1. Introduction
Climate change is an escalating crisis, causing global warming by rising levels of CO
2 in the atmosphere, largely due to burning fossil fuels [
1,
2,
3]. Reducing greenhouse gas emissions has become a top priority for industries, especially the iron and steel sector, which is the world’s largest consumer of energy [
4]. In 2020 alone, global steel production reached a staggering 1.878 billion tons, as reported by the World Steel Association (WSA) [
5]. Despite steel’s recyclability, its production process remains notorious for its immense energy consumption and carbon intensity [
6]. Remarkably, the greenhouse gases emitted from steelmaking activities account for a substantial portion, approximately 2.8 billion tons, constituting 5.5–6% of the world’s annual greenhouse gas emissions [
7,
8].
One potential solution to reduce these emissions is mineral carbonation, a technology that captures CO
2 [
9,
10]. The fundamental idea behind mineral carbon dioxide sequestration is to replicate natural weathering processes, where silicate-based minerals, such as steelmaking slags, containing calcium or magnesium transform into calcium or magnesium carbonates [
3,
11,
12]:
Mineral CO
2 sequestration, while effective, requires substantial amounts of calcium/magnesium minerals, although global sources exist [
13,
14]. An overlooked solution lies in using industrial solid waste, such as slags and combustion residues, rich in calcium and readily available near CO
2 sources [
3]. Unlike primary minerals, these waste materials are highly reactive, making them cost-efficient options that could reduce the energy consumption associated with mineral CO
2 sequestration [
15,
16]. This study focuses on a multifaceted approach to integrating slags from steelmaking with the modified Solvay process from saline brines in CO
2 sequestration.
Saline brines, which are abundant and often considered waste, can be utilized effectively in industrial processes, reducing environmental impact as they have an enormous capacity for industrial CO
2 storage [
17,
18,
19]. CO
2-saturated brine descends to the lower depth of the aquifer because of the density variance after the CO
2 injection phase [
20]. One propitious method for capturing CO
2 in saline brine is using chemical solvents, such as liquid ammonia [
21]. While liquid NH
3 helps in CO
2 capture while utilizing the saline water and the production of useful carbonates like baking soda (NaHCO
3) by ammonia-based Solvay process, its presence poses health and environmental risks [
22,
23]. Therefore, efforts have been made to modify the Solvay process, aiming to replace ammonia and address these concerns [
22,
24,
25,
26]. Ali et al. (2023), replaced liquid NH
3 in the Solvay process with different additives like Ca(OH)
2, KOH, and NH
4HCO
3 and compared their results using advanced techniques like XRD and SEM [
27]. The analysis revealed that using NH
4HCO
3 as an additive produced the highest-quality NaHCO
3, along with valuable Na
2CO
3. The present work was conducted to combine the modified Solvay process and steelmaking waste elements to create a synergistic system. The novelty of the work is exploring the multifaceted utility of steelmaking slags, aiming to leverage their properties in (1) investigating the potential of slags to efficiently regenerate ammonium from the spent brine; (2) experimenting with slag as co-reagents in the bicarbonate production process, strategically designed to decrease the reliance on ammonium, thus optimizing the overall production efficiency; (3) exploring the direct mineral carbonation of slags using residual brine from the bicarbonate synthesis.
In the context of sodium bicarbonate production and steelmaking, slags, rich in calcium oxide (CaO), are hypothesized in this study to potentially play a pivotal role in one or more of three key processes. Firstly, in the regeneration of ammonium from spent brine, ammonium chloride in the brine reacts with calcium oxide in the slags to form ammonium (NH
4+) and chloride (Cl
-). This regenerated ammonium could then be utilized in the bicarbonate synthesis reaction, minimizing the need for additional ammonium production.
Secondly, slags could act as a co-reagent by reacting with CO
2 and brine, forming sodium bicarbonate alongside ammonium. This dual action can significantly reduce the requirement for ammonium, cutting both costs and environmental impact associated with ammonium production, which often involves CO
2-emitting processes like natural gas burning. Additionally, these slags would facilitate mineral carbonation, a method of converting CO
2 into stable forms like calcium carbonate (CaCO
3).
Thirdly, by combining post-bicarbonate synthesis residual brine with slags, it would be expected that CO
2 would be extracted from the solution, reacting with the slags to form solid carbonates. This process could effectively sequester CO
2 from steelmaking emissions, presenting a viable carbon capture and storage (CCS) strategy for hard-to-abate sectors such as ironmaking and steelmaking.
Integrating these components is aimed to optimize resource utilization, minimize waste, and significantly reduce the carbon footprint of industrial processes, aligning with the broader goals of sustainable development and environmental responsibility. The primary objective of the study was to evaluate the reactivity and the pH buffering capabilities of the steelmaking electric arc furnace (EAF) slag and compare it with two other abundant natural minerals, olivine, and kimberlite, to showcase its potential applications for the three aforementioned purposes, with particular emphasis on the ammonium regeneration step. Geochemical modeling was further employed to predict the reactivity of the minerals in the study and to correlate to experimental findings for furthering mechanistic understanding.
2. Materials and Methods
2.1. Materials
The reactivity of three distinct minerals, EAF slag, olivine, and kimberlite, was examined in the presence of three aqueous solutions: ultrapure water (UPW; 18.2 MΩ·cm), brine containing ammonium bicarbonate (ABB), and brine containing potassium bicarbonate (PBB). The EAF slag utilized in this study was obtained from NRCan and required crushing to achieve a particle size <2 mm due to its coarse nature. Olivine, chosen for its relative purity and natural origin, was sourced from Italy, and used in its finely milled form. Kimberlite, a complex mine tailing known for its lower reactivity compared to olivine, was obtained from Northern Ontario and was sieved to <2 mm. The initial stage of the experiments involved preparing the brine solution. The chemicals used for brine preparation were sodium chloride NaCl (Fisher Scientific with a purity level ≥ 90%), potassium hydroxide KOH (Fisher Scientific with a purity level ≥ 85-100%), sulfuric acid HCl (Fisher Scientific with a purity level ≥ 36.5-38%), and ammonium bicarbonate NH
4HCO
3 (Acros Organics with a purity level ≥ 98%). These solutions were meticulously prepared to mirror the conditions explored in prior research (Ali et al., 2023), with brines containing an equivalent of 1.258 mol/L NaCl and reagents NH
4HCO
3 and KHCO
3 at the same concentration [
27]. The objective was to assess the minerals’ reactivity concerning their influence on solution pH control and ion delivery, crucial factors in sodium bicarbonate NaHCO
3 synthesis. To simulate the varied pH levels these solutions might encounter during NaHCO
3 synthesis, the solutions were made more acidic by adding up to 1 mol/L HCl or made more basic by adding up to 1 mol/L NaOH. The pH stock solutions were prepared with ultrapure water and the chemicals used were sulfuric acid HCl (Fisher Scientific with a purity level ≥ 36.5-38%), and sodium hydroxide NaOH (Fisher Scientific with a purity level ≥ 50%).
