1. Introduction
With the global challenges of climate change and increasing energy demand, the search for alternative renewable energy sources has become a global priority. In this context, bio-oils derived from biomass pyrolysis are gaining importance as alternatives to the liquid fuels produced using fossil fuels [
1,
2,
3]. Biomass pyrolysis is the process of thermochemical decomposition of organic materials under anaerobic conditions. This process produces three products: bio-oil, combustible gas, and char [
4,
5,
6]. Among these products, bio-oil is of particular interest because of its potential use as fuel (either directly or after refinement using various methods) [
7,
8]. It contains defragmented components of the original biomass structure, mainly cellulose, hemicellulose, and lignin. However, the chemical composition and fuel properties of crude bio-oil differ from those of liquid fuels derived from fossil fuels, making it a subject of intensive research to optimize its preparation and processing.
Bio-oils obtained by fast biomass pyrolysis are characterized by a high content of reactive oxygenates and a significant water content (up to 60 wt.% [
9,
10]), and the presence of acidic compounds [
11]. These products are extremely complex mixtures of organic compounds that are difficult to unambiguously identify. They contain approximately 400 identified organic compounds, which represent approximately 50 wt.% of the total composition of bio-oils. Their composition greatly depends on the type of biomass used for the pyrolysis process, process conditions, purification method, and storage conditions. From a chemical point of view, they are highly polar liquids, containing ~40-50 wt.% oxygen in the dry state, whereas their content in mineral oils remains at the ppm level. Their significant water and polar oxygen compounds contents make them poorly miscible with other mineral oils and unstable, especially at elevated temperatures [
12,
13]. The typical oxygen content of biomass pyrolysis bio-oils is 35-40 wt.% (dry state) [
14]. The oxygen content of bio-oils is the main feature that distinguishes them from liquid petroleum fuels. Unfortunately, oxygen in bio-oils adversely affects their energy density and impairs their miscibility with hydrocarbon fuels.
Bio-oils can be considered as microemulsions consisting of a dispersed phase in the form of water and organic compounds dissolved in it, and a dispersed phase in the form of water-insoluble lignin oligomers. The aqueous fraction of bio-oils mainly consists of alcohols, carboxylic acids, carbonyl compounds (aldehydes and ketones), and sugars [
15]. The water-insoluble phase mainly contains compounds derived from the breakdown of lignins such as higher fatty acids, terpenes, triglycerides, phenolic derivatives, and acidic resins [
13].
Biomass pyrolysis oil is typically a reddish-brown liquid with a pungent odor. Owing to the presence of reactive components, oil is unstable and tends to phase-separate and form solid by-products during storage. Phase separation was more intense at higher temperatures and appeared to be faster when the amount of water exceeded 30 wt.%. Distillation (even vacuum distillation) causes undesirable chemical changes in bio-oil (strong polymerization of oil components at elevated temperatures), leading to the unfavorable formation of large amounts of solid carbonaceous material – char.
In recent years, many studies have been conducted on the preparation and properties of bio-oils from the pyrolysis of different types of biomass [
16,
17,
18]. For example, Adegoke et al. [
19] investigated the properties of bio-oil obtained from the pyrolysis of Gmelina arborea biomass, highlighting the low sulfur content of this product and highlighting the potential environmental benefits of its use. Other studies have focused on the quality of bio-oil obtained by pyrolysis of waste materials, such as corncobs, and the improvement of its functional properties with conversions carried out using different catalysts [
20,
21,
22]. Bio-oils with good functional properties have also been obtained by the co-pyrolysis of various biomass feedstocks, such as hardwood, pressed mustard oil cake and corncob[
23]. The number of studies presented in the literature on this topic is large because of the enormous implementation potential of the studied solutions.
Biomass pyrolysis bio-oils are promising candidates as alternative energy sources. Its production and use can contribute to reducing greenhouse gas emissions and increasing sustainable energy production worldwide. To enable the use of biomass pyrolysis bio-oils as transport fuels, the following adjustments to their properties are necessary:
– reduction of acidity,
– increase in energy density,
– lowering of viscosity,
– increasing the miscibility with liquid fossil fuels,
– lowering the content of the solid products formed during heating or storage.
Hydrodeoxygenation (HDO) is one of the methods by which these objectives can be achieved.
This paper presents selected results from research conducted at the Institute of Energy and Fuel Processing Technology as part of the EnCat project [
24,
25]. This research involved quality assessment of crude biomass pyrolysis bio-oil samples and preliminary hydrodeoxygenation tests of selected bio-oil samples. Mixing tests of bio-oil samples with other fuel components were also carried out. This study was intended to enable an assessment of the rationality of using such products as motor liquid fuels.
2. Materials and Methods
2.1. Bio-oil samples
This paper presents the results of crude bio-oil samples from biomass pyrolysis produced by EnCat project partners [
24] and a reference bio-oil sample produced by BTG Bioliquids B.V. (The Netherlands). The bio-oil samples produced in the EnCat project were stripped of a part of the aqueous phase separated using a cone separatory funnel; hence, the remaining water content was slightly lower than the typical water content of crude bio-oil samples from biomass pyrolysis. The tested samples are summarized in
Table 1, along with the basic characteristics of their production processes.
Analyses of the physicochemical properties of the bio-oil samples were performed in accordance with the accredited procedures of the ITPE laboratories and the applicable Polish and European standards. The results of the analysis of the bio-oil samples are summarized in
Table 2.
