Submitted:
30 October 2023
Posted:
31 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pain and the Gut Microbiome
3. Surgical Operation and the Microbiome
4. Probiotics in post-operative pain management
4.1. Probiotics in relation to the inflammation-induced pain of surgical trauma
4.2. Probiotics in relation to gut distension-induced visceral pain
5. Discussion - Conclusions
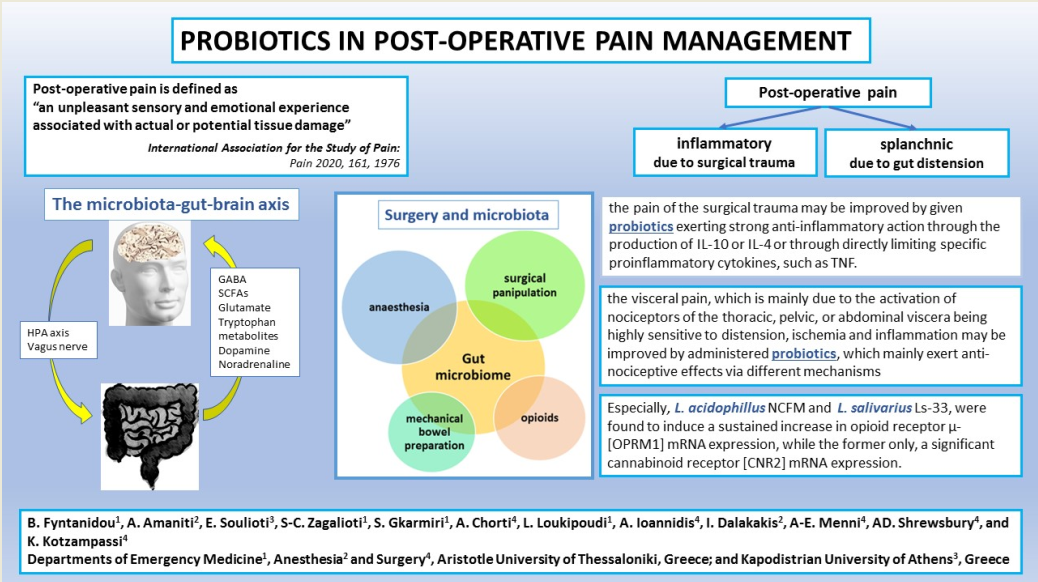
- the pain of the surgical trauma on the abdominal wall which is of an inflammatory etiology may be improved by given probiotics exerting strong anti-inflammatory action through the production of IL-10 or IL-4 or through directly limiting specific pro-inflammatory cytokines, such as TNF. Such benefits have been recognized after treatments mainly with Lactiplantibacillus plantarum, and to a lesser extent with L. acidophilus LA-5, L. rhamnosus GG ATCC 53103 and UBLR-58, L. fermentum SGL10, L. brevis GQ4237768, SGL 12, and CECT7480, L. paracasei SGL04, and MSMC39, B. longum UBBL-64 and Reuter and L. casei Shirota. Additionally, Lactobacillus spp., B. dentium and Bifidobacterium spp. are able to modify pain signaling by producing GABA, the most important inhibitory neurotransmitter.
- the visceral pain, which is mainly due to the activation of nociceptors of the thoracic, pelvic, or abdominal organs being extremely sensitive to distension, tissue ischemia and inflammation may be improved by administered probiotics, which mainly exert anti-nociceptive effects via different mechanisms: L. plantarum PS128, L. acidophilus NCFM, L. rhamnosus GG ATCC53103, L. reuteri DSM 17938, L. paracasei, B. infantis 35624, B. longum and L. helveticus in combination, Bifidobacterium lactis CNCM I-2494 and Lactococcus lactis CNCM I-1631 in combination, and the less known L. farciminis, Roseburia hominis, a species of butyrate-producing Lachnospiraceae family, and Faecalibacterium prausnitzii.
- Finally, particular mention must be made of the extraordinary action of L. acidophillus NCFM and of L. salivarius Ls-33, which induce a sustained increase in opioid receptor μ- [OPRM1] mRNA expression, while the former only, also induce significant cannabinoid receptor [CNR2] mRNA expression.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Astyrakaki, E.; Papaioannou, A.; Askitopoulou, H. References to anesthesia, pain, and analgesia in the Hippocratic Collection. Anesth Analg 2010, 110, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.wikidoc.org/index.