2.2. Experimental setup
The first experiment procedure involved mixing 5 grams of each mineral with 100 ml of the corresponding solution. For each combination of mineral and solution (total of 9 combinations), 11 samples were prepared: one unaltered, devoid of any acidic or basic modifications, and 10 others subject to the influence of precisely controlled acid (0.001, 0.01, 0.05, 0.2, and 1 mol/L) and base (0.001, 0.01, 0.05, 0.2, and 1 mol/L) concentrations. This methodical approach yielded a total of 99 distinct compositions.
The resulting mixtures were shaken for 24 hours in sealed bottles (Thermo Scientific Nalgene HDPE Centrifuge Bottles) using an orbital shaker. Following each experiment, the pH levels of the solutions were meticulously determined. The mixtures were then filtered through analytical filter paper, separating the solids from the liquid phase. The pH of each filtered liquid was measured to examine the change in pH levels. The collected solids underwent a thorough purification process, involving washing with ultrapure water to remove residual salts. Subsequently, the purified solids were dried at 105°C for 24 hours in the oven, ensuring the removal of any remaining moisture. The resulting dry mass was precisely measured, providing valuable insights into the reactivity and composition of the mineral-solution interactions. The residual solids underwent mineralogical analysis through X-ray diffraction (XRD) to identify their composition. The specific composition was quantified using the Rietveld refinement technique. Meanwhile, the liquid filtrate was acidified with 2% nitric acid and subjected to analysis via inductively coupled plasma optical emission spectroscopy (ICP-OES) to determine the concentrations of calcium, magnesium, and iron.
The second experiment was to observe the rate of regeneration of ammonium from spent brine. The experiment focused on creating an ammonium bicarbonate brine solution with a significantly reduced sodium concentration of 0.01258 mol/L, representing a 99% reduction compared to standard levels. In this procedure, 250 ml of the brine solution was heated on a hot plate to 50 °C and stirred at a constant rate of 400 rpm using a magnetic stirrer. Sequential additions of 2.5 g of the designated mineral were made at 5-minute intervals, and pH measurements were taken before each addition. This process continued until a total of 50 g of the mineral had been added, maintaining a consistent liquid-to-solid (L/S) ratio of 5:1.
Another ammonium regeneration experiment was conducted to examine the necessary calcium leaching from slag to achieve optimal reactivity. Employing a setup similar to the second experiment but only with slag, the experiment maintained a similar sodium concentration where a total of 250 ml of brine solution was heated to 50 °C. In this setup, the slag used was milled using a ball mill to achieve a particle size of <2 μm). In intervals of 30 minutes, 2.5 g portions of slag were systematically introduced until the total reached 50 g. Afterward, increments of 5 g of Ca(OH)2 were added at 30-minute intervals until a cumulative total of 10 g was reached, representing 20% of the weight concerning the initial slag used. Before each addition, the pH levels were recorded, providing data on the reaction dynamics.
2.3. Geochemical modeling
The Geochemist’s Workbench (GWB) software was utilized for geochemical modeling purposes. Specifically, the Phase2 application within GWB was employed to generate phase diagrams for six minerals, namely chrysotile (Mg3Si2O5(OH)4), fayalite (Fe2SiO4), forsterite (Mg2SiO4), gehlenite (Ca2Al2SiO7), larnite (Ca2SiO4), and talc (Mg3Si4O10(OH)2). These minerals were chosen to represent the compositions found in EAF slag, olivine, and kimberlite. The modeling considered a solution composition equivalent to brine with ammonium bicarbonate, with variations in temperature from 0 to 100 °C and pH from the unadjusted value when the brine was mixed with 1 mol/L HCl, up to a pH of 14. As GWB performs equilibrium modeling, to ensure that the target mineral formed in at least a part of the phase diagram, competing silicate and carbonate minerals were suppressed.
3. Results and discussion
Previous studies have indicated useful modified Solvay processes by using NaHCO3, Ca(OH)2, and KOH as buffering agents. In the present study, the most effective modified Solvay processes identified by Ali et al. (2023) were combined with steelmaking slags to observe the reactivity of the mineral. The experiments would explore the reactivity of EAF slags in contrast to their industrial counterparts. Olivine, and kimberlite, were meticulously chosen to represent a broad spectrum of compositions found in industrial waste minerals. The use of XRD and ICP-OES techniques enhances the precision of process characterization, while geochemical modeling provides insights into the precipitate behavior of the minerals.
In the mineral reactivity experiments, the observed outcomes align closely with theoretical expectations.