Gas chromatography (GC/MS) analyses of the volatile organic fractions of the pyrolytic bio-oils tested were also carried out. The analytical results obtained are presented in
Table 3. Qualitative analysis was performed using an Agilent 7890B gas chromatograph coupled to an MSD 5977A mass spectrometer, while quantitative analysis was performed using a Thermo-Scientific TRACE Gas Chromatograph with FID detector.
2.2. Bio-oil hydrodeoxygenation tests
Hydrodeoxygenation tests were performed in a batch reactor (autoclave) with a working volume of 1290 cm
3. Due to the amount of bio-oil required for the hydrodeoxygenation process, the tests were only carried out for BTL sample and the mixture of UT1 and UT2 samples (it was necessary to mix the samples due to their insufficient individual amount). The autoclave used in this study allowed the reaction to be carried out at pressures of up to 20 MPa and temperatures of up to 220°C. The procedure for testing the hydrodeoxygenation of bio-oils was to place the catalyst and a portion of the bio-oil in the working space of the autoclave and then fill it with hydrogen at a pressure of 10 MPa. The prepared reactor charge was heated with intensive stirring to a temperature of approximately 200°C (above which bio-oil coking could occur). The heating rate of the reactor charge is approximately 4 K/min. Hydrodeoxygenation of the bio-oil samples was performed for 3 h. At the end of the test, the reactor was cooled, and the resulting products were removed: a light liquid product and a high-density liquid product (heavier product). The basic parameters of the hydrodeoxygenation tests are listed in
Table 4.
A cobalt-molybdenum catalyst (HDMax200) from Clariant was chosen to study the hydrodeoxygenation of bio-oils. The catalyst was in the form of rollers with a diameter of 2.5 mm. It contained 3.5 wt.% cobalt oxide and 10 wt.% molybdenum oxide placed on an alumina support. To carry out the hydrodeoxygenation process, it was necessary to bring the catalyst into an active form. It was therefore subjected to a drying and desulfurization process. The catalyst was then dried under a pure nitrogen stream. In turn, catalyst activation consists of catalyst reduction and sulfidation in a hydrogen sulfide atmosphere. Sulfidation was performed using 2.4% H2S in a mixture of hydrogen (mixture flow of 2 dm3/min) to convert cobalt and molybdenum oxides into sulfides. Due to the high reactivity of hydrogen sulfide, catalyst activation was carried out from room temperature to 250°C at atmospheric pressure.
Table 5 summarizes the physicochemical characteristics of the products obtained from the hydrodeoxygenation tests of the bio-oil samples.
2.3. Bio-oil blending tests
One of the most promising methods for valorizing biomass pyrolysis bio-oils is to produce mixtures/emulsions of bio-oils with other liquid fuels. The miscibility studies focused on obtaining homogenous, stable oil-fuel emulsions. Selected bio-oil samples were used, along with diesel (D), rapeseed methyl ester (RME), and butyl alcohol (BuOH). The prepared liquid mixtures with different compositions were magnetically stirred in a laboratory beaker for 30 min and then poured into a measuring cylinder to observe the stability of the resulting fuel. The fuel blends were considered stable when the components did not delaminate within 48 h. Blends of the following compositions were tested in this study: BTL/D/BuOH at ingredient weight shares of 10-90/10-90/0-60, BTL/RME/BuOH at ingredient weight shares of 10-90/5-90/0-80, KTH/D/BuOH at ingredient weight shares of 7-67/25-77/0-47, and UT2/D/BuOH at ingredient weight shares of 7-67/17-77/0-56.
3. Results and Discussion
3.1. Bio-oil samples
The biomass pyrolysis bio-oil samples tested were brown coloured liquids with a characteristic, intense, pungent odour, containing relatively small amounts of water (11.6-22.3 wt.%) for such products. The water content of bio-oils can have both negative and positive effects on combustion. This results in a low energy density for these fuels, low flame adiabatic temperatures, and a reduction in their combustion rate. The water content causes difficulties in ignition, which can be problematic when conducting bio-oil combustion in compression-ignition engines [
13,
26]. However, the significant water content of bio-oils facilitates their atomization by lowering their viscosity.
A large number of factors determine the composition of the obtained bio-oils, but the importance of the use of catalysts in the pyrolysis of biomass can already be seen from the elemental carbon content of the samples tested. The samples obtained by catalytic pyrolysis (KTH, UT1 and UT2) had a significantly higher carbon content (66.93-70.31 wt.%) compared to the sample obtained by non-catalytic pyrolysis (57.13 wt.%). As described earlier, the bio-oil samples KTH, UT1, and UT2 were initially stripped of a part of the water fraction separated after phase stratification in the separating funnel. The high water content of the tested samples is obviously reflected in the determined calorific values of the tested bio-oils (16.57-24.26 MJ/kg), whose values are significantly lower than the typical values of this parameter for hydrocarbon liquid fuels (e.g., gasoline – ~44-46 MJ/kg, diesel – ~42-46 MJ/kg). The initial removal of the aqueous fraction from the KTH, UT1 and UT2 samples (along with the organic compounds dissolved in it) is also evident from the oxygen content, which was significantly lower in these samples (22.39-25.71 wt.%) compared to the BTL commercial bio-oil sample (35.92 wt.%). The tested samples had relatively low sulfur contents, although the samples obtained at the University of Twente (UT1 and UT2) contained more than twice as much sulfur as the BTL and KTH samples. The large difference in the contents of these samples also applies to nitrogen. This, of course, has consequences for the possible further combustion of such fuels in terms of SOx and NOx emissions.