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Backhed, F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat Med 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu Rev Med 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mazmanian, S.K. Microbiota-brain axis: Context and causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr Med Chem 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Felice, V.D.; O'Mahony, S.M. The microbiome and disorders of the central nervous system. Pharmacol Biochem Behav 2017, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth 2019, 123, 637–654. [Google Scholar] [CrossRef]
- Alizadeh, N.; Naderi, G.; Kahrizi, M.S.; Haghgouei, T.; Mobed, A.; Shah-Abadi, M.E. Microbiota-Pain Association; Recent Discoveries and Research Progress. . Curr Microbiol. 2022, 80, 29. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The microbiota-gut-brain axis: Focus on the fundamental communication pathways. Prog Mol Biol Transl Sci 2020, 176, 43–110. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.; Fernández-Ciganda, S.; González Revello, Á.; Hirigoyen, D.; Martínez, M.; Scorza, C.; Zunino, P. Probiotic potential of GABA-producing lactobacilli isolated from Uruguayan artisanal cheese starter cultures. J Appl Microbiol. 2022, 133, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Attar, N. Good for the gut, good for the brain. Nature Reviews Microbiology 2016, 14, 269–269. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int J Tryptophan Res 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Lagomarsino, V.N.; Kostic, A.D.; Chiu, I.M. Mechanisms of microbial-neuronal interactions in pain and nociception. Neurobiol Pain 2021, 9, 100056. [Google Scholar] [CrossRef]
- Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Liang, Y.; Cui, L.; Gao, J.; Zhu, M.; Zhang, Y.; Zhang, H.L. Gut Microbial Metabolites in Parkinson's Disease: Implications of Mitochondrial Dysfunction in the Pathogenesis and Treatment. Mol Neurobiol 2021, 58, 3745–3758. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.J.; Li, Y.; Han, Y.; Zhou, D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J Clin Cases 2022, 10, 1754–1763. [Google Scholar] [CrossRef]
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J Psychiatr Res 2015, 61, 114–121. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, L.; Machaj, F.; Goracy, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Wendisch, V.F.; Jorge, J.M.P.; Perez-Garcia, F.; Sgobba, E. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J Microbiol Biotechnol 2016, 32, 105. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Hashimoto, K.I.; Sawada, Y.; Sokabe, M.; Kawasaki, H.; Martinac, B. Corynebacterium glutamicum mechanosensitive channels: towards unpuzzling "glutamate efflux" for amino acid production. Biophys Rev 2018, 10, 1359–1369. [Google Scholar] [CrossRef]
- Zareian, M.; Ebrahimpour, A.; Bakar, F.A.; Mohamed, A.K.S.; Forghani, B.; Ab-Kadir, M.S.B.; Saari, N. A glutamic acid-producing lactic acid bacteria isolated from Malaysian fermented foods. Int J Mol Sci 2012, 13, 5482–5497. [Google Scholar] [CrossRef] [PubMed]
- Neunlist, M.; Michel, K.; Reiche, D.; Dobreva, G.; Huber, K.; Schemann, M. Glycine activates myenteric neurones in adult guinea-pigs. J Physiol. 2001, 536, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Caputi, V.; Marsilio, I.; Filpa, V.; Cerantola, S.; Orso, G.; Bistoletti, M.; Paccagnella, N.; De Martin, S.; Montopoli, M.; Dall'Acqua, S.; et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol 2017, 174, 3623–3639. [Google Scholar] [CrossRef] [PubMed]
- Gronier, B.; Savignac, H.M.; Di Miceli, M.; Idriss, S.M.; Tzortzis, G.; Anthony, D.; Burnet, P.W.J. Increased cortical neuronal responses to NMDA and improved attentional set-shifting performance in rats following prebiotic (B-GOS((R))) ingestion. Eur Neuropsychopharmacol 2018, 28, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lin, W.J.; Salton, S.R. Role of a VGF/BDNF/TrkB Autoregulatory Feedback Loop in Rapid-Acting Antidepressant Efficacy. J Mol Neurosci 2019, 68, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Minerbi, A.; Shen, S. Gut Microbiome in Anesthesiology and Pain Medicine. Anesthesiology 2022, 137, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J Nutr Biochem 2021, 95, 108631. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.C.; O'Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci Ther 2016, 22, 102–117. [Google Scholar] [CrossRef] [PubMed]
- O' Mahony, S.M.; Dinan, T.G.; Cryan, J.F. The gut microbiota as a key regulator of visceral pain. Pain 2017, 158 Suppl 1, S19–S28. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Moloney, R.D.; Johnson, A.C.; Vicario, M. Mechanisms of Stress-Induced Visceral Pain: Implications in Irritable Bowel Syndrome. J Neuroendocrinol 2016, 28. [Google Scholar] [CrossRef]
- Tap, J.; Derrien, M.; Tornblom, H.; Brazeilles, R.; Cools-Portier, S.; Dore, J.; Storsrud, S.; Le Neve, B.; Ohman, L.; Simren, M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017, 152, 111–123. [Google Scholar] [CrossRef]