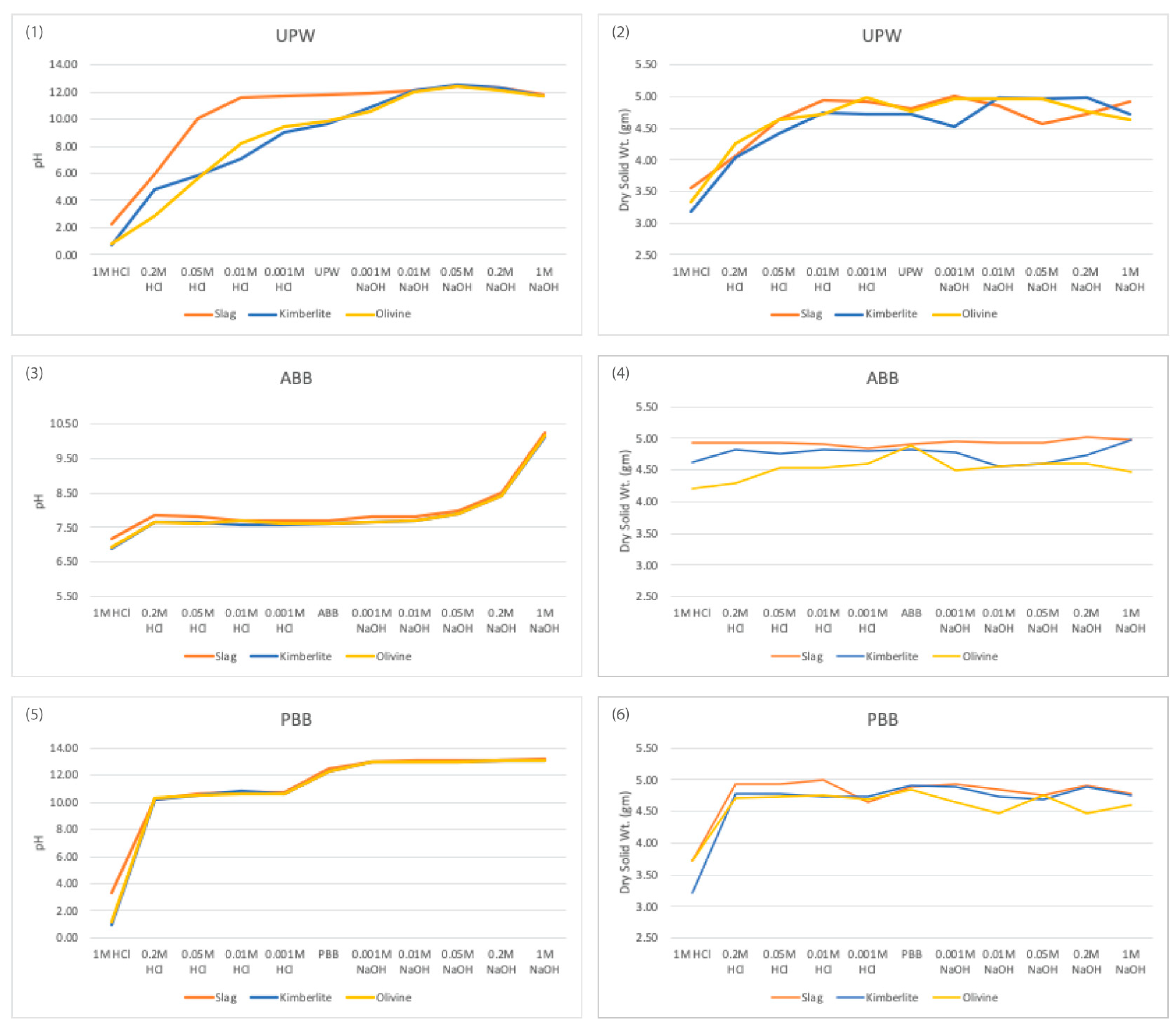
Figure 1 illustrates the change in pH and loss of mass at different acidic or basic concentrations for all three aqueous solutions. Minerals exhibited greater mass loss at lower pH levels and reduced mass loss at higher pH levels. Lower pH is known to facilitate the extraction of calcium, magnesium, and iron from silicate minerals, whereas higher pH enables the extraction of aluminum and silica, but to a lesser extent [
28,
29]. The observed mass changes also show these tendencies. The mass change of slag was similar to that of natural minerals but slightly lower. This difference might be attributed to the coarser particle size of the crushed slag (<2 mm), whereas the other minerals were already finely milled, with olivine being finer than kimberlite. However, slag exhibited superior pH buffering capabilities compared to natural minerals. Consequently, mineral reactivity appears to be more influenced by pH effects than mere mass loss.
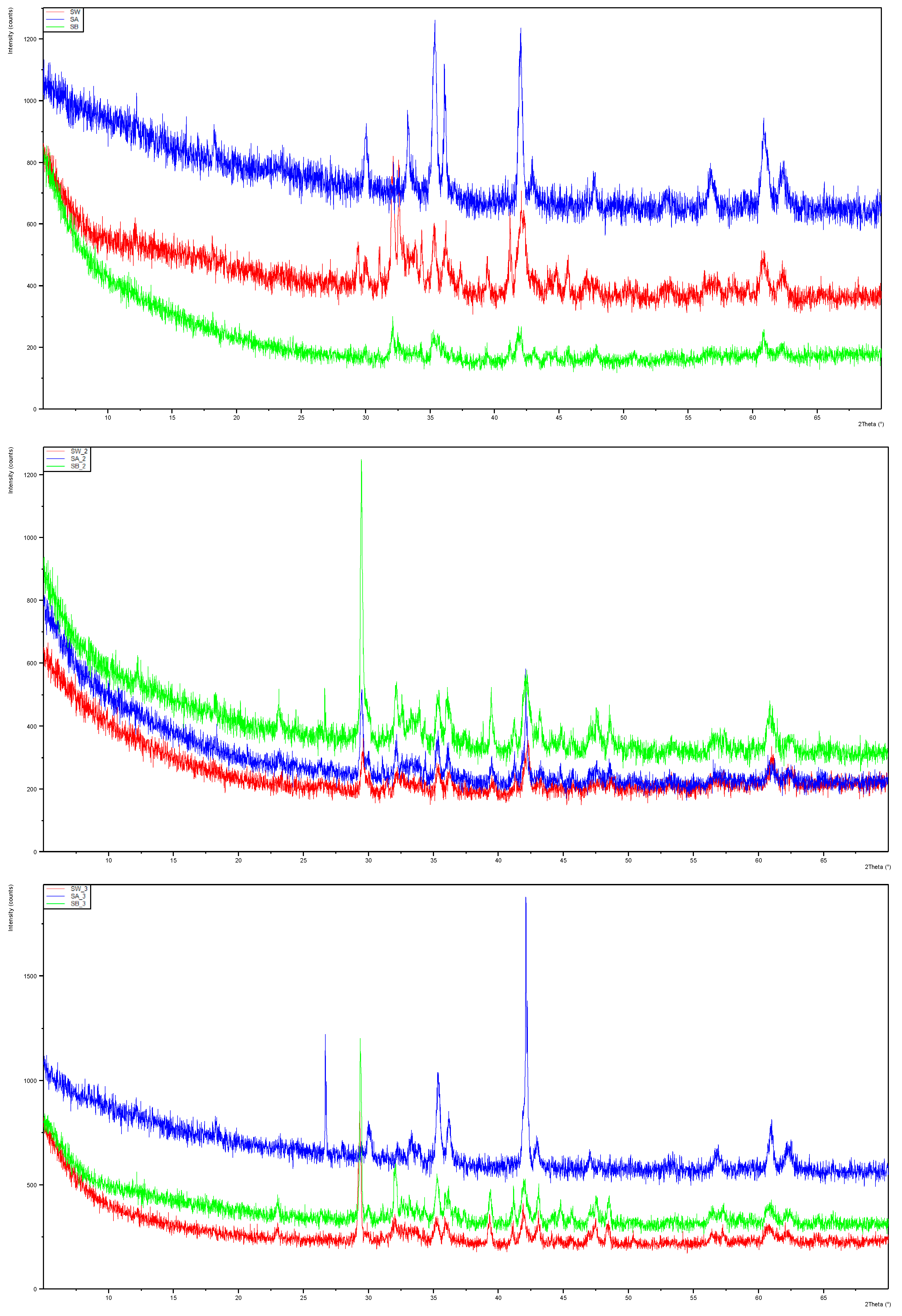
XRD analysis in
Figure 2 indicated that the mineralogy undergoes changes in the reactivity experiments, although a more intricate examination is required to precisely discern the alterations in each mineral component. The complexity of these mineral samples diminishes the accuracy of Rietveld refinement analysis due to their intricate composition, and particle size discrepancies may lead to preferential orientation and packing issues, impacting peak ratios and intensities. A suggestion for future analyses involves re-examining the samples using McCrone mill pre-treatment to enhance accuracy.
