Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Virus. Notes on the Structure and Pathogenetic Mechanisms
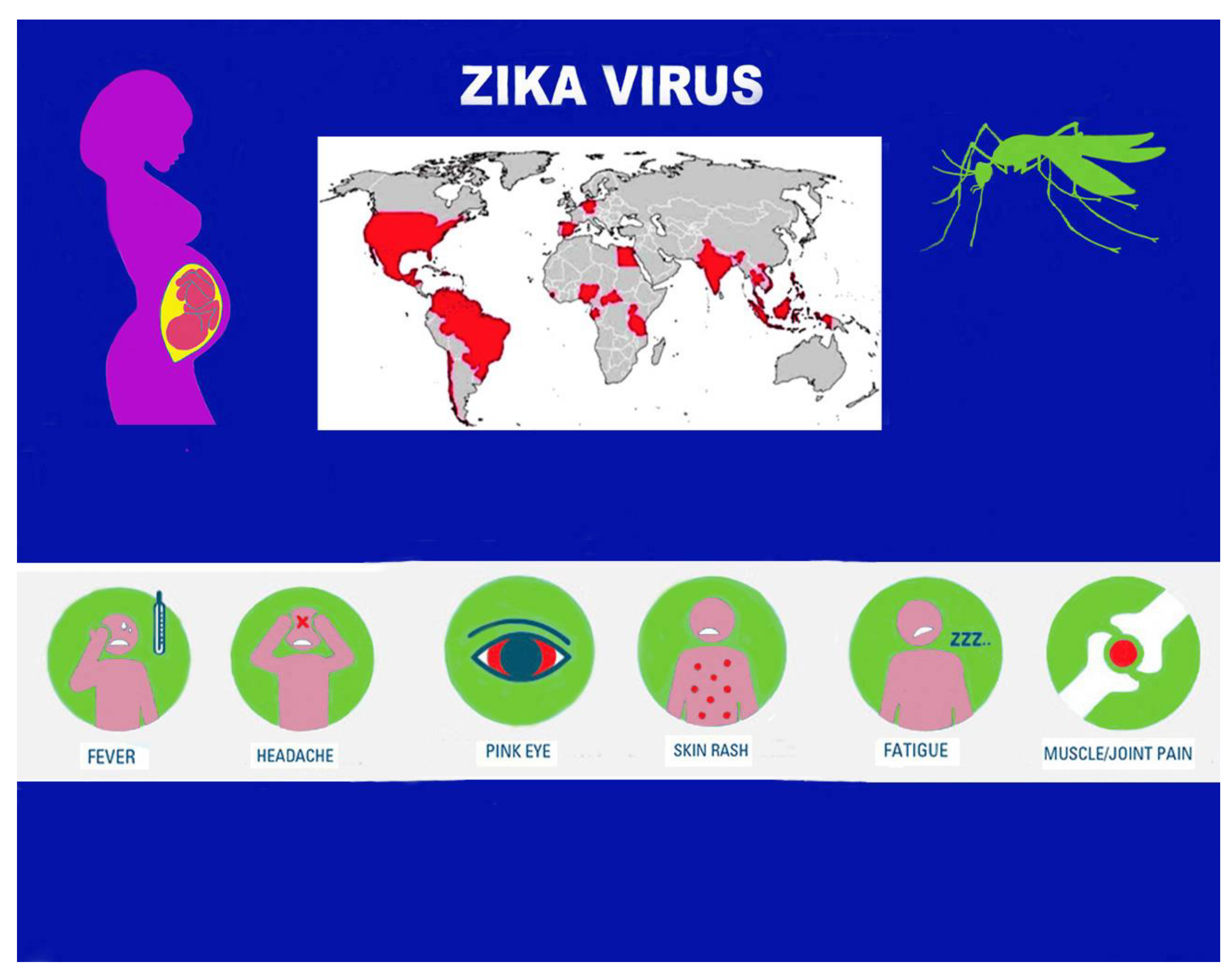
3. Epidemiological Aspects
4. Transmission Mode
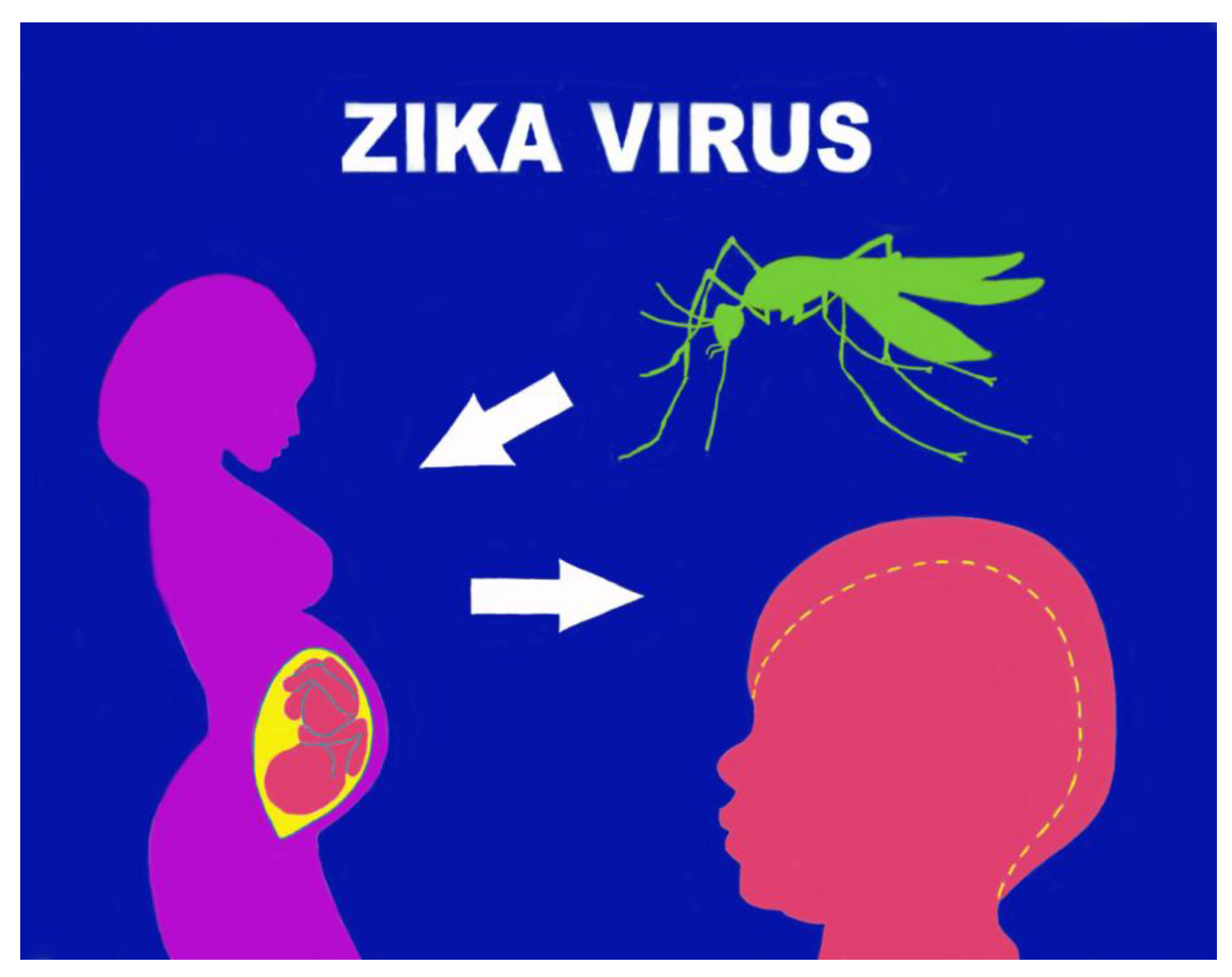
5. Pathogenetic Mechanisms in Pregnancy
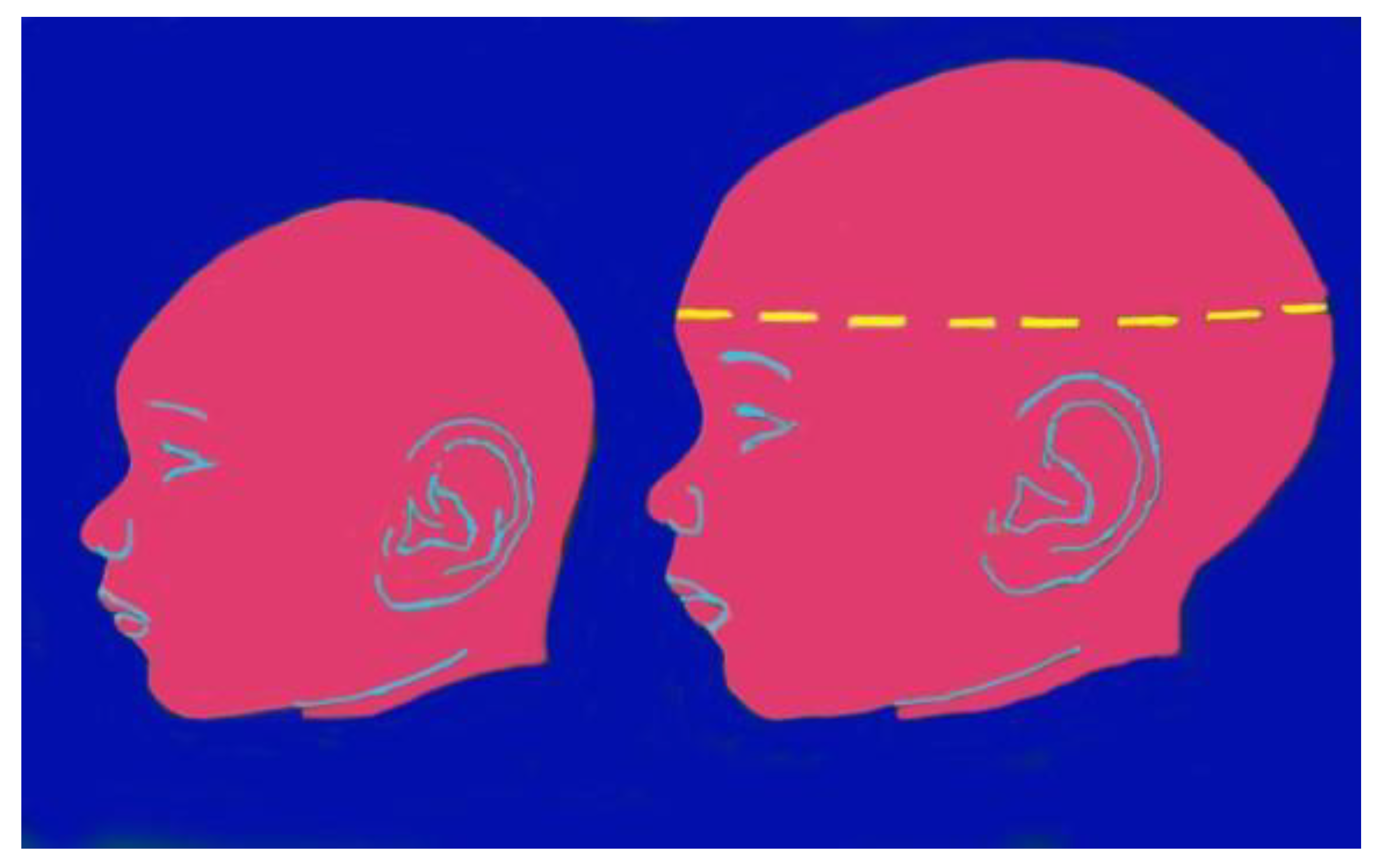
7. Microcephaly and Other Brain Anomalies Due to CZS
7.1. Microcephaly
7.2. Orofacial Anomalies
8. Conclusions
References
- Musso D, Rodriguez-Morales AJ, Levi JE, et al. Unexpected outbreaks of arbovirus infections: lessons learned from the Pacific and tropical America. Lancet Infect Dis. 2018;18:e355-e361. [CrossRef]
- Huntington MK, Allison J, Nair D. Emerging Vector-Borne Diseases. Am Fam Physician. 2016; 94:551-7.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952;46: 509-20. [CrossRef]
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol 2016;161: 665 8. [CrossRef]
- de Oliveira Melo AS, Malinger G, Ximenes R, et al. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet Gynecol 2016;47: 67. [CrossRef]
- Postler TS, Beer M, Bradley J, et al. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae Arch Virol. 2023 Aug 10;168(9):224. [CrossRef]
- Sirohi D, Kuhn RJ. Share Zika Virus Structure, Maturation, and Receptors. J Infect Dis. 2017 Dec 16;216(suppl_10):S935-S944. [CrossRef]
- Silva LR, Souza AM. Zika virus: what do we know about the viral structure, mechanisms of transmission, and neurological outcomes? Rev Soc Bras Med Trop. 2016 May-Jun;49(3):267-73. [CrossRef]
- Hu Y, Sun L. Systematic Analysis of Structure Similarity between Zika Virus and Other Flaviviruses. ACS Infect Dis. 2019 Jul 12;5(7): 1070-1080. [CrossRef]
- Gabaglia, CR. Zika virus and diagnostics. Curr Opin Pediatr. 2017 Feb;29(1):107-113. [CrossRef]