- Mars, R.A.T.; Yang, Y.; Ward, T.; Houtti, M.; Priya, S.; Lekatz, H.R.; Tang, X.; Sun, Z.; Kalari, K.R.; Korem, T.; et al. Longitudinal Multi-omics Reveals Subset-Specific Mechanisms Underlying Irritable Bowel Syndrome. Cell 2020, 182, 1460–1473. [Google Scholar] [CrossRef]
- Maeda, T.; Kishioka, S. PPAR and Pain. Int Rev Neurobiol 2009, 85, 165–177. [Google Scholar]
- Galiazzo, G.; De Silva, M.; Giancola, F.; Rinnovati, R.; Peli, A.; Chiocchetti, R. Cellular distribution of cannabinoid-related receptors TRPV1, PPAR-gamma, GPR55 and GPR3 in the equine cervical dorsal root ganglia. Equine Vet J 2021, 54, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of active, inactive, and derivatives of Akkermansia muciniphila on the expression of the endocannabinoid system and PPARs genes. Sci Rep 2022, 12, 10031. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci U S A 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, D.A.; Wang, H.; Polackwich, A.S.; Tucky, B.; Altemus, J.; Eng, C. Analysis of Gut Microbiome Reveals Significant Differences between Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls. J Urol. 2016, 196, 435–441. [Google Scholar] [CrossRef]
- Brenner, D.; Cherry, P.; Switzer, T.; Butt, I.; Stanton, C.; Murphy, K.; McNamara, B.; Iohom, G.; O'Mahony, S.M.; Shorten, G. Pain after upper limb surgery under peripheral nerve block is associated with gut microbiome composition and diversity. Neurobiol Pain 2021, 10, 100072. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. . Arthritis Rheumatol 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Puchimada, B.; Kadouri, D.; Zusman, T.; Javed, F.; Eliav, E. The anti-nociceptive effects of Porphyromonas gingivalis lipopolysaccharide. . Arch Oral Biol. 2019, 102. [Google Scholar] [CrossRef]
- Cruz-Aguliar, R.M.; Wantia, N.; Clavel, T.; Vehreschild, M.J.G.T.; Buch, T.; Bajbouj, M.; Haller, D.; Busch, D.; Schmid, R.M.; Stein-Thoeringer, C.K. An Open-Labeled Study on Fecal Microbiota Transfer in Irritable Bowel Syndrome Patients Reveals Improvement in Abdominal Pain Associated with the Relative Abundance of Akkermansia Muciniphila. . Digestion. 2019, 100, 127–138. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Kolios, G.; Manousou, P.; Kazamias, P.; Paramythiotis, D.; Papavramidis, T.; Heliadis, S.; Kouroumalis, E.; Eleftheriadis, E. Oxidative stress due to anesthesia and surgical trauma: importance of early enteral nutrition. . Mol Nutr Food Res. 2009, 53, 770–779. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. . Dig Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Bolton, L. Feed Abdominal Surgery Patients to Improve Outcomes. . Wounds. 2021, 33, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sindberg, G.; Wang, F.; Meng, J.; Sharma, U.; Zhang, L.; Dauer, P.; Chen, C.; Dalluge, J.; Johnson, T.; et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 2016, 9, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.J.; Alverdy, J.C. Influence of the Microbiome on Anastomotic Leak. Clin Colon Rectal Surg 2021, 34, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Salonen, A.; Salojarvi, J.; Ritari, J.; Immonen, O.; Marciani, L.; Gowland, P.; Hoad, C.; Garsed, K.; Lam, C.; et al. Effects of bowel cleansing on the intestinal microbiota. Gut 2015, 64, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, G.; Kotzampassi, K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol 2017, 30, 45–53. [Google Scholar] [CrossRef]
- Gorkiewicz, G.; Thallinger, G.G.; Trajanoski, S.; Lackner, S.; Stocker, G.; Hinterleitner, T.; Gully, C.; Hogenauer, C. Alterations in the colonic microbiota in response to osmotic diarrhea. PLoS One 2013, 8, e55817. [Google Scholar] [CrossRef]
- Harrell, L.; Wang, Y.; Antonopoulos, D.; Young, V.; Lichtenstein, L.; Huang, Y.; Hanauer, S.; Chang, E. Standard colonic lavage alters the natural state of mucosal-associated microbiota in the human colon. PLoS One 2012, 7, e32545. [Google Scholar] [CrossRef]
- Watanabe, M.; Murakami, M.; Nakao, K.; Asahara, T.; Nomoto, K.; Tsunoda, A. Randomized clinical trial of the influence of mechanical bowel preparation on faecal microflora in patients undergoing colonic cancer resection. Br J Surg 2010, 97, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Toscano, M.; De Grandi, R.; Casini, V.; Pace, F. Persisting changes of intestinal microbiota after bowel lavage and colonoscopy. . Eur J Gastroenterol Hepatol 2016, 28, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut microbiota, dysbiosis and colon lavage. Dig Liver Dis 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, L.; Jin, D.; Wu, X.; Long, B. General anesthesia bullies the gut: a toxic relationship with dysbiosis and cognitive dysfunction. Psychopharmacology (Berl). 2022, 239, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Serrano, M.; Gerónimo-Pardo, M.; Martínez-Monsalve, A.; Crespo-Sánchez, M.D. Antibacterial effect of sevoflurane and isoflurane. Rev Esp Quimioter 2017, 30, 84–89. [Google Scholar] [PubMed]