The ICP-OES data presented in
Table 1 shows the pivotal role of pH in the extraction of Ca, Fe, and Mg from the minerals. Due to its Ca-rich composition [
30,
31], slag exhibited the highest concentrations of Ca. Despite superior pH control, more Ca was extracted from slag than from natural minerals, validating the superior reactivity of the slag. Also, when the slag reacted with the acid, it extracted calcium and simultaneously helped to maintain the solution’s pH level, showcasing the buffering capacity of the slag. Consequently, the ICP-OES findings align with the pH results. The mobilization of Fe from slag was notable, while Mg extraction was comparatively lower. It’s worth mentioning that Periclase, identified in XRD analysis, might be iron oxide due to their similar crystal structures. Kimberlite, richer in Mg than Ca, displayed relatively high Ca mobilization, indicating Ca speciation as carbonates rather than silicates. As anticipated, Mg mobilization from Mg-rich olivine [
32] was the highest, followed by Fe, given olivine’s solid solution of forsterite and fayalite, and then Ca due to olivine’s low content [
33] of Ca-silicates or carbonates. Interestingly, olivine didn’t outperform kimberlite significantly due to kimberlite’s complex mineral structure [
34], which contains some highly reactive minerals despite its coarser texture.
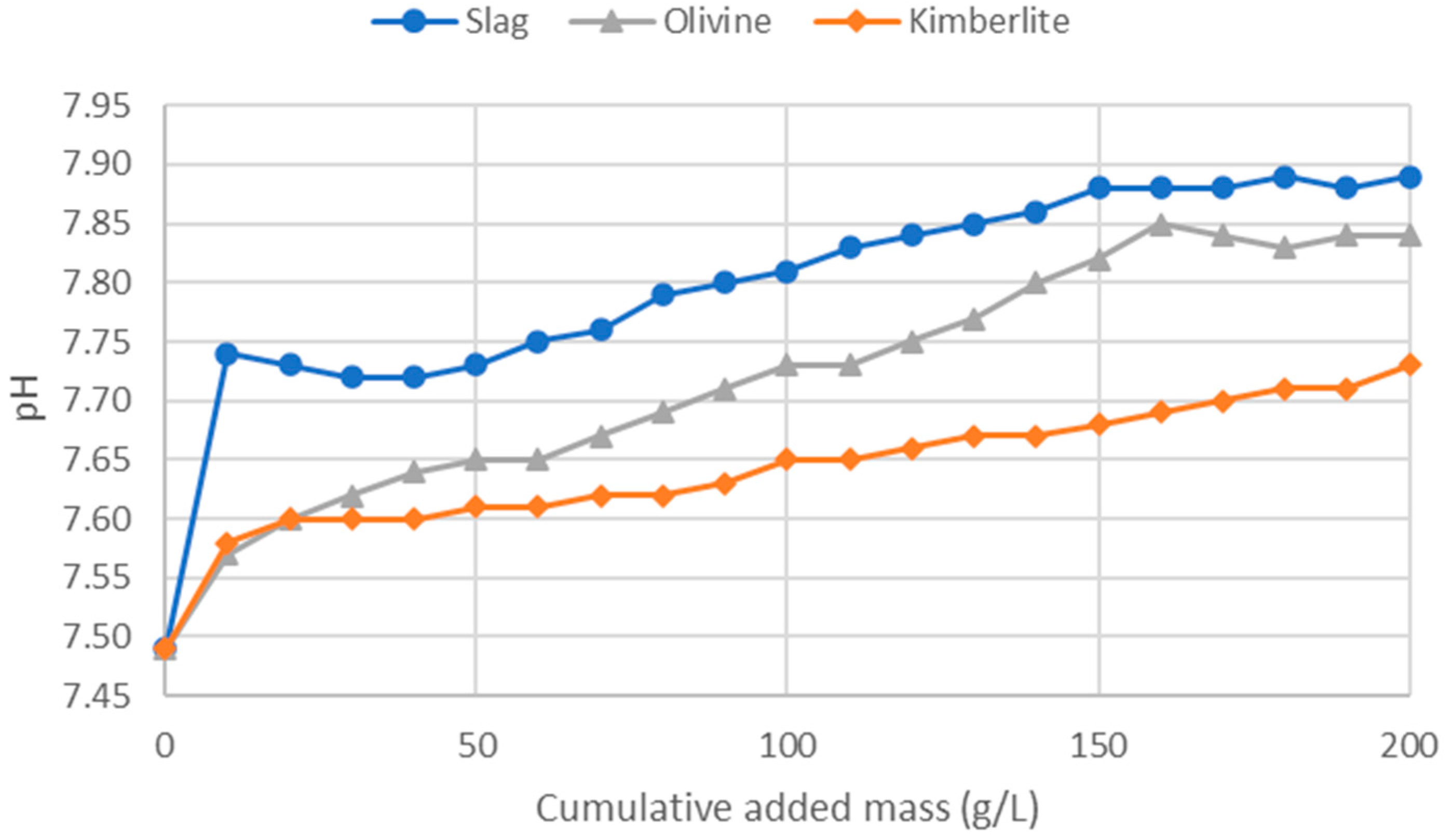
In the ammonium regeneration experiments, the results confirmed the earlier findings. From
Figure 3, it can be observed that the slag, which is the most reactive mineral, effectively raised the solution’s pH quickly and significantly. Olivine performed next in terms of pH increase, while kimberlite, despite having reactive components, couldn’t buffer the pH as effectively as olivine. The final pH of the olivine solution was only slightly lower than that of the slag, indicating olivine’s potential as a buffering agent, although slag remained superior.
With a hypothesis that finer slag particles might perform better, potentially raising the pH above 8, the level needed to convert ammonium into ammonia according to Huang et al. (2006) the next ammonium regeneration experiment was conducted [
35]. Thus, a finer slag (<2 μm) was used, and it raised the pH above 8 after a cumulative added mass of 100 g/L as illustrated in
Figure 4. After adding Ca(OH)
2 to examine the necessary calcium leaching from slag to achieve optimal reactivity the pH raised to 11 which indicates that the addition of Ca(OH)
2 successfully promoted the desired chemical reactions, emphasizing the significance of calcium leaching in enhancing the reactivity of the slag.
