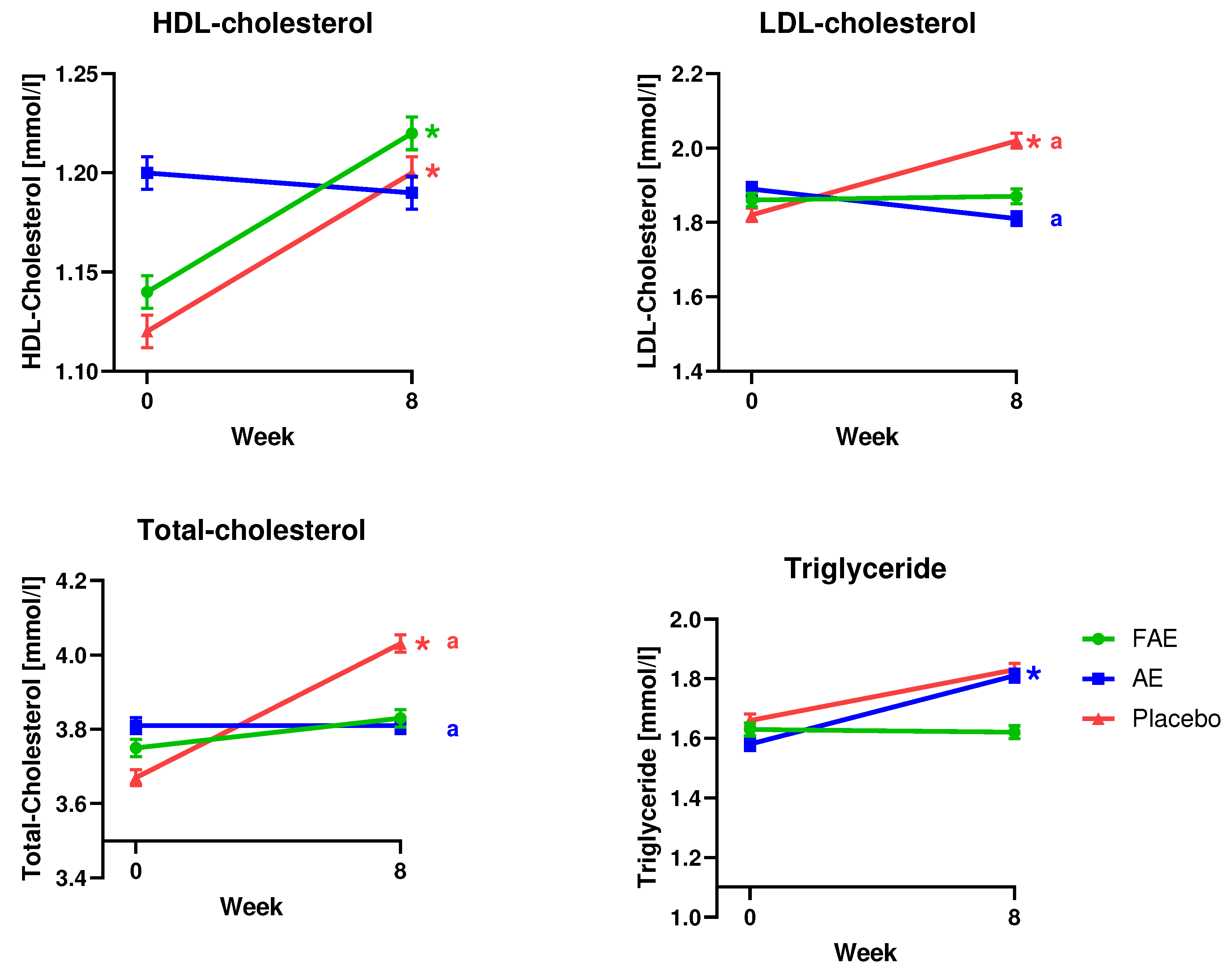
3.3. Blood analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federation, I.D. IDF Diabetes Atlas, 10th edn. Available online: https://www.diabetesatlas.org (accessed on April).
- (WHO), W.h.o. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on.