Among the oxygen-based organic compounds that are components of bio-oils, there are always compounds of acidic nature, mainly acetic acid and phenols, which are responsible for the pH of the bio-oil. The pH values determined for the 1% aqueous solutions of the bio-oil samples tested were 4-5, and were higher than the pH values of other bio-oils from rapid biomass pyrolysis reported in the literature (pH=2-3) [
11]. The acidic compound content has a significant effect on the pH value of bio-oils, but the research methodology adopted to determine it also has an influence. Owing to the possibility of corrosion phenomena under the influence of bio-oils, particularly at elevated temperatures, it is necessary to provide suitable acid-resistant construction materials when building apparatus and equipment used for their transport, storage, and use.
Biomass pyrolysis oils tend to coke at elevated temperatures, typically above 200°C. The phenomenon of bio-oil coking was reflected in the determined coking numbers of the bio-oil samples tested, which determine the percentage yield of the solid residue of the sample after pyrolysis under standardized conditions. The determined coking numbers for the bio-oil samples tested were, respectively: BTL – 14.70 wt.%, KTH – 6.85 wt.%, UT1 – 26.23 wt.% and UT2 – 30.71 wt.%. None of these values are favorable, especially the high coking number values of UT1 and UT2 bio-oils, from the point of view of the possible combustion of such fuels in reciprocating engines.
Analysis of the volatile organic compound content in the bio-oil samples showed significant differences in their chemical compositions. The high content of volatile organic compounds in oils produced by biomass pyrolysis causes their physicochemical properties to differ significantly from those of petroleum-based fuels. Bio-oils are characterized by a lack of miscibility with nonpolar solvents and limited stability. They are mixtures of several hundred organic compounds, including hydrocarbons, fats, waxes, sterols, acids, aldehydes, ketones, alcohols, cyclic compounds, phenols, catechols, guaiacol, sugars, and lignin derivatives. It is estimated that approximately 20 wt.% of the total bio-oil is composed of reactive oxygen compounds responsible for its limited stability during storage and processing, particularly at elevated temperatures [
12,
27].
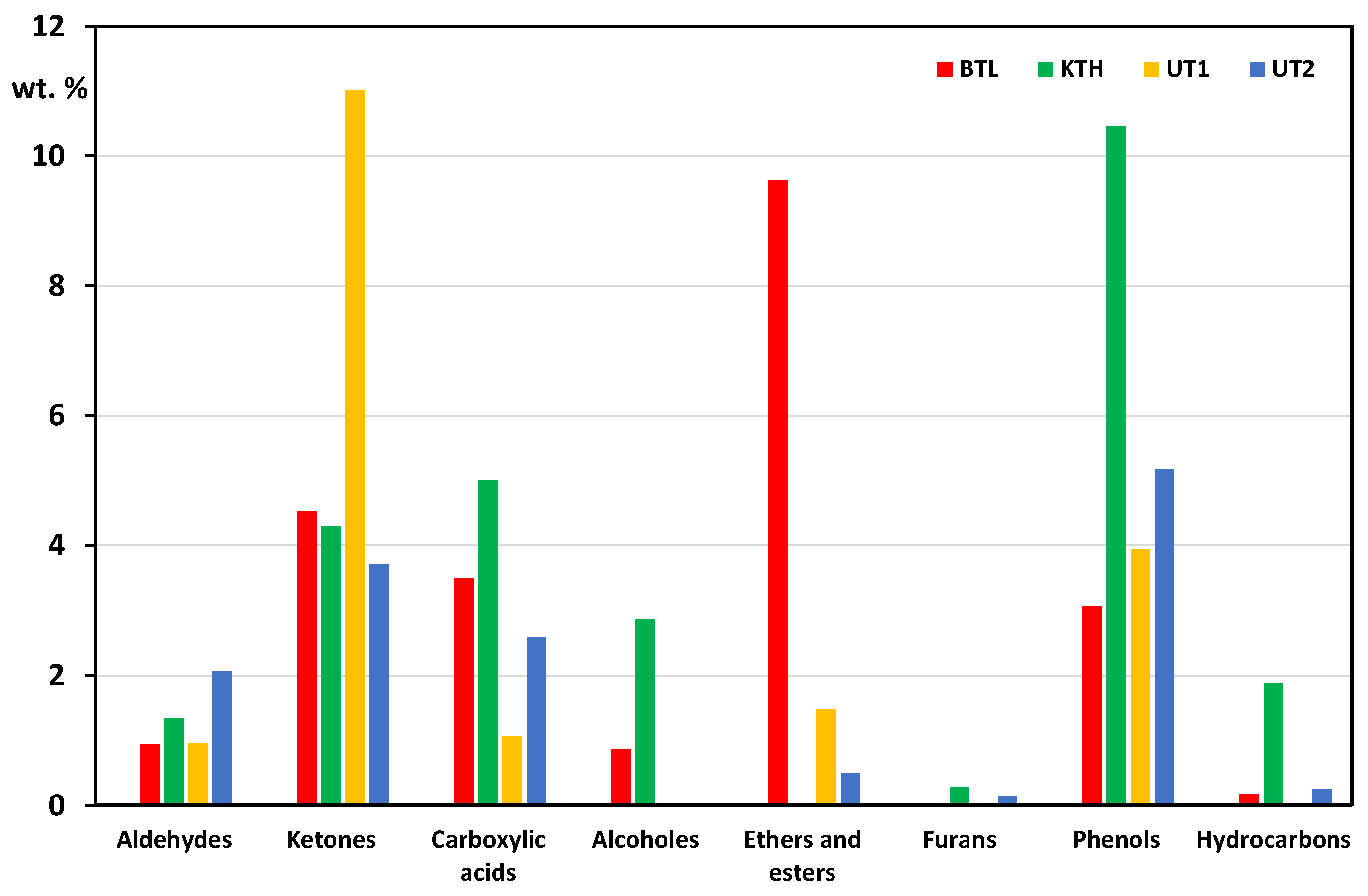
The oxygen content of the bio-oils, as well as the type of oxygenated compounds, is a key parameter when assessing pyrolysis liquids for use, storage and transport. Considering the data presented in
Table 3, the identified volatile organic compounds in the studied bio-oils were divided into the following groups: aldehydes, ketones, acids, ethers and esters, furans, phenols and hydrocarbons. The results of this division are shown in
Figure 1.