- Song BH, Yun SI, Woolley M, et al. Lee YM. Zika virus: History, epidemiology, transmission, and clinical presentation. J Neuroimmunol. 2017 Jul 15;308:50-64. [CrossRef]
- Pielnaa P, Al-Saadawe M, Saro A, et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology. 2020 Apr; 543:34-42. [CrossRef]
- Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev. 2016 Jul;29(3):487-524. [CrossRef]
- Younger DS. Epidemiology of Zika Virus. Neurol Clin. 2016 Nov;34(4):1049-1056. [CrossRef]
- Hills SL, Fischer M, Petersen LR. Epidemiology of Zika Virus Infection. J Infect Dis. 2017 Dec 16;216(suppl_10):S868-S874. [CrossRef]
- Runge-Ranzinger S, Morrison AC, Manrique-Saide P, et al. Zika transmission patterns: a meta-review.Trop Med Int Health. 2019 May;24(5): 523-529. [CrossRef]
- Gregory CJ, Oduyebo T, Brault AC, et al. Modes of Transmission of Zika Virus. J Infect Dis. 2017 Dec 16;216(suppl_10): S875-S883. [CrossRef]
- Gutiérrez-Bugallo G, Piedra LA, Rodriguez M, et al. Vector-borne transmission and evolution of Zika virus. Nat Ecol Evol. 2019 Apr;3(4):561-569. [CrossRef]
- Brasil P, Vasconcelos Z, Kerin T, et al. Zika virus vertical transmission in children with confirmed antenatal exposure. Nat Commun. 2020 Jul 14;11(1):3510. [CrossRef]
- Marbán-Castro E, Goncéb A, Romero-Acevedo L et al. Zika virus infection in pregnant women and their children: A review. European Journal of Obstetrics & Gynecology and Reproductive Biology 265 (2021) 162–168. [CrossRef]
- Mysorekar IU, Diamond MS. Modeling Zika Virus Infection in Pregnancy. N Engl J Med. 2016 Aug 4;375(5):481-4. [CrossRef]
- Rosenberg AZ, Yu W, Hill DA, et al. Placental Pathology of Zika Virus: Viral infection of the placenta Induces Villous Stromal Macrophage (Hofbauer Cell) proliferation and hyperplasia. Arch Pathol Lab Med 2017;141:43–8. [CrossRef]