- Koutsogiannaki, S.; Schaefers, M.M.; Okuno, T.; Ohba, M.; Yokomizo, T.; Priebe, G.P.; DiNardo, J.A.; Sulpicio, S.G.; Yuki, K. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol Sci 2017, 156, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.; Koutsogiannaki, S.; Schaefers, M.; Babazada, H.; Liu, R.; Yuki, K. The Differential Effects of Anesthetics on Bacterial Behaviors. PLoS One 2017, 12, e0170089. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. . Nat Rev Gastroenterol Hepatol 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Raybould, H.E. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci 2010, 153, 41–46. [Google Scholar] [CrossRef]
- Mazzotta, E.; Villalobos-Hernandez, E.C.; Fiorda-Diaz, J.; Harzman, A.; Christofi, F.L. Postoperative Ileus and Postoperative Gastrointestinal Tract Dysfunction: Pathogenic Mechanisms and Novel Treatment Strategies Beyond Colorectal Enhanced Recovery After Surgery Protocols. Front Pharmacol 2020, 11, 583422. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol 2021, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis 2020, 11, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ding, Y.; Wang, L.; Xiao, Y. Gut microbiome improves postoperative cognitive function by decreasing permeability of the blood-brain barrier in aged mice. Brain Res Bull 2020, 164, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, X.; Wu, H. Juvenile Rats Show Altered Gut Microbiota After Exposure to Isoflurane as Neonates. Neurochem Res. 2019, 44, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhang, Z.; Guo, N.; Li, X.; Yang, M.; Peng, Y.; Ma, X.; Yu, K.; Wang, C. Effects of Sevoflurane Inhalation Anesthesia on the Intestinal Microbiome in Mice. Front Cell Infect Microbiol 2021, 11, 633527. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, Z.; Han, C.; Chen, L.; Zheng, X.; Yu, K.; Zhang, Z.; Wang, C. Effects of continuous intravenous infusion of propofol on intestinal flora in rats. Biomed Pharmacother 2021, 134, 111080. [Google Scholar] [CrossRef] [PubMed]
- Liufu, N.; Liu, L.; Shen, S.; Jiang, Z.; Dong, Y.; Wang, Y.; Culley, D.; Crosby, G.; Cao, M.; Shen, Y.; et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging (Albany NY) 2020, 12, 1965–1986. [Google Scholar] [CrossRef]
- Yang, S.; Gu, C.; Mandeville, E.T.; Dong, Y.; Esposito, E.; Zhang, Y.; Yang, G.; Shen, Y.; Fu, X.; Lo, E.H.; et al. Anesthesia and Surgery Impair Blood-Brain Barrier and Cognitive Function in Mice. Front Immunol 2017, 8, 902. [Google Scholar] [CrossRef]
- Ren, Q.; Peng, M.; Dong, Y.; Zhang, Y.; Chen, M.; Yin, N.; Marcantonio, E.R.; Xie, Z. Surgery plus anesthesia induces loss of attention in mice. Front Cell Neurosci 2015, 9, 346. [Google Scholar] [CrossRef]
- Miao, H.; Dong, Y.; Zhang, Y.; Zheng, H.; Shen, Y.; Crosby, G.; Culley, D.J.; Marcantonio, E.R.; Xie, Z. Anesthetic Isoflurane or Desflurane Plus Surgery Differently Affects Cognitive Function in Alzheimer's Disease Transgenic Mice. Mol Neurobiol 2018, 55, 5623–5638. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Zhang, C.; Dong, Y.; Zhang, Y.; Nakazawa, H.; Kaneki, M.; Zheng, H.; Shen, Y.; Marcantonio, E.R.; Xie, Z. Battery of behavioral tests in mice to study postoperative delirium. Sci Rep 2016, 6, 29874. [Google Scholar] [CrossRef]
- Rodino-Janeiro, B.K.; Alonso-Cotoner, C.; Pigrau, M.; Lobo, B.; Vicario, M.; Santos, J. Role of Corticotropin-releasing Factor in Gastrointestinal Permeability. J Neurogastroenterol Motil 2015, 21, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Matzaras, R.; Anagnostou, N.; Nikopoulou, A.; Tsiakas, I.; Christaki, E. The Role of Probiotics in Inflammation Associated with Major Surgery: A Narrative Review. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Agnes, A.; Puccioni, C.; D'Ugo, D.; Gasbarrini, A.; Biondi, A.; Persiani, R. The gut microbiota and colorectal surgery outcomes: facts or hype? A narrative review. BMC Surg 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat Immunol 2020, 21, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu Rev Pathol 2020, 15, 493–518. [Google Scholar] [CrossRef] [PubMed]
- Defaye, M.; Gervason, S.; Altier, C.; Berthon, J.Y.; Ardid, D.; Filaire, E.; Carvalho, F.A. Microbiota: a novel regulator of pain. J Neural Transm (Vienna) 2020, 127, 445–465. [Google Scholar] [CrossRef]
- Morowitz, M.J.; Babrowski, T.; Carlisle, E.M.; Olivas, A.; Romanowski, K.S.; Seal, J.B.; Liu, D.C.; Alverdy, J.C. The human microbiome and surgical disease. Ann Surg 2011, 253, 1094–1101. [Google Scholar] [CrossRef]
- Kotzampassi, K. What Surgeon Should Know about Probiotics. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Ohigashi, S.; Sudo, K.; Kobayashi, D.; Takahashi, T.; Nomoto, K.; Onodera, H. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg. 2013, 17, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, N.; Dang, Q.; Liu, L.; Wang, L.; Li, H.; Han, X. Exploring the roles of intestinal flora in enhanced recovery after surgery. iScience 2023, 26, 105959. [Google Scholar] [CrossRef] [PubMed]