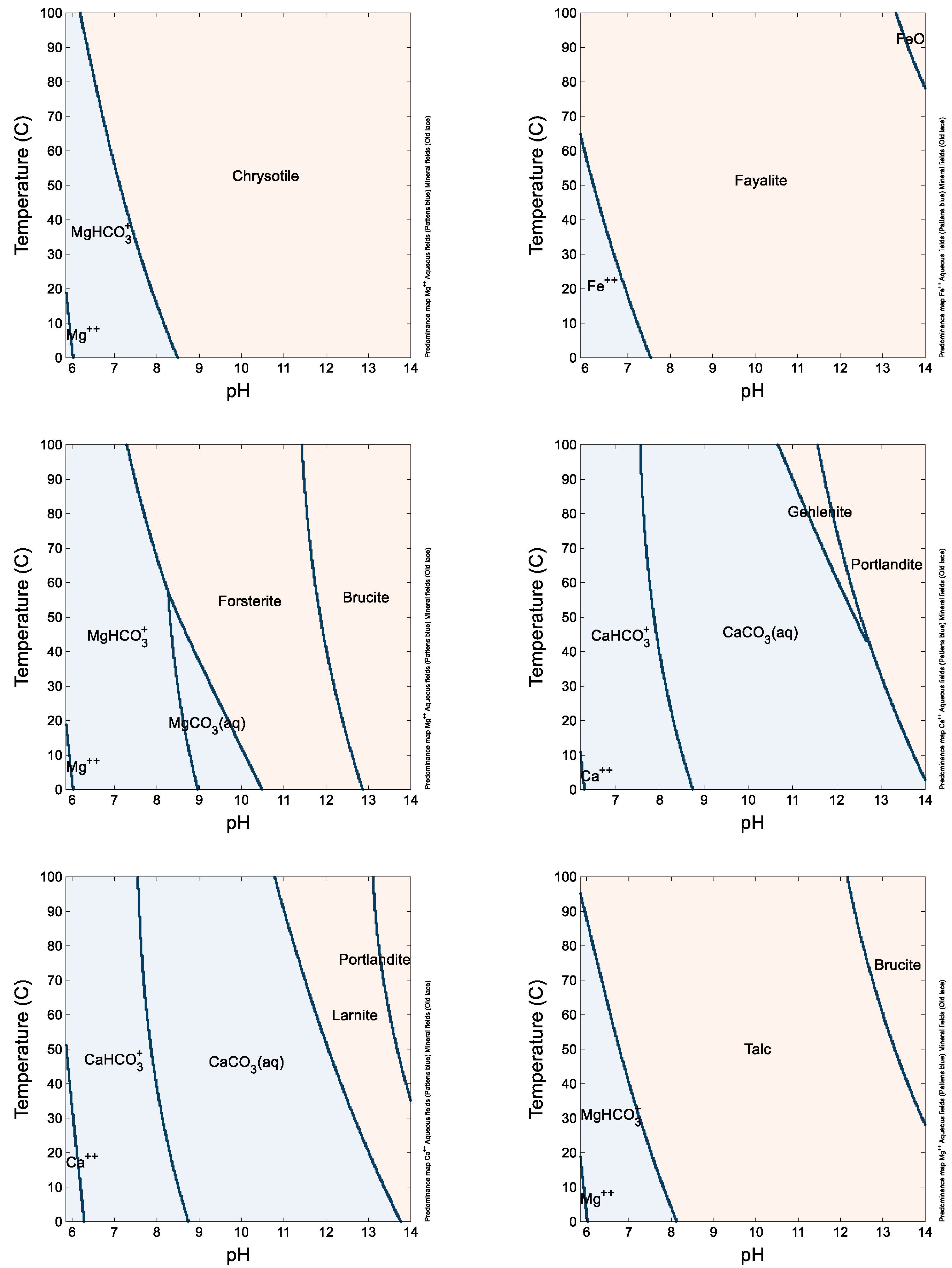
Geochemical modeling models tend to overestimate reactivity, but they still yield comparable results (listed in
Figure 5). Notably, larnite and gehlenite, minerals commonly found in slags [
36], exhibit the least stability, explaining their reactivity below a pH of approximately 11. Forsterite, reactive below a pH of about 9, follows in terms of reactivity, while fayalite, also present in olivine, demonstrates lesser reactivity [
37]. However, due to the solid solutions formed by these minerals, the reaction of solid solutions releases both Mg and Fe congruently, aligning with the ICP-OES results. Hydrated magnesium silicate minerals like chrysotile and talc exhibit lower reactivity compared to calcium silicate minerals, reacting only at a pH below approximately 8 or even 7, as per the equilibrium modeling results and kinetic modeling studies [
12]. When these minerals coexist within olivine, they are known to impede its reactivity, showing that reactivity hindrances at the particle scale require specific intensification approaches that cannot be overcome at the process scale, when considering the multi-scale nature of mineral carbonation reactions [
38]. Kimberlite, rich in these minerals, exhibits adequate reactivity in the experiments, likely due to the presence of minor reactive phases like carbonates.
4. Conclusion
In conclusion, this study presents a pioneering approach that integrates the modified Solvay process for sodium bicarbonate synthesis with steelmaking slags, addressing both CO2 sequestration and reagent regeneration in a synergistic system. The pressing need to mitigate industrial CO2 emissions, especially from the steel sector, finds a potential solution in mineral carbonation. Utilizing industrial solid waste, such as slags, in mineral carbonation processes can significantly reduce the carbon footprint associated with CO2 capture and storage.
The reactivity experiments revealed intricate insights into the behavior of EAF slag, olivine, and kimberlite in varied pH conditions. The results demonstrated that mineral reactivity is intricately linked to pH effects, with pH playing a pivotal role in the extraction of key elements like calcium, magnesium, and iron from these minerals. The superior buffering capacity of EAF slag, especially in ammonium regeneration experiments, showcases its potential as a versatile and efficient material for CO2 sequestration processes. Additionally, the finer particle size of the slag exhibited enhanced reactivity, indicating the importance of particle size in reactivity enhancement. Incorporating advanced analytical techniques such as XRD and ICP-OES further enhanced the precision of our study, enabling a comprehensive understanding of mineralogical alterations and elemental concentrations. Geochemical modeling also provided valuable insights into the behavior of minerals under varied pH conditions, aligning well with the experimental results. Notably, the presence of minerals like larnite and gehlenite in slags contributes significantly to their reactivity, suggesting potential avenues for optimizing industrial waste materials for CO2 sequestration. These findings highlight the importance of understanding mineralogical compositions and their influence on reactivity, paving the way for tailored approaches in industrial CO2 capture and storage strategies. In summary, the project findings suggest that using slag as a buffering agent in ammonium regeneration is a more effective application than its use in the sodium bicarbonate synthesis process. Additionally, since the slag only partially reacts, the remaining solid could be utilized to accelerate mineral carbonation processes, aiding in the sequestration of more CO2. To advance this research, future studies should delve into these processes in detail and assess the overall energy requirements and carbon footprint associated with them.