By gas chromatography, 14.6-26.1 wt.% of volatile organic compounds were identified. The residue consisted of organic substances that could not be analysed by this method. The identified compounds included products of polysaccharide fragmentation processes and lignin degradation products. The first group includes acetic acid, hydroxyacetone, methyl alcohol, 2-hydroxy-3-methyl-2-cyclopenten-1-one, 2-furaldehyde, acetone, and furan, among others. The second group are substituted aromatic compounds, mainly phenolic derivatives, including: phenol, 2-methoxyphenol, 2-methoxy-4-methylphenol, 2-methoxy-4-allylphenol, 2,6-dimethoxyphenol, 2,6-dimethoxy-4-methylphenol, 2-methoxy-4-vinylphenol, and hydrocarbons.
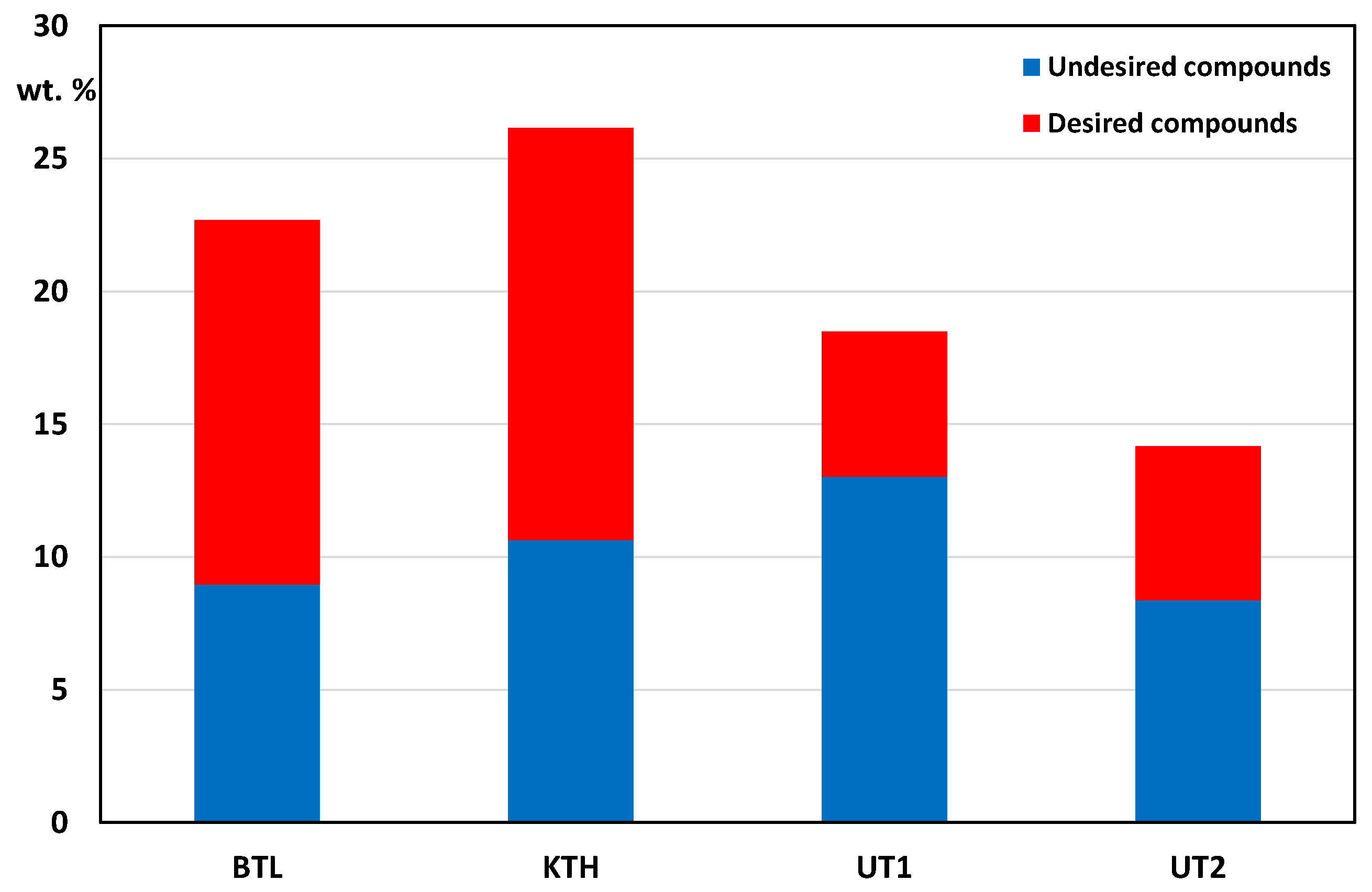
The pyrolytic oils tested contained undesirable reactive carbonyl compounds in their composition, which mainly include aldehydes: 2-furaldehyde, 4-hydroxy-3-ethoxybenzaldehyde, coniferyl aldehyde and ketones: acetone, hydroxyacetone, 1-hydroxy-2-butanone, 2,3-butanedione, 2-cyclopenten-1-one, 2-methyl-2-cyclopenten-1-one, 2-furanone, 3-methyl-1,2-cyclopentanedione. Undesirable organic compounds are also carboxylic acids (mainly acetic acid) responsible for corrosion processes, especially in the presence of water at high temperatures. A comparison of the content of desirable and undesirable VOCs is shown in
Figure 2. All bio-oils tested contained significant amounts of undesirable compounds (aldehydes, ketones and acids). The best ratio of the proportions of desirable to undesirable components was characterised by BTL and KTH bio-oils, the worst by UT1 bio-oil.