- Lederer, A.K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: are surgical complications related to the gut flora? A systematic review. BMC Surg 2017, 17, 125. [Google Scholar] [CrossRef]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Tabata, S.; et al. Effects of perioperative synbiotic treatment on infectious complications, intestinal integrity, and fecal flora and organic acids in hepatic surgery with or without cirrhosis. JPEN J Parenter Enteral Nutr. 2011, 35, 317–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery - a double-blind study. Aliment Pharmacol Ther 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Lu, H.; He, J.; Wu, Z.; Xu, W.; Zhang, H.; Ye, P.; Yang, J.; Zhen, S.; Li, L. Assessment of microbiome variation during the perioperative period in liver transplant patients: a retrospective analysis. Microb Ecol. 2013, 65, 781–791. [Google Scholar] [CrossRef]
- Shogan, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. . Microbiome. 2014, 3, 35. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, C.; Tang, C.; Li, J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One 2012, 7, e42027. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, E.; Kotzampassi, K.; Botsios, D.; Tzartinoglou, E.; Farmakis, H.; Dadoukis, J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996, 10, 324–326. [Google Scholar] [CrossRef]
- Eleftheriadis, E.; Kotzampassi, K.; Papanotas, K.; Heliadis, N.; Sarris, K. Gut ischemia, oxidative stress, and bacterial translocation in elevated abdominal pressure in rats. . World J Surg. 1996, 20, 11–16. [Google Scholar] [CrossRef]
- Ioannidis, A.; Arvanitidis, K.; Filidou, E.; Valatas, V.; Stavrou, G.; Michalopoulos, A.; Kolios, G.; Kotzampassi, K. The Length of Surgical Skin Incision in Postoperative Inflammatory Reaction. JSLS 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.; Nicholson, G.; Hall, G. Endocrine and metabolic response to surgery. Continuing Education in Anaesthesia Critical Care & Pain 2004, 4, 144–147. [Google Scholar] [CrossRef]
- Schricker, T.; Lattermann, R. Perioperative catabolism. Can J Anaesth. 2015, 62, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.T.; Sperandio, V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol 2008, 6, 111–120. [Google Scholar] [CrossRef]
- Kendall, M.M.; Sperandio, V. What a Dinner Party! Mechanisms and Functions of Interkingdom Signaling in Host-Pathogen Associations. mBio 2016, 7, e01748. [Google Scholar] [CrossRef] [PubMed]
- Biaggini, K.; Barbey, C.; Borrel, V.; Feuilloley, M.; Déchelotte, P.; Connil, N. The pathogenic potential of Pseudomonas fluorescens MFN1032 on enterocytes can be modulated by serotonin, substance P and epinephrine. . Arch Microbiol. 2015, 197, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 2018, 8, 3596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, J.; Ban, Y.; Jalodia, R.; Chupikova, I.; Fernandez, I.; Brito, N.; Sharma, U.; Abreu, M.T.; Ramakrishnan, S.; et al. Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A 2019, 116, 13523–13532. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Menni, A.; Moysidis, M.; Tzikos, G.; Stavrou, G.; Tsetis, J.K.; Shrewsbury, A.D.; Filidou, E.; Kotzampassi, K. Looking for the Ideal Probiotic Healing Regime. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Xiang, Q.; Tang, X.; Zhang, Q.; Liu, X.; Zhao, J.; Cui, S.; Zhang, H. Lactobacillus reuteri CCFM1175 and Lactobacillus paracasei CCFM1176 Could Prevent Capsaicin-Induced Ileal and Colonic Injuries.. Probiotics Antimicrob Proteins. 2023, 15:797-812. [CrossRef]
- Meenakshi, S.; Santhanakumar, R. The role of probiotics as wound healers: an overall view. . J Wound Care 2023, 32, 318–328. [Google Scholar] [CrossRef]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A Four-Probiotics Regimen Reduces Postoperative Complications After Colorectal Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. . World J Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, O.; Chatzakis, C.; Tirta, M.; Anestiadou, E.; Zapsalis, K.; Symeonidis, S.; Bitsianis, S.; Kotidis, E.; Pramateftakis, M.G.; Mantzoros, I.; et al. The Efficacy of Probiotics, Prebiotics, and Synbiotics in Patients Who Have Undergone Abdominal Operation, in Terms of Bowel Function Post-Operatively: A Network Meta-Analysis. J Clin Med 2023, 12. [Google Scholar] [CrossRef]
- Tang, G.; Huang, W.; Tao, J.; Wei, Z. Prophylactic effects of probiotics or synbiotics on postoperative ileus after gastrointestinal cancer surgery: A meta-analysis of randomized controlled trials. PLoS One 2022, 17, e0264759. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Li, J.; Feng, M.; Zhang, Y.; Yao, W.; Zhang, C.; Wan, L. Celastrol attenuates incision-induced inflammation and pain associated with inhibition of the NF-κB signalling pathway via SARM. . Life Sci. 2018, 205, 136–144. [Google Scholar] [CrossRef]
- Kim, M.K.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Choi, G.J.; Shin, H.Y.; Kim, K.S. Antinociceptive and anti-inflammatory effects of ginsenoside Rf in a rat model of incisional pain. J Ginseng Res 2018, 42, 183–191. [Google Scholar] [CrossRef]
- Sommer, C.; Kress, M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. . Neurosci Lett. 2004, 361, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Brennan, T.J. Comparison of skin incision vs. skin plus deep tissue incision on ongoing pain and spontaneous activity in dorsal horn neurons. Pain 2009, 144, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Takeuchi, H.; Fujii, K.; Shiraishi, N.; Adachi, Y.; Kitano, S. Length of laparotomy incision and surgical stress assessed by serum IL-6 level. Injury. 2006, 37, 247–251. [Google Scholar] [CrossRef]