- Quicke KM, Bowen JR, Johnson EL, et al. Zika virus infects human placental macrophages. Cell Host Microbe 2016;20(1):83–90. [CrossRef]
- Merfeld E, Ben-Avi L, Kennon M, et al. Potential mechanisms of Zika-linked microcephaly. Wiley Interdiscip Rev Dev Biol. 2017 Jul;6(4): e273. [CrossRef]
- Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384: 857-868. [CrossRef]
- Venceslau EM, Guida JP, Amaral E, et al. Characterization of placental infection by Zika virus in humans: A review of the literature. Rev Bras Ginecol e Obstet Rev da Fed Bras das Soc Ginecol e Obstet 2020;42(09):577–85. [CrossRef]
- Pomar L, Musso D, Malinger G, et al. Zika virus during pregnancy: From maternal exposure to congenital Zika virus syndrome. Prenat Diagn. 2019; 39: 420-430. [CrossRef]
- von der Hagen M, Pivarcsi M, Liebe J, et al. Diagnostic approach to microcephaly in childhood: a two-center study and review of the literature. Dev Med Child Neurol 2014; 56: 732- 741. 20140312. [CrossRef]
- Pool KL, Adachi K, Karnezis S, et al. Association between neonatal neuroimaging and clinical outcomes in Zika-Exposed infants From Rio de Janeiro, Brazil. JAMA Netw Open. 2019; 2: e198124. [CrossRef]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol 2013, 5: a009076. [CrossRef]
- Rui Ji, Meng L, Jiang X, et al. TAM receptors support neural stem cell survival, proliferation and neuronal differentiation. PLoS One 2014, 9: e115140. [CrossRef]
- Nowakowski TJ, Pollen AA, Di Lullo E, et al. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 2016, 18:591–596. [CrossRef]
- Bhagat R, Prajapati B, Narwal S, et al. Zika virus E protein alters the properties of human fetal neural stem cells by modulating microRNA circuitry. Cell Death Differ. 2018 Nov;25(10):1837-1854. [CrossRef]