- Wang, C.C.L.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa) - A review on the characteristic components and potential health effects. Planta medica 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa on Cardiometabolic Diseases: A Systematic Review of Quasi-Design Studies and Randomized Controlled Trials. Rev Diabet Stud 2022, 18, 76–92. [Google Scholar] [CrossRef]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials. Journal of Dietary Supplements 2021, 18, 517–530. [Google Scholar] [CrossRef]
- Rahmani, J.; Clark, C.; Kord Varkaneh, H.; Lakiang, T.; Vasanthan, L.T.; Onyeche, V.; Mousavi, S.M.; Zhang, Y. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Phytotherapy research: PTR 2019, 33, 1981–1990. [Google Scholar] [CrossRef]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic Effects of Aronia melanocarpa and Its Other Therapeutic Properties. Frontiers in nutrition 2017, 4, 53–53. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Brandrup-Wognsen, G.; Palmer, M.K.; Nicholls, S.J. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER Database. Journal of Lipid Research 2010, 51, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.K.; Wang, T.Y.; Li, S.; Robinson, J.G.; Roger, V.L.; Goldberg, A.C.; Virani, S.S.; Louie, M.J.; Lee, L.V.; Peterson, E.D. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. Journal of the American Heart Association 2019, 8, e011765. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Zanchetti, A.; Grassi, G.; Mancia, G. When should antihypertensive drug treatment be initiated and to what levels should systolic blood pressure be lowered? A critical reappraisal. Journal of hypertension 2009, 27, 923–934. [Google Scholar] [CrossRef] [PubMed]
- ANDERSSON, O.K.; NELDAM, S. The antihypertensive effect and tolerability of candesartan cilexetil, a new generation angiotensin II antagonist, in comparison with losartan. Blood Pressure 1998, 7, 53–59. [Google Scholar]
- Barrios, V.; Escobar, C.; Navarro, A.; Barrios, L.; Navarro-Cid, J.; Calderón, A.; INVESTIGATORS, L. Lercanidipine is an effective and well tolerated antihypertensive drug regardless the cardiovascular risk profile: The LAURA study. International journal of clinical practice 2006, 60, 1364–1370. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Laniewska, I.; Millo, B.; Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–184. [Google Scholar] [CrossRef]
- Banach, M.; Wiloch, M.; Zawada, K.; Cyplik, W.; Kujawski, W. Evaluation of Antioxidant and Anti-Inflammatory Activity of Anthocyanin-Rich Water-Soluble Aronia Dry Extracts. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Valentina, B.; Minodora, A.; Antal, D.; Florina, A.; Ioana Zinuca, P.; Cristina, D.; Codruta, S.; Roxana, F.; Felicia, A.; Corina, D. Cardioprotective Effects of Cultivated Black Chokeberries (Aronia spp.): Traditional Uses, Phytochemistry and Therapeutic Effects. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; Kavita, S., Kanchan, M., Kula Kamal, S., Corina, D., Eds.; IntechOpen: Rijeka, 2020; p. Ch. 9. [Google Scholar]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and Antioxidant Activity of Black Chokeberry (Aronia melanocarpa) Polyphenols: in vitro and in vivo Evidences and Possible Mechanisms of Action: A Review. Comprehensive Reviews in Food Science and Food Safety 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin Absorption and Metabolism by Human Intestinal Caco-2 Cells--A Review. Int J Mol Sci 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, E.; Piskula, M.K. Bioavailability of cyanidin glycosides from natural chokeberry (Aronia melanocarpa) juice with dietary-relevant dose of anthocyanins in humans. Journal of agricultural and food chemistry 2010, 58, 12130–12136. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological properties of protocatechuic Acid and its potential roles as complementary medicine. Evid Based Complement Alternat Med 2015, 2015, 593902. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacological Research 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Jeppesen, P.B.; Hermansen, K.; Gregersen, S. Aronia in the Type 2 Diabetes Treatment Regimen. Nutrients 2023, 15, 4188. [Google Scholar] [CrossRef]
- Reinhard, M.; Frystyk, J.; Jespersen, B.; Bjerre, M.; Christiansen, J.S.; Flyvbjerg, A.; Ivarsen, P. Effect of hyperinsulinemia during hemodialysis on the insulin-like growth factor system and inflammatory biomarkers: a randomized open-label crossover study. BMC Nephrology 2013, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Knopfholz, J.; Disserol, C.C.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Massucheto Ledesma, P.; Faria-Neto, J.R.; Olandoski, M.; da Cunha, C.L.; et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol 2014, 2014, 261878. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia medica 2002, 44, 20–23. [Google Scholar]
- Milutinović, M.; Radovanović, R.V.; Šavikin, K.; Radenković, S.; Arvandi, M.; Pešić, M.; Kostić, M.; Miladinović, B.; Branković, S.; Kitić, D. Chokeberry juice supplementation in type 2 diabetic patients-impact on health status. 2019. [CrossRef]
- Cozlea, D.L.; Farcas, D.M.; Nagy, A.; Keresztesi, A.A.; Tifrea, R.; Cozlea, L.; Carașca, E. The impact of C reactive protein on global cardiovascular risk on patients with coronary artery disease. Curr Health Sci J 2013, 39, 225–231. [Google Scholar]
- Boncheva, M.; Turnovska, T. Administration of bioflavonoides improves plasma levels of adipocyte hormones. Acta Medica Bulgarica 2014, 41, 5–11. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Association, A.D. Standards of Medical Care in Diabetes. Diabetes Care 2005, 28, s4–s36. [Google Scholar] [CrossRef]
- Lopez-Alvarenga, J.C.; Ebbesson, S.O.; Ebbesson, L.O.; Tejero, M.E.; Voruganti, V.S.; Comuzzie, A.G. Polyunsaturated fatty acids effect on serum triglycerides concentration in the presence of metabolic syndrome components. The Alaska-Siberia Project. Metabolism 2010, 59, 86–92. [Google Scholar] [CrossRef]
- Esfahani, A.; Lam, J.; Kendall, C.W. Acute effects of raisin consumption on glucose and insulin reponses in healthy individuals. J Nutr Sci 2014, 3, e1. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Huitrón-Bravo, G.; Talavera, J.O.; Castañón, S.; Gallegos-Carrillo, K.; Flores, Y.; Salmerón, J. Dietary glycemic index, dietary glycemic load, blood lipids, and coronary heart disease. J Nutr Metab 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-H.; Hwang, I.-G.; Lee, Y.-M. Effects of anthocyanin supplementation on blood lipid levels: a systematic review and meta-analysis. Frontiers in Nutrition 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLOS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef] [PubMed]