- Ronchetti, S.; Migliorati, G.; Delfino, D.V. Association of inflammatory mediators with pain perception. . Biomed Pharmacother. 2017, 96, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front Immunol 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Penzkover, K.R.; Soderquist, R.G.; Mahoney, M.J. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation 2012, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Tanga, F.Y.; Nutile-McMenemy, N.; DeLeo, J.A. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. . Proc Natl Acad Sci U S A. 2005, 102, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. . Biotechnol Appl Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S. ; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight Into the Beneficial Role of Lactiplantibacillus plantarum Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front Pharmacol 2021, 12, 728614. [Google Scholar] [CrossRef]
- Panagiotou, D.; Filidou, E.; Gaitanidou, M.; Tarapatzi, G.; Spathakis, M.; Kandilogiannakis, L.; Stavrou, G.; Arvanitidis, K.; Tsetis, J.K.; Gionga, P.; et al. Role of Lactiplantibacillus plantarum UBLP-40, Lactobacillus rhamnosus UBLR-58 and Bifidobacterium longum UBBL-64 in the Wound Healing Process of the Excisional Skin. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Tarapatzi, G.; Filidou, E.; Kandilogiannakis, L.; Spathakis, M.; Gaitanidou, M.; Arvanitidis, K.; Drygiannakis, I.; Valatas, V.; Kotzampassi, K.; Manolopoulos, V.G.; et al. The Probiotic Strains Bifidomicronbacterium lactis, Lactobacillus acidophilus, Lactiplantibacillus plantarum and Saccharomyces boulardii Regulate Wound Healing and Chemokine Responses in Human Intestinal Subepithelial Myofibroblasts. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci Rep 2020, 10, 11572. [Google Scholar] [CrossRef]
- Chen, X.; Thibeault, S.L. Role of tumor necrosis factor-alpha in wound repair in human vocal fold fibroblasts. Laryngoscope 2010, 120, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Bastien, P.; Ovigne, J.M.; Kermici, M.; Courchay, G.; Chevalier, V.; Breton, L.; Castiel-Higounenc, I. Bifidobacterium longum lysate, a new ingredient for reactive skin. Exp Dermatol 2010, 19, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Banjonjit, S.; Taweechotipatr, M.; Rungsiyanont, S. Effect of probiotic Lactobacillus paracasei on tumor necrosis factor-alpha level in gingival crevicular fluid of patients undergoing impacted third molar removal. J Oral Sci 2022, 64, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ferrés-Amat, E.; Espadaler-Mazo, J.; Calvo-Guirado, J.L.; Ferrés-Amat, E.; Mareque-Bueno, J.; Salavert, A.; Aguiló-García, M.; Moreno-Centeno, J.; Ferrés-Padró, E. Probiotics diminish the post-operatory pain following mandibular third molar extraction: a randomised double-blind controlled trial (pilot study). Benef Microbes. 2020, 11, 631–639. [Google Scholar] [CrossRef]
- Lei, M.; Guo, C.; Wang, Y.; Hua, L.; Xue, S.; Yu, D.; Zhang, C.; Wang, D. Oral administration of probiotic Lactobacillus casei Shirota relieves pain after single rib fracture: a randomized double-blind, placebo-controlled clinical trial. Asia Pac J Clin Nutr 2018, 27, 1252–1257. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, C.; Wang, J.; Guo, Q.; Zou, W. Oral Lactobacillus reuteri LR06 or Bifidobacterium BL5b supplement do not produce analgesic effects on neuropathic and inflammatory pain in rats. Brain Behav 2019, 9, e01260. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Versalovic, J. Probiotics-host communication: Modulation of signaling pathways in the intestine. . Gut Microbes. 2010, 1, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Drabik, K.A.; Waypa, T.S.; Musch, M.W.; Alverdy, J.C.; Schneewind, O.; Chang, E.B.; Petrof, E.O. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 2006, 290, C1018–C1030. [Google Scholar] [CrossRef]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Effect of Probiotics Lactobacillus acidophilus and Lacticaseibacillus rhamnosus on Antibacterial Response Gene Transcription of Human Peripheral Monocytes. Probiotics Antimicrob Proteins. 2023, 15, 264–274. [Google Scholar] [CrossRef]
- Du, X.; Hao, H.; Yang, Y.; Huang, S.; Wang, C.; Gigout, S.; Ramli, R.; Li, X.; Jaworska, E.; Edwards, I.; et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 2017, 127, 1741–1756. [Google Scholar] [CrossRef]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain - a randomised clinical study. Aliment Pharmacol Ther 2014, 40, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Agostini, S.; Goubern, M.; Tondereau, V.; Salvador-Cartier, C.; Bezirard, V.; Lévèque, M.; Keränen, H.; Theodorou, V.; Bourdu-Naturel, S.; Goupil-Feuillerat, N.; et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil. 2012, 24, 376–e172. [Google Scholar] [CrossRef] [PubMed]
