Submitted:
31 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Non-covalent complexes of Apiol-PPh3 and analogues with cyclodextrins and heparin
2.3. Determination of the dissociation constants of complexes of Apiol-PPh3 and analogues with cyclodextrins and heparin
2.4. Cell cultivation and determination of cytotoxic activity
2.5. Phenotypic Sea Urchin Embryo Assay
2.6. Study of the safety of formulations (hemolytic activity and thrombogenicity)
2.7. Statistical Analysis
3. Results and Discussion
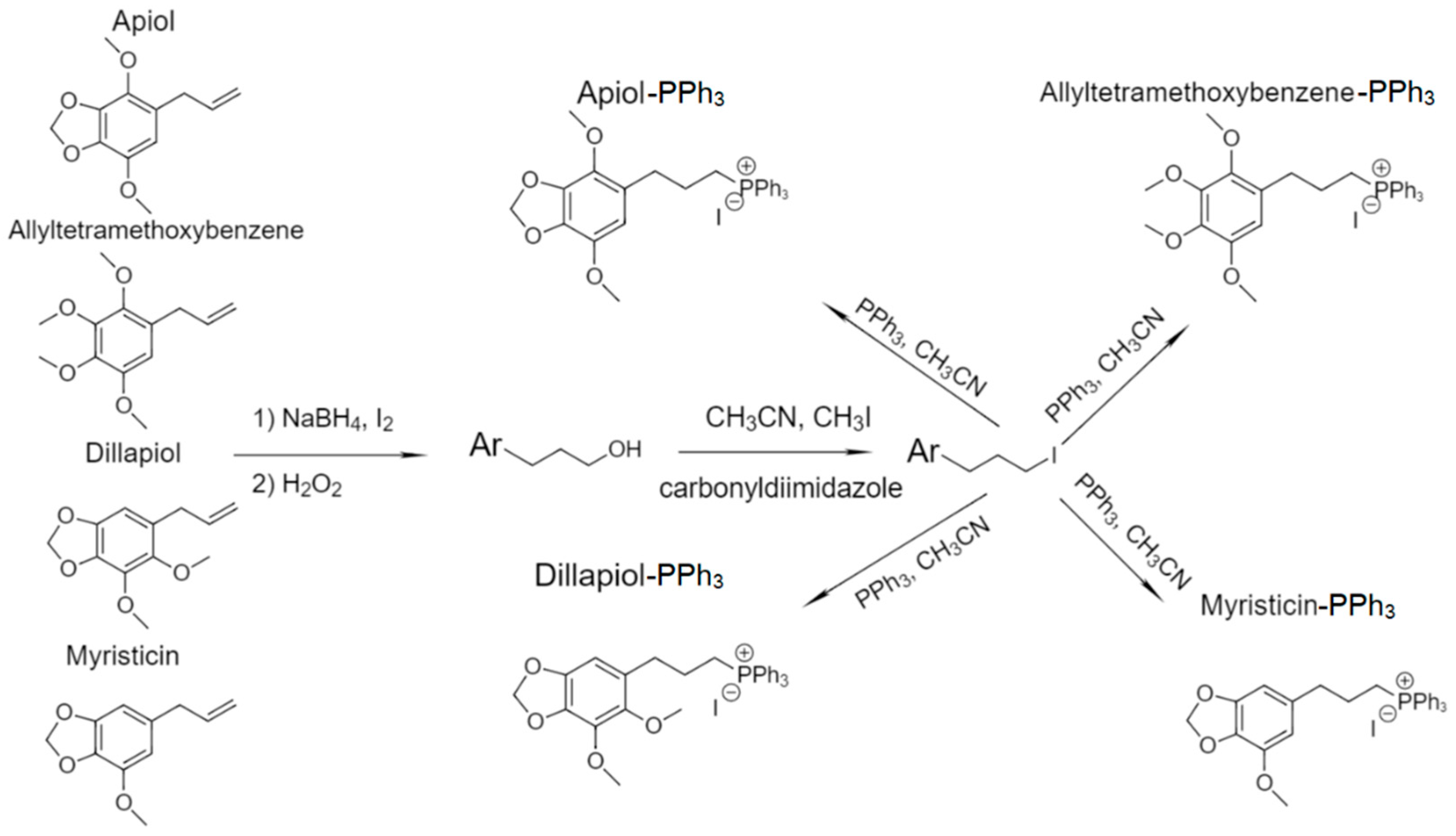
3.1. Article Design
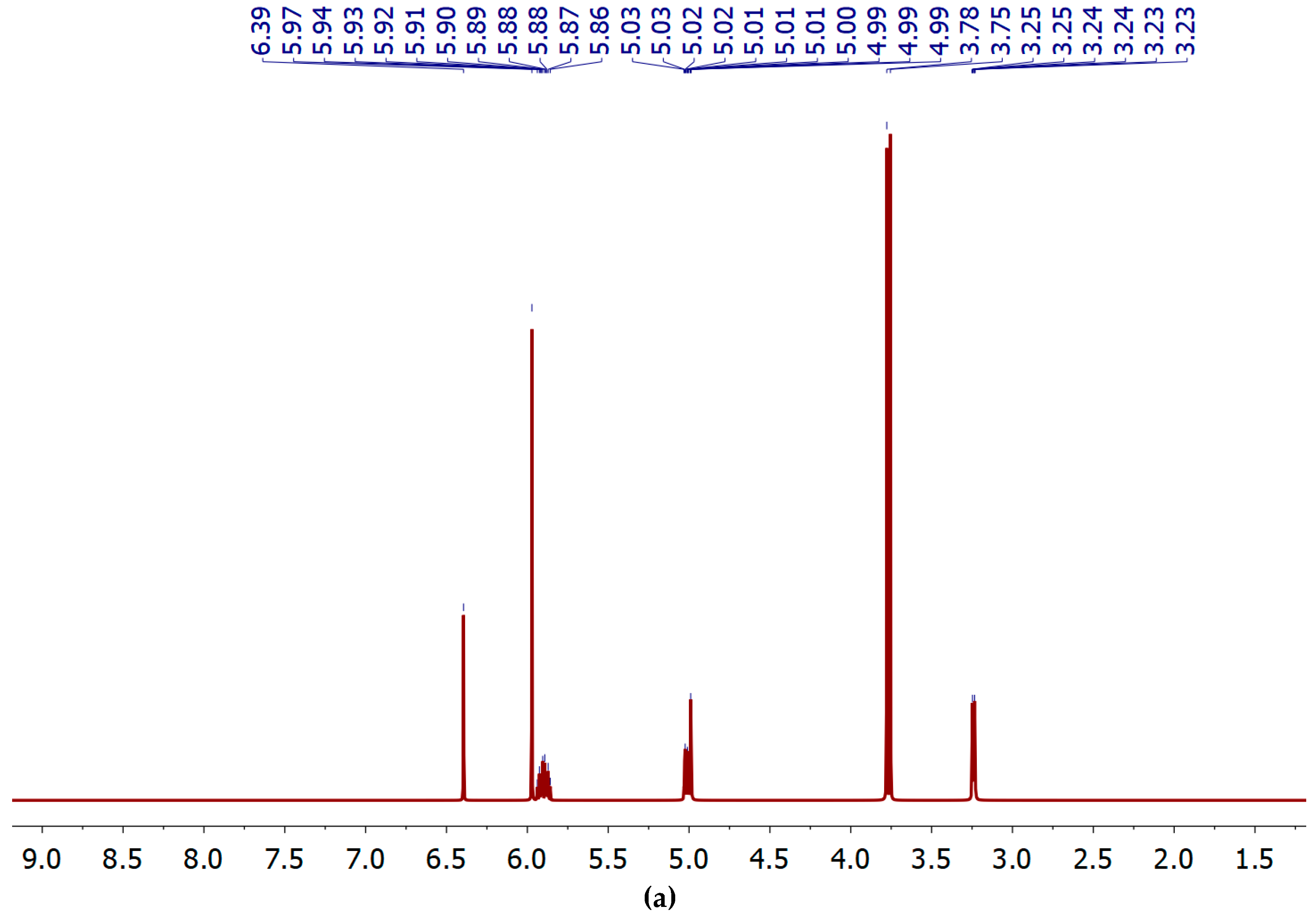
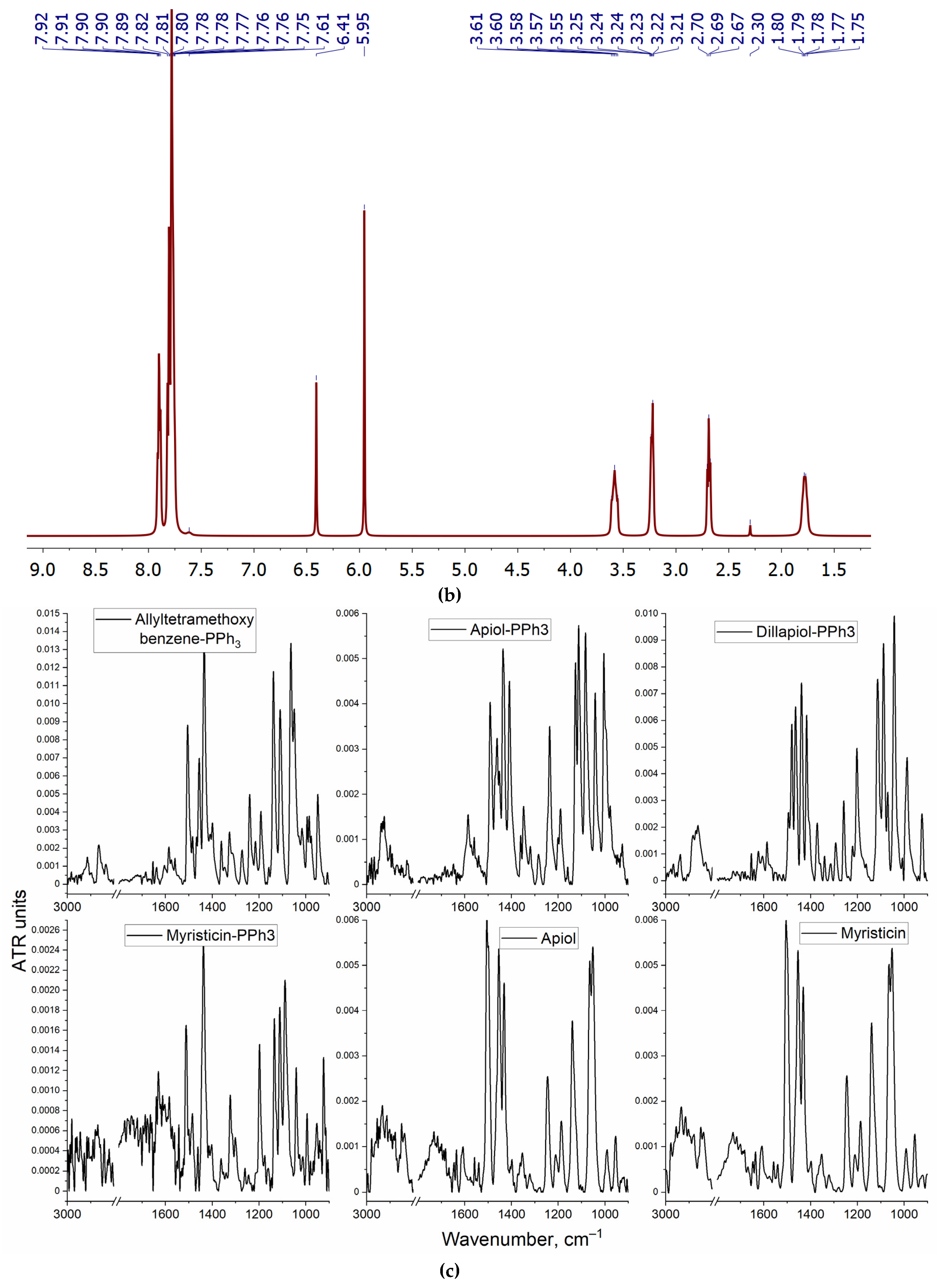
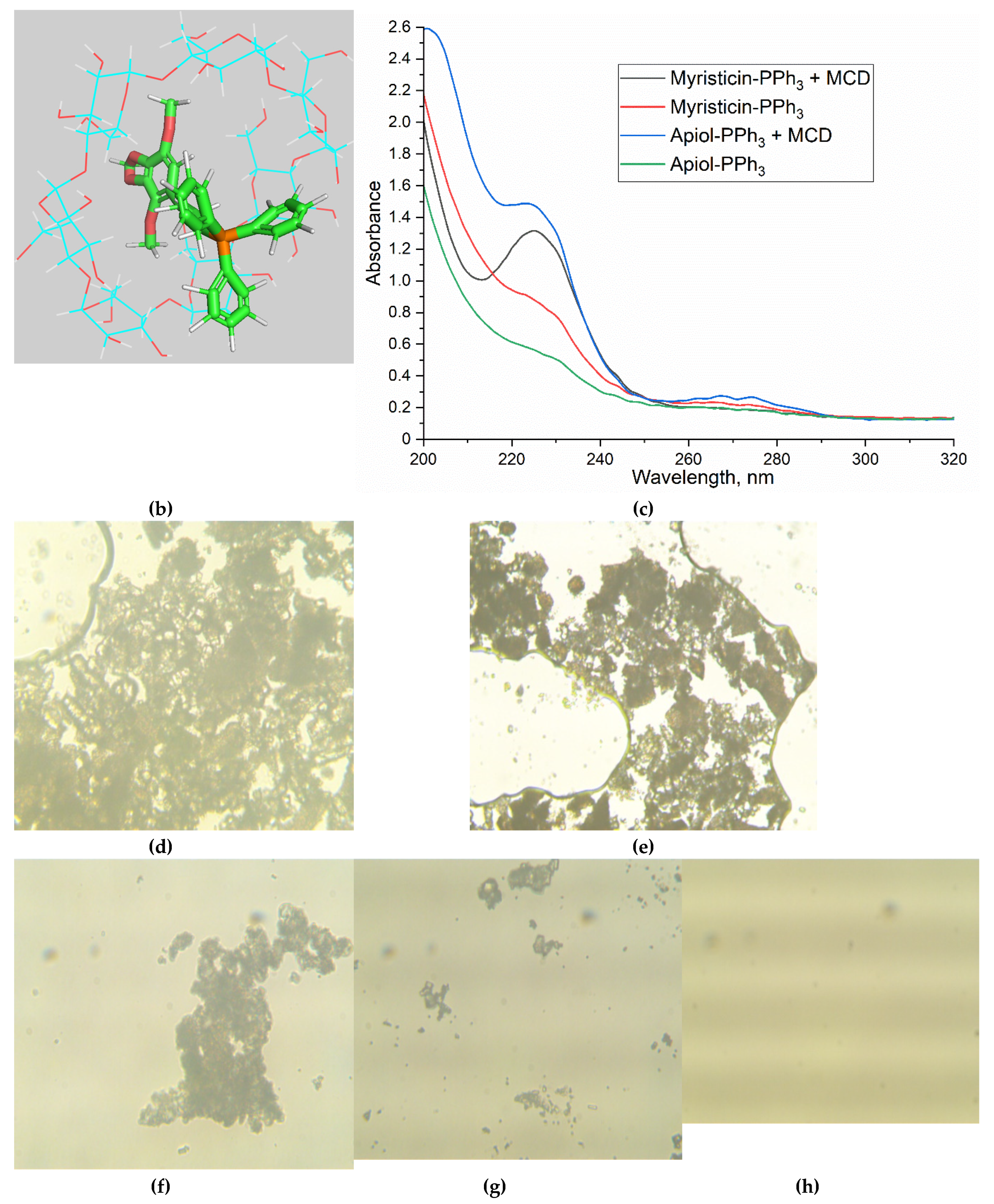
3.2. The spectral characteristics of PPh3-derivatives of allylbenzenes
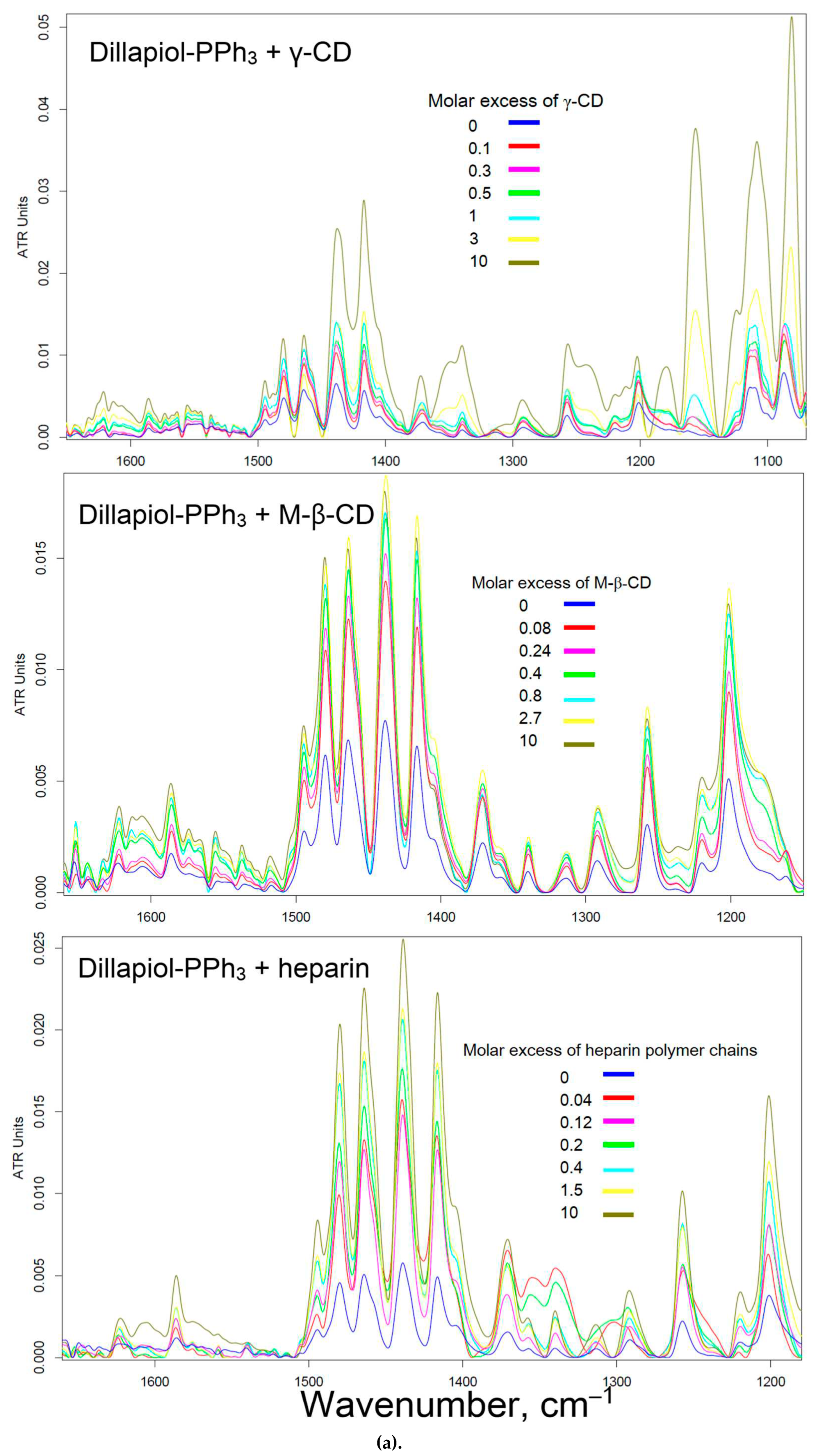
3.3. Solubility of PPh3-modified allylbenzenes adjuvants and complex formation with cyclodextrins and heparin
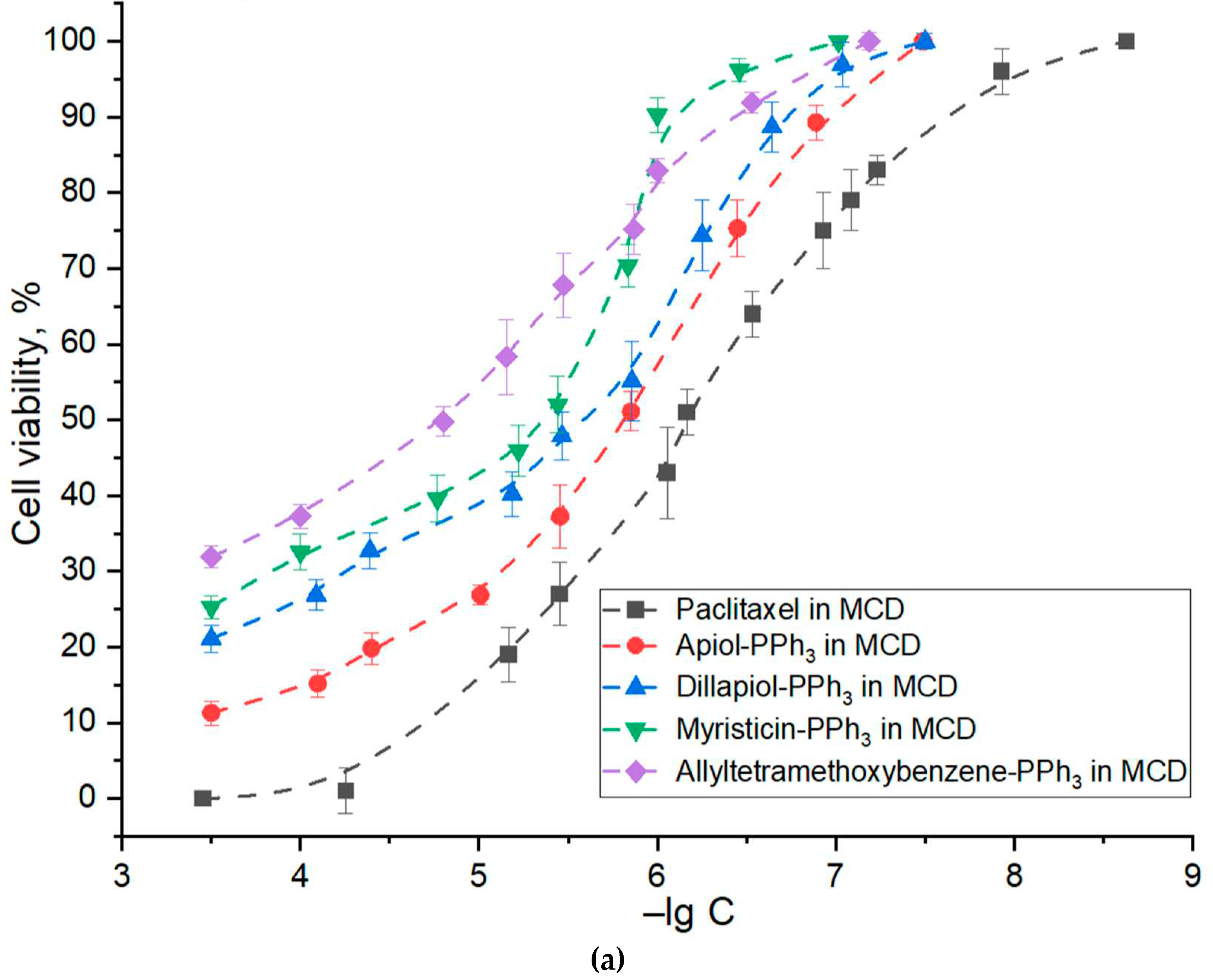
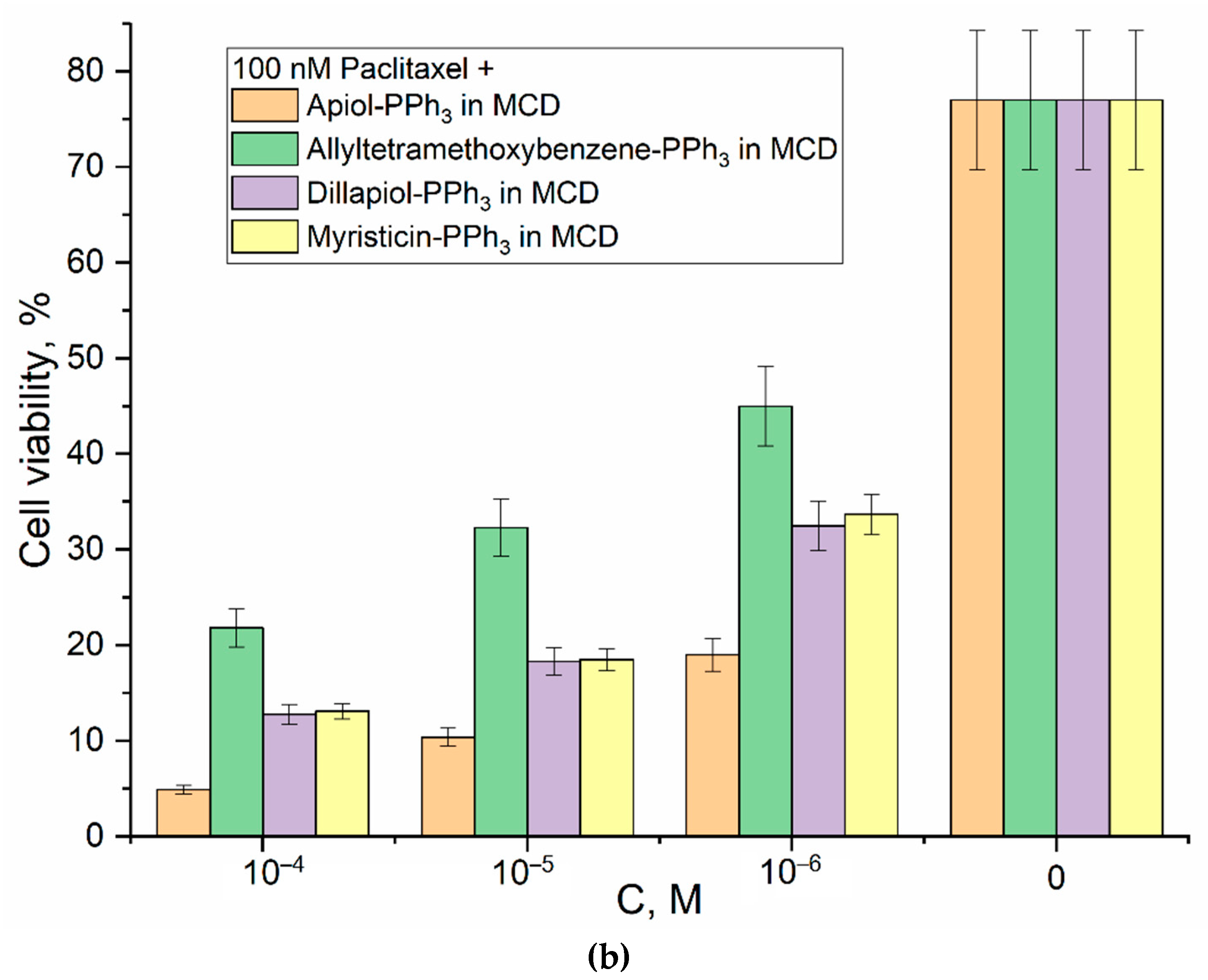
3.4. Anticancer activity of PPh3-derivatives and formulations
3.5. Selectivity of Action and Safety of Cytotoxic Formulations Developed
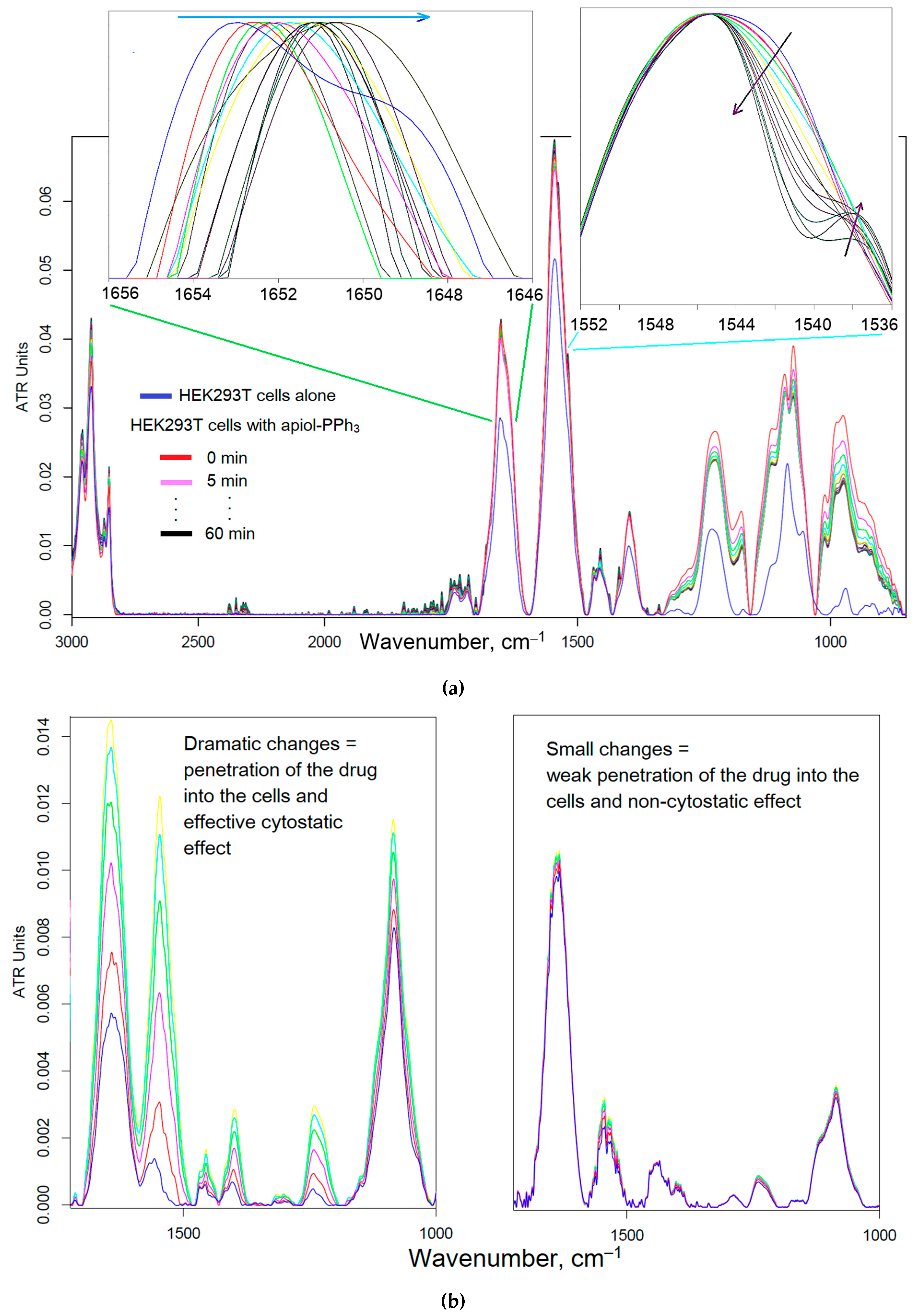
3.5.1. HEK293T as normal cell model
3.5.2. Hemolytic Activity, Thrombogenicity and Phenotypic Sea Urchin Embryo Assay
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| γ-CD | γ-cyclodextrin |
| M-β-CD or MCD | methyl β-cyclodextrin |
References