Lastly, by minimizing waste, optimizing reagent usage, and enhancing CO2 sequestration efficiency, this integrated approach not only contributes to mitigating climate change but also aligns with the principles of circular economy and sustainable industrial practices. In essence, these findings represent a significant step towards more sustainable, efficient, and economically viable solutions for reducing industrial carbon emissions, ultimately contributing to global efforts in combatting climate change.
Author Contributions
Conceptualization, R.M.S.; methodology, S.M.A., A.A., and R.M.S.; investigation, S.M.A., and A.A.; writing—original draft preparation, S.M.A.; writing—review and editing, R.M.S.; supervision, R.M.S.; project administration, R.M.S.; funding acquisition, R.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Program of Energy Research and Development of Canada. The authors acknowledge funding received from the Natural Science and Engineering Research Council (Discovery Grant).
Data Availability Statement
Data available upon request.
Acknowledgments
The authors are grateful for the laboratory assistance provided by doctoral students Reza Khalidy and Hiral Jariwala, and for the slag sourcing and funding securement facilitated by Dr. Jinsheng Wang of CanmetENERGY (Natural Resources Canada).
Conflicts of Interest
The authors declare no conflict of interest.
References
- T. M. Letcher, “1 - Global warming—a complex situation,” in Climate Change (Third Edition), Elsevier, 2021, pp. 3-17. [CrossRef]
- E. Miao, Y. Du, H. Wang, Z. Xiong, Y. Zhao and J. Zhang, “Experimental study and kinetics on CO2 mineral sequestration by the direct aqueous carbonation of pepper stalk ash,” Fuel, vol. 303, p. 121230, 2021. [CrossRef]
- W. J. Huijgen, G.-J. Witkamp and R. N. Comans, “Mineral CO2 Sequestration by Steel Slag Carbonation,” Environ. Sci. Technol., vol. 39, no. 24, pp. 9676-9682, 2005. [CrossRef]
- B. Rammer, R. Millner and C. Boehm, “Comparing the CO2 Emissions of Different Steelmaking Routes,” BHM Berg- und Hüttenmännische Monatshefte, vol. 162, no. 1, pp. 7-13, 2017. [CrossRef]
- World Steel Association, “World Steel in Figures 2021,” [Online]. Available: https://worldsteel.org/steel-topics/statistics/World-Steel-in-Figures/. [Accessed 10 October 2023].
- C. Mapelli, G. Dall’Osto, D. Mombelli, S. Barella and A. Gruttadauria, “Future Scenarios for Reducing Emissions and Consumption in the Italian Steelmaking Industry,” Steel Research International, vol. 93, no. 5, p. 2100631, 2022. [CrossRef]
- International Energy Agency, “Greenhouse gas emissions from major industrial sources III-Iron and Steel Production Report PH3/30,” [Online]. Available: https://ieaghg.org/docs/General_Docs/Reports/PH3-30%20iron-steel.pdf. [Accessed 10 October 2023].
- International Energy Agency, “Iron and Steel Technology Roadmap Towards more sustainable steelmaking Part of the Energy Technology Perspectives series,” [Online]. Available: https://www.iea.org/reports/iron-and-steel-technology-roadmap. [Accessed 23 October 2023].
- K. S. Lackner, “A Guide to CO2 Sequestration,” Science, vol. 300, no. 5626, pp. 1677-1678, 2003. [CrossRef]
- Y. Chai, S. Chalouati, H. Fantucci and R. Santos, “Accelerated weathering and carbonation (mild to intensified) of natural Canadian silicates (kimberlite and wollastonite) for CO2 sequestration,” Crystals, vol. 11, p. 1584, 2021. [CrossRef]
- S. Eloneva, S. Teir, H. Revitzer, J. Salminen, A. Said, C.-J. Fogelholm and R. Zevenhoven, “Reduction of CO2 Emissions from Steel Plants by Using Steelmaking Slags for Production of Marketable Calcium Carbonate,” Steel Research International, vol. 80, no. 6, pp. 415-421, 2010. [CrossRef]
- F. Haque, R. Khalidy, Y. Chiang and R. Santos, “Constraining the capacity of global croplands to CO2 drawdown via mineral weathering,” ACS Earth and Space Chemistry, vol. 7, no. 7, pp. 1294-1305, 2023. [CrossRef]
- G. Gadikota, J. Matter, P. Kelemen, P. Brady and A.-H. Park, “Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage,” Fuel, vol. 277, p. 117900, 2020. [CrossRef]
- C. Woodall, X. Lu, G. Dipple and J. Wilcox, “Carbon Mineralization with North American PGM Mine Tailings—Characterization and Reactivity Analysis.,” Minerals, vol. 11, p. 844, 2021. [CrossRef]
- J. A. Meima, R. van der Weijden, T. T. Eighmy and R. N. J. Comans, “Carbonation processes in municipal solid waste incinerator bottom ash and their effect on the leaching of copper and molybdenum,” Applied Geochemistry, vol. 17, no. 12, pp. 1503-1513, 2002. [CrossRef]
- S. Chalouati, A. Yoosefdoost, Y. Chiang and R. Santos, “Intensified mineral carbonation of natural Canadian silicates using simultaneous ball milling,” International Journal of Coal Geology, vol. 277, p. 104332. [CrossRef]
- F. Al Hameli, H. Belhaj and M. Al Dhuhoori, “CO2 Sequestration Overview in Geological Formations: Trapping Mechanisms Matrix Assessment,” Energies, vol. 15, p. 7805, 2022. [CrossRef]
- Y. Sun, R. L. Payton, S. Hier-Majumder and A. Kingdon, “Geological Carbon Sequestration by Reactive Infiltration Instability,” Front. Earth Sci., vol. 8, p. 533588, 2020. [CrossRef]
- N. Zhang, Y. Chai, R. Santos and L. Šiller, “Advances in process development of aqueous CO2 mineralisation towards scalability,” Journal of Environmental Chemical Engineering, vol. 8, no. 6, p. 104453, 2020. [CrossRef]
- D. Alexander and D. Boodlal, “Evaluating the effects of CO2 Injection in Faulted Saline Aquifers,” Energy Procedia, vol. 63, pp. 3012-3021, 2014. [CrossRef]
- H. Bai and A. C. Yeh, “Removal of CO2 Greenhouse Gas by Ammonia Scrubbing,” Ind. Eng. Chem. Res., vol. 36, no. 6, pp. 2490-2493, 1997. [CrossRef]
- A.-M. Cormos, C. Dinca, L. Petrescu, D. A. Chisalita, S. Szima and C.-C. Cormos, “Carbon capture and utilisation technologies applied to energy conversion systems and other energy-intensive industrial applications,” Fuel, vol. 211, pp. 883-890, 2018. [CrossRef]
- P. Carson and C. Mumford, Hazardous Chemicals Handbook, Library of Congress Cataloguing in Publication Data, 2002. Available online: http://ccc.chem.pitt.edu/wipf/Web/HCH (accessed on 10 October 2023).