3.2. Bio-oil hydrodeoxygenation tests
Oxygen removal from bio-oils in order to improve their physico-chemical properties and enable their use as transport fuels is carried out through a hydrodeoxygenation process. The hydrodeoxygenation of pyrolysis oils is a complex process due to the complex and ambiguous chemical composition of bio-oils, strongly dependent on both the type of biomass being pyrolysed and the process conditions. Hydrodeoxygenation of pyrolysed bio-oils is carried out at elevated temperatures, in a hydrogen atmosphere and in the presence of various catalysts (e.g., sulphided NiMo/Al
2O
3 and CoMo/Al
2O
3, Ru, Pd, Rh, Pt, CuCr, CuO, NiO). Experiments have shown that high process performance requires temperatures in the range ~300-400°C. Unfortunately, the test system used in the present study only allowed temperatures of max. 220°C. However, it should be borne in mind that above 200°C, bio-oils can already undergo coking with the formation of carbon deposits, which hinder the hydrodeoxygenation process by deactivating the catalyst. The use of high pressure (above 10 MPa) is necessary to maintain the water in a liquid state, as well as to promote the hydrogenation reaction and limit the occurrence of carbonisation reactions [
28], and such pressure was used in the present study.
Hydrodeoxygenation tests on bio-oil samples yielded liquid products: a light liquid product (aqueous fraction) and a heavier liquid product (oil fraction). The elemental composition of these fractions is presented in
Table 5. The light fractions (HBTL1 and HUT1) are not important from the point of view of fuel applications due to their very high water content. From a practical point of view, they represent a post-production effluent that must undergo further complex mechanical-chemical-biological treatment processes. The organic parts contained in the light fractions obtained were characterised by molar ratios of H/C and O/C (in the waterless state) locating these substances on the classical van Krevelen diagram on the section connecting cellulose and ethanol/DME. The relative oxygen content of the organic parts of the produced light fractions increased relative to its content in the initial samples, hence the negative values of the calculated deoxidation degrees.
The molar ratios of H/C and O/C in the heavier (oil) fractions in both cases decreased relative to the raw materials, indicating that partial conversion of crude bio-oils to high-molecular-weight organic compounds has occurred and that some oxygen has been removed from the converted bio-oils. In the case of BTL bio-oil, a deoxidation degree for the heavier fraction HBTL2 of 53.6 wt.% was obtained, while for the UT1/UT2 oil blend, the determined deoxidation degree for the heavier fraction HUT2 was only 7.15 wt.%. The likely reason for the significant difference in the determined deoxidation rates for the two bio-oils tested was the significant difference in their coking numbers and the more intensive coking of the catalyst during the test with the UT1/UT2 bio-oil blend. Hence the significantly lower yield of the heavier fraction HUT2. In the classical van Krevelen diagram, the heavier fraction produced from BTL oil (HBTL2), characterised by H/C and O/C molar ratios of 1.16 and 0.17, respectively, is located in the hydrothermal liquefaction oil region. In contrast, the heavier fraction from the UT1/UT2 oil blend (HUT2), with H/C and O/C molar ratios of 0.76 and 0.23, respectively, was located in the coal area on the same graph.
Despite the occurrence of partial hydrodeoxidation of samples of the tested bio-oils, it must be concluded that it was not possible to carry out more efficient deoxidation under the process conditions adopted in the study. The oxygen content of the heavier fractions remained at a relatively high level, differing significantly from the composition of hydrocarbon fuels. Further work is needed to test and/or develop effective catalytic systems for the hydrodeoxidation of pyrolytic bio-oils.
3.3. Bio-oil blending tests
The blending of pyrolytic bio-oils with other hydrocarbon fuels characterised by a higher cetane number is the most widely used method to improve and stabilise the properties of bio-oils that can be used as fuel for conventional diesel engines. Such blends generally have better ignition and combustion characteristics and better corrosion properties. Possible polymerisation reactions of reactive components of bio-oils (which also occur under the influence of various surfactants) can be limited by the addition of polar solvents, such as methanol, ethanol, butanol, furfural, ethyl acetate, methyl isobutyl ketone or acetone, which inhibit the ageing processes of bio-oils, while reducing their viscosity and increasing their homogeneity and calorific value. The aim of the work on bio-oil valorisation was to obtain homogeneous, stable blends of bio-oils with other liquid fuels, which will ultimately avoid or minimise changes to liquid fuel storage and transport systems, and ultimately enable the use of existing fuel systems currently used for hydrocarbon fuels.