- Bhagat R, Rajpara P, Kaur G, et al. Zika virus E protein dysregulate mir-204/WNT2 signalling in human fetal neural stem cells. Brain Res Bull. 2021 Nov;176: 93-102. [CrossRef]
- Lottini G, Baggiani M, Chesi G, et al. Zika virus induces FOXG1 nuclear displacement and downregulation in human neural progenitors. Stem Cell Reports. 2022 Jul 2;17(7):1683-1698. [CrossRef]
- Ribeiro RA, Mattos A, Meneghim MC, et al. Oral and maxillofacial outcomes in children with microcephaly associated with the congenital Zika syndrome. Eur J Orthod 2021; 43: 346-352. [CrossRef]
- Gomes PN, do Amaral BA, Azevedo ID, et al. Association of congenital Zika syndrome with dental alterations in children with microcephaly. PLoS One 2022; 17: e0276931. 20221101. [CrossRef]
- D’Agostino ES, Chagas J, Cangussu MCT, et al. Chronology and sequence of deciduous teeth eruption in children with microcephaly associated to the Zika virus. Spec Care Dentist 2020; 40: 3-9. 20191203. [CrossRef]
- Carvalho A, Brites C, Mochida G, et al. Clinical and neurodevelopmental features in children with cerebral palsy and probable congenital Zika. Brain Dev 2019; 41: 587-594. 20190323. [CrossRef]
- Carvalho IF, Alencar PNB, Carvalho de Andrade MD, et al. Clinical and x-ray oral evaluation in patients with congenital Zika Virus. J Appl Oral Sci 2019; 27: e20180276. [CrossRef]
- Cavalcanti AFC, Aguiar YPC, de Oliveira Melo AS, et al. Teething symptoms in children with congenital Zika syndrome: A 2-year follow-up. Int J Paediatr Dent 2019; 29: 74-78. 20181009. [CrossRef]
- Gusmao TPL, Faria ABS, Leao Filho JC, et al. Dental changes in children with congenital Zika syndrome. Oral Dis 2020; 26: 457-464. 20191217. [CrossRef]