- Darbaky, Y.; Evrard, B.; Patrier, S.; Falenta, J.; Garcin, S.; Tridon, A.; Dapoigny, M.; Silberberg, C.; Nivoliez, A.; Diop, L. Oral probiotic treatment of Lactobacillus rhamnosus Lcr35® prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. J Appl Microbiol. 2017, 122, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kannampalli, P.; Pochiraju, S.; Chichlowski, M.; Berg, B.M.; Rudolph, C.; Bruckert, M.; Miranda, A.; Sengupta, J.N. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol Motil. 2014, 26, 1694–1704. [Google Scholar] [CrossRef]
- Roman, P.; Abalo, R.; Marco, E.M.; Cardona, D. Probiotics in digestive, emotional, and pain-related disorders. Behav Pharmacol. 2018, 29, 103–119. [Google Scholar] [CrossRef]
- McVey Neufeld, K.A.; Strain, C.R.; Pusceddu, M.M.; Waworuntu, R.V.; Manurung, S.; Gross, G.; Gerry, M.M.; Hoban, A.E.; Murphy, K.; Stanton, C.; et al. Lactobacillus rhamnosus GG soluble mediators ameliorate early life stress-induced visceral hypersensitivity and changes in spinal cord gene expression. Neuronal Signal 2020, 4, NS20200007. [Google Scholar] [CrossRef]
- Vanhoutvin, S.A.; Troost, F.J.; Kilkens, T.O.; Lindsey, P.J.; Hamer, H.M.; Jonkers, D.M.; Venema, K.; Brummer, R.J. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. 2009, 21, 952–e976. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Theodorou, V.; Tompkins, T.A. Bifidobacterium longum and Lactobacillus helveticus Synergistically Suppress Stress-related Visceral Hypersensitivity Through Hypothalamic-Pituitary-Adrenal Axis Modulation. J Neurogastroenterol Motil 2018, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Eutamene, H.; Houdeau, E.; Bueno, L.; Fioramonti, J.; Theodorou, V. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol Motil. 2009, 21, 567–573. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Han, W.; Lamine, F.; Eutamene, H.; Fioramonti, J.; Bueno, L.; Theodorou, V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut 2006, 55, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Wang, Y.P.; Yen, H.F.; Liu, P.Y.; Tzeng, W.J.; Tsai, C.F.; Lin, H.C.; Lee, F.Y.; Jeng, O.J.; Lu, C.L.; et al. Lactobacillus plantarum PS128 Ameliorated Visceral Hypersensitivity in Rats Through the Gut-Brain Axis. Probiotics Antimicrob Proteins. 2020, 12, 980–993. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Lashermes, A.; Gillet, M.; Meleine, M.; Gelot, A.; Eschalier, A.; Ardid, D.; Bermudez-Humaran, L.G.; Sokol, H.; et al. Anti-nociceptive effect of Faecalibacterium prausnitzii in non-inflammatory IBS-like models. Sci Rep 2016, 6, 19399. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- van Praagh, J.B.; de Goffau, M.C.; Bakker, I.S.; van Goor, H.; Harmsen, H.J.M.; Olinga, P.; Havenga, K. Mucus Microbiome of Anastomotic Tissue During Surgery Has Predictive Value for Colorectal Anastomotic Leakage. Ann Surg 2019, 269, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Leclerc, M.; Martin, R.; Chain, F.; Lenoir, M.; Raguideau, S.; Hudault, S.; Bridonneau, C.; Northen, T.; Bowen, B.; et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio 2015, 6. [Google Scholar] [CrossRef]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermudez-Humaran, L.G.; Langella, P. The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm Bowel Dis 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, A.V.; Söderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef]
- Laval, L.; Martin, R.; Natividad, J.N.; Chain, F.; Miquel, S.; Desclee de Maredsous, C.; Capronnier, S.; Sokol, H.; Verdu, E.F.; van Hylckama Vlieg, J.E.; et al. Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. TRPV1: a new target for treatment of visceral pain in IBS? Gut 2008, 57, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.; Shenoy, M.; Medley, D.; Naniwadekar, A.; Pasricha, P.J. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 2007, 132, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Boesmans, W.; Owsianik, G.; Tack, J.; Voets, T.; Vanden Berghe, P. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol 2011, 162, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Vay, L.; Gu, C.; McNaughton, P.A. The thermo-TRP ion channel family: properties and therapeutic implications. Br J Pharmacol 2012, 165, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Perez-Burgos, A.; Wang, L.; McVey Neufeld, K.A.; Mao, Y.K.; Ahmadzai, M.; Janssen, L.J.; Stanisz, A.M.; Bienenstock, J.; Kunze, W.A. The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J Physiol 2015, 593, 3943–3957. [Google Scholar] [CrossRef] [PubMed]
- Zholos, A.V.; Dryn, D.O.; Melnyk, M.I. General anaesthesia-related complications of gut motility with a focus on cholinergic mechanisms, TRP channels and visceral pain. Front Physiol 2023, 14, 1174655. [Google Scholar] [CrossRef]
- Melnyk, M.I.; Dryn, D.O.; Al Kury, L.T.; Dziuba, D.O.; Zholos, A.V. Suppression of mI(CAT) in Mouse Small Intestinal Myocytes by General Anaesthetic Ketamine and its Recovery by TRPC4 Agonist (-)-englerin A. Front Pharmacol 2020, 11, 594882. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. . Pediatrics. 2010, 126, e526–e533. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Pautasso, S.; Palumeri, E.; Tullio, V.; Roana, J.; Silvestro, L.; Oggero, R. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr. 2004, 93, 825–829. [Google Scholar] [CrossRef]