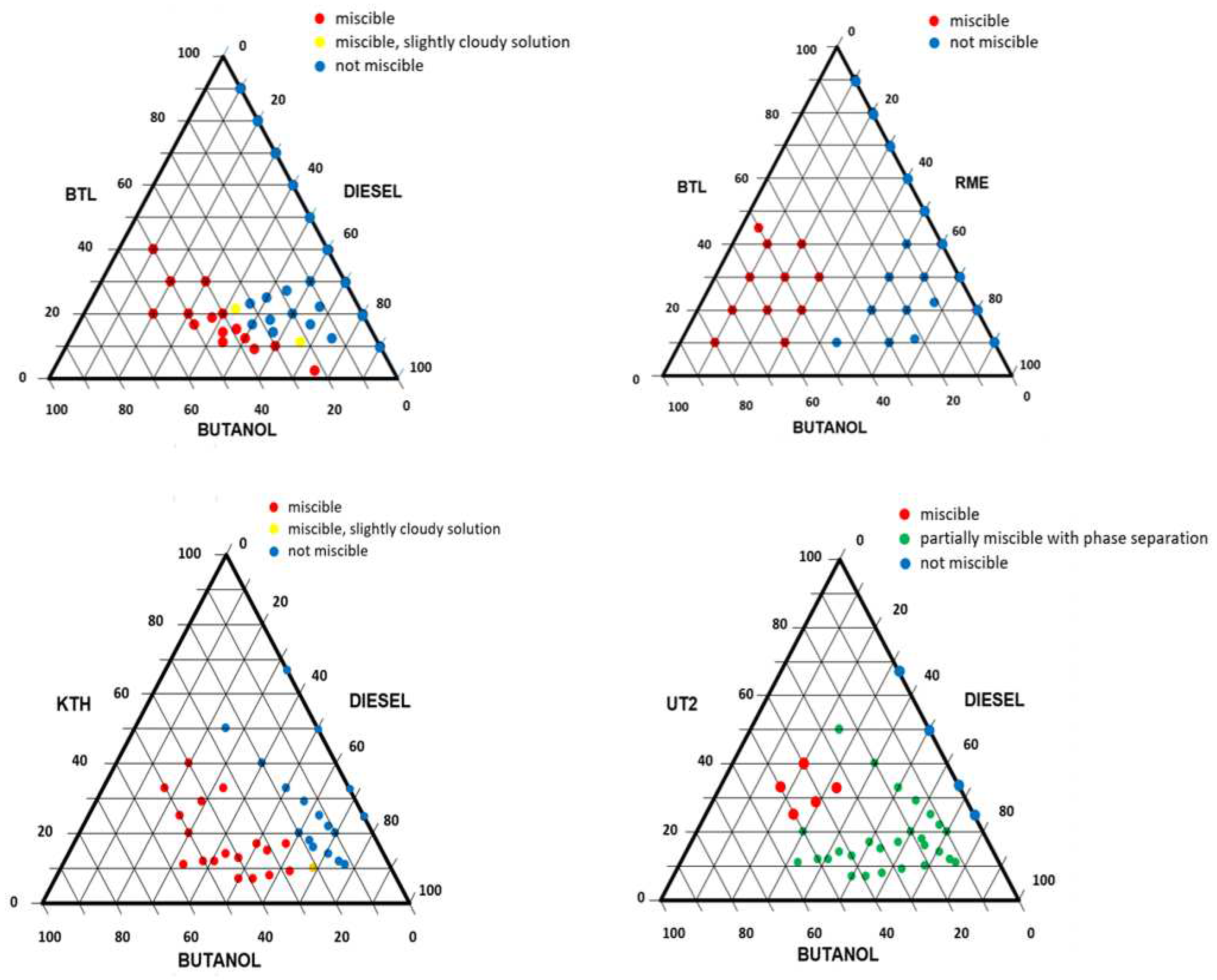
Figure 3 shows the solubility diagrams of the tested three-component mixtures developed on the basis of the experiments performed: BTL/D/BuOH, BTL/RME/BuOH; KTH/D/BuOH and UT2/D/BuOH. Red points indicate homogeneous solutions, blue – heterogeneous solutions that separate into two phases, yellow – cloudy solutions, and green – partially soluble solutions, accompanied by precipitation of probably heavy lignin fractions.
The concentration ranges of individual components of the produced fuel mixtures were, of course, different for each sample of the tested bio-oil. In the case of BTL bio-oil, 15 homogeneous, stable fuel mixtures were obtained for various mass fractions of components, with the fuel mixture with the highest bio-oil content having a mass composition of 40/10/50 (BTL/D/BuOH). For BTL/RME/BuOH mixtures (11 homogeneous, stable fuel mixture compositions), the mixture with the mass composition of 45/5/50 had the highest content of BTL bio-oil.
Figure 3.
Solubility diagrams of three-component mixtures BTL/D/BuOH, BTL/RME/BuOH; KTH/D/BuOH and UT2/D/BuOH.
Figure 3.