- Malebari, A.M.; Wang, S.; Greene, T.F.; O’boyle, N.M.; Fayne, D.; Khan, M.F.; Nathwani, S.M.; Twamley, B.; McCabe, T.; Zisterer, D.M.; и др. Synthesis and antiproliferative evaluation of 3-chloroazetidin-2-ones with antimitotic activity: Heterocyclic bridged analogues of combretastatin a-4. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Muniz, D.F.; dos Santos Barbosa, C.R.; de Menezes, I.R.A.; de Sousa, E.O.; Pereira, R.L.S.; Júnior, J.T.C.; Pereira, P.S.; de Matos, Y.M.L.S.; da Costa, R.H.S.; de Morais Oliveira-Tintino, C.D.; и др. In vitro and in silico inhibitory effects of synthetic and natural eugenol derivatives against the NorA efflux pump in Staphylococcus aureus. Food Chem. 2021, 337, 127776. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, N.S.; Silveira, Z.D.S.; Patrícia, P.; Cordeiro, M.; Douglas, H.; Coutinho, M.; Pinto, J.; Júnior, S.; José, L.; Júnior, Q.; и др. Inhibition of Staphylococcus aureus Efflux Pump by O-Eugenol and Its Toxicity in Drosophila melanogaster Animal Model. 2022, 2022. [Google Scholar]
- Demchuk, D. V.; Samet, A. V.; Chernysheva, N.B.; Ushkarov, V.I.; Stashina, G.A.; Konyushkin, L.D.; Raihstat, M.M.; Firgang, S.I.; Philchenkov, A.A.; Zavelevich, M.P.; и др. Synthesis and antiproliferative activity of conformationally restricted 1,2,3-triazole analogues of combretastatins in the sea urchin embryo model and against human cancer cell lines. Bioorganic Med. Chem. 2014, 22, 738–755. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alkahtani, H.M.; AlQuadeib, B.T. Paclitaxel. Profiles Drug Subst. Excipients Relat. Methodol. 2019, 44, 205–238. [Google Scholar] [CrossRef]