- de Oliveira AMM, de Melo EGM, Mendes MLT, et al. Oral and maxillofacial conditions, dietary aspects, and nutritional status of children with congenital Zika syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2020; 130: 71-77. 20200531. [CrossRef]
- Silva M, Arnaud MA, Lyra MCA, et al. Dental development in children born to Zikv-infected mothers: a case-based study. Arch Oral Biol 2020; 110: 104598. 20191116. [CrossRef]
- da Silva Sobrinho AR, Ramos LFS, Maciel YL, et al. Orofacial features in children with microcephaly associated with Zika virus: A scoping review. Oral Dis 2022; 28: 1022-1028. 20210228. [CrossRef]
- Medina DT, Santos A, Rodrigues F, et al. Oral manifestations of congenital Zika virus infection in children with microcephaly: 18-month follow-up case series. Spec Care Dentist 2022; 42: 343-351. 20211122. [CrossRef]
- Siqueira RMP, Santos M and Cabral GMP. Alterations in the primary teeth of children with microcephaly in Northeast Brazil: a comparative study. Int J Paediatr Dent 2018 20180702. [CrossRef]
- da Costa CCG, Dias VO, Martelli DRB, et al. First cases of oligodontia as a manifestation of the Zika virus congenital syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2022; 134: e261-e266. 20220425. [CrossRef]
- Bruggink R, Baan F, Kramer GJC, et al. Symmetry of palatal shape during the first year of life in healthy infants. Clin Oral Investig 2021; 25: 1069-1076. 20200624. [CrossRef]
- Snider TN and Mishina, Y. Cranial neural crest cell contribution to craniofacial formation, pathology, and future directions in tissue engineering. Birth Defects Res C Embryo Today 2014; 02: 324-332. 20140916. [CrossRef]
- Rios D, Rios M, Nobrega AC, et al. Alterations in deglutition in children with congenital Zika virus syndrome. Codas 2023; 35: e20210270. 20230106. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