Solubility diagrams of three-component mixtures BTL/D/BuOH, BTL/RME/BuOH; KTH/D/BuOH and UT2/D/BuOH.
For KTH bio-oil, 18 homogeneous, stable mixtures containing diesel oil and butanol were obtained. The composition of the mixture with the highest share of bio-oil was 40/20/40 (KTH/D/BuOH). A similar thing happened in the case of UT2 bio-oil (5 homogeneous, stable fuel mixtures), where the highest mass share of bio-oil was obtained in a mixture with a composition of 40/20/40 (UT2/D/BuOH).
In the tests carried out, it was not possible to produce homogeneous mixtures containing more than 45 wt.% of bio-oil. The obtained homogeneous, stable fuel mixtures of bio-oils with other additives met the criteria for long-term storage.
4. Conclusions
Bio-oils produced by the pyrolysis of organic materials such as wood, agricultural waste or plant residues can potentially be used as a feedstock for the production of biofuels or chemicals. They have many advantages, chiefly their renewability, the reduction in CO2 emissions resulting from their use, and the multitude of possible applications.
Bio-oils from biomass pyrolysis, due to their extremely complex chemical composition and resulting physicochemical properties, are not easy products to use. Research into their production and valorisation has been ongoing for many years, yet the path to their widespread use remains distant. Nonetheless, in light of the ever-increasing environmental awareness and the vital need for climate protection, bio-oils present an attractive alternative to traditional hydrocarbon fuels. Moreover, advancements in pyrolysis technology and methods to refine crude bio-oils could render their production more economically viable.
In the study, the properties of four different bio-oil samples obtained under varying process conditions were tested, revealing their distinct physicochemical properties. The variations in bio-oil compositions and their resulting properties pose one of the primary challenges that must be addressed for their broader practical application. A significant concern with these fuels is their high content of oxygenated compounds. Trials have confirmed that the hydrodeoxygenation process (HDO) can significantly enhance bio-oil properties by reducing their oxygen content. Proper implementation of this process hinges on the selection of an appropriate catalyst and conversion conditions, which were only partially achieved in the conducted experiments.
From the perspective of efficiently utilizing bio-oils as motor fuels, the current viable approach is blending them with other liquid fuels, whether derived from fossil fuels or biofuels (e.g., ethanol). Experiments exploring the feasibility of creating ternary, homogeneous, stable bio-oil blends with diesel, rapeseed methyl ester (RME), and butanol have demonstrated the potential to produce fuel blends with long storage stability. However, due to the limited quantities of fuel blends produced during the tests, comprehensive combustion evaluations in engines were not feasible and will be addressed in future research. The maximum bio-oil content in the produced fuel blends reached 45 wt.%. It seems that producing usable liquid fuels with significantly higher proportions of bio-oil in the end product presents a substantial technological challenge that remains elusive.
In conclusion, although bio-oils from biomass pyrolysis are difficult materials to handle, they represent a promising direction for the development of sustainable energy production, and have considerable potential to become an important element of a global CO2 reduction strategy.
Author Contributions
conceptualization, S.S., K.I., A.C. and J.B.; methodology, S.S. and A.C.; validation, S.S., K.I. and A.C.; investigation, S.S., K.I., A.C. and J.B.; resources, S.S. and K.I.; data curation, K.I., A.C. and J.B.; writing—original draft preparation, S.S. and A.C.; writing—review and editing, S.S., K.I. and J.B.; visualization, A.C.; supervision, S.S.; project administration, A.C.; funding acquisition, A.C. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded and carried out in the core of the ERA-NET Bioenergy programme “10th Joint Call for Research and Development Proposals of the ERA-NET Bioenergy” with financial support provided by: the Austrian Federal Ministry of Climate Action, Environment, Energy, Mobility, Innovation and Technology (BMK); the Netherlands Enterprise Agency (RvO); the Polish National Centre for Research and Development (NCBR) and the Swedish Energy Agency.
Acknowledgments
The authors would like to thank all participants of the EnCat project for their fruitful cooperation during the project and after its completion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eladnani, I.; Bracciale, M.P.; Damizia, M.; Mousavi, S.; De Filippis, P.; Lakhmiri, R.; de Caprariis, B. Catalytic Hydrothermal Liquefaction of Brachychiton Populneus Biomass for the Production of High-Value Bio-Crude. Processes 2023, 11, 324. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Muys, B.; Hermy, M. Lignocellulosic Biomass for Bioenergy beyond Intensive Cropland and Forests. Renewable and Sustainable Energy Reviews 2019, 102, 139–149. [Google Scholar] [CrossRef]
- Salman, B.; Ong, M.Y.; Nomanbhay, S.; Salema, A.A.; Sankaran, R.; Show, P.L. Thermal Analysis of Nigerian Oil Palm Biomass with Sachet-Water Plastic Wastes for Sustainable Production of Biofuel. Processes 2019, 7, 475. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic Study of Lignocellulosic Biomass Oxidative Pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Sheth, P.; B.V. Babu, G. Kinetic Modeling of the Pyrolysis of Biomass. Presented at the National Conference on Environmental Conservation, 2006.