- Iyer, A.K. V Ionophores : Potential Use as Anticancer Drugs and Chemosensitizers. 2018, 1–21. [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: the most critical pathogens. Pathogens 2021, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Huang, W.C.; Hsiao, M.H.; Wang, Y.J.; Nydén, M.; Chiou, S.H.; Liu, D.M. Biomedical applications and colloidal properties of amphiphilically modified chitosan hybrids. Prog. Polym. Sci. 2013, 38, 1307–1328. [Google Scholar] [CrossRef]
- Seneme, E.F.; dos Santos, D.C.; de Lima, C.A.; Zelioli, Í.A.M.; Sciani, J.M.; Longato, G.B. Effects of Myristicin in Association with Chemotherapies on the Reversal of the Multidrug Resistance (MDR) Mechanism in Cancer. Pharmaceuticals 2022, 15. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.B.; Lin, S.Y.; Arya, H.; Fu, I.H.; Yeh, T.K.; Charles, M.R.C.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Overcoming vincristine resistance in cancer: Computational design and discovery of piperine-inspired P-glycoprotein inhibitors. Chem. Biol. Drug Des. 2021, 97, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Wright, C.D.; Ishizuka, H. GS-X pump is functionally overexpressed in cis- diamminedichloroplatinum(II)-resistant human leukemia HL-60 cells and down- regulated by cell differentiation. J. Biol. Chem. 1994, 269, 29085–29093. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Taranejoo, S.; Monemian, S.A.; Moghaddam, Z.S.; Daliri, K.; Derakhshankhah, H.; Derakhshani, Z. Classification of stimuli-responsive polymers as anticancer drug delivery systems. Drug Deliv. 