- T. Setayeshmanesh, M. M. Parivazh, M. Abbasi, S. Osfouri, M. J. Dianat and M. Akrami, “Reducing the Environmental Impacts of Desalination Reject Brine Using Modified Solvay Process Based on Calcium Oxide,” Sustainability, vol. 14, p. 2298, 2022. [CrossRef]
- Q. Wang and Z. Li, “A modified Solvay process with low-temperature calcination of NaHCO3 using monoethanolamine: Solubility determination and thermodynamic modeling,” AIChE Journal, vol. 65, no. 10, p. e16701, 2019. [CrossRef]
- A. A.-H. Mourad, A. F. Mohammad, A. H. Al-Marzouqi, M. H. El-Naas, M. H. Al-Marzouqi and M. Altarawneh, “CO2 capture and ions removal through reaction with potassium hydroxide in desalination reject brine: Statistical optimization,” Chemical Engineering and Processing - Process Intensification, vol. 170, p. 108722, 2022. [CrossRef]
- A. Ali, C. E. Mendes, L. G. de Melo, J. Wang and R. M. Santos, “Production of Sodium Bicarbonate with Saline Brine and CO2 Co-Utilization: Comparing Modified Solvay Approaches,” Crystals, vol. 13, p. 470, 2023. [CrossRef]
- M. Owais, M. Järvinen, P. Taskinen and A. Said, “Experimental study on the extraction of calcium, magnesium, vanadium and silicon from steelmaking slags for improved mineral carbonation of CO2,” Journal of CO2 Utilization, vol. 31, pp. 1-7, 2019. [CrossRef]
- D. Hermosilla, R. Ordóñez, L. Blanco, E. de la Fuente and Á. Blanco, “pH and Particle Structure Effects on Silica Removal by Coagulation,” Chemical Engineering & Technology, vol. 35, no. 9, pp. 1632-1640, 2012. [CrossRef]
- N. M. Piatak, M. B. Parsons and R. R. Seal, “Characteristics and environmental aspects of slag: A review,” Applied Geochemistry, vol. 57, pp. 236-266, 2015. [CrossRef]
- A. Said, H.-P. Mattila, M. Järvinen and R. Zevenhoven, “Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2,” Applied Energy, vol. 112, pp. 765-771, 2013. [CrossRef]
- J. M. Sunshine and C. M. Pieters, “Determining the composition of olivine from reflectance spectroscopy,” Journal of Geophysical Research: Planets, vol. 103, no. E6, p. 1367513688, 1998. [CrossRef]
- V. S. Kamenetsky, M. Elburg, R. Arculus and R. Thomas, “Magmatic origin of low-Ca olivine in subduction-related magmas: Co-existence of contrasting magmas,” Chemical Geology, vol. 233, no. 3-4, pp. 346-357, 2006. [CrossRef]
- L. Wilson and J. W. Head III, “An integrated model of kimberlite ascent and eruption,” Nature, vol. 447, pp. 53-57, 2007. [CrossRef]
- J.-C. Huang and C. Shang, “Air Stripping. In: Wang, L.K., Hung, YT., Shammas, N.K. (eds) Advanced Physicochemical Treatment Processes,” in Handbook of Environmental Engineering, vol. 4, Humana Press, 2006. [CrossRef]
- D. Mombelli, C. Mapelli, S. Barella, C. D. Cecca, G. L. Saout and E. Garcia-Diaz, “The effect of microstructure on the leaching behaviour of electric arc furnace (EAF) carbon steel slag,” Process Safety and Environmental Protection, vol. 102, pp. 810-821, 2016. [CrossRef]
- H. Béarat, M. J. McKelvy, A. V. G. Chizmeshya, D. Gormley, R. Nunez, R. W. Carpenter, K. Squires and G. H. Wolf, “Carbon Sequestration via Aqueous Olivine Mineral Carbonation: Role of Passivating Layer Formation,” Environ. Sci. Technol., vol. 40, no. 15, p. 4802–4808, 2006. [CrossRef]
- R. Santos, N. Zhang and R. Bakhshoodeh, “Multiscale process intensification of waste valorization reactions,” Accounts of Chemical Research, vol. 56, no. 19, pp. 2606-2619, 2023. [CrossRef]
Figure 1.
Change in pH (left) and change of mass of minerals after filtration (right) in different concentrations of acid (0.001, 0.01, 0.05, 0.2, and 1 mol/L), unaltered aqueous solution (UPW/ ABB/ PBB) and base (0.001, 0.01, 0.05, 0.2, and 1 mol/L) for EAF slag, olivine, and kimberlite in UPW, ABB and PBB.
Figure 1.
Change in pH (left) and change of mass of minerals after filtration (right) in different concentrations of acid (0.001, 0.01, 0.05, 0.2, and 1 mol/L), unaltered aqueous solution (UPW/ ABB/ PBB) and base (0.001, 0.01, 0.05, 0.2, and 1 mol/L) for EAF slag, olivine, and kimberlite in UPW, ABB and PBB.
Figure 2.
Stacked diffractograms from XRD analysis of the EAF slag reacted in UPW (SW), UPW with 1 mol/L HCl (SA), and UPW with 1 mol/L NaOH (SB). Samples with suffix _2 reacted in ABB and with suffix _3 in PBB.
Figure 2.
Stacked diffractograms from XRD analysis of the EAF slag reacted in UPW (SW), UPW with 1 mol/L HCl (SA), and UPW with 1 mol/L NaOH (SB). Samples with suffix _2 reacted in ABB and with suffix _3 in PBB.
Figure 3.
Ammonium regeneration experiment using slag, olivine, and kimberlite.
Figure 3.