- de Weerth, C.; Fuentes, S.; de Vos, W.M. Crying in infants: on the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013, 4, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Garro, M.; Montanari, P.; Galliano, I.; Bergallo, M. Crying Time and RORgamma/FOXP3 Expression in Lactobacillus reuteri DSM17938-Treated Infants with Colic: A Randomized Trial. J Pediatr 2018, 192, 171–177. [Google Scholar] [CrossRef]
- Verdú, E.F.; Bercík, P.; Bergonzelli, G.E.; Huang, X.X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthésy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology. 2004, 127, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.; Altomare, A.; Stasi, E.; Marignani, M.; Severi, C.; Alloni, R.; Dicuonzo, G.; Morelli, L.; Coppola, R.; Cicala, M. Effect of acute mucosal exposure to Lactobacillus rhamnosus GG on human colonic smooth muscle cells. J Clin Gastroenterol. 2008, 42, S185–S190. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Wang, L.; Forsythe, P.; Goettsche, G.; Mao, Y.; Wang, Y.; Tougas, G.; Bienenstock, J. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut 2006, 55, 191–196. [Google Scholar] [CrossRef]
- Duncker, S.C.; Kamiya, T.; Wang, L.; Yang, P.; Bienenstock, J. Probiotic Lactobacillus reuteri alleviates the response to gastric distension in rats. J Nutr 2011, 141, 1813–1818. [Google Scholar] [CrossRef]
- McKernan, D.P.; Fitzgerald, P.; Dinan, T.G.; Cryan, J.F. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil. 2010, 22, 1029–1035. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O'Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- OMahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O'Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005, 128, 541–551. [Google Scholar] [CrossRef]
| Probiotics | Action | Type of operation | Type of study |
|---|---|---|---|
| L. plantarum (128,135) | ↑ IL-10 and TGF1 ↓ TNF |
1. dorsal wound 2. mandibular 3rd molar excision |
1. diabetic rats 2. humans |
| L. rhamnosus UBLR-58 (130-132) | ↑ IL-10 | Skin Wound healing | Rats and humans |
|
L. acidophilus LA-5 (130-132) |
↑ IL-10 and MAPKs | Skin Wound healing | Rats and humans |
| L. fermentum SGL10 (130-132) | ↑ IL-10 and TGF1 | Skin Wound healing | Rats and humans |
| L. brevis GQ4237768 (130-132, 135) | ↑ IL-10 ↓ TNF |
mandibular 3rd molar excision | Humans |
| L. brevis SGL 12 (130-132) | ↑ IL-10 | Skin Wound healing | Rats and humans |
| L. paracasei SGL 04 (130-132, 135) | ↑ Increase of IL-10 | Skin Wound healing | Rats and humans |
| B. longum UBBL-64 (130-132) | ↑ Increase of IL-10 ↓ TNF |
mandibular 3rd molar excision | Humans |
| L. casei Shirota (137) | NA | Rib fracture pain modulation | Humans |
| L. rhamnosus GG ATCC 53103 (140-141) | MAPKs | Skin Wound healing | Rats |
| Lactobacillus spp (142) | GABA | Skin Wound healing | Rats |
| Bifidobacterium spp (142) | GABA | Skin Wound healing | Rats |
| B. dentium (142) | GABA | Skin Wound healing | Rats |
| Probiotics | Action | Type of operation |
|---|---|---|
| Experimental studies | ||
| L. acidophillus NCFM (143) | Decrease in μ-opioid receptor and cannabinoid receptor R2 | Colorectal distention Reduction of stress-induced visceral hypersensitivity and pain |
| L. salivarius Ls-33 (143) | Decrease in opioid receptor μ | Colorectal distention |
| L. rhamnosus Lcr35 (145) | IL-13/Th 17 immune activation | Colorectal distention |
| L. rhamnosus GG ATCC53103 (145) | NA | Colorectal distention |
| Roseburia hominis (151-152) | Butyrate activation | Reduction of stress-induced visceral hypersensitivity and pain |
| B. longum (153) | regulating glucocorticoid negative feedback on the HPA axis | Reduction of stress-induced visceral hypersensitivity and pain |
| L. helveticus (153) | regulating glucocorticoid negative feedback on the HPA axis | Reduction of stress-induced visceral hypersensitivity and pain |
| L. farciminis (154-155) | Fos downregulation | Reduction of stress-induced visceral hypersensitivity and pain |
| L. plantarum PS128 (156) | 5-HTP regulation and altering signaling in DRG fibres | Reduction of stress-induced visceral hypersensitivity and pain |
| Faecalibacterium prausnitzii (157,160-163) | intestinal epithelial barrier enhancement/ modulation of tight junctions in inflammation models | Reduction of stress-induced visceral hypersensitivity and pain |
| L. reuteri DSM 17938 (168,171) | TRPV1 channel antagonist modulation | Reduction of stress-induced visceral hypersensitivity and pain |
|
L. johnsonii, B. lactis, or B. longum (175) |
GF-1, COX-2, and PGE2 levels transformation | Reduction of muscle hypercontractility |
| L. rhamnosus JB-1 (177) | altering signaling in DRG fibres | Reduction of stress-induced visceral hypersensitivity and pain |
| B. infantis 35624 (179) | NA | Reduction of stress-induced visceral hypersensitivity and pain |
| Clinical studies | ||
| B. lactis CNCM I-2494 (144) | NA | Reduction of stress-induced visceral hypersensitivity and pain |
| Lactococcus lactis CNCM I-1631 (144) | NA | Reduction of stress-induced visceral hypersensitivity and pain |
| L. reuteri DSM 17938 (168,171) | TRPV1 channel antagonist modulation | Reduction of stress-induced visceral hypersensitivity and pain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