2015, 22, 145–155. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Petrov, R.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Belogurova, N.G.; Kudryashova, E. V. Mannosylated Polymeric Ligands for Targeted Delivery of Antibacterials and Their Adjuvants to Macrophages for the Enhancement of the Drug Efficiency. Pharmaceuticals 2022, 15, 1172. [Google Scholar] [CrossRef]
- Zheng, H.; He, W.; Jiao, W.; Xia, H.; Sun, L.; Wang, S.; Xiao, J.; Ou, X.; Zhao, Y.; Shen, A. Molecular characterization of multidrug-resistant tuberculosis against levofloxacin, moxifloxacin, bedaquiline, linezolid, clofazimine, and delamanid in southwest of China. BMC Infect. Dis. 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The «Old» and the «New» antibiotics for MDR Gram-negative pathogens: For whom, when, and how. Front. Public Heal. 2019, 7, 1–25. [Google Scholar] [CrossRef]
- Marquez, B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie 2005, 87, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Lai, S.; Piras, A.; Marongiu, B.; Lai, A. 13C-CPMAS and 1H-NMR study of the inclusion complexes of β-cyclodextrin with carvacrol, thymol, and eugenol prepared in supercritical carbon dioxide. Chem. Biodivers. 2004, 1, 1354–1366. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—a review. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. FOOD Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, U.; Matwijczuk, A.; Dródz, T.; Zabinski, A.; Niemczynowicz, A. Spectroscopic Examination and Chemometric Analysis of Essential Oils Obtained from Peppermint. Processes 2019, 7, 1–16. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 1–29. [Google Scholar] [CrossRef]
- Boire, N.A.; Riedel, S.; Parrish, N.M. Essential Oils and Future Antibiotics: New Weapons against Emerging’Superbugs’? Journal of Ancient Diseases & Preventive Remedies. J Anc Dis Prev Rem 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 2014, 28, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Taylan, O.; Cebi, N.; Sagdic, O. Rapid screening of mentha spicata essential oil and l-menthol in mentha piperita essential oil by atr-ftir spectroscopy coupled with multivariate analyses. Foods 2021, 10. [Google Scholar] [CrossRef]
- Cardoso, N.N.R.; Alviano, C.S.; Blank, A.F.; Romanos, M.T. V.; Fonseca, B.B.; Rozental, S.; Rodrigues, I.A.; Alviano, D.S. Synergism Effect of the Essential Oil from Ocimum basilicum var. Maria Bonita and Its Major Components with Fluconazole and Its Influence on Ergosterol Biosynthesis. Evidence-based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential oil quality and purity evaluation via ft-ir spectroscopy and pattern recognition techniques. Appl. Sci. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Samet, A. V.; Shevchenko, O.G.; Rusak, V. V.; Chartov, E.M.; Myshlyavtsev, A.B.; Rusanov, D.A.; Semenova, M.N.; Semenov, V. V. Antioxidant Activity of Natural Allylpolyalkoxybenzene Plant Essential Oil Constituents. J. Nat. Prod. 2019, 82, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Weisheimer, V.; Miron, D.; Silva, C.B.; Guterres, S.S.; Schapoval, E.E.S. Microparticles containing lemongrass volatile oil: Preparation, characterization and thermal stability. Pharmazie 2010, 65, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated inβ-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Tadtong, S.; Watthanachaiyingcharoen, R.; Kamkaen, N. Antimicrobial constituents and synergism effect of the essential oils from Cymbopogon citratus and Alpinia galanga. Nat. Prod. Commun. 2014, 9, 277–280. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; deLampasona, M.P.; Catalan, C.A.N. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C. Bin; Han, K.T.; Cho, K.S.; Ha, J.; Park, H.J.; Nam, J.H.; Kil, U.H.; Lee, K.T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S. V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.C.; Kovrizhina, A.R.; и др. Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evidence-based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT - Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, C.; Sun, Y. Effect of Selective Encapsulation of Hydroxypropyl-β-cyclodextrin on Components and Antibacterial Properties of Star Anise Essential Oil. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Davydova, M.P.; Danilov, M.R.; Krylov, S.S.; Belogurova, N.G. Covalent Conjugates of Allylbenzenes and Terpenoids as Antibiotics Enhancers with the Function of Prolonged Action. 2023, 1–34.