Ammonium regeneration experiment using slag, olivine, and kimberlite.
Figure 4.
Ammonium regeneration experiment using finer slag before and after adding Ca(OH)2.
Figure 4.
Ammonium regeneration experiment using finer slag before and after adding Ca(OH)2.
Figure 5.
Phase diagrams of chrysotile (as Mg), fayalite (as Fe), forsterite (as Mg), gehlenite (as Ca), larnite (as Ca), and talc (as Mg) obtained from Geochemist’s Workbench. Tan-colored regions indicate the dominant speciation of the element is a mineral, and blue-colored regions indicate the soluble species is dominant. Lines indicate transition regions.
Figure 5.
Phase diagrams of chrysotile (as Mg), fayalite (as Fe), forsterite (as Mg), gehlenite (as Ca), larnite (as Ca), and talc (as Mg) obtained from Geochemist’s Workbench. Tan-colored regions indicate the dominant speciation of the element is a mineral, and blue-colored regions indicate the soluble species is dominant. Lines indicate transition regions.
Table 1.
The composition of the solutions used in the mineral reactivity experiments, and the elemental concentrations of Ca, Fe, and Mg in the filtrate of the filtered solids, as determined by ICP-OES (samples with low concentration below the detection limit are shown as DT).
Table 1.
The composition of the solutions used in the mineral reactivity experiments, and the elemental concentrations of Ca, Fe, and Mg in the filtrate of the filtered solids, as determined by ICP-OES (samples with low concentration below the detection limit are shown as DT).
| |
Conc (M) |
Ca |
Fe |
Mg |
Ca |
Fe |
Mg |
Ca |
Fe |
Mg |
| UPW |
ABB |
PBB |
| Slag |
HCl |
1.000 |
16081.20 |
5546.17 |
2097.62 |
262.53 |
DT |
287.28 |
5415.03 |
1002.84 |
483.66 |
| 0.200 |
3199.02 |
180.48 |
198.12 |
38.19 |
37.52 |
370.29 |
DT |
2.47 |
DT |
| 0.050 |
789.45 |
DT |
8.18 |
41.03 |
59.18 |
422.27 |
2.14 |
0.43 |
5.67 |
| 0.010 |
241.52 |
DT |
DT |
66.45 |
73.86 |
385.30 |
14.36 |
459.43 |
22.60 |
| 0.001 |
99.11 |
DT |
DT |
57.68 |
67.09 |
341.63 |
9.15 |
0.71 |
6.12 |
| Brine |
0.000 |
138.90 |
0.40 |
0.90 |
45.20 |
38.40 |
189.70 |
0.60 |
1.40 |
1.00 |
| NaOH |
0.001 |
82.35 |
0.09 |
0.04 |
36.66 |
40.34 |
228.28 |
3.09 |
0.89 |
1.05 |
| 0.010 |
73.42 |
DT |
DT |
24.13 |
34.63 |
169.24 |
1.90 |
0.00 |
0.26 |
| 0.050 |
39.28 |
DT |
DT |
15.73 |
33.75 |
183.41 |
2.88 |
0.35 |
DT |
| 0.200 |
8.29 |
0.01 |
DT |
7.89 |
27.74 |
76.79 |
3.23 |
0.35 |
DT |
| 1.000 |
23.88 |
DT |
DT |
2.05 |
1.15 |
72.33 |
8.77 |
0.91 |
DT |
| Kimberlite |
HCl |
1.000 |
4862.14 |
963.88 |
4713.14 |
22.20 |
DT |
245.99 |
2783.84 |
248.36 |
1769.26 |
| 0.200 |
3118.89 |
4.86 |
977.25 |
4.30 |
1.07 |
227.30 |
7.07 |
7.74 |
58.11 |
| 0.050 |
732.13 |
DT |
164.36 |
5.94 |
DT |
8.18 |
5.79 |
0.30 |
8.37 |
| 0.010 |
166.71 |
DT |
34.93 |
0.05 |
2.29 |
168.68 |
0.81 |
DT |
3.99 |
| 0.001 |
21.77 |
DT |
3.86 |
1.10 |
5.09 |
136.78 |
1.88 |
DT |
5.76 |
| Brine |
0.000 |
12.20 |
0.20 |
3.60 |
6.50 |
1.20 |
127.00 |
0.70 |
0.10 |
0.50 |
| NaOH |
0.001 |
41.03 |
0.18 |
7.01 |
5.98 |
1.37 |
120.70 |
3.06 |
DT |
DT |
| 0.010 |
4.60 |
DT |
0.59 |
6.50 |
2.34 |
121.58 |
2.32 |
DT |
DT |
| 0.050 |
5.20 |
DT |
0.05 |
6.24 |
2.63 |
115.23 |
2.12 |
DT |
DT |
| 0.200 |
1.64 |
DT |
DT |
4.47 |
2.26 |
105.04 |
2.46 |
DT |
DT |
| 1.000 |
37.36 |
DT |
DT |
4.05 |
1.24 |
88.88 |
5.60 |
0.08 |
DT |
| Olivine |
HCl |
1.000 |
295.62 |
2791.75 |
7377.88 |
13.51 |
DT |
254.28 |
151.75 |
832.65 |
3163.50 |
| 0.200 |
159.37 |
390.42 |
1872.18 |
0.50 |
0.99 |
238.24 |
7.88 |
18.70 |
199.77 |
| 0.050 |
662.64 |
181.81 |
540.39 |
0.69 |
1.63 |
236.15 |
1.55 |
0.17 |
38.73 |
| 0.010 |
47.32 |
DT |
95.48 |
DT |
1.26 |
226.25 |
DT |
DT |
DT |
| 0.001 |
DT |
DT |
14.99 |
DT |
1.46 |
229.66 |
2.37 |
0.05 |
DT |
| Brine |
0.000 |
3.80 |
0.1 |
10.10 |
5.20 |
1.40 |
209.00 |
0.90 |
DT |
1.60 |
| NaOH |
0.001 |
8.28 |
DT |
1.43 |
5.52 |
1.33 |
205.78 |
3.06 |
DT |
0.00 |
| 0.010 |
14.75 |
1.70 |
22.08 |
5.57 |
1.34 |
201.89 |
2.69 |
DT |
DT |
| 0.050 |
3.67 |
DT |
0.38 |
4.57 |
1.86 |
187.82 |
2.30 |
DT |
DT |
| 0.200 |
0.13 |
DT |
DT |
4.13 |
2.25 |
103.21 |
2.72 |
DT |
DT |
| 1.000 |
4.30 |
DT |
DT |
4.10 |
1.49 |
109.56 |
6.11 |
0.10 |
DT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).