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends in Biotechnology 2019, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Ratnasari, D.K.; Horn, A.; Brunner, T.; Yang, W.; Jönsson, P.G. The Thermal Degradation of Lignocellulose Biomass with an Acid Leaching Pre-Treatment Using a H-ZSM-5/Al-MCM-41 Catalyst Mixture. Fuel 2019, 257, 116086. [Google Scholar] [CrossRef]
- Rout, P.K.; Naik, M.K.; Naik, S.N.; Goud, V.V.; Das, L.M.; Dalai, A.K. Supercritical CO 2 Fractionation of Bio-Oil Produced from Mixed Biomass of Wheat and Wood Sawdust. Energy & Fuels 2009, 23, 6181–6188. [Google Scholar]
- Zaini, I.N.; Sophonrat, N.; Sjöblom, K.; Yang, W. Creating Values from Biomass Pyrolysis in Sweden: Co-Production of H2, Biocarbon and Bio-Oil. Processes 2021, 9, 415. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Fuel Oil Quality and Combustion of Fast Pyrolysis Bio-Oils; VTT Technology; VTT Technical Research Centre of Finland: Espoo, 2013. [Google Scholar]
- Oasmaa, A.; Kuoppala, E.; Gust, S.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 1. Effect of Extractives on Phase Separation of Pyrolysis Liquids. Energy Fuels 2003, 17, 1–12. [Google Scholar] [CrossRef]
- Oasmaa, A.; Kuoppala, E.; Solantausta, Y. Fast Pyrolysis of Forestry Residue. 2. Physicochemical Composition of Product Liquid. Energy Fuels 2003, 17, 433–443. [Google Scholar] [CrossRef]
- Patel, A.D.; Zabeti, M.; Seshan, K.; Patel, M.K. Comparative Technical Process and Product Assessment of Catalytic and Thermal Pyrolysis of Lignocellulosic Biomass. Processes 2020, 8, 1600. [Google Scholar] [CrossRef]
- Sánchez-Borrego, F.J.; Álvarez-Mateos, P.; García-Martín, J.F. Biodiesel and Other Value-Added Products from Bio-Oil Obtained from Agrifood Waste. Processes 2021, 9, 797. [Google Scholar] [CrossRef]
- Chernova, N.I.; Grigorenko, A.V.; Kiseleva, S.V.; Larina, O.M.; Kumar, V.; Vlaskin, M.S. Comparative Evaluation of Pyrolysis and Hydrothermal Liquefaction for Obtaining Biofuel from a Sustainable Consortium of Microalgae Arthrospira Platensis with Heterotrophic Bacteria. Processes 2022, 10, 2202. [Google Scholar] [CrossRef]
- Charis, G.; Danha, G.; Muzenda, E. Optimizing Yield and Quality of Bio-Oil: A Comparative Study of Acacia Tortilis and Pine Dust. Processes 2020, 8, 551. [Google Scholar] [CrossRef]
- Sarkar, J.K.; Wang, Q. Characterization of Pyrolysis Products and Kinetic Analysis of Waste Jute Stick Biomass. Processes 2020, 8, 837. [Google Scholar] [CrossRef]
- Adegoke, I.A.; Ogunsanwo, O.Y.; Ige, A.R. Bio-Fuel Properties and Elemental Analysis of Bio-Oil Produced from Pyrolysis of Gmelina Arborea. Acta Chemica Malaysia 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Abatyough, M.T.; Ajibola, V.O.; Agbaji, E.B.; Yashim, Z.I. Properties of Upgraded Bio-Oil from Pyrolysis of Waste Corn Cobs. J. Stabil. Environ. Mgt 2022, 1, 120–128. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Kinetic Study of an H-ZSM-5/Al–MCM-41 Catalyst Mixture and Its Application in Lignocellulose Biomass Pyrolysis. Energy Fuels 2019, 33, 5360–5367. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Yang, W.; Jönsson, P.G. Two-Stage Ex-Situ Catalytic Pyrolysis of Lignocellulose for the Production of Gasoline-Range Chemicals. Journal of Analytical and Applied Pyrolysis 2018, 134, 454–464. [Google Scholar] [CrossRef]
- Madhu, P.; Vidhya, L.; Vinodha, S.; Wilson, S.; Sekar, S.; Patil, P.P.; Kaliappan, S.; Prabhakar, S. Co-Pyrolysis of Hardwood Combined with Industrial Pressed Oil Cake and Agricultural Residues for Enhanced Bio-Oil Production. Journal of Chemistry 2022, 2022, e9884766. [Google Scholar] [CrossRef]
- Łabojko, G.; Czardybon, A.; Bigda, J.; Popowicz, J.; Fryza, R.; Billig, T. Enhanced Catalytic Fast Pyrolysis of Biomass for Maximum Production of High-Quality Biofuels; Report no 165/2020; IChPW: Zabrze, 2020. [Google Scholar]
- Czardybon, A.; Ignasiak, K.; Iluk, T.; Szul, M. Enhanced Catalytic Fast Pyrolysis of Biomass for Maximum Production of High-Quality Biofuels; Report no 166/2020; IChPW: Zabrze, 2020. [Google Scholar]
- Moloodi, S. Experimental Investigation of The Effects of Fuel Properties on Combustion Performance and Emissions of Biomass Fast Pyrolysis Liquid-Ethanol Blends in a Swirl Burner. Master of Applied Science, University of Toronto, 2011.
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Dómine, M.E.; Fernández-Arroyo Naranjo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy & Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Venderbosch, R. h.; Ardiyanti, A. r.; Wildschut, J.; Oasmaa, A.; Heeres, H. j. Stabilization of Biomass-Derived Pyrolysis Oils. Journal of Chemical Technology & Biotechnology 2010, 85, 674–686. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).