- Tsyganov, D. V.; Yakubov, A.P.; Konyushkin, L.D.; Firgang, S.I.; Semenov, V. V. Polyalkoxybenzenes from plant sources 2. Synthesis of isoxazoline analogs of combretastatin from natural allyl(methylenedioxy)methoxybenzenes. Russ. Chem. Bull. 2007, 56, 2460–2465. [Google Scholar] [CrossRef]
- Tsyganov, D. V.; Samet, A. V.; Silyanova, E.A.; Ushkarov, V.I.; Varakutin, A.E.; Chernysheva, N.B.; Chuprov-Netochin, R.N.; Khomutov, A.A.; Volkova, A.S.; Leonov, S. V.; и др. Synthesis and Antiproliferative Activity of Triphenylphosphonium Derivatives of Natural Allylpolyalkoxybenzenes. ACS Omega 2022, 7, 3369–3383. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V. V.; Rusak, V. V.; Chartov, E.M.; Zaretskii, M.I.; Konyushkin, L.D.; Firgang, S.I.; Chizhov, A.O.; Elkin, V. V.; Latin, N.N.; Bonashek, V.M.; и др. Polyalkoxybenzenes from plant raw materials 1. Isolation of polyalkoxybenzenes from CO2 extracts of Umbelliferae plant seeds. Russ. Chem. Bull. 2007, 56, 2448–2455. [Google Scholar] [CrossRef]
- Neuhaus-Carlisle, K.; Vierling, W.; Wagner, H. Screening of plant extracts and plant constituents for calcium-channel blocking activity. Phytomedicine 1997, 4, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Dobryakova, N. V; Ezhov, A.A.; Kudryashova, E. V Achievement of the selectivity of cytotoxic agents against can- cer cells by creation of combined formulation with terpenoid adjuvants as prospects to overcome multidrug resistance. 2022, 1–34. [Google Scholar]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Phytochemistry Menthol : A simple monoterpene with remarkable biological properties. 2013, 1–11. [Google Scholar]
- Russin, W.A.; Hoesly, J.D.; Elson, C.E.; Tanner, M.A.; Gould, M.N. Inhibition of rat mammary carcinogenesis by monoterpenoids. 1989, 10, 2161–2164. [Google Scholar] [CrossRef] [PubMed]
- Pereira de Lira, M.H.; Fernandes Queiroga Moraes, G.; Macena Santos, G.; Patrício de Andrade Júnior, F.; De Oliveira Pereira, F.; Oliveira Lima, I. Synergistic antibacterial activity of monoterpenes in combination with conventional antimicrobials against Gram-positive and Gram-negative bacteria. Rev. Ciências Médicas e Biológicas 2020, 19, 258. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Jiso, A.; Khemawoot, P.; Techapichetvanich, P.; Soopairin, S.; Phoemsap, K.; Damrongsakul, P.; Wongwiwatthananukit, S.; Vivithanaporn, P. Drug-Herb Interactions among Thai Herbs and Anticancer Drugs: A Scoping Review. Pharmaceuticals 2022, 15, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- The Rumen Microbial Ecosystem; 1997; ISBN 9789401071499.
- Zlotnikov, I.D.; Kudryashova, E. V Spectroscopy Approach for Highly - Efficient Screening of Lectin - Ligand Interactions in Application for Mannose Receptor and Molecular Containers for Antibacterial Drugs. 2022. [Google Scholar] [CrossRef] [PubMed]
- MacGowan, A.; Macnaughton, E. Antibiotic resistance. Med. (United Kingdom) 2017, 45, 622–628. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Zari, A.T.; Zari, T.A.; Hakeem, K.R. Anticancer Properties of Eugenol : A Review. 2021. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Gupta, B.; Prakash, R.; Singh, S. Preparation and characterization of hydroxypropyl-β-cyclodextrin inclusion complex of eugenol: Differential pulse voltammetry and 1H-NMR. Chem. Pharm. Bull. 2010, 58, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Rojas, D.F.; Souza, C.R.F.; Oliveira, W.P. Encapsulation of eugenol rich clove extract in solid lipid carriers. J. Food Eng. 2014, 127, 34–42. [Google Scholar] [CrossRef]
- Akshaya, R.; Anjali, A.K. Eugenol as Potential Medicine- Review. 2021, 25, 6250–6260. [Google Scholar]
- Sampaio, C.; Moriwaki, C.; Claudia, A.; Sato, F.; Luciano, M.; Medina, A.; Matioli, G. Curcumin – b -cyclodextrin inclusion complex : Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. FOOD Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef]
- Schneider, H.J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 1998, 98, 1755–1785. [Google Scholar] [CrossRef] [PubMed]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87. [Google Scholar] [CrossRef]
- Cavalli, R.; Trotta, F.; Tumiatti, W. Cyclodextrin-based nanosponges for drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 209–213. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin-cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, T.; Chen, F.; Duan, X.; Yuan, Y.; Zhang, D.; Jiang, Y. An inclusion complex of eugenol into β-cyclodextrin: Preparation, and physicochemical and antifungal characterization. Food Chem. 2016, 196, 324–330. [Google Scholar] [CrossRef]
- Seo, S.J.; Kim, S.H.; Sasagawa, T.; Choi, Y.J.; Akaike, T.; Cho, C.S. Delivery of all trans -retinoic acid ( RA ) to hepatocyte cell line from RA / galactosyl a -cyclodextrin inclusion complex. 2004, 58, 681–687. [CrossRef]
- Fernandes, C.M.; Carvalho, R.A.; Pereira da Costa, S.; Veiga, F.J.B. Multimodal molecular encapsulation of nicardipine hydrochloride by β-cyclodextrin, hydroxypropyl-β-cyclodextrin and triacetyl-β-cyclodextrin in solution. Structural studies by 1H NMR and ROESY experiments. Eur. J. Pharm. Sci. 2003, 18, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Belogurova, N.G.; Krylov, S.S.; Semenova, M.N.; Semenov, V. V; Kudryashova, E. V Plant Alkylbenzenes and Terpenoids in the Form of Cyclodextrin Inclusion Complexes as Antibacterial Agents and Levofloxacin Synergists. 2022. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V. V.; Kiselyov, A.S.; Titov, I.Y.; Sagamanova, I.K.; Ikizalp, N.N.; Chernysheva, N.B.; Tsyganov, D. V.; Konyushkin, L.D.; Firgang, S.I.; Semenov, R. V.; и др. Synthesis of antimitotic polyalkoxyphenyl derivatives of combretastatin using plant allylpolyalkoxybenzenes (1). J. Nat. Prod. 2010, 73, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Zlotnikov, I.D.; Vigovskiy, M.A.; Davydova, M.P.; Danilov, M.R.; Dyachkova, U.D.; Grigorieva, O.A.; Kudryashova, E. V Mannosylated Systems for Targeted Delivery of Antibacterial Drugs to Activated Macrophages. 2022, 1–29. [Google Scholar] [CrossRef]