| Nutritional content per daily dose | FAE | AE | Placebo |
| Energy (kcal) | 234.6 | 240.1 | 227.3 |
| Total fats (g) [%] | 3.7 [14.2] | 4.0 [15.0] | 3.5 [13.9] |
| - Saturated (g) | 2.9 | 3.0 | 2.4 |
| - Unsaturated (g) | 0.4 | 0.6 | 0.5 |
| - Polyunsaturated | 0.2 | 0.2 | 0.5 |
| Total carbohydrates (g) [%] | 43.2 [73.7] | 45.1 [75.1] | 41.1 [72.3] |
| - Free sugars (g) | 38.6 | 38.6 | 34.7 |
| Dietary fibers (g) | 9.2 | 6.6 | 8.1 |
| Protein (g) [%] | 2.5 [4.3] |
2.5 [4.2] |
3.7 [6.5] |
| Variable (unit) | Completers (n=23). Value in mean ± SD or median (IQR) | Randomized (n=36). Value in mean ± SD or median (IQR) |
| Gender | 15 (M) 8 (W) |
21 (M) 15 (W) |
| Age (years) | 67.6±5.5 | 66.9±6.0 |
| Bodyweight (kg) | 82.0±16.2 | 85.9 (72.6-95.5) |
| Body mass index (kg/m2) | 26.7 (23.2-29.8) | 28.6 (24.3-32.0) |
| Hemoglobin A1c (mmol/mol) | 50.0 (47.5-54) | 50.5 (47.0-55.0) |
| Medication/compound | Completers (n=23). Received by [number participants (%)] | Randomized (n=36). Received by [number participants (%)] |
| Metformin | 21 (91.3) | 33 (91.7) |
| Insulin | 1 (4.3) | 3 (8.3) |
| GLP-1 receptor agonist | 4 (17.4) | 6 (16.7) |
| Dipeptidyl peptidase-4 inhibitor | 2 (8.7) | 2 (5.6) |
| Sodium-glucose Cotransporter-2 inhibitor | 8 (34.8) | 9 (25.0) |
| Sulfonylurea | 2 (8.7) | 3 (8.3) |
| Statins | 15 (65.2) | 25 (69.4) |
| ACE inhibitor | 10 (43.5) | 15 (41.7) |
| Angiotensin II receptor blocker | 4 (17.4) | 9 (25.0) |
| Beta blocker | 8 (34.8) | 11 (30.6) |
| Antiplatelet/anticoagulant treatment | 6 (26.1) | 9 (25.0) |
| Calcium channel blocker | 6 (26.1) | 10 (27.8) |
| Cardiac glycosides | 0 (0.0) | 1 (2.8) |
| Diuretics | 5 (21.7) | 8 (22.2) |
| Levothyroxine treatment | 2 (8.7) | 2 (5.6) |
| Dietary supplement | 12 (52.2) | 20 (55.6) |
| Variable (unit) | Δ-mean for FAE | Δ-mean for AE | Δ-mean for placebo | p value | ||||
| Pre | Post | Pre | Post | Pre | Post | |||
| 24-hours systolic BP (mmHg) n=35 |
-0.71 ± 1.18 | 0.03 ± 1.07 | -0.30 ± 1.17 | |||||
| 125 ± 2.14 | 125 ± 2.19 | 124 ± 2.14 | 124 ± 2.14 | 124 ± 2.12 | 124 ± 2.14 | |||
| 24-hours diastolic BP (mmHg) n=35 |
0.16 ± 0.77 | 0.03 ± 0.70 | -1.40 ± 0.77 | |||||
| 71 ± 1.47 | 71 ± 1.49 | 71 ± 1.43 | 71 ± 1.46 | 71 ± 1.46 | 71 ± 1.49 | |||
| Hs-CRP n=23 | (ratio) | 1.10 (0.69-1.76) | 0.83 (0.52-1.34) | 1.05 (0.66-1.68) | ||||
| (mg/l) | 0.56 ± 0.11 | 0.62 ± 0.12 | 0.69 ± 0.14 | 0.58 ± 0.12 | 0.53 ± 0.10 | 0.55 ± 0.11 | ||
| Adiponectin (mg/l) n=23 |
0.14 ± 0.26 | -0.59 ± 0.26 | 0.06 ± 0.26 | |||||
| 8.44 ± 0.87 | 8.58 ± 0.87 | 9.14 ± 0.87 | 8.55 ± 0.87 | 8.38 ± 0.87 | 8.43 ± 0.87 | |||
| HDL-cholesterol (mmol/l) n=36 |
0.08 ± 0.03 | -0.01 ± 0.03 | 0.08 ± 0.03 | |||||
| 1.14 ± 0.05a | 1.22 ± 0.05a | 1.2 ± 0.05 | 1.19 ± 0.05 | 1.12 ± 0.05b | 1.2 ± 0.05b |
a0.03 b0.02 |
||
| LDL-cholesterol (mmol/l) n=36 |
0.01 ± 0.08 | -0.09 ± 0.07* | 0.20 ± 0.08* | 0.04 | ||||
| 1.86 ± 0.11 | 1.87 ± 0.12 | 1.89 ± 0.11 | 1.81 ± 0.11 | 1.82 ± 0.11* | 2.02 ± 0.12* | 0.01 | ||
| Total-cholesterol (mmol/l) n=36 |
0.08 ± 0.09 | 0.002 ± 0.08* | 0.36 ± 0.09* | 0.01 | ||||
| 3.75 ± 0.14 | 3.83 ± 0.14 | 3.81 ± 0.13 | 3.81 ± 0.13 | 3.67 ± 0.13* | 4.03 ± 0.14* | 0.0003 | ||
| Triglyceride (mmol/l) n=36 |
-0.01 ± 0.09 | 0.23 ± 0.08 | 0.17 ± 0.09 | |||||
| 1.63 ± 0.13 | 1.62 ± 0.13 | 1.58 ± 0.13* | 1.81 ± 0.13* | 1.66 ± 0.13 | 1.83 ± 0.13 | 0.03 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