- Chernysheva, N.B.; Tsyganov, D. V.; Philchenkov, A.A.; Zavelevich, M.P.; Kiselyov, A.S.; Semenov, R. V.; Semenova, M.N.; Semenov, V. V. Synthesis and comparative evaluation of 4-oxa- and 4-aza-podophyllotoxins as antiproliferative microtubule destabilizing agents. Bioorganic Med. Chem. Lett. 2012, 22, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Chudin, A.A.; Kudryashova, E. V. Improved Enzymatic Assay and Inhibition Analysis of Redox Membranotropic Enzymes, AtGALDH and TcGAL, Using a Reversed Micellar System. Analytica 2022, 3, 36–53. [Google Scholar] [CrossRef]
- Chudin, A.A.; Zlotnikov, I.D.; Krylov, S.S.; Semenov, V. V.; Kudryashova, E. V. Allylpolyalkoxybenzene Inhibitors of Galactonolactone Oxidase from Trypanosoma cruzi. Biochem. 2023, 88, 131–141. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Ferberg, A.S.; Krylov, S.S.; Semenova, M.N.; Semenov, V. V; Kudryashova, E. V Polymeric Micelles Formulation of Combretastatin Derivatives with Enhanced Solubility, Cytostatic Activity and Selectivity against Cancer Cells. 2023. [Google Scholar] [CrossRef]
- Ghosh, P.; Vidal, C.; Dey, S.; Zhang, L. Mitochondria targeting as an effective strategy for cancer therapy. Int. J. Mol. Sci. 2020, 21, 1–19. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer Douglas. Nat Rev, Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Semenova, M.N.; Demchuk, D. V.; Tsyganov, D. V.; Chernysheva, N.B.; Samet, A. V.; Silyanova, E.A.; Kislyi, V.P.; Maksimenko, A.S.; Varakutin, A.E.; Konyushkin, L.D.; и др. Sea Urchin Embryo Model As a Reliable in Vivo Phenotypic Screen to Characterize Selective Antimitotic Molecules. Comparative evaluation of Combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as Tubulin-Binding Agents. ACS Comb. Sci. 2018, 20, 700–721. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Ezhov, A.A. Smart pH- and Temperature-Sensitive Micelles Based on Chitosan Grafted with Fatty Acids to Increase the Efficiency and Selectivity of Doxorubicin and Its Adjuvant Regarding the Tumor Cells. 2023. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Dyachkova, U.D.; Belogurova, N.G.; Kudryashova, E. V Application Prospects of FTIR Spectroscopy and CLSM to Monitor the Drugs Interaction with Bacteria Cells Localized in Macrophages for Diagnosis and Treatment Control of Respiratory Diseases. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Gheran, C.V.; Voicu, S.N.; Galateanu, B.; Callewaert, M.; Moreau, J.; Cadiou, C.; Chuburu, F.; Dinischiotu, A. In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Plenagl, N.; Duse, L.; Seitz, B.S.; Goergen, N.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Photodynamic therapy–hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv. 2019, 26, 23–33. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological Investigations and Spectroscopic Studies of New Moxifloxacin/Glycine-Metal Complexes. Chem. Biodivers. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- E.M.Frantsiyants, I.V.Neskubina, E.A.S. Mitochondria of transformed cell as a target of antitumor influence. Res. Pract. Med. J. 2020, 7, 92–108. [Google Scholar] [CrossRef]
| Compound | Functional group | Position of the characteristic peak in the FTIR spectra, cm–1 | |
| octane-ethanol (50:50 v:v) | water-ethanol (50:50 v:v) | ||
| Dillapiol | O–CH2–O | 2917 | 2924 |
| =C–O–C | 1065 | 1045 | |
| –O–CH3 | 2848 | 2858 | |
| С–С aromatic | 1464 | 1448 | |
| Allyltetramethoxybenzene | Aryl–CH2–CH=CH2 | 2956 | 2930 |
| –O–CH3 | 2924 | 2901 | |
| С–С aromatic | 1492 и 1466 | 1488 и 1449–1456 | |
| Propyl-PPh3 | С–С aromatic | 1421 | 1414–1420 |
| 1440 и 1455 | 1455 | ||
| Dillapiol-PPh3 | O–CH2–O | 2937–2952 | 2927–2932 (2928) |
| =C–O–C | 1082–1087 | 1086 (1088) | |
| –O–CH3 | 2848 | – | |
| Aryl–CH2–CH2–CH2–PPh3 | 2970 | 2981 (2974) | |
| С–С aromatic | 1502 | 1485 | |
| 1455 и 1465 | 1448-1457 | ||
| Allyltetramethoxybenzene-PPh3 | =C–O–C | 1086 | 1089 (1088) |
| –O–CH3 | 2855 | 2900 (2880–2900) | |
| Aryl–CH2–CH2–CH2–PPh3 | 2993 и 2957 | 2980 (2974) | |
| С–С aromatic | 1467 | 1482–1488 (1486) | |
| Substance X-PPh3 | –lg Kd (X – M-β-CD)* | –lg Kd (X – γ-CD)** | –lg Kd (X – heparin)*** | Solubility in PBS, mM | Solubility in the presence of 0.05 M MCD, mM |
| Apiol-PPh3 | 2.9±0.3 | 1.2±0.2 | 2.7±0.2 | 0.08 ± 0.01 | 15 ± 2 |
| Dillapiol-PPh3 | 2.6±0.2 | 1.4±0.3 | 3.0±0.3 | 0.09 ± 0.01 | 8 ± 1 |
| Myristicin-PPh3 | 3.0±0.3 | 1.3±0.1 | 2.6±0.2 | 0.04 ± 0.005 | 12 ± 3 |
| Allyltetramethoxybenzene-PPh3 | 3.1±0.2 | 2.1±0.2 | 3.2±0.1 | 0.07± 0.01 | 17 ± 5 |
| Substance X | –lg Kd (X – M-β-CD)**** | Solubility in PBS, mM | Solubility in the presence of 0.05 M MCD, mM | ||
| Apiol | 2.6±0.3 | 0.13 ± 0.01 | 22 ± 4 | ||
| Dillapiol | 2.7±0.5 | 0.24 ± 0.05 | 27 ± 3 | ||
| Myristicin | 3.5±0.2 | 0.030 ± 0.007 | 41 ± 5 | ||
| Allyltetramethoxybenzene | 3.4±0.3 | 0.16 ± 0.02 | 38 ± 2 | ||
| Substance X in M-β-CD | –lg (IC50) against A549 | Synergy coefficients of PPh3 – adjuvants with paclitaxel* |
| Paclitaxel | 6.2±0.2 | - |
| Apiol-PPh3 | 5.8±0.1 | 2.2±0.2 |
| Dillapiol-PPh3 | 5.6±0.2 | 1.5±0.1 |
| Myristicin-PPh3 | 5.3±0.2 | 1.8±0.3 |
| Allyltetramethoxybenzene-PPh3 | 4.8±0.1 | 1.3±0.1 |
| Apiol | 3.6±0.3 | 1.3±0.2 |
| Dillapiol | 3.2±0.1 | 1.1±0.1 |
| Myristicin | 2.9±0.3 | 0.9±0.2 |
| Allyltetramethoxybenzene | 3.5±0.2 | 1.4±0.2 |
| Substance X in M-β-CD | HEK293T viability (%) at CX = 300 µM | HEK293T viability (%) at CX = 100 µM | HEK293T viability (%) at CX = 10 µM |
| Apiol-PPh3 | 71±2 | 82±3 | 93±2 |
| Dillapiol-PPh3 | 70±5 | 84±5 | 95±3 |
| Myristicin-PPh3 | 75±3 | 91±2 | 97±3 |
| Allyltetramethoxybenzene-PPh3 | 83±4 | 88±3 | 98±1 |
| Substance X in M-β-CD | Hemolysis index*, % | Thrombosis index**, % | Concentration causing changes in sea urchin embryos, μM |
| Paclitaxel | <0.5 (p = 0.012) | 0.6±0.1 | >4*** |
| Apiol-PPh3 | 0.8±0.2 | 1.1±0.2 | |
| Dillapiol-PPh3 | 0.9±0.2 | 1.0±0.1 | |
| Myristicin-PPh3 | 0.5±0.1 | 1.5±0.2 | |
| Allyltetramethoxybenzene-PPh3 | 0.7±0.1 | 0.7±